Abstract
The role of MRI differs considerably between the three main groups of hematological malignancies: lymphoma, leukemia, and myeloma. In myeloma, whole‐body MRI (WB‐MRI) is recognized as a highly sensitive test for the assessment of myeloma, and is also endorsed by clinical guidelines, especially for detection and staging. In lymphoma, WB‐MRI is presently not recommended, and merely serves as an alternative technique to the current standard imaging test, [18F]FDG‐PET/CT, especially in pediatric patients. Even for lymphomas with variable FDG avidity, such as extranodal mucosa‐associated lymphoid tissue lymphoma (MALT), contrast‐enhanced computed tomography (CT), but not WB‐MRI, is presently recommended, despite the high sensitivity of diffusion‐weighted MRI and its ability to capture treatment response that has been reported in the literature. In leukemia, neither MRI nor any other cross‐sectional imaging test (including positron emission tomography [PET]) is currently recommended outside of clinical trials. This review article discusses current clinical applications as well as the main research topics for MRI, as well as PET/MRI, in the field of hematological malignancies, with a focus on functional MRI techniques such as diffusion‐weighted imaging and dynamic contrast‐enhanced MRI, on the one hand, and novel, non‐FDG PET imaging probes such as the CXCR4 radiotracer [68Ga]Ga‐Pentixafor and the amino acid radiotracer [11C]methionine, on the other hand.
Level of Evidence: 5
Technical Efficacy Stage: 3
J. Magn. Reson. Imaging 2020;51:1325–1335.
Keywords: lymphoma, leukemia, myeloma, MRI, PET
IMAGING OF HEMATOLOGICAL MALIGNANCIES, which account for about 10% of all newly diagnosed cancers in the United States, is a complex topic. Despite their common origin—the hematopoietic and lymphoid tissues—there is a high level of heterogeneity within this group of cancers, and even within its three main subgroups: lymphoma (48% of all new cases), leukemia (35% of all new cases), and myeloma (18% of all new cases; also known as plasma cell disorders).1 This heterogeneity does not only affect prognosis and choice of treatment, but also choice of imaging technique and imaging features.
The role of imaging as part of the clinical workup is also fundamentally different between the three main groups of hematological malignancies. While imaging is the main tool for staging as well as treatment response assessment in lymphoma,2, 3, 4 it represents one of several key criteria for the diagnosis and follow‐up of myeloma5; whereas in leukemia, imaging (and even more so, cross‐sectional techniques such as magnetic resonance imaging [MRI]) is presently just an adjunct test of questionable clinical relevance outside of clinical trials.6, 7, 8, 9
Computed tomography (CT), MRI, and positron emission tomography (PET) using the radiotracer [18F]FDG (2‐[18F]‐fluoro‐2‐deoxy‐D‐glucose) have very different applications in hematological malignancies; generally, while CT and PET/CT clearly dominate imaging of lymphomas,2, 3, 4 in myeloma whole‐body (WB‐)MRI and [18F]FDG‐PET/CT are of similar importance, but used for different clinical questions.5 Regardless of the type of hematological cancer, the lack of exposure to ionizing radiation with MRI could represent an advantage in pediatric patients who may require life‐long follow‐up. Reduced radiation exposure relative to PET/CT, but mainly the possibility to obtain truly multiparametric information that may potentially improve treatment response assessment and outcome prognostication, are the arguments in favor of the novel hybrid imaging test, PET/MRI.
The aim of this article is to review the current imaging state‐of‐the‐art in the three main groups of hematological malignancies, with a special focus on the role of MRI, alone or in combination with PET, and to discuss its possible future applications.
MRI and PET/MRI Protocols for Hematological Malignancies
MRI Pulse Sequences for Whole‐Body Imaging
In systemic diseases such as hematological malignancies that lack a true "primary" tumor, whole‐body sequences represent the backbone of MRI. The most commonly used whole‐body MR sequences for cancer imaging are:
a STIR or fat‐saturated T2‐weighted sequence, in the coronal (for lymphoma) and/or sagittal (for myeloma) plane;
an axial 3D gradient‐echo Dixon or fast spin‐echo T1‐weighted sequence with multiplanar reconstructions;
an axial diffusion‐weighted imaging (DWI) sequence, obtained during free breathing and with background suppression, utilizing SPIR‐ or STIR‐based techniques; at least two b‐values (0–50; and 800–1000) should be used;
a fast spin‐echo T2‐weighted sequence (such as half‐Fourier acquisition single shot / half‐acquisition turbo spin‐echo [HASTE]) in the coronal or axial plane.
DWI, in which the imaging signal depends on the degree of compression of extracellular space due to cell enlargement and increased cell density in tumor tissue, has become especially popular due to its high lesion‐to‐background contrast and its ability to quantify treatment‐induced changes in diffusivity through calculation of apparent diffusion coefficients (ADCs). Increased diffusivity on DWI as reflected by an ADC increase has been shown to correspond to treatment‐induced cell death (necrosis) in melanoma as well as colon cancer xenografts.10, 11 Although limited by some technical artifacts—especially in the mediastinum, where the combination of cardiac and respiratory motion represents a challenge; and in the lower neck, where RF field inhomogeneity is frequently observed—DWI has largely replaced gadolinium‐based contrast‐enhanced (CE‐) T1‐weighted sequences for whole‐body imaging of hematological malignancies in clinical practice. This is because CE‐MRI does not appear to have clear advantages over unenhanced MR sequences in terms of lesion detection or staging12, 13—eg, in the study by Arendt et al,13 agreement between unenhanced and contrast‐enhanced MRI for lymph node assessment was high, with κ = 0.81—but introduces the small but recognized risk of contrast media side effects, and even nephrogenic systemic fibrosis in patients with impaired renal function,14 as is common in myeloma. The risk introduced by gadolinium deposits in the brain is currently being investigated.15 Nevertheless, CE‐MRI is still being recommended as an alternative to CE‐CT in many clinical trials.
PET Radiotracers
There is no doubt that the radiolabeled glucose analog [18F]FDG is the dominant radiotracer for PET imaging of hematological malignancies (see Lymphoma and Myeloma sections, below). Nevertheless, alternative tracers to [18F]FDG have been investigated, mainly to differentiate tumor tissue from inflammation, or to predict therapy response, but also to improve image quality in areas of high [18F]FDG uptake such as the central nervous system (CNS). These include the cellular proliferation tracer [18F]FLT (3‐[18F]‐fluoro‐3‐deoxythymidine), which has mainly been applied posttreatment, and appears to be effective in differentiating residual lymphoma from areas of inflammation. There are also studies indicating that pretreatment imaging with [18F]FLT could be a predictor of survival and of therapy response to CD20 targeting monoclonal antibody treatments (eg, rituximab).16, 17
There has been interest in the use of the radiolabeled amino acid [11C]methionine (L‐methyl‐[11C]‐methionine) in hematological malignancies, as it can, for instance, delineate CNS lymphoma due to the low uptake in normal brain compared with [18F]FDG, and changes in tracer uptake have been detected postradiotherapy.18 [11C]methionine has increased cellular uptake in neoplasms via the large amino acid transporter (LAT1). In a study in pediatric lymphoma, both Hodgkin's and non‐Hodgkin lymphomas were effectively visualized, although high physiologic activity in liver and bone marrow was a major limitation.19
Chemokine receptor‐targeted PET is another highly interesting approach for imaging of hematological malignancies. Chemokines are signaling molecules that bind to chemokine receptors on the surface of immune cells, which migrate in response to the increasing chemokine concentration gradient. CXCR4 is a member of this family of receptors; it is involved in organogenesis at the embryonic stage and has many other physiological roles; importantly, the receptor also has a role in tumorigenesis in some cancers, enhancing proliferation, migration, and invasion.20 Clinically, CXCR4 PET imaging has been shown to be feasible in leukemia, lymphoma, and multiple myeloma, as well as some solid tumors. For instance, [68Ga]Ga‐Pentixafor has been developed as a cyclic peptide imaging agent with DO3A (1,4,7,10‐Tetraazacyclododecane‐1,4,7‐triacetic acid) chelated gallium‐68 with imaged receptor expression levels acting as a prognostic marker.21 In multiple myeloma (MM) and leukemia, CXCR4 overexpression in terms of pathologically increased uptake was observed in about two‐thirds of patients imaged.22, 23, 24 This agent has been used to image therapy response, for example in a patient with extranodal marginal zone lymphoma of the orbital cavities.25 There are, however, challenges with dynamic and variable CXCR4 expression levels, indicating that further investigation of the receptor biology is required to fully understand the prognostic value and therapy response data. Small molecule alternatives to the peptidic agent, radiolabeled with either copper‐64 or gallium‐68, are undergoing preclinical evaluation and are likely to have future clinical impact.26, 27, 28
Lymphoma
Overview and Current Recommendations for Imaging
Lymphomas represent the group of hematological malignancies with the highest prevalence and incidence,1 and arguably also the highest degree of heterogeneity in terms of histology and prognosis. Based on their histological features, they can be divided into B‐cell and T/NK (natural killer)‐cell lymphomas on the one hand, and Hodgkin (HL) and non‐Hodgkin (NHL) lymphomas on the other hand. In addition, the REAL classification that very roughly subdivides these neoplasms into fast‐ and very fast‐growing "aggressive" and slowly‐growing but incurable "indolent" subtypes is also still widely used.29 Notably, histology is not just important for assessment of prognosis and treatment decisions—for instance, HL has a long‐term cure rate of up to 90% with standard chemotherapy regimens30—but also imaging.
For the initial detection of lymphoma, imaging is frequently used early on in the diagnostic workup: enlarged palpable superficial lymph nodes (eg, of the neck, axilla, or groin) are often evaluated by ultrasound sonography ahead of more specific laboratory tests, to determine the extent, morphology, and likelihood of malignancy. Depending on the anatomic location of the lymph node or mass, CT or ultrasound‐guided biopsies may also be performed instead of surgical resection to establish the diagnosis.
For both staging and treatment response assessment, [18F]FDG‐PET/CT is considered the technique of choice for the vast majority of lymphoma subtypes, according to the guidelines of the International Conference on Malignant Lymphoma (ICML), because they show a high glucose metabolism.2, 3 These FDG‐avid lymphomas include HL, diffuse large B‐cell lymphoma (DLBCL) as the most common aggressive NHL (33%), and follicular lymphoma (FL) as the most common indolent NHL (25%). However, other lymphoma subtypes such as the family of marginal zone lymphomas (MZL), of which extranodal mucosa‐associated lymphoid tissue lymphoma (MALT) is the most common representative, are not reliably FDG‐avid,3, 31, 32 at least when PET is performed at the standard timepoint (ie, 60 min) after tracer injection. For such lymphoma subtypes, the ICML presently recommends the use of contrast‐enhanced CT, rather than MRI or [18F]FDG‐PET,3 for both staging and treatment response assessment, despite the fact that, at least in MALT lymphoma, [18F]FDG‐PET has been shown to predict survival.33, 34
Treatment response assessment—arguably the most relevant task for imaging tests in lymphoma patients from a clinical point of view—is currently based on the Lugano classification,2, 3 or alternatively, the more novel RECIL classification.4 Both response classification systems share the same approach for evaluation of [18F]FDG‐PET: the semiquantitative 5‐point Deauville scale that is based on comparison of the posttherapeutic [18F]FDG uptake (maximum standardized uptake, SUVmax) of lesions compared with that of the liver, and, of lesser importance, the mediastinal blood pool. Any uptake higher than that of the liver (Deauville scores 4 and 5) is considered residual disease, regardless of lesion size. To achieve complete remission, on the other hand, Deauville scores 1–3 (uptake equal to, or less than the liver) is required2, 3, 4; and with RECIL, an additional ≥30% decrease in tumor size.4
Notably, many previous studies have also shown that pretherapeutic SUVs, metabolic tumor volumes (MTV), and total lesion glycolysis (TLG) extracted from [18F]FDG‐PET enable outcome prognostication in a variety of histological subtypes, including HL, DLBCL, FL, and also T‐cell lymphomas.35, 36, 37, 38, 39, 40 In addition, [18F]FDG‐PET has also been evaluated as a tool for risk stratification in clinical trials,41, 42 and several studies to investigate this topic are still ongoing.
MRI is currently only recommended by the guidelines for specific scenarios: assessment of suspected lymphomatous CNS involvement, where CE‐MRI is the established standard of care; and assessment of primary bone involvement,3, 4 or when minimization of exposure to ionizing radiation is desirable4; for instance, in pediatric patients. PET/MRI is currently not included in the Lugano or RECIL guidelines.
Research Applications and Future Directions for MRI and PET/MRI
A substantial number of comparative studies in FDG‐avid lymphomas have shown good agreement between WB‐MRI and [18F]FDG‐PET/CT in terms of lesion assessment and staging, most of them reporting an overall small‐to‐moderate inferiority for WB‐MRI.43, 44, 45, 46, 47, 48 For instance, Abdulqadr et al reported identical staging for DWI and [18F]FDG‐PET/CT in 90.3%,43 whereas in a study by van Ufford et al, agreement in terms of staging was only 77%,44 in mixed lymphoma populations, respectively. In our own study, concordance with a reference standard that relied chiefly on [18F]FDG‐PET/CT was observed in 94% of patients with FDG‐avid lymphoma subtypes.45 However, in patients with lymphomas with variable FDG avidity (eg, MALT lymphoma and small lymphocytic lymphoma [SLL]) (see Fig. 1), WB‐MRI with/without DWI has proven to be superior to both [18F]FDG‐PET/CT and the officially recommended standard technique, CE‐CT43, 45, 49—in the largest study so far, with staging accuracies of 92.5%, 65%, and 60% for WB‐DWI, PET/CT and CE‐CT, respectively.45 The drawbacks of WB‐DWI are imaging of the mediastinum and the head/neck region (which are typical sites of involvement in lymphoma patients) due to artifacts, as discussed above; and also the fact that small inflammatory lymph nodes frequently show a diffusion restriction pattern.45, 50 No generally accepted ADC cutoff has been established to distinguish inflammatory from malignant lymph nodes so far, and thus, the established morphological criterion of 1.5 cm long axis diameter, or novel techniques such as histogram analyses,50 may need to be taken into account (see Fig. 2). Despite the known strength of MRI for bone marrow assessment, neither T1‐weighted MRI nor DWI enabled reliable assessment of diffuse marrow infiltration in previous studies.51, 52 Asenbaum et al reported that, while sensitivity was reasonably good (87.5%) when using a fixed ADC cutoff, specificity for diffuse bone marrow involvement was poor, with only 57%.51
Figure 1.
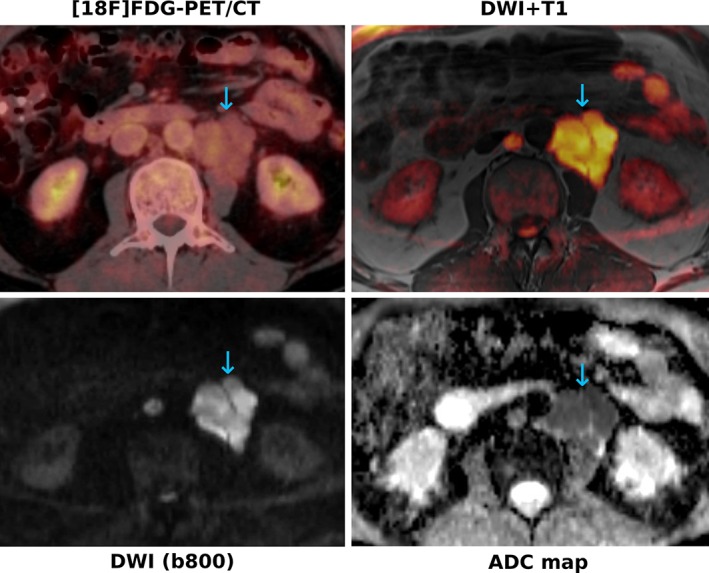
A 62‐year‐old patient with small‐cell lymphocytic lymphoma (SLL). The paraaortic nodal manifestations (blue arrows) show only very subtle [18F]FDG uptake on PET, whereas they show a clearly restricted diffusivity, with high signal on the b‐800 DWI and low signal on the corresponding ADC map. The color‐coded b‐800 DWI fused with T1‐weighted images enable better anatomic localization of the lesions, similar to fused PET/CT images.
Figure 2.
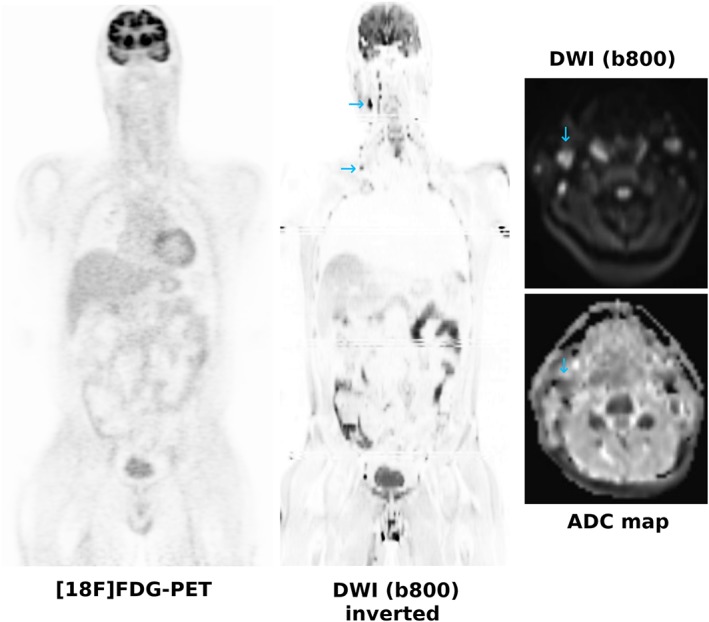
A 42‐year‐old patient referred with a clinical suspicion of lymphoma. [18F]FDG‐PET is negative, whereas DWI shows several small cervical lymph nodes with a long axis diameter <1.5 cm (blue arrows) that show a moderately restricted diffusivity, and which were proven to be inflammatory.
WB‐MRI has also shown good sensitivity and specificity for response assessment of lymphomas, especially when including DWI, with results that are only slightly inferior to those of [18F]FDG‐PET/CT in the majority of studies.53, 54, 55 In one study, Maggialetti et al reported a concordance of WB‐MRI/DWI with [18F]FDG‐PET/CT in 94% of their patients who were classified as showing either treatment response or no response55; whereas in a different study that used the classic treatment response categories of complete remission, partial remission, stable disease, and progressive disease, agreement between the two techniques was 97%.54 Contrary to morphological MRI sequences, and similar to [18F]FDG‐PET, DWI has been shown to enable (semi)quantitative assessment of treatment effects as early as 48–72 hours after treatment initiation in lymphomas.56 However, while it is generally recognized that ADCs reflect treatment response per se, no ADC cutoff values are presently established for separating partial from complete response, which is of high clinical significance, and there is also no MRI‐based scoring system comparable to the Deauville score (ie, no reference tissue definitions). Therefore, since [18F]FDG‐PET/CT is currently so well established as the imaging technique of choice for response assessment, WB‐MRI may need to establish its role in clinical situations where PET/CT is currently not recommended in clinical routine, such as in the monitoring of lymphoma patients who have achieved complete remission, or in patients with indolent NHL subtypes that undergo "watchful waiting"—a recent publication showed that WB‐MRI seems to be well suited for this task.57
Comparing [18F]FDG‐PET/MRI and PET/CT, a limited number of studies have either suggested a similar performance of the two techniques,58, 59, 60, 61 and in lymphomas with variable FDG avidity a superiority of PET/MRI over PET/CT for disease detection.62, 63 Furthermore, due to the longer examination time of PET/MRI, dual timepoint [18F]FDG‐PET may be more easily implemented for patients with indolent, variably FDG‐avid lymphomas such as MALT, whose visualization is possibly improved by prolonged radiotracer uptake time in the tumor and concurrent tracer washout from surrounding tissues.64 In pediatric lymphoma patients, [18F]FDG‐PET/MRI performed moderately better than WB‐MRI (with diagnostic accuracies of 96–97% vs. 86%, respectively), which is frequently used as a radiation‐free alternative imaging test in this age group.65 Since no study on [18F]FDG‐PET/MRI in lymphomas has suggested an inferiority of the technique relative to PET/CT (or WB‐MRI), it would seem reasonable to include PET/MRI as an alternative to PET/CT in management guidelines, at least for certain populations. Whether PET/MRI has advantages over PET/CT in terms of treatment response assessment and outcome prediction—for instance, by combining SUV and ADC measurements, or PET and DWI radiomic features—is presently unclear.
Myeloma
Overview and Current Recommendations for Imaging
Of the three large families of hematological malignancies, myeloma is the one with the overall poorest prognosis.66 Characterized by clonal proliferation of plasma cells and associated proteins, myeloma has three consecutive stages: the precursor condition, termed monoclonal gammopathy of undetermined significance (MGUS); the asymptomatic smoldering myeloma (SM); and the symptomatic MM, or plasmacytoma in case of single‐site involvement.
While the diagnosis of the asymptomatic MGUS and SM are purely based on laboratory findings in the blood and urine,67 the International Myeloma Working Group's (IMWG) updated criteria for the diagnosis of MM include any M‐spike or urinary M protein, clonal bone marrow plasma cell infiltration >10%, or biopsy‐proven bony or extramedullary plasmacytoma, and one or more of the following myeloma‐defining events (the so‐called CRAB criteria)5:
Hypercalcemia
Renal insufficiency
Anemia
Osteolytic bone lesions, as depicted on skeletal radiography, CT, or [18F]FDG‐PET/CT;
OR one of the following biomarkers of malignancy:
Clonal bone marrow plasma cell percentage of at least 60%
Involved‐to‐uninvolved serum free light chain ratio of at least 100
More than one focal lesion on MRI with 5 mm diameter.
Therefore, imaging tests play an important role for establishing the diagnosis of MM, although no single cross‐sectional imaging test is preferred. This contrasts with national guidelines from the British Society of Haematology as well as NICE, according to which WB‐MRI is preferred as the first‐line imaging test for suspected and newly diagnosed myeloma.68, 69 Notably, unlike CT and PET/CT, the IMWG lists MRI as a "biomarker," albeit without providing details on the type(s) of MRI sequences that should be used. With regard to the comparative performances of WB‐MRI and [18F]FDG‐PET/CT, the IMWG states that MRI is the best technique for detection of diffuse marrow involvement, whereas [18F]FDG‐PET/CT is recommended to distinguish active MM from SM; in addition, [18F]FDG PET/CT at the onset of MM is endorsed because it may have prognostic value.5 While the guidelines state that [18F]FDG‐PET/CT should be considered the preferred imaging technique to evaluate and monitor metabolic response to therapy in MM, there are presently no studies that directly compare [18F]FDG‐PET/CT with WB‐DWI or DCE‐MRI. A recent systematic review concluded that data on this topic are too heterogeneous, with biased accrual, and lack an independent reference standard, which also precluded a meta‐analysis.70 Therefore, it remains unclear which test may be best for response assessment. For reasons that are presently not quite clear (the main hypothesis being a low hexokinase‐2 expression), a nonnegligible number (up to 11%) of MM patients show no [18F]FDG‐uptake71, 72; MRI and [11C]‐Methionine‐PET may be helpful in this subset of cases.
Minimal residual disease (MRD) status is an important predictor of clinical outcome in MM.73, 74 Several highly sensitive techniques such as flow cytometry and next‐generation DNA sequencing have been introduced for detection of MRD in the bone marrow.74, 75, 76, 77 Outside of the bone marrow, [18F]FDG‐PET is regarded to be promising for the monitoring of MM patients with MRD,78 even though a previous study in 45 newly diagnosed MM or SM patients found no relationship between [18F]FDG‐PET/CT‐based response and MRD status, progression‐free survival, or clinical response; whereas DWI and DCE‐MRI are largely untested in the setting of MRD.79
Research Applications and Future Directions for MRI and PET/MRI
The role of WB‐MRI, and in particular, WB‐DWI and DCE‐MRI for response assessment, monitoring, and prognostication in myeloma remains unclear. In some studies, MRI could capture treatment effects and separate responding from nonresponding lesions using ADC measurements80, 81 or Dixon‐based bone signal fat fraction.81 For instance, Messiou et al observed significantly higher ADC values for active bone marrow disease than for bone marrow in remission (761.2 vs. 601.8 × 10‐6mm2s‐1).80 On the other hand, DCE‐MRI parameters, which capture different aspects of microvascularity, showed prognostic potential in patients with SM.82 However, in contrast to DWI‐MRI, DCE‐MRI is not a whole‐body technique and in general can only be performed for a single anatomic region.
One of the main obstacles for generating reproducible results and evidence on the value of WB‐MRI for response assessment and prognostication is the current lack of standardization, including the choice of MR sequences and their interpretation. Messiou et al recently proposed the so‐called MY‐RADS criteria that provide guidelines for initial assessment and response assessment of myeloma patients.83 Similar to PI‐RADS for prostate cancer, DWI plays an important role in MY‐RADS. Specifically, an ADC increase of at least 40% with a corresponding decrease in normalized high b‐value signal intensity (relative to muscle tissue, which was used as reference tissue), or an ADC increase from below to above 1400 μm2/s indicates responding disease with a high likelihood, also in the presence of morphologically stable disease.83 These authors also proposed a scoring system for use in clinical trials.
The combination of DWI and [18F]FDG‐PET may prove useful in myeloma, especially following treatment: Rasche et al84 reported that WB‐DWI identified a higher number of lesions than [18F]FDG‐PET (21% vs. 6%), but not all PET‐positive lesions were visible on DWI, indicating complementary roles for the two imaging tests; in addition, patients with positive findings on both tests after completion of treatment had a particularly poor prognosis84 (see Fig. 3). PET with other radiotracers, such as [11C]methionine or [68Ga]Ga‐Pentixafor21 may perhaps also provide useful information.
Figure 3.
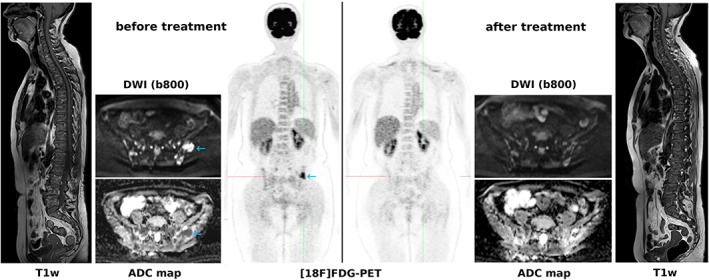
A 60‐year‐old patient with multiple myeloma. While DWI captures multiple focal lesions with restricted diffusivity in the pelvic bones, [18F]FDG‐PET captures just a single lesion with extension through the cortical bone into the soft tissues (blue arrows). After treatment, [18F]FDG‐PET shows complete remission in good accordance with clinical findings, whereas DWI shows residual changes with increasing ADCs as a clear sign of treatment response; whether these DWI/ADC changes represent partial or complete response is unclear. T1‐weighted images of the spine show pathologically decreased signal with multiple focal lesions before as well as after treatment, without any obvious changes.
Leukemia
Overview and Current Recommendations for Imaging
Leukemia is the second largest family of hematological malignancies after the lymphomas, and, depending on the subtype, may show a considerable overlap of histological features with the latter. The four main kinds of leukemia are, in the order of their prevalence:
chronic lymphocytic leukemia (CLL; the most common form, affecting mainly elderly patients),
acute myeloid leukemia (AML),
chronic myeloid leukemia (CML), and
acute lymphoblastic leukemia (ALL, the most common form in pediatric patients).
Other notable subtypes include hairy cell leukemia (HCL), acute promyelocytic leukemia (APML), and the extremely aggressive and frequently therapy‐refractory T‐cell prolymphocytic leukemia (T‐PLL), the most common type of mature T‐cell leukemia, and the leukemia with the poorest prognosis.
Imaging has traditionally played a limited role in the work‐up of leukemias, with regard to detection, staging, and response assessment. Imaging—typically plain film radiographs of the chest8 and rarely CT—are mostly used to rule out complications such as lung involvement or infection, or a mediastinal mass. Indeed, the only leukemia guideline to mention cross‐sectional imaging techniques is the International Workshop for Chronic Lymphocytic Leukemia (iwCLL), which explicitly advises against the use of CT for detection of enlarged lymph nodes and splenomegaly in routine clinical practice, suggesting that physical examination is sufficient for this task, and should be the basis of the Binet and Rai staging systems, even though it has been reported that patients with Rai stage 0 but abdominal disease detectable by CT scans may have a more aggressive course.6 For CLL patients participating in clinical trials, on the other hand, contrast‐enhanced CT of the neck, chest, abdomen, and pelvis is recommended by the iwCLL, both for staging and treatment response assessment, using a slightly simplified version of the Lugano criteria that rely on bidimensional measurements of up to six enlarged lymph nodes, as well as the vertical diameter of the spleen.6 Whole‐body MRI is not recommended as an alternative to CT outside of clinical trials, because according to the guidelines, it does not offer a clear advantage over CT—the ability of MRI to evaluate bone marrow directly is not addressed. [18F]FDG‐PET is not recommended for routine evaluation of CLL, because the disease shows low uptake in the majority of cases. The only exception is suspected Richter's transformation into an aggressive lymphoma, such as DLBCL.6
The clinical utility of MRI lies in the detection of bone marrow abnormalities that are suspicious for leukemia in adult and pediatric patients with unclear musculoskeletal symptoms. These findings include replacement of the fatty bone marrow by leukemic cells, leading to a decreased signal on T1‐weighted, increased signal on STIR, or an abnormal enhancement pattern on contrast‐enhanced fat‐saturated sequences. In children, the classic band‐like abnormalities along the metaphysis of long bones, especially at the level of the knee or wrist, may be seen in ALL.85 PET/MRI, on the other hand, has no real clinical justification behind it at present, but due to its reduced radiation dose (in comparison with PET/CT), it may become more attractive with the use of newer, non‐FDG PET radiotracers.
Research Applications and Future Directions for MRI and PET/MRI
Apart from the classic T1‐weighted and STIR sequences that are well established for visualization of the bone marrow, two other MRI sequence types—particularly DCE‐MRI, and DWI and its variants—have been subject to research in adult and pediatric leukemia patients.
A decade ago, Shih et al reported that bone marrow angiogenesis, as assessed by two quantitative parameters (pretherapeutic peak enhancement and amplitude) derived from DCE‐MRI, enables prediction of overall and disease‐free survival in AML patients who received induction chemotherapy; in addition, higher peak enhancement was shown to be an independent predictor for overall survival, with a hazard ratio of 9.2.86 In a second study, the same author group reported that a reduction in peak enhancement 7 days after treatment was associated with a more favorable treatment outcome in AML: 87% of patients with a decrease in peak enhancement achieved complete remission, as compared with 71% with an increase in peak enhancement, and 80% vs. 44% remained disease‐free, respectively.87 However, no further studies on DCE‐MRI in AML or other leukemias were performed, and so the technique was never implemented in clinical practice.
Nishi et al were the first to describe the successful application of DWI to predict bone marrow infiltration in pediatric patients with different leukemia subtypes, using the clivus‐to‐pons signal intensity ratios.88 Cao et al reported the successful use of DWI to capture treatment response in pediatric ALL patients, based on normalization of ADC values measured in the skull.89 Finally, Niu et al investigated the predictive value of pretherapeutic intravoxel‐incoherent motion (IVIM) MRI—a variation of DWI that enables the assessment of the perfusion component in addition to true molecular diffusion—in patients with AML. Their results suggested that true molecular diffusion (D) and the perfusion fraction (f) differ significantly between AML patients achieving, and those not achieving, complete remission after induction chemotherapy.90
More recently, [68Ga]Ga‐Pentixafor‐PET/MRI with DWI has been investigated in a small series of patients with CLL, to determine whether this imaging technique can capture bone marrow involvement based on CXCR4 expression or diffusivity91 (see Fig. 4). Here, [68Ga]Ga‐Pentixafor uptake, which was significantly higher than in the two nonleukemic control groups, and ADC values showed no significant correlation, suggesting a complementary role for the two imaging tests. In addition, there was a significant negative correlation between ADCs and the white blood cell count (r = –0.78), and ADCs and the lymphocyte percentage (r = –0.81), but not between [68Ga]Ga‐Pentixafor uptake and laboratory findings in that study. The feasibility of [68Ga]Ga‐Pentixafor‐PET has also been demonstrated for AML.22 Whether the combination of DWI with [68Ga]Ga‐Pentixafor is clinically useful in the sense of providing a multimodal oncologic "signature" for leukemias is yet to be determined. The two main arguments for the use of [68Ga]Ga‐Pentixafor‐PET in CLL are that high CXCR4 expression is known to be associated with poor prognosis in CLL,92 and that—similar to myeloma—[68Ga]Ga‐Pentixafor‐PET might enable the selection of patients for systemic treatment with [177Lu]Pentixather. In this setting, ADCs might serve as a surrogate marker for treatment response whose biologic correlate—ie, cell density—is at least partly independent of CXCR4 expression.
Figure 4.
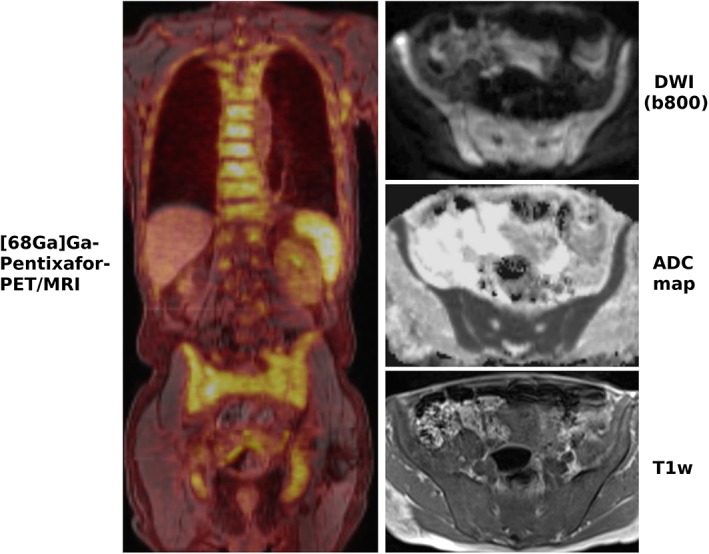
A 70‐year‐old patient with chronic lymphocytic leukemia (CLL) with clearly increased CXCR4 expression in the entire skeleton as well as axillary lymph nodes on [68Ga]Ga‐Pentixafor‐PET/MRI. DWI shows diffusion restriction within the bone marrow, with high signal on the b‐800 DWI and low signal on the ADC map. While this finding alone is unreliable for the diagnosis of bone marrow involvement, the low signal on the T1‐weighted image (T1w) that reflects the replacement of fatty bone marrow by leukemic cells supports the diagnosis.
The proliferation marker [18F]FLT, on the other hand, is a promising PET tracer for acute leukemias, as it has been shown to carry diagnostic as well as prognostic value in a small number of studies.93, 94 So far, only a combination of [18F]FLT with dual‐energy CT,95 but no combination with MRI has been attempted.
Summary and Outlook
The role of WB‐MRI and PET/MRI in hematological malignancies is subject to intensive research. WB‐MRI is already recognized as a highly sensitive test for the assessment of myeloma, and endorsed by clinical guidelines, since it is the only technique that can directly visualize bone marrow. For lymphoma, on the other hand, WB‐MRI is presently not recommended by clinical guidelines, and is merely regarded as an alternative technique to [18F]FDG‐PET/CT when radiation exposure is a concern, as for instance in pediatric patients, or when CNS involvement is suspected. Despite their high sensitivity, WB‐MRI, and especially DWI, is currently not recommended in patients with non‐FDG‐avid lymphoma subtypes such as MALT lymphoma and SLL. Here, active promotion by the MRI community is needed to raise the awareness of clinicians with regard to the superiority of WB‐MRI with DWI over the standard test, CE‐CT.
For response assessment, [18F]FDG‐PET/CT is the established imaging test for lymphoma, and is also gaining momentum for this indication in myeloma. On the other hand, the recently published MY‐RADS guidelines propose the use of WB‐MRI with DWI for this purpose. Prospective studies focusing on changes in clinical management and survival are required to generate evidence for the noninferiority of advanced MRI techniques such as DWI, and possibly also DCE‐MRI.
If supported by sufficient data, PET/MRI may be able to establish itself as a uniformly applicable and truly multiparametric imaging test for hematological malignancies. In particular for leukemia and myeloma, the combination of PET with non‐FDG radiotracers (such as [68Ga]Ga‐Pentixafor or [11C]methionine) and WB‐MRI has high potential.
References
- 1. American Cancer Society: Cancer Facts & Figures 2019 . URL: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
- 2. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: The Lugano classification. J Clin Oncol 2014;32:3059–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017;28:1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavo M, Terpos E, Nanni C, et al. Role of (18)F‐FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol 2017;18:e206–e217. [DOI] [PubMed] [Google Scholar]
- 6. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018;131:2745–2760. [DOI] [PubMed] [Google Scholar]
- 7. Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv41–iv51. [DOI] [PubMed] [Google Scholar]
- 8. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C; ESMO Guidelines Committee. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v69–v82. [DOI] [PubMed] [Google Scholar]
- 10. Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 2000;43:828–836. [DOI] [PubMed] [Google Scholar]
- 11. Papaevangelou E, Almeida GS, Jamin Y, Robinson SP, deSouza NM. Diffusion‐weighted MRI for imaging cell death after cytotoxic or apoptosis‐inducing therapy. Br J Cancer 2015;112:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahmouni A, Divine M, Mathieu D, et al. Detection of multiple myeloma involving the spine: Efficacy of fat‐suppression and contrast‐enhanced MR imaging. AJR Am J Roentgenol 1993;160:1049–1052. [DOI] [PubMed] [Google Scholar]
- 13. Arendt CT, Beeres M, Leithner D, et al. Gadolinium‐enhanced imaging of pediatric thoracic lymphoma: Is intravenous contrast really necessary? Eur Radiol 2019;29:2553–2559. [DOI] [PubMed] [Google Scholar]
- 14. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium‐based contrast media: Updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2013;23:307–318. [DOI] [PubMed] [Google Scholar]
- 15. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 16. Herrmann K, Buck AK, Schuster T, et al. Week one FLT‐PET response predicts complete remission to R‐CHOP and survival in DLBCL. Oncotarget 2014;5:4050–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schöder H, Zelenetz AD, Hamlin P, et al. Prospective study of 3'‐Deoxy‐3'‐18F‐Fluorothymidine PET for early interim response assessment in advanced‐stage B‐cell lymphoma. J Nucl Med 2016;57:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogawa T, Kanno I, Hatazawa J, et al. Methionine PET for follow‐up of radiation therapy of primary lymphoma of the brain. Radiographics 1994;14:101–110. [DOI] [PubMed] [Google Scholar]
- 19. Kaste SC, Snyder SE, Metzger ML, et al. Comparison of C‐11‐methionine and F‐18‐FDG PET/CT for staging and follow‐up of pediatric lymphoma. J Nucl Med 2017;58:419–424. [DOI] [PubMed] [Google Scholar]
- 20. Ratajczak MZ, Serwin K, Schneider G. Innate immunity derived factors as external modulators of the CXCL12‐CXCR4 axis and their role in stem cell homing and mobilization. Theranostics 2013;3:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buck AK, Stolzenburg A, Hanscheid H, et al. Chemokine receptor — Directed imaging and therapy. Methods 2017;130:63–71. [DOI] [PubMed] [Google Scholar]
- 22. Herhaus P, Habringer S, Philipp‐Abbrederis K, et al. Targeted positron emission tomography imaging of CXCR4 expression in patients with acute myeloid leukemia. Haematologica 2016;101:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lapa C, Schreder M, Schirbel A, et al. Ga‐68 Pentixafor‐PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma — Comparison to F‐18 FDG and laboratory values. Theranostics 2017;7:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Philipp‐Abbrederis K, Herrmann K, Knop S, et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. Embo Mol Med 2015;7:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herhaus P, Habringer S, Vag T, et al. Response assessment with the CXCR4‐directed positron emission tomography tracer [(68)Ga]Pentixafor in a patient with extranodal marginal zone lymphoma of the orbital cavities. EJNMMI Res 2017;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan A, Nicholson G, Greenman J, et al. Binding optimization through coordination chemistry: CXCR4 chemokine receptor antagonists from ultrarigid metal complexes. J Am Chem Soc 2009;131:3416–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miranda CS, Burke BP, Lee RE, et al. CXCR4 chemokine receptor imaging: Evaluation and validation of a new configurationally restricted tetraazamacrocyclic CXCR4 antagonist, Cu‐64‐CB‐bicyclam. Eur J Nucl Med Mol Imaging 2016;43:S146–S147. [Google Scholar]
- 28. Lee RE, Burke BP, Miranda CS. Gallium‐68 PET imaging of CXCR4 expression in vivo/vitro: Optimised small molecule azamacrocyclic chemokine receptor antagonists. J Labelled Comp Radiopharm 2018;61:463–464. [Google Scholar]
- 29. Harris NL, Jaffe ES, Stein H, et al. A revised European‐American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994;84:1361–1392. [PubMed] [Google Scholar]
- 30. Johnson PW. Response‐adapted frontline therapy for Hodgkin lymphoma: Are we there yet? Hematology Am Soc Hematol Educ Program 2016;2016:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beal KP, Yeung HW, Yahalom J. FDG‐PET scanning for detection and staging of extranodal marginal zone lymphomas of the MALT type: A report of 42 cases. Ann Oncol 2005;16:473–480. [DOI] [PubMed] [Google Scholar]
- 32. Park SH, Lee JJ, Kim HO, et al. 18F‐Fluorodeoxyglucose (FDG)‐positron emission tomography/computed tomography in mucosa‐associated lymphoid tissue lymphoma: Variation in 18F‐FDG avidity according to site involvement. Leuk Lymphoma 2015;56:3288–3294. [DOI] [PubMed] [Google Scholar]
- 33. Qi S, Huang MY, Yang Y, et al. Uptake of [(18)F]fluorodeoxyglucose in initial positron‐emission tomography predicts survival in MALT lymphoma. Blood Adv 2018;2:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayerhoefer ME, Staudenherz A, Kiesewetter B, et al. Pre‐therapeutic total lesion glycolysis on [(18)F]FDG‐PET enables prognostication of 2‐year progression‐free survival in MALT lymphoma patients treated with CD20‐antibody‐based immunotherapy. Mol Imaging Biol 2019. [Epub ahead of print] doi: 10.1007/s11307-019-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moskowitz AJ, Schöder H, Gavane S, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood 2017;130:2196–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meignan M, Cottereau AS, Versari A, et al. Baseline metabolic tumor volume predicts outcome in high‐tumor‐burden follicular lymphoma: A pooled analysis of three multicenter studies. J Clin Oncol 2016;34:3618–3626. [DOI] [PubMed] [Google Scholar]
- 37. Mikhaeel NG, Smith D, Dunn JT, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression‐free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 2016;43:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cottereau AS, Lanic H, Mareschal S, et al. Molecular profile and FDG‐PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B‐cell lymphoma. Clin Cancer Res 2016;22:3801–3809. [DOI] [PubMed] [Google Scholar]
- 39. Akhtari M, Milgrom SA, Pinnix CC, et al. Reclassifying patients with early‐stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood 2018;131:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cottereau AS, El‐Galaly TC, Becker S, et al. Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T‐cell lymphoma patients. J Nucl Med 2018;59:589–595. [DOI] [PubMed] [Google Scholar]
- 41. Borchmann P, Goergen H, Kobe C, et al. PET‐guided treatment in patients with advanced‐stage Hodgkin's lymphoma (HD18): Final results of an open‐label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2018;390:2790–2802. [DOI] [PubMed] [Google Scholar]
- 42. Yhim HY, Park Y, Han YH, et al. A risk stratification model for nodal peripheral T‐cell lymphomas based on the NCCN‐IPI and posttreatment Deauville score. Eur J Nucl Med Mol Imaging 2018;45:2274–2284. [DOI] [PubMed] [Google Scholar]
- 43. Abdulqadhr G, Molin D, Aström G, et al. Whole‐body diffusion‐weighted imaging compared with FDG‐PET/CT in staging of lymphoma patients. Acta Radiol 2011;52:173–180. [DOI] [PubMed] [Google Scholar]
- 44. van Ufford HM, Kwee TC, Beek FJ, et al. Newly diagnosed lymphoma: Initial results with whole‐body T1‐weighted, STIR, and diffusion‐weighted MRI compared with 18F‐FDG‐PET/CT. AJR Am J Roentgenol 2011;196:662–669. [DOI] [PubMed] [Google Scholar]
- 45. Mayerhoefer ME, Karanikas G, Kletter K, et al. Evaluation of diffusion‐weighted MRI for pretherapeutic assessment and staging of lymphoma: Results of a prospective study in 140 patients. Clin Cancer Res 2014;20:2984–2993. [DOI] [PubMed] [Google Scholar]
- 46. Albano D, Patti C, La Grutta L, et al. Comparison between whole‐body MRI with diffusion‐weighted imaging and PET/CT in staging newly diagnosed FDG‐avid lymphomas. Eur J Radiol 2016;85:313–318. [DOI] [PubMed] [Google Scholar]
- 47. Tsuji K, Kishi S, Tsuchida T, et al. Evaluation of staging and early response to chemotherapy with whole‐body diffusion‐weighted MRI in malignant lymphoma patients: A comparison with FDG‐PET/CT. J Magn Reson Imaging 2015;41:1601–1607. [DOI] [PubMed] [Google Scholar]
- 48. Baranska D, Matera K, Podgorski M, et al. Feasibility of diffusion‐weighted imaging with DWIBS in staging Hodgkin lymphoma in pediatric patients: Comparison with PET/CT. MAGMA 2019;32:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwee TC, Vermoolen MA, Akkerman EA, et al. Whole‐body MRI, including diffusion‐weighted imaging, for staging lymphoma: Comparison with CT in a prospective multicenter study. J Magn Reson Imaging 2014;40:26–36. [DOI] [PubMed] [Google Scholar]
- 50. De Paepe KN, De Keyzer F, Wolter P, et al. Improving lymph node characterization in staging malignant lymphoma using first‐order ADC texture analysis from whole‐body diffusion‐weighted MRI. J Magn Reson Imaging 2018;48:897–906. [DOI] [PubMed] [Google Scholar]
- 51. Asenbaum U, Nolz R, Karanikas G, et al. Bone marrow involvement in malignant lymphoma: Evaluation of quantitative PET and MRI biomarkers. Acad Radiol 2018;25:453–460. [DOI] [PubMed] [Google Scholar]
- 52. Asenbaum U, Nolz R, Karanikas G, et al. Evaluation of [18F]‐FDG‐based hybrid imaging combinations for assessment of bone marrow involvement in lymphoma at initial staging. PLoS One 2016;11:e0164118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Paepe K, Bevernage C, De Keyzer F, et al. Whole‐body diffusion‐weighted magnetic resonance imaging at 3 Tesla for early assessment of treatment response in non‐Hodgkin lymphoma: A pilot study. Cancer Imaging 2013;13:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mayerhoefer ME, Karanikas G, Kletter K, et al. Evaluation of diffusion‐weighted magnetic resonance imaging for follow‐up and treatment response assessment of lymphoma: Results of an 18F‐FDG‐PET/CT‐controlled prospective study in 64 patients. Clin Cancer Res 2015;21:2506–2513. [DOI] [PubMed] [Google Scholar]
- 55. Maggialetti N, Ferrari C, Minoia C, et al. Role of WB‐MR/DWIBS compared to (18)F‐FDG PET/CT in the therapy response assessment of lymphoma. Radiol Med 2016;121:132–143. [DOI] [PubMed] [Google Scholar]
- 56. Mayerhoefer ME, Raderer M, Jaeger U, et al. Ultra‐early response assessment in lymphoma treatment: [(18)F]FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging 2018;45:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galia M, Albano D, Tarella C, et al. Whole body magnetic resonance in indolent lymphomas under watchful waiting: The time is now. Eur Radiol 2018;28:1187–1193. [DOI] [PubMed] [Google Scholar]
- 58. Atkinson W, Catana C, Abramson JS, et al. Hybrid FDG‐PET/MR compared to FDG‐PET/CT in adult lymphoma patients. Abdom Radiol 2016;41:1338–1348. [DOI] [PubMed] [Google Scholar]
- 59. Afaq A, Fraioli F, Sidhu H, et al. Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med 2017;42:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herrmann K, Queiroz M, Huellner MW, et al. Diagnostic performance of FDG‐PET/MRI and WB‐DW‐MRI in the evaluation of lymphoma: A prospective comparison to standard FDG‐PET/CT. BMC Cancer 2015;15:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sher AC, Seghers V, Paldino MJ, et al. Assessment of sequential PET/MRI in comparison with PET/CT of pediatric lymphoma: A prospective study. AJR Am J Roentgenol 2016;206:623–631. [DOI] [PubMed] [Google Scholar]
- 62. Heacock L, Weissbrot J, Raad R, et al. PET/MRI for the evaluation of patients with lymphoma: Initial observations. AJR Am J Roentgenol 2015;204:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Giraudo C, Raderer M, Karanikas G, et al. 18F‐fluorodeoxyglucose positron emission tomography/magnetic resonance in lymphoma: Comparison with 18F fluorodeoxyglucose positron emission tomography/computed tomography and with the addition of magnetic resonance diffusion‐weighted imaging. Invest Radiol 2016;51:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mayerhoefer ME, Giraudo C, Senn D, et al. Does delayed‐time‐point imaging improve 18F‐FDG‐PET in patients with MALT lymphoma: Observations in a series of 13 patients. Clin Nucl Med 2016;41:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kirchner J, Deuschl C, Schweiger B, et al. Imaging children suffering from lymphoma: An evaluation of different (18)F‐FDG PET/MRI protocols compared to whole‐body DW‐MRI. Eur J Nucl Med Mol Imaging 2017;44:1742–1750. [DOI] [PubMed] [Google Scholar]
- 66. Noone AM, Howlader N, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975‐2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 67. Ghobrial IM, Landgren O. How I treat smoldering multiple myeloma. Blood 2014;124:3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chantry A, Kazmi M, Barrington S, et al. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 2017;178:380–393. [DOI] [PubMed] [Google Scholar]
- 69. National Institute for Health and Care Excellence . Myeloma: Diagnosis and management. NICE guideline [NG35]. Available at: https://www.nice.org.uk/ guidance/ng35 [PubMed]
- 70. Gariani J, Westerland O, Natas S, Verma H, Cook G, Goh V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or (18)F‐fluorodeoxyglucose positron emission tomography/CT ((18)F‐FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit Rev Oncol Hematol 2018;124:66–72. [DOI] [PubMed] [Google Scholar]
- 71. Rasche L, Angtuaco E, McDonald JE, et al. Low expression of hexokinase‐2 is associated with false‐negative FDG‐positron emission tomography in multiple myeloma. Blood 2017;130:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kircher S, Stolzenburg A, Kortüm KM, et al. Hexokinase‐2 expression in (11)C‐methionine‐positive, (18)F‐FDG‐negative multiple myeloma. J Nucl Med 2019;60:348–352. [DOI] [PubMed] [Google Scholar]
- 73. Landgren O. MRD testing in multiple myeloma: From a surrogate marker of clinical outcomes to an every‐day clinical tool. Semin Hematol 2018;55:1–3. [DOI] [PubMed] [Google Scholar]
- 74. Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: Role of minimal residual disease in multiple myeloma. Blood 2015;125:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paiva B, Cedena MT, Puig N, et al; Grupo Español de Mieloma/Programa para el Estudio de la Terapéutica en Hemopatías Malignas (GEM/PETHEMA) Cooperative Study Groups. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016;127:3165–3174. [DOI] [PubMed] [Google Scholar]
- 76. Martinez‐Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 2014;123:3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ladetto M, Brüggemann M, Monitillo L, et al. Next‐generation sequencing and real‐time quantitative PCR for minimal residual disease detection in B‐cell disorders. Leukemia 2014;28:1299–1307. [DOI] [PubMed] [Google Scholar]
- 78. Sundaram S, Driscoll J, Fernandez‐Ulloa M, de Lima M, Malek E. FDG PET imaging in multiple myeloma: Implications for response assessments in clinical trials. Am J Nucl Med Mol Imaging 2018;8:421–427. [PMC free article] [PubMed] [Google Scholar]
- 79. Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib‐lenalidomide‐dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol 2015;1:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Messiou C, Giles S, Collins DJ, et al. Assessing response of myeloma bone disease with diffusion‐weighted MRI. Br J Radiol 2012;85:e1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Latifoltojar A, Hall‐Craggs M, Bainbridge A, et al. Whole‐body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur Radiol 2017;27:5325–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hillengass J, Ritsch J, Merz M, et al. Increased microcirculation detected by dynamic contrast enhanced magnetic resonance imaging is of prognostic significance in asymptomatic myeloma. Br J Haematol 2016;174:127–135. [DOI] [PubMed] [Google Scholar]
- 83. Messiou C, Hillengass J, Delorme S, et al. Guidelines for acquisition, interpretation, and reporting of whole‐body MRI in myeloma: Myeloma response assessment and diagnosis system (MY‐RADS). Radiology 2019. [Epub ahead of print] doi: 10.1148/radiol.2019181949. [DOI] [PubMed] [Google Scholar]
- 84. Rasche L, Alapat D, Kumar M, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia 2018. [Epub ahead of print] doi: 10.1038/s41375-018-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Averill LW, Acikgoz G, Miller RE, Kandula VV, Epelman M. Update on pediatric leukemia and lymphoma imaging. Semin Ultrasound CT MR 2013;34:578–599. [DOI] [PubMed] [Google Scholar]
- 86. Shih TT, Hou HA, Liu CY, et al. Bone marrow angiogenesis magnetic resonance imaging in patients with acute myeloid leukemia: Peak enhancement ratio is an independent predictor for overall survival. Blood 2009;113:3161–3167. [DOI] [PubMed] [Google Scholar]
- 87. Hou HA, Shih TT, Liu CY, et al. Changes in magnetic resonance bone marrow angiogenesis on day 7 after induction chemotherapy can predict outcome of acute myeloid leukemia. Haematologica 2010;95:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nishii T, Kono AK, Akasaka Y, et al. Bone marrow magnetic resonance imaging of the clivus in pediatric leukemia patients and normal controls. Jpn J Radiol 2015;33:146–152. [DOI] [PubMed] [Google Scholar]
- 89. Cao W, Liang C, Gen Y, Wang C, Zhao C, Sun L. Role of diffusion‐weighted imaging for detecting bone marrow infiltration in skull in children with acutelymphoblastic leukemia. Diagn Interv Radiol 2016;22:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Niu J, Li W, Wang H, et al. Intravoxel incoherent motion diffusion‐weighted imaging of bone marrow in patients with acute myeloid leukemia: A pilot study of prognostic value. J Magn Reson Imaging 2017;46:476–482. [DOI] [PubMed] [Google Scholar]
- 91. Mayerhoefer ME, Jaeger U, Staber P, et al. [68Ga]Ga‐Pentixafor PET/MRI for CXCR4 imaging of chronic lymphocytic leukemia: Preliminary results. Invest Radiol 2018;53:403–408. [DOI] [PubMed] [Google Scholar]
- 92. Ganghammer S, Gutjahr J, Hutterer E, et al. Combined CXCR3/CXCR4 measurements are of high prognostic value in chronic lymphocytic leukemia due to negative co‐operativity of the receptors. Haematologica 2016;101:e99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res 2011;35:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Han EJ, Lee BH, Kim JA, Park YH, Choi WH. Early assessment of response to induction therapy in acute myeloid leukemia using (18)F‐FLT PET/CT. EJNMMI Res 2017;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Magome T, Froelich J, Holtan SG, et al. Whole‐body distribution of leukemia and functional total marrow irradiation based on FLT‐PET and dual‐energy CT. Mol Imaging 2017;16 10.1177/1536012117732203 [DOI] [PMC free article] [PubMed] [Google Scholar]


