Summary
Background
Azole resistance in Aspergillus fumigatus is an emerging problem and reported from all continents. As triazole antifungals are the mainstay of therapy in the management of invasive aspergillosis, azole‐resistant A fumigatus has become a major medical concern and with complicated clinical management.
Objective
Screening of environmental presence of azole‐resistant A fumigatus in Iran.
Methods
Compost from Northern Iran, collected between 2017 and 2018, was screened for the presence of azole‐resistant A fumigatus with azole‐containing agar. Phenotypic MICs were obtained from selected, molecularly confirmed isolates. cyp51A gene sequencing and genotyping of azole‐resistant isolates were done.
Results
Among 300 compost samples, three A fumigatus isolates had high voriconazole MICs (≥16 mg/L) and harboured the TR46/Y121F/T289A mutation in the cyp51A gene. Microsatellite typing of these isolates showed that two strains had the same allele across all nine examined microsatellite loci and were genotypically related to Indian azole‐resistant strains. The other isolate had a different genotype.
Conclusion
This is the first report of A fumigatus with TR46/Y121F/T289A mutation from the region. Monitoring and surveillance of antifungal susceptibility of clinical A fumigatus is warranted in Iran and elsewhere in the region.
Keywords: Aspergillus fumigatus, azole resistance, compost, TR34/L98H, TR46/Y121F/T289A
1. INTRODUCTION
Invasive aspergillosis due to azole‐resistant A fumigatus has become a major medical concern associated with high mortality in immunocompromised individuals.1, 2, 3 Azole resistance in A fumigatus emerges due to long‐term treatment with azole antifungals, but also extensive exposure of the fungus to azole compounds in the environment is a major driver of resistance selection.4, 5, 6, 7 Resistance to azole drugs in A fumigatus is mainly linked to multiple amino acid substitutions in the cyp51A gene. The most described cyp51A‐mediated resistance mechanism is a 34‐basepair (bp) sequence tandem repeat (TR34) in the promoter region of cyp51A gene combination with the L98H substitution followed by a double substitution combined with a 46 bp tandem duplication in the cyp51A promoter (TR46/Y121F/T289A).8, 9 These mutations lead to high‐level resistance to triazole antifungals of A fumigatus isolates from both azole‐naive and azole‐treated patients.10 Furthermore, several institutions report a rise in resistant strains found in environmental niches such as soil samples, paddy fields, aerial samples of hospitals and compost.11, 12, 13, 14, 15, 16, 17, 18, 19 Compost (decaying plant waste material) is an important source of A fumigatus. It is known that high concentrations of azole‐resistant A fumigatus spores are released during incomplete composting processes, especially when azole residues from agricultural waste are present.11, 13, 14, 15, 20, 21, 22, 23 Azole‐resistant A fumigatus with the TR34/L98H mutation in the environment has been reported earlier in Iran.24 Here, we report for the first time the presence of resistance due to the TR46/Y121F/T289A mutation.
2. MATERIAL AND METHODS
2.1. Sample collection and identification of azole resistent A fumigatus isolates
Three hundred compost samples from different regions of Iran (Mazandaran province (n = 200) and Tehran province (n = 100), located about 300 km apart, were collected during 2017‐2018. To recover A fumigatus strains, 1 cm2 compost was dissolved in 5 mL sterile saline solution containing Tween 40 (0.05%), vortexed, and allowed to settle. Briefly, according to a previously described protocol, for primary screening of azole‐resistant A fumigatus strains from the supernatant, 100 μL was plated on a Sabouraud dextrose agar plate (SDA; Difco), supplemented with 4 and 1 mg/L itraconazole and voriconazole, respectively, and incubated at 45°C for 72 hours in the dark.23 Identification of Aspergillus section Fumigati was performed based on both macroscopic and microscopic characteristics. Subsequently, molecular identification with DNA sequencing of the partial b‐tubulin gene, using TUB2a (5‐ TGACCCAGCAGATGTT‐3) and TUB2b (5‐GTTGTTGGGAATCCACTC‐3), was done.25
2.2. In vitro antifungal susceptibility testing
To detect azole‐resistant A fumigatus, colonies that grew on screening media supplemented with itraconazole and voriconazole and confirmed by DNA sequencing as A fumigatus were selected and evaluated with microbroth dilution minimum inhibitory concentration (MIC) testing using the Clinical and Laboratory Standards Institute document for filamentous fungi.26 Paecilomyces variotii (ATCC 22319) and Candida parapsilosis (ATCC 22019) were used as quality controls.
2.3. Detection of cyp51A gene mutations
Isolates with reduced susceptibility to voriconazole (MIC >2 µg/mL) were tested with a mixed‐format real‐time PCR assay as described before.27 Isolates with TR46/Y121F/T289A mutations were further confirmed by sequencing the cyp51A gene as described previously.28
2.4. Microsatellite typing
Genotyping of A fumigatus isolates was performed with a panel of nine short tandem repeats (STRs) loci (namely STRAf 2A, 2B, 2C, 3A, 3B, 3C, 4A, 4B and 4C), as previously described.29
Genetic relatedness between the environmental Iranian azole‐resistant TR46/Y121F/T289A isolates was compared with already barcoded clinical and environmental isolates from different countries (The Netherlands, India, Tanzania, France, Colombia, Ireland, China and Germany) using BioNumerics software version 7.6.1.(Applied Maths NV).
3. RESULTS
A total of 63 samples from both Mazandaran (n = 44) and Tehran province (n = 19) yielded A fumigatus on SDA supplemented with itraconazole and voriconazole. A total of 57 A fumigatus isolates had high MICs level of itraconazole (>8 mg/L) (55/57) or voriconazole (>2 mg/L) (51/57) by in vitro antifungal susceptibility testing. Exploring the mechanisms of resistance by mixed‐format real‐time PCR and sequencing of cyp51A and its promoter in these isolates showed that resistant isolates had different resistance mechanisms including forty‐four with TR34/L98H and three isolates with TR46/Y121F/T289A (1 isolate with TR46/Y121F/M172I/T289A/G448S and two isolates with TR46/Y121F/T289A mutations in the cyp51 region). All three isolates with TR46/Y121F/T289A mutation in cyp51A and high voriconazole MIC (≥16 mg/L) were isolated from Mazandaran province. Microsatellite analysis revealed two different genotypes among isolates harbouring TR46/Y121F/T289A. Two strains had the same alleles across all nine examined microsatellite loci and the other differed by seven loci (2C, 3A, 3B, 3C, 4A, 4B and 4C). For comparison of the genotype of these isolates with other isolates from different countries, a minimum‐spanning tree was constructed based on nine STRAf loci of different azole‐resistant isolates.29 Two Iranian A fumigatus TR46/Y121F/T289A isolates were related to resistant isolates originating from India while the third was genetically different from other strains (Figure 1).
Figure 1.
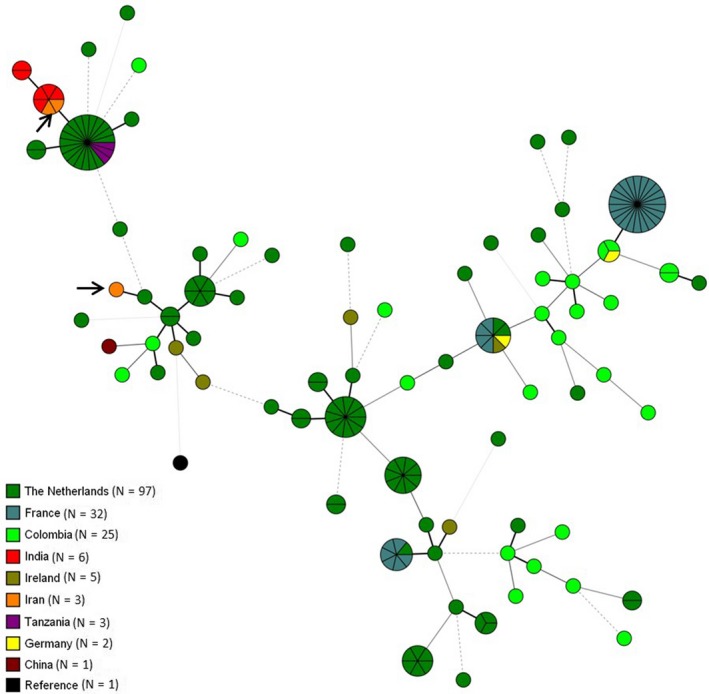
Minimum‐spanning tree showing the genetic relationship of A fumigatus genotypes harbouring TR46/Y121F/T289A mutation in cyp51A allele. Genotypic relationship of three Iranian isolates is illustrated by arrows
4. DISCUSSION
Azole resistance in A fumigatus due to TR34/L98H and more recent TR46/Y121F/T289A mutations in cyp51A has been described from wide geographical areas among both clinical and environmental isolates.30, 31 This is linked to the widespread use of azole fungicides in agriculture rather than the clinical use of antifungal drugs.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 19, 20, 21 The fungicide‐driven mutation TR46/Y121F/T289A has been first detected in the Netherlands in 2009.32 Since then, several reports have demonstrated that, particularly this mutation, corresponded to voriconazole resistance in A fumigatus in European, African, American and Asian countries. We report the presence of TR46/Y121F/T289A now also from Iran, where until now only TR34/L98H had been isolated.24, 33, 34, 35 This mutation, conferring voriconazole resistance, has been reported from both environmental (China,17, 21 Taiwan,13 United Kingdom,11 Colombia,12, 14 France,16, 36 Germany,37 India,19 the Netherlands32 and Tanzania18) and clinical sources (Spain,38, 39 United Kingdom,40 France,41 Portugal,42, 43 Argentina,44 Taiwan,45 Germany,46, 47, 48, 49 China,50, 51 Japan,52 United States,53, 54 Denmark,10 Belgium 7, 55, 56, 57 and the Netherlands7, 32, 58, 59, 60) (Table 1). All previous studies conducted in Iran for monitoring the mechanism of resistance among azole‐resistant A fumigatus in both clinical and environmental samples showed that the TR34/L98H mutation was reported with increasing frequency from 3.3% in 2013 to 6.6% in 2016.24, 34 Microsatellite typing of the current isolates showed that two A fumigatus isolates with TR46/Y121F/T289A isolates were related to resistant isolates from India in 2014. It is noteworthy that the first isolates with TR34/L98H in Iran in 2013 were also genotypically related to some resistant clinical and environmental TR34/L98H isolates from India and the Netherlands.24 It is anticipated that strains with the TR46/Y121F/T289A or TR34/L98H mutation will spread rapidly to other geographical regions especially due to the use of azole‐based agricultural fungicides which is a significant factor in the increase of multiple‐triazole‐resistant A fumigatus.4 In our study, most of the azole‐resistant A fumigatus isolated from Iranian compost were from Mazandaran province located in Northern Iran where agricultural activity and subsequently the usage of fungicides are generally higher than in other regions of Iran. Compost is a main ecological niche for A fumigatus, and azole residues have been reported to be present in commercial compost.20 Snelders et al61 showed that 86% of triazole‐resistant A fumigatus isolates recovered from environmental sources such as compost had the same resistance mechanism as found in clinical isolates. Compost is used widely in gardens and indoor plants in Iran, and has a key role in the exposure of azole‐resistant A fumigatus to susceptible hosts. Although CLSI and EUCAST have published standardised antifungal susceptibility testing methods to determine the in vitro efficacy of antifungal drugs, most clinical microbiology laboratories in Iran do not routinely perform antifungal susceptibility testing of Aspergillus isolates as recommended by ESCMID guidelines.62 Therefore, the true prevalence of resistance and mechanism of resistance in clinical A fumigatus isolates in Iran is unknown. Reports of clinical isolates harbouring this mutation are mainly limited to developed countries in Europe, East Asia and the USA, maybe because of the lack of routine antifungal susceptibility testing in less developed countries (Table 1). With this background in mind, the emergence of a new azole resistance mechanism in A fumigatus isolated from environmental sources in Iran is concerning as this mechanism has been associated with therapeutic failure with voriconazole, the first‐line treatment for invasive aspergillosis. Nevertheless, correlations between in vitro susceptibility results and clinical outcomes are not really estimated for A fumigatus. Compost used for planting surroundings of immunocompromised patient's houses could be a source of invasive aspergillosis due to azole‐resistant A fumigatus. 21, 22 Our study reinforces the importance of surveillance studies to monitor antifungal susceptibility of clinical isolates in Iran.
Table 1.
Reports of azole resistant Aspergillus fumigatus isolates carrying TR46/Y121F/T289A mutation
| Number | Country | Year of publishing | Number of environmental isolates | Number of clinical isolates |
|---|---|---|---|---|
| Present report | Iran | 2020 | 3 | – |
| 1 | China | 202021 | 6 | – |
| 2 | Taiwan | 201913 | 3 | – |
| 3 | Spain | 201938 | – | 1 |
| 4 | Belgium ‐ Netherlands | 20197 | – | 12 |
| 5 | United kingdom | 201911 | 6 | – |
| 6 | France | 201941 | – | 2 |
| 7 | Colombia | 201914 | 8 | – |
| 8 | Netherlands | 201858 | – | 5 |
| 9 | France | 201816 | 9 | – |
| 10 | Portugal | 201842 | – | 1 |
| 11 | Argentina | 201844 | – | 1 |
| 12 | Taiwan | 201845 | – | 3 |
| 13 | Portugal | 201843 | – | 1 |
| 14 | Germany | 201846 | – | 1 |
| 15 | Germany | 201747 | – | 1 |
| 16 | Belgium | 201755 | – | 4 |
| 17 | Colombia | 201712 | 17 | – |
| 18 | United kingdom | 201740 | – | 1 |
| 19 | France | 201736 | 21 | 1 |
| 20 | China | 201617 | 2 | – |
| 21 | Netherlands | 201659 | – | 4 |
| 22 | Japan | 201652 | – | 1 |
| 23 | China | 201650 | – | 1 |
| 24 | United States | 201653 | – | 2 |
| 25 | United States | 201554 | – | 1 |
| 26 | China | 201551 | – | 1 |
| 27 | Spain | 201539 | – | 1 |
| 28 | Germany | 201537 | 6 | – |
| 29 | France | 201541 | – | 2 |
| 30 | Germany | 201548 | – | 2 |
| 31 | Netherlands | 201560 | – | 1 |
| 32 | Belgium | 201456 | – | 1 |
| 33 | Denmark | 201410 | – | 1 |
| 34 | Germany | 201449 | – | 1 |
| 35 | Tanzania | 201418 | 4 | – |
| 36 | India | 201419 | 6 | – |
| 37 | Netherlands | 201332 | 14 | 21 |
| 38 | Belgium | 201257 | – | 1 |
CONFLICT OF INTEREST
JFM received grants from Pulmozyme and F2G. He has been a consultant to Scynexis and received speaker's fees from United Medical, TEVA and Gilead Sciences. The other authors report no conflicts of interest.
AUTHORS CONTRIBUTIONS
FA and HB conceived this study. MN, SK, MM, ZS and MLK were responsible for collection of the isolates and data acquisition. FA and YP did the molecular analysis. FA and JFM wrote the concept paper and all authors critically reviewed the manuscript prior to submission.
ETHICS APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as the research in this article related to micro‐organisms.
ACKNOWLEDGMENTS
The authors are grateful to Masume Farhadi, Miad Banaye Gholrizi and Fatemeh Barimani for their supportive help during our sampling. We thank Iman Haghani for excellent technical assistance and help with antifungal susceptibility testing. FA was the recipient of an ESCMID observership grant to visit ESCMID observership centre 58, CWZ.
Ahangarkani F, Puts Y, Nabili M, et al. First azole‐resistant Aspergillus fumigatus isolates with the environmental TR46/Y121F/T289A mutation in Iran. Mycoses. 2020;63:430–436. 10.1111/myc.13064
Funding information
This study was supported by National Institutes for Medical Research Development (NIMAD), Grant/Award Number: 982677; Mazandaran University of Medical Sciences, Sari, Iran, Grant/Award Number: 1352.
REFERENCES
- 1. Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: Can we retain the clinical use of mold‐active antifungal azoles? Clin Infect Dis. 2016;62:362‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus . Philos Trans R Soc Lond B Biol Sci. 2016;371:20150460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect. 2019;25:799‐806. [DOI] [PubMed] [Google Scholar]
- 4. Chowdhary A, Kathuria S, Xu J, Meis JF. Emergence of azole‐resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013;9:e1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Snelders E, Zwaan BJ, et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. MBio. 2017;8:e00791‐e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhary A, Meis JF. Emergence of azole resistant Aspergillus fumigatus and One Health: time to implement environmental stewardship. Environ Microbiol. 2018;20:1299‐1301. [DOI] [PubMed] [Google Scholar]
- 7. Resendiz‐Sharpe A, Mercier T, Lestrade P, et al. Prevalence of voriconazole‐resistant invasive aspergillosis and its impact on mortality in haematology patients. J Antimicrob Chemother. 2019;74:2759‐2766. [DOI] [PubMed] [Google Scholar]
- 8. Chowdhary A, Sharma C, Hagen F, Meis JF. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 2014;9:697‐711. [DOI] [PubMed] [Google Scholar]
- 9. Chowdhary A, Sharma C, Meis JF. Azole‐resistant aspergillosis: epidemiology, molecular mechanisms and treatment. J Infect Dis. 2017;216(suppl_3):S436‐S444. [DOI] [PubMed] [Google Scholar]
- 10. Astvad KM, Jensen RH, Hassan TM, et al. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole‐naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother. 2014;58:5096‐5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sewell TR, Zhang Y, Brackin AP, Shelton JM, Rhodes J, Fisher MC. Elevated prevalence of azole resistant Aspergillus fumigatus in urban versus rural environments in the United Kingdom. Antimicrob Agents Chemother. 2019;63:e00548‐e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alvarez‐Moreno C, Lavergne R‐A, Hagen F, Morio F, Meis JF, Le Pape P. Azole‐resistant Aspergillus fumigatus harboring TR34/L98H, TR 46/Y121F/T289A and TR 53 mutations related to flower fields in Colombia. Sci Rep. 2017;7:45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen YC, Kuo SF, Wang HC, et al. Azole resistance in Aspergillus species in Southern Taiwan: an epidemiological surveillance study. Mycoses. 2019;62:1174‐1181. [DOI] [PubMed] [Google Scholar]
- 14. Alvarez‐Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, Le Pape P. Fungicide‐driven alterations in azole‐resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia. 2019;111:217‐224. [DOI] [PubMed] [Google Scholar]
- 15. Prigitano A, Esposto MC, Romanò L, Auxilia F, Tortorano AM. Azole‐resistant Aspergillus fumigatus in the Italian environment. J Glob Antimicrob Resist. 2019;16:220‐224. [DOI] [PubMed] [Google Scholar]
- 16. Rocchi S, Ponçot M, Morin‐Crini N, et al. Determination of azole fungal residues in soils and detection of Aspergillus fumigatus‐resistant strains in market gardens of Eastern France. Environ Sci Pollut Res Int. 2018;25:32015‐32023. [DOI] [PubMed] [Google Scholar]
- 17. Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu Y. Fungicides induced triazole‐resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater. 2017;326:54‐60. [DOI] [PubMed] [Google Scholar]
- 18. Chowdhary A, Sharma C, van den Boom M, et al. Multi‐azole‐resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother. 2014;69:2979‐2983. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Azole‐resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother. 2014;69:555‐557. [DOI] [PubMed] [Google Scholar]
- 20. Schoustra SE, Debets AJM, Rijs AJMM, et al. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, the Netherlands. Emerg Infect Dis. 2019;25:1347‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Dong J, Zhao J, et al. High azole resistance in Aspergillus fumigatus isolates from strawberry fields, China, 2018. Emerg Infect Dis. 2020;26:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godeau C, Reboux G, Scherer E, et al. Azole‐resistant Aspergillus fumigatus in the hospital: Surveillance from flower beds to corridors. Am J Infect Control. 2019. pii: S0196‐6553(19)30894‐6. [DOI] [PubMed] [Google Scholar]
- 23. Vanni A, Fontana F, Gamberini R, Calabria A. Occurrence of dicarboximide fungicides and their metabolites’ residues in commercial compost. Argonomie. 2004;24:7‐12. [Google Scholar]
- 24. Badali H, Vaezi A, Haghani I, et al. Environmental study of azole‐resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses. 2013;56:659‐663. [DOI] [PubMed] [Google Scholar]
- 25. Mellado E, Garcia‐Effron G, Alcázar‐Fuoli L, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross‐resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi—Third Edition: M38. Wayne, PA, USA: CLSI; 2017. [Google Scholar]
- 27. Klaassen CH, de Valk HA, Curfs‐Breuker IM, Meis JF. Novel mixed‐format real‐time PCR assay to detect mutations conferring resistance to triazoles in Aspergillus fumigatus and prevalence of multi‐triazole resistance among clinical isolates in the Netherlands. J Antimicrob Chemother. 2010;65:901‐905. [DOI] [PubMed] [Google Scholar]
- 28. Lavergne RA, Morio F, Favennec L, et al. First description of azole‐resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother. 2015;59:4331‐4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Valk HA, Meis JF, Curfs IM, Muehlethaler K, Mouton JW, Klaassen CH. Use of a novel panel of nine short tandem repeats for exact and high‐resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol. 2005;43:4112‐4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sewell TR, Zhu J, Rhodes J, et al. Nonrandom distribution of azole resistance across the global population of Aspergillus fumigatus . MBio. 2019;10:e00392‐e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ashu EE, Hagen F, Chowdhary A, Meis JF, Xu J. Global population genetic analysis of Aspergillus fumigatus . MSphere. 2017;2:e00019‐e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Der Linden JW, Camps SM, Kampinga GA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513‐520. [DOI] [PubMed] [Google Scholar]
- 33. Seyedmousavi S, Hashemi SJ, Zibafar E, et al. Azole‐resistant Aspergillus fumigatus, Iran. Emerg Infect Dis. 2013;19:832‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nabili M, Shokohi T, Moazeni M, et al. High prevalence of clinical and environmental triazole‐resistant Aspergillus fumigatus in Iran: is it a challenging issue? J Med Microbiol. 2016;65:468‐475. [DOI] [PubMed] [Google Scholar]
- 35. Falahatinejad M, Vaezi A, Fakhim H, et al. Use of cell surface protein typing for genotyping of azole‐resistant and ‐susceptible Aspergillus fumigatus isolates in Iran. Mycoses. 2018;61:143‐147. [DOI] [PubMed] [Google Scholar]
- 36. Lavergne RA, Chouaki T, Hagen F, et al. Home environment as a source of life‐threatening azole‐resistant Aspergillus fumigatus in immunocompromised patients. Clin Infect Dis. 2017;64:76‐78. [DOI] [PubMed] [Google Scholar]
- 37. Bader O, Tünnermann J, Dudakova A, Tangwattanachuleeporn M, Weig M, Groß U. Environmental isolates of azole‐resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother. 2015;59:4356‐4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivero‐Menendez O, Soto‐Debran JC, Medina N, Lucio J, Mellado E, Alastruey‐Izquierdo A. Molecular identification, antifungal susceptibility testing and mechanisms of azole resistance in Aspergillus spp. received within a surveillance program on antifungal resistance in Spain. Antimicrob Agents Chemother. 2019;63:00865‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pelaez T, Monteiro MC, Garcia‐Rubio R, Bouza E, Gomez‐Lopez A, Mellado E. First detection of Aspergillus fumigatus azole‐resistant strain due to Cyp51A TR46/Y121F/T289A in an azole‐naive patient in Spain. New Microbes New Infect. 2015;6:33‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore CB, Novak‐Frazer L, Muldoon E, et al. First isolation of the pan‐azole‐resistant Aspergillus fumigatus cyp51A TR46/Y121F/T289A mutant in a UK patient. Int J Antimicrob Agents. 2017;49:512‐514. [DOI] [PubMed] [Google Scholar]
- 41. Lavergne RA, Morio F, Danner‐Boucher I, et al. One year prospective survey of azole resistance in Aspergillus fumigatus at a French cystic fibrosis reference centre: prevalence and mechanisms of resistance. J Antimicrob Chemother. 2019;74:1884‐1889. [DOI] [PubMed] [Google Scholar]
- 42. Monteiro C, Faria MA, Pinheiro D, Lameiras C, Pinto E. First description of clinical Aspergillus fumigatus cyp51A TR46/Y121F/T289A mutant in Portugal. J Glob Antimicrob Resist. 2018;13:190‐191. [DOI] [PubMed] [Google Scholar]
- 43. Pinto E, Monteiro C, Maia M, et al. Aspergillus species and antifungals susceptibility in clinical setting in the North of Portugal: cryptic species and emerging azoles resistance in A fumigatus . Front Microbiol. 2018;9:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Isla G, Leonardelli F, Tiraboschi IN, et al. First clinical isolation of an azole resistant Aspergillus fumigatus isolate harboring a TR46/Y121F/T289A mutation in South America. Antimicrob Agents Chemother. 2018;62:e00872‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y, Wang H, Zhao YP, Xu YC, Hsueh PR. Antifungal susceptibility of clinical isolates of 25 genetically confirmed Aspergillus species collected from Taiwan and Mainland China. J Microbiol Immunol Infect. 2020;53:125‐132. [DOI] [PubMed] [Google Scholar]
- 46. Seufert R, Sedlacek L, Kahl B, et al. Prevalence and characterization of azole‐resistant Aspergillus fumigatus in patients with cystic fibrosis: a prospective multicentre study in Germany. J Antimicrob Chemother. 2018;73:2047‐2053. [DOI] [PubMed] [Google Scholar]
- 47. Rößler S, Bader O, Stölzel F, et al. Progressive dispersion of azole resistance in Aspergillus fumigatus: fatal invasive aspergillosis in a patient with acute myeloid leukemia infected with an A fumigatus strain with a cyp51A TR46 Y121F M172I T289A allele. Antimicrob Agents Chemother. 2017;61:e00270‐e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steinmann J, Hamprecht A, Vehreschild MJ, et al. Emergence of azole‐resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522‐1526. [DOI] [PubMed] [Google Scholar]
- 49. Fischer J, van Koningsbruggen‐Rietschel S, Rietschel E, et al. Prevalence and molecular characterization of azole resistance in Aspergillus spp. isolates from German cystic fibrosis patients. J Antimicrob Chemother. 2014;69:1533‐1536. [DOI] [PubMed] [Google Scholar]
- 50. Chen Y, Lu Z, Zhao J, et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother. 2016;60:5878‐5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Y, Wang H, Lu Z, et al. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolate from a Chinese patient. Antimicrob Agents Chemother. 2015;59:7148‐7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hagiwara D, Takahashi H, Fujimoto M, et al. Multi‐azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother. 2016;22:577‐579. [DOI] [PubMed] [Google Scholar]
- 53. Wiederhold NP, Gil VG, Gutierrez F, et al. First detection of TR34/L98H and TR46/Y121F/T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol. 2016;54:168‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vazquez JA, Manavathu EK. Molecular characterization of a voriconazole‐resistant, posaconazole‐susceptible Aspergillus fumigatus isolate in a lung transplant recipient in the United States. Antimicrob Agents Chemother. 2015;60:1129‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montesinos I, Argudin MA, Hites M, et al. Culture‐based methods and molecular tools for azole‐resistant Aspergillus fumigatus detection in a Belgian university hospital. J Clin Microbiol. 2017;55:2391‐2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montesinos I, Dodemont M, Lagrou K, Jacobs F, Etienne I, Denis O. New case of azole‐resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in Belgium. J Antimicrob Chemother. 2014;69:3439‐3440. [DOI] [PubMed] [Google Scholar]
- 57. Vermeulen E, Maertens J, Schoemans H, Lagrou K. Azole‐resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro Surveill. 2012;17:20326. [PubMed] [Google Scholar]
- 58. Valdes ID, van den Berg J, Haagsman A, et al. Comparative genotyping and phenotyping of Aspergillus fumigatus isolates from humans, dogs and the environment. BMC Microbiol. 2018;18:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Paassen J, Russcher A, In 't Veld‐van Wingerden AW, Verweij PE, Kuijper EJ. Emerging aspergillosis by azole‐resistant Aspergillus fumigatus at an intensive care unit in the Netherlands, 2010 to 2013. Euro Surveill. 2010;21 10.2807/1560-7917.ES.2016.21.30.30300 [DOI] [PubMed] [Google Scholar]
- 60. Chong GL, van de Sande WW, Dingemans GJ, et al. Validation of a new Aspergillus real‐time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53:868‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Snelders E, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol. 2009;75:4053‐4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ullmann AJ, Aguado JM, Arikan‐Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID‐ECMM‐ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1‐e38. [DOI] [PubMed] [Google Scholar]


