Abstract
Head‐of‐bed (HOB) elevation is a common clinical practice in hospitals causing the patient's body to slide down in bed because of gravity. This migration effect likely results in tissue shearing between the sacrum and the support surface, which increases the risk for pressure injuries. StayInPlace (HillRom Inc.) is a commercial migration‐reduction technology (MRT) incorporated in intensive care bedframes. Yet, the effects of migration‐reduction on tissue shear stresses during HOB elevation are unknown. We analysed relationships between migration and resulting sacral soft tissue stresses by combining motion analysis and three‐dimensional finite element modelling of the buttocks. Migration data were collected for 10 subjects, lying supine on two bedframe types with and without MRT, and at HOB elevations of 45°/65°. Migration data were used as displacement boundary conditions for the modelling to calculate tissue stress exposures. Migration values for the conventional bed were 1.75‐ and 1.6‐times greater than those for the migration‐reduction bed, for elevations of 45° and 65°, respectively (P < .001). The modelling showed that the farther the migration, the greater the tissue stress exposures. Internal stresses were 1.8‐fold greater than respective skin stresses. Our results, based on the novel integrated experimental‐computational method, point to clear biomechanical benefits in minimising migration using MRT.
Keywords: deep tissue injury, finite element modelling, head of bed elevation, motion capture analysis, pressure ulcer prophylaxis
Abbreviations
- BMI
body mass index
- DTI
deep tissue injury
- FE
finite element
- HOB
head of bed
- HAPI
hospital‐acquired pressure injury
- ICU
intensive care unit
- MRI
magnetic resonance imaging
- MIB
migration in bed
- MRT
migration reduction technology
- MCS
motion capture system
- PI
pressure injury
- SED
strain energy density
- VOI
volume of interest
- WHO
World Health Organisation
1. INTRODUCTION
Pressure injuries (PIs), also termed pressure ulcers, severely compromise the quality of life of affected individuals and can be life‐threatening.1 These wounds, comprising deep tissue injuries (DTIs) which are a sub‐category of PIs where tissue damage develops near a bony prominence under intact skin, form primarily because of cell and tissue exposure to sustained deformations which are typically caused by unrelieved bodyweight forces.2, 3, 4 In supine patients with impaired mobility or sensory functions (that are either permanent or temporary), the sacral region is a common site for PIs, including sacral DTIs.5, 6
A common clinical practice in hospitals—especially in intensive care units (ICUs)—is to elevate the head‐of‐bed (HOB) to at least 30° for lowering pulmonary aspiration and ventilator‐associated pneumonia risks,7 improving the hemodynamic stability8 or for better comfort.9 However, when hospital beds are articulated to raise the HOB section as recommended earlier, the body of the patient will often slide downwards in the bed, because of the gravity forces pulling the body towards the foot of the bed. This is a well‐known phenomenon, which is termed here “migration in bed” (MIB).10, 11, 12, 13 Because of MIB, hospital staffs are often required to pull patients up towards the HOB in cases where these patients cannot reposition themselves; Vasihadou et al reported that nurses may need to pull patients up in their bed 10 times per shift.14 MIB can be measured and quantified using a motion capture system (MCS) which determines the magnitudes of the displacements of anatomical landmarks in the patient's body relative to the bedframe, because of this gradual sliding motion effect.10 The extent of the MIB depends on the biomechanical patient‐bed interactions which are specific to the individual (eg, the levels of immersion and envelopment and the body‐support contact pressure distribution), as well as on the specific bed design and the materials of the clothing and bedsheets.11 The MIB generates kinetic friction and associated frictional forces between the body and the contacting materials and surfaces (ie, the clothing, bedsheet, and mattress). Specifically in the sacral region, these frictional forces cause shearing deformations in the soft tissues of the buttocks. In extreme cases where the skin is fragile, visible skin tears may develop.15 The internal shearing loads that buildup in tissues as the MIB progresses intensify near the rigid and highly curved sacral bone which promotes a “stress concentration” effect internally in the distorted soft tissues.4, 16 This tissue shearing effect that occurs between the sharp sacral bone and support is the primary factor causing the development of a sacral hospital‐acquired pressure injury (HAPI) in patients, on either ICU beds or conventional hospital beds.4, 16
From a health care system perspective, such HAPIs are a major concern, as they radically impact hospital quality measures, eg, safety of care, patient experience, and readmissions, as well as expenditures. In the USA alone, the annual direct costs of HAPIs exceed $26.8‐billion.17 The associated indirect costs of HAPIs, eg, on litigation and insurance premia, are also substantial, and are growing. For example, in the UK, most HAPI cases (80%–90%) are settled out‐of‐court for approximately £20 000–£30 000, but are trending upward, with some cases reaching as much as £1 to £3‐million.18 Accordingly, from a cost–benefit standpoint, the ideal strategy is primary prevention of HAPIs. The latest (2019) International Guidelines for Pressure Ulcer Prevention & Treatment elucidates that medical technologies demonstrating effective alleviation of tissue deformations and distortions should be beneficial in prophylaxis.4
The StayInPlace bed mechanism (HillRom Inc., Batesville, Indiana) is a new migration‐reduction technology (MRT) incorporated in the bedframe, which reduces the MIB by extending the head section of the bedframe and bed surface, in unison, as the HOB elevates.11, 12 This MRT technology is an optional feature in commercial hospital beds such as the Progressa ICU bed system (HillRom). Although the aforementioned MRT has already been shown to reduce the MIB in subject trials using MCS methodology,10, 11, 12, 13 the effects of reducing the MIB on sacral soft tissue loads during HOB elevation are currently unknown.
In the present study, we analysed the complex relationships between MIB and the resulting sacral soft tissue stresses, by combining MCS and three‐dimensional (3D) anatomically realistic biomechanical computer modelling. Specifically, we used digital motion analysis to calculate the net MIB resulting from elevation of the HOB to different inclination levels while subjects were lying in a bed that incorporated MRT, versus a bed without this new technology. Based on these experimental MIB data, we further developed a novel computational modelling framework for determining the effects of the MIB magnitude on the sacral soft tissue stresses.
2. METHODS
2.1. Motion capture measurements
A digital MCS (MotionAnalysis, Santa Rosa, California) was used to track the location of the trochanter of subjects who were lying supine on a mattress, with respect to the position of the mattress. The motion tracking was performed continuously during multiple tests (as detailed in the following), to calculate the net MIB during elevation of the HOB. Markers were placed on the skin of subjects, as well as on the mattress. The location of each marker was recorded as the HOB was elevated. The MIB was calculated for each subject relative to the mattress, as the difference between the initial and final spatial locations of the markers attached at the trochanter site, along the length of the bed (x‐axis) as described by Kotowski et al10 (Figure 1).
Figure 1.
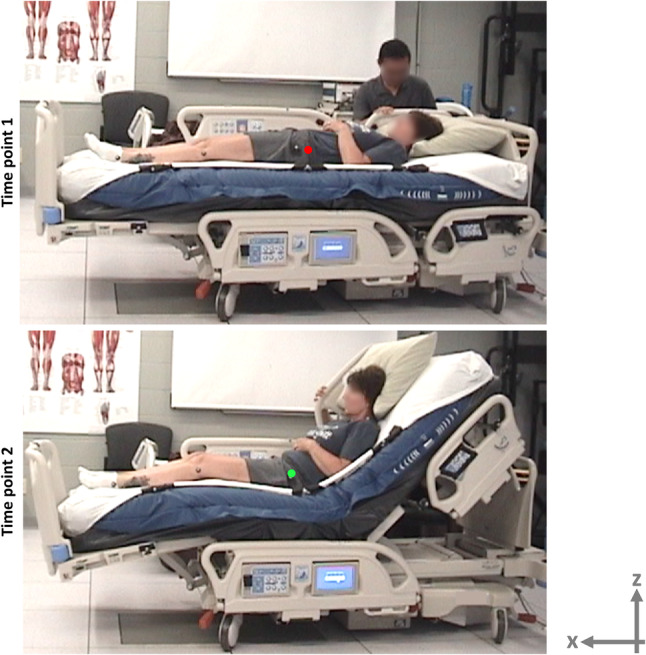
Acquisition of experimental body migration data in a motion capture laboratory: Markers were placed on the skin of subjects, as well as on the mattress. The location of each marker was recorded as the head of bed was elevated to either 45° or 65° inclination level. The red and green dots depict the location of the skin markers. The “trochanter migration” was defined along the length of the bed (x‐axis) and was calculated relative to the mattress as the difference between the initial and final spatial locations of the markers attached at the trochanter site
2.1.1. Participants
A convenience sample of 10 healthy subjects (five males, five females) participated in the study. All subjects provided their informed consent, and the protocol and informed consent form were reviewed by the IntegReview (Austin, Texas) Institutional Review Board (IRB approval no. ERGO‐2019‐01). The body characteristics of all participants are reported in Table 1. The bodyweight of participants ranged between 62 and 144 kg (median = 86 kg) and their height was 1.60–1.85 m (median = 1.73 m). Hence, the body mass index (BMI) of participants ranged between 24.3 and 41.8 kg/m2 (median = 30.6 kg/m2).
Table 1.
Gender (F = female, M = male), height, weight, and BMI data of the 10 subjects who participated in the motion capture measurements
| Gender | Height (m) | Weight (kg) | BMI (kg/m2) | |
|---|---|---|---|---|
| F | 1.60 | 62.1 | 24.3 | |
| F | 1.63 | 81.2 | 30.7 | |
| F | 1.65 | 68 | 25.0 | |
| F | 1.65 | 98 | 35.9 | |
| F | 1.75 | 74.8 | 24.4 | |
| M | 1.70 | 88.5 | 30.5 | |
| M | 1.75 | 83.9 | 27.3 | |
| M | 1.77 | 115.2 | 36.7 | |
| M | 1.80 | 102.1 | 31.4 | |
| M | 1.85 | 143.8 | 41.8 | |
| Mean | 1.72 | 91.8 | 30.8 | |
| SD | 0.08 | 24.3 | 5.9 |
2.1.2. Protocol
An experiment session involving a specific subject was divided into four tests, in which participants were lying on one of the two bed types (MRT bed or a conventional bed) as the HOB was raised to either 45° or 65°. Each test was repeated twice, which formed a total of eight tests per participant (two bed types, two HOB angles, and two repetitions). The MIB of each subject, per each test condition, was determined based on the MCS data as explained earlier.
2.1.3. Statistical analyses of the experimental data
We used a two‐way analysis of variance for the factors of (a) bed type and (b) HOB elevation in order to identify potential significant differences in the extent of MIB between the experimental conditions. Tukey pairwise comparisons were followed, to identify the specific combination of conditions affecting the MIB. A P < .05 was considered statistically significant.
2.2. Finite element model
2.2.1. Geometry
To examine the effects of the extent of MIB on the resulting soft tissue stresses near the sacrum, a 3D anatomical model of the buttocks, previously developed and experimentally validated by our group, has been used here.19, 20 This anatomical model is based on 76 magnetic resonance imaging slices of the buttocks of a 28‐year‐old healthy woman, which were segmented to pelvic/sacral bony structures versus soft tissues, and then 3D‐reconstructed using the Synopsys' Simpleware software package (Synopsis Inc, Mountain View, California). A 4‐cm‐thick standard medical foam mattress has also been generated and was located under the buttocks (Figure 2A).
Figure 2.
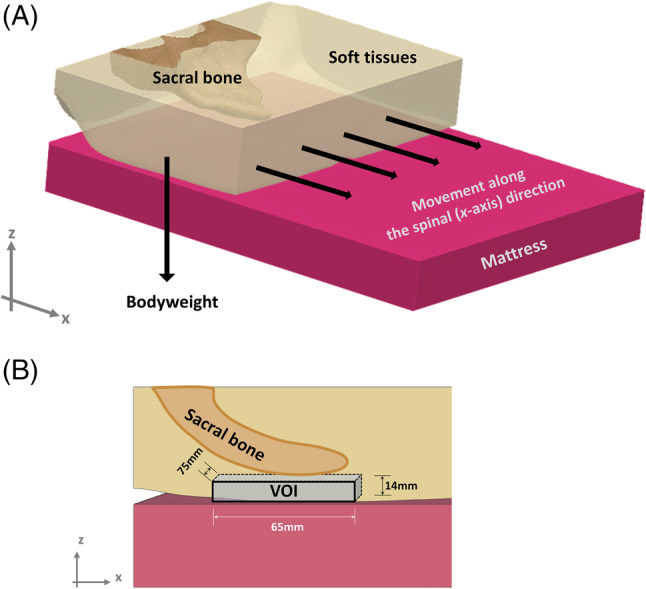
Geometry and boundary conditions applied to the computational model. A, The buttocks, deformed by the bodyweight during supine lying, incorporates a stiff sacral bone which distorts the soft tissues in its vicinity as the body migrates on the mattress, along the spinal (x‐axis) direction. B, The volume of interest of the soft tissues surrounding the sacrum, for computational analyses of deep soft tissue exposure to stress
2.2.2. Mechanical properties of model components
Constitutive laws and mechanical properties of the tissue components and the mattress were adopted from the literature. Specifically, the sacral bone was assumed to be a linear‐elastic isotropic material with elastic modulus of 7 GPa and a Poisson's ratio of 0.3.21, 22, 23 The soft tissues were assumed to be nearly incompressible (Poisson's ratio of 0.49) non‐linear isotropic, with their large deformation behaviour described by an uncoupled Neo‐Hookean model with a strain energy density function W (Equation (1)):
| (1) |
where Gins is the instantaneous shear modulus (2 kPa), λi(i = 1, 2, 3) are the principal stretch ratios, and K is the bulk modulus (1 kPa) and J = def(F) where F is the deformation gradient tensor.
Specifically, the material constants reported by Oomens et al24 were used to calculate an effective soft tissue instantaneous shear modulus Gins comprised of 60% skin and 40% fat, as in our previous publications of this modelling.20, 25 The mattress was considered as an isotropic linear‐elastic material, with an elastic modulus of 50 kPa and a Poisson's ratio of 0.3, likewise based on literature and consistent with our previous published work.25, 26, 27, 28
2.2.3. Boundary conditions
A downward displacement of 5 mm and horizontal displacements of 0, 4, 8, 12, and 16 cm were applied on the top surface of the model to simulate the descent of the weight‐bearing sacrum because of gravity and the MIB resulting from HOB elevation, respectively (Figure 2A). The above‐simulated MIB range included values that were experimentally measured using the MCS, with an addition of approximately 6 cm to account for any potential cases of more slippery support surfaces or clothing, or, possibly, greater HOB elevations than those set in our experiments. With regards to the latter point, it should be mentioned that patients often also slide in bed over time without HOB elevation, which has been termed “passive” MIB.12 A total reaction force of 40 N was obtained in all simulations; this force represented approximately 7% of the total bodyweight of the subject which were assumed to be transferred through the restricted modelled portion of the pelvic region that envelops the sacral bone, as detailed in our published work20 (Figure 2). Comparisons between all simulation cases were therefore conducted under the same (7% bodyweight) boundary conditions for consistency of outcome measures.20 Other boundary conditions that applied (identically across all simulation cases) were as follows. The bottom surface of the mattress was fixed for all motions, and tied interfaces were defined at the bone‐soft tissue boundaries. Frictional sliding was defined between the buttocks and the mattress, with the coefficient of friction set to 0.35.26, 29
2.2.4. Numerical method
Meshing of the model components (soft tissues, sacral bone, and mattress) was performed using the ScanIP module of Simpleware (Synopsis Inc). Four‐node linear tetrahedral elements were used in all model components. Finer meshes were used locally in specific regions where bony curvature is higher, or where soft tissues are known to be at a risk for a DTI, ie, near the sacral bone. The meshed model included approximately 500 000 elements.
The finite element (FE) simulations were set up using PreView of FEBio (Ver.1.19.0, University of Utah, Salt Lake City, Utah), analysed using the Pardiso linear solver of FEBio (Ver.2.5.0), and post‐processed using the PostView module of FEBio (Ver.1.10.2).30 The runtime of each model variant ranged between 2 and 9 hours using a 64‐bit Windows 10‐based workstation with an Intel Core i9‐7900X 3.30 GHz CPU and 64 GB of RAM.
2.2.5. Biomechanical outcome measures
Sliding in bed is directly related to shear stresses in skin and deeper soft tissues. Hence, we chose to specifically analyse shear stresses in superficial and deep soft tissues near the sacrum. However, for completeness, and in particular to account for the compressive and tensile stress components that also apply, we further calculated the effective stresses σe in soft tissues, which are defined as follows (at each tissue point):
| (EQ.2) |
where σc, σt, and σs are the compressive, tensile, and shear stress components, respectively.
We analysed and compared average shear and effective stresses in two volumes of interest (VOIs) of soft tissues near the sacrum: (a) a 3‐mm‐thin skin region of 270 × 170 mm, projected directly under the sacral bone and cantered at approximately the lowest point on the surface of the sacral bone, and (b) the skin (in the former VOI) and additionally deeper soft tissues contained in a block under the sacrum which was cantered as explained earlier (65 mm × 75 mm × 14 mm) (Figure 2B). The two aforementioned VOIs represented superficial and deep soft tissues under the sacrum, respectively, for analyses of their exposures to stresses depending on the MIB level. Accordingly, we compared volumetric exposures of soft tissues to elevated shear and effective stresses using stress exposure histogram charts, where the distribution of stress magnitudes in each VOI is presented.
3. RESULTS
3.1. Motion capture data
The trochanter migration values for the conventional bed were 7 ± 2.7 cm (mean ± SD) and 7.5 ± 3.7 cm, for the HOB elevation levels of 45° and 65°, respectively. For the MRT bed, however, these values were significantly lower, being 4.0 ± 1.9 cm and 4.7 ± 1.6 cm for HOB elevations of 45° and 65°, respectively (Figure 3; P < .001).
Figure 3.
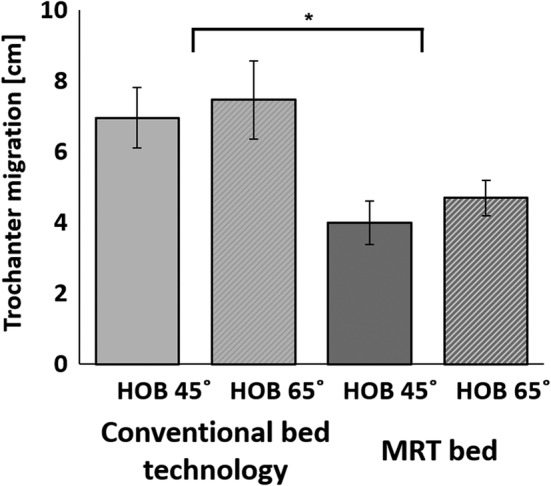
The average trochanter migration in a conventional bed versus that in a migration reduction technology (MRT) bed, for head‐of‐bed elevation of 45° and 65°. The MRT bed significantly reduced the extent of migration in bed with respect to the conventional bed technology. *P < .001, N = 10 subjects, two repetitions were conducted for each subject trial; error bars are the SEs
3.2. FE model
The present FE simulations demonstrated considerable soft tissue stress concentrations under the sacral bone for both the effective and shear stresses, and these stresses increased as MIB increased (Figure 4 and video provided in the Supplementary Materials). Near the skin surface, the magnitude of shear and effective stresses increased as MIB increased, as did the volume of skin tissue subject to elevated stresses (Figure 5). For the second VOI inclusive of the deeper tissues under the sacrum, increasing MIB was associated with increased effective/shear stresses and greater volume of soft tissues at elevated stress concentrations (Figure 6). The magnitudes of internal tissue shear stresses were on average 1.8‐fold greater than the respective shear stresses on skin (Figures 5 and 6).
Figure 4.
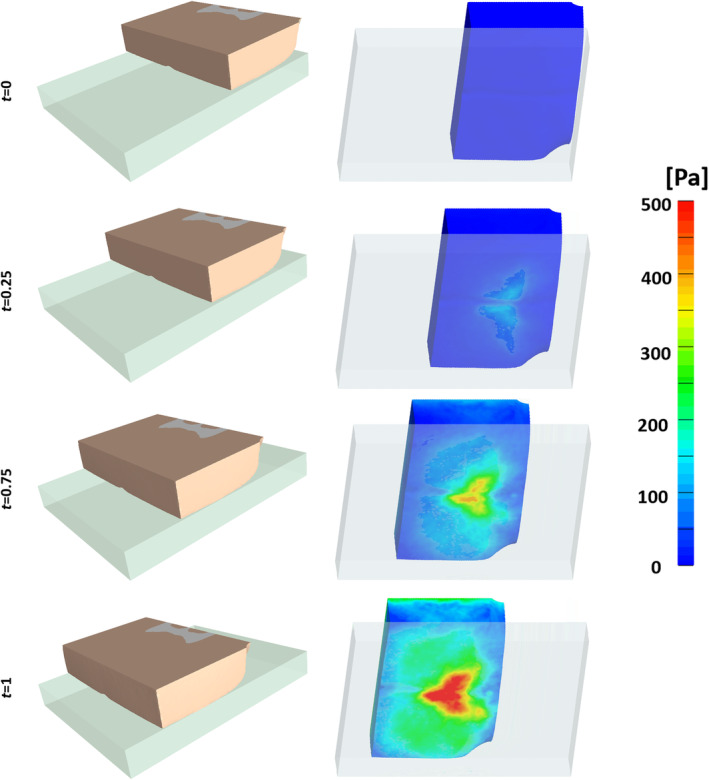
Shear stress distributions on the surface (skin) of the buttocks model as the body migrates farther on the mattress (dimensionless time units)
Figure 5.
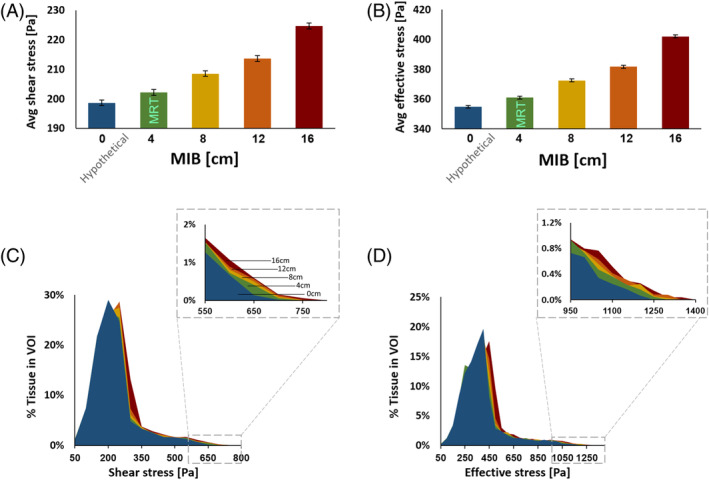
Average surface (skin) shear (A) and effective (B) stresses versus the extent of body migration in bed (MIB). The volumetric exposures to skin stresses exhibit a similar trend, where a growing skin tissue volume within the pre‐defined volume of interest is exposed to greater shear (C) and effective (D) stresses as the MIB increases
Figure 6.
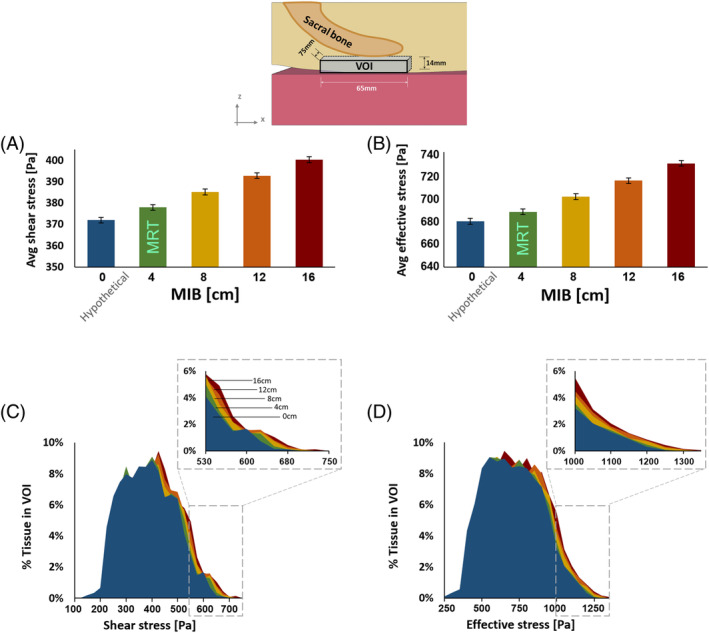
Average internal soft tissue shear (A) and effective (B) stresses at the vicinity of the sacral bone, ie, in a block of skin and subdermal tissues (taken together), versus the extent of body migration in bed (MIB). Volumetric exposures to the deeper tissue stresses exhibit a similar trend, where a growing tissue volume within the pre‐defined volume of interest (VOI) is exposed to greater shear (C) and effective (D) stresses as the MIB increases. The top frame depicts the tissue VOI which has been used here to determine deep tissue exposures to stresses, as function of the MIB
4. DISCUSSION
In the present study, we used a digital MCS to calculate the net MIB resulting from elevation of the HOB to different inclination levels (45° or 65°) while subjects were lying in a bed that incorporated MRT versus a conventional bed (Figure 1). Our data demonstrated that the trochanter migration values for the MRT bed were significantly lower than for the conventional bed, and that an HOB inclination of 45° yielded lower MIB than inclination of 65° (Figure 3), which was in agreement with published work.10, 11, 12, 13
Kotowski and Davis used an MCS to study potential factors that impact the MIB.31 Among the factors analysed in their work, namely the bed type (technology), HOB elevation level, the number of sequential bed articulations, gender, body height, and body mass, the bed type and HOB elevation level were found to be substantially more influential than the gender and body habitus. This recent publication thereby supports the generalizability of our present MIB findings. In addition, Sopher and colleagues discussed the World Health Organisation (WHO) definition of the “healthy” BMI range, and noted that the ‘healthy’ WHO BMI range does not necessarily apply globally, ie, across different countries.32 For example, the BMIs of the healthy population in Singapore may be lower than the WHO definition of ‘healthy’ whereas in the South and Midwest parts of the US, the BMI distributions of populations are shifted to the higher domains. In fact, large‐scale Center of Disease Control studies of the US population found that only 1 of 3 Americans has a ‘healthy’ BMI and 1 of 3 is obese; the BMI for the “average” adult American male is 28.6, which is above the WHO ‘healthy’ range.33 Hence, although the majority of our subjects were overweight as per the WHO definitions, the group was definitely representative of the US adult population in terms of bodyweights.
Based on the experimental MIB data, we further developed a novel computational modelling framework for determining the effects of the MIB on sacral soft tissue stresses. Our computational modelling results showed that the farther the MIB, the greater the surface and volumetric tissue stress exposures (Figure 4). Although this relationship is intuitive, this research is the first to investigate and quantify this effect. Specifically, we showed that during MIB, internal tissue shear is, on average, 1.8‐fold greater than the surface (interface) shear, which implies that MIB substantially increases risk for a sacral DTI34, 35, 36 (Figures 5 and 6). Although the increase in both shear and effective stresses because of increased MIB (between displacement levels of 0, 4, 8, 12, and 16 cm) is small as a percentage of baseline values, the consequences may be significant. The patient exposure to these stresses is cumulative over days and a modest increase can make the difference between non‐injury and an HAPI outcome in fragile individuals.3, 37
The present computational results are in agreement with previously published work of the authors with regards to sacral soft tissue stresses developed in a supine position, in the context of prophylactic sacral dressing studies.19, 25, 38, 39 Taken together, the body of literature suggests that MRT beds and biomechanically effective prophylactic dressing technologies may complement each other in protecting the sacral area, and this could be a topic of future work.
At‐risk patients are more exposed to HAPIs initiated or exacerbated because of MIB. Such individuals are, for example, mechanically ventilated patients who need substantial HOB elevation to improve respiratory function, improve hemodynamics, and lower their risk of developing pneumonia.7, 40, 41 Elevation of the HOB in such ventilated patients increases the loading on the sacral area42, 43 but every individual will experience different tissue loads depending on their anatomy and tissue composition and architecture. Moreover, the presence of any pathoanatomical and pathophysiological factors (of any kind) will interact differently with MIB‐induced tissue shear in different individuals, as it has been established now that there is no “universal” injury threshold.4, 44 For that reason, future work should incorporate other at‐risk anatomies, such as atrophied (bony), cachectic, and obese body structures.
The mobility of the individual is an important consideration affecting the buildup of shear stresses in soft tissues near bony prominences during MIB, as demonstrated in the present work. If a patient can self‐adjust or relief soft tissue loads by self‐repositioning, then, even if they cannot pull themselves back up in the bed, the detachment of contact between the skin and bed may be sufficient for “resetting” the shear forces, and therefore, much of the internal tissue stresses. In previous modelling of posterior heel loads of a patient who is sliding in bed, we found indeed that alleviating tissue shear by repositioning the heels after elevating, the HOB is critical for limiting the increase in tissue stresses and the subsequent risk of HAPIs.27
Computer modelling inevitably involves assumptions and simplifications. In order to facilitate direct comparisons between different extents of MIB, we have omitted some anatomical details and did not consider anatomical variants, which, as explained earlier, could potentially intensify the shear stresses in sacral soft tissues but would require additional numerical complexity and computing power to represent. The 3D model used here was previously developed and experimentally validated by our group in Levy et al19 and Levy and Gefen.20 This application of this model in the current study is further limited to the initial tissue response to MIB, ie, it does not consider the stress relaxation which is expected at the deformed tissues, with individual time characteristics that would again depend on age, tissue composition, and health status.
In conclusion, based on digital MCS analysis, we demonstrated that MRT beds can significantly reduce the MIB resulting from HOB elevation. In addition, we have used a state‐of‐the‐art biomechanical modelling framework for evaluating the effects of minimising the MIB on alleviation of sacral soft tissue stresses. This new integrated quantitative experimental‐computational method points to the strong prophylactic benefit in minimising the MIB, particularly using the MRT technology embedded in the bedframe, which is shown here to reduce the biomechanical risk for HAPIs.
5. IN MEMORIAM
The authors N.W. and A.G. wish to honour the memory of the late Dr Charlie Lachenbruch, a friend and colleague of ours who, as Chief Scientist at HillRom, envisioned this work, but passed away before its realisation. We know that Charlie, a world‐renowned expert of medical support surface technology, would be happy to see this study come to fruition and our research collaboration continued, to reduce the incidence of PIs.
Supporting information
Video S1 in the Supplementary MaterialsThe finite element simulations in this video file demonstrate considerable soft tissue stress concentrations near the sacral bone which intensify and spread as migration in bed occurs.
ACKNOWLEDGMENTS
The study was supported by an unrestricted educational grant from HillRom Inc. (Batesville, Indiana).
Lustig M, Wiggermann N, Gefen A. How patient migration in bed affects the sacral soft tissue loading and thereby the risk for a hospital‐acquired pressure injury. Int Wound J. 2020;17:631–640. 10.1111/iwj.13316
Funding information The study was supported by an unrestricted educational grant from HillRom Inc. (Batesville, IN, USA).
REFERENCES
- 1. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care. 2005;18(7):367‐372. [DOI] [PubMed] [Google Scholar]
- 2. Gefen A. How medical engineering has changed our understanding of chronic wounds and future prospects. Med Eng Phys. 2019;72:13‐18. [DOI] [PubMed] [Google Scholar]
- 3. Gefen A. The future of pressure ulcer prevention is here: detecting and targeting inflammation early. EWMA J. 2018;19(2):7‐13. [Google Scholar]
- 4. Gefen A, Brienza D, Edsberg L, et al. The etiology of pressure injuries. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP) and the Pan Pacific Pressure Injury Alliance (PPPIA). 3rd ed. Westford, MA: EPUAP‐NPIAP‐PPPIA; 2019. [Google Scholar]
- 5. Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manag. 2008;54(2):40‐54. [PubMed] [Google Scholar]
- 6. Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract. 2007;13(2):227‐235. [DOI] [PubMed] [Google Scholar]
- 7. Niël‐Weise BS, Gastmeier P, Kola A, Vonberg RP, Wille JC, van den Broek PJ. An evidence‐based recommendation on bed head elevation for mechanically ventilated patients. Crit Care 2011;15(2): R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Laat E, Schoonhoven L, Grypdonck M, et al. Early postoperative 30° lateral positioning after coronary artery surgery: influence on cardiac output. J Clin Nurs. 2007;16(4):654‐661. [DOI] [PubMed] [Google Scholar]
- 9. Coyne C, Baier W, Perra B, Sherer BK. Controlled trial of backrest elevation after coronary angiography. Am J Crit Care. 1994;3(4):282‐288. [PubMed] [Google Scholar]
- 10. Kotowski SE, Davis KG, Wiggermann N, Williamson R. Quantification of patient migration in bed. Hum Factors J Hum Factors Ergon Soc. 2013;55(1):36‐47. [DOI] [PubMed] [Google Scholar]
- 11. Davis KG, Kotowski SE. Role of bed design and head‐of‐bed articulation on patient migration. J Nurs Care Qual. 2015;30(3):E1‐E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis KG, Kotowski SE, Coombs MT. Stopping the slide. J Nurs Care Qual. 2017;32(1):E11‐E19. [DOI] [PubMed] [Google Scholar]
- 13. Wiggermann N, Kotowski S, Davis K, VanGilder C. The effect of patient migration in bed on torso elevation. Nurs Res. 2015;64(3):221‐225. [DOI] [PubMed] [Google Scholar]
- 14. Vasihadou A, Karvountzis GG, Soumilas A, Roumehotis D, Theodosopoulou E. Occupational low‐back pain in nursing staff in a Greek hospital. J Adv Nurs. 1995;21(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 15. Sterner E, Lindholm C, Berg E, Stark A, Fossum B. Category I pressure ulcers how reliable is clinical assessment? Orthop Nurs. 2011;30(3):194‐205. [DOI] [PubMed] [Google Scholar]
- 16. Gefen A, Farid KJ, Shaywitz I, et al. A review of deep tissue injury development, detection, and prevention: shear savvy. Ostomy Wound Manag. 2013;59(2):26‐35. [PubMed] [Google Scholar]
- 17. Padula WV, Delarmente BA. The national cost of hospital‐acquired pressure injuries in the United States. Int Wound J. 2019;16(3):634‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White R, Bennett D, Bree‐Aslan C, Downie F. Pressure ulcers, negligence and litigation. Wounds UK. 2015;11(1):8‐14. [Google Scholar]
- 19. Levy A, Schwartz D, Gefen A. The contribution of a directional preference of stiffness to the efficacy of prophylactic sacral dressings in protecting healthy and diabetic tissues from pressure injury: computational modelling studies. Int Wound J. 2017;14(6):1370‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy A, Gefen A. Assessment of the biomechanical effects of prophylactic sacral dressings on tissue loads: a computational modeling analysis. Ostomy Wound Manag. 2017;63(10):48‐55. [PubMed] [Google Scholar]
- 21. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129(6):924‐930. [DOI] [PubMed] [Google Scholar]
- 22. Palevski A, Glaich I, Portnoy S, Linder‐Ganz E, Gefen A. Stress relaxation of porcine gluteus muscle subjected to sudden transverse deformation as related to pressure sore modeling. J Biomech Eng. 2006;128(5):782‐787. [DOI] [PubMed] [Google Scholar]
- 23. Linder‐Ganz E, Shabshin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub‐dermal tissues during sitting: a combined experimental‐MRI and finite element approach. J Biomech. 2007;40(7):1443‐1454. [DOI] [PubMed] [Google Scholar]
- 24. Oomens CWJ, Zenhorst W, Broek M, et al. A numerical study to analyse the risk for pressure ulcer development on a spine board. Clin Biomech. 2013;28(7):736‐742. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz D, Levy A, Gefen A. A computer modeling study to assess the durability of prophylactic dressings subjected to moisture in biomechanical pressure injury prevention. Ostomy Wound Manag. 2018;64(7):18‐26. [PubMed] [Google Scholar]
- 26. Levy A, Frank MBO, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 27. Levy A, Gefen A. Computer modeling studies to assess whether a prophylactic dressing reduces the risk for deep tissue injury in the heels of supine patients with diabetes. Ostomy Wound Manag. 2016;62(4):42‐52. [PubMed] [Google Scholar]
- 28. Sopher R, Nixon J, McGinnis E, Gefen A. The influence of foot posture, support stiffness, heel pad loading and tissue mechanical properties on biomechanical factors associated with a risk of heel ulceration. J Mech Behav Biomed Mater. 2011;4(4):572‐582. [DOI] [PubMed] [Google Scholar]
- 29. Call E, Pedersen J, Bill B, et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J. 2015;12(4):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maas SA, Ellis BJ, Ateshian GA, FEBio WJA. Finite elements for biomechanics. J Biomech Eng. 2012;134(1):011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kotowski SE, Davis KG. Factors impacting the patient migration in hospital beds: pathway to reduce patient handling injuries. Int J SPHM. 2019;9(3):102‐110. [Google Scholar]
- 32. Sopher R, Nixon J, Gorecki C, Gefen A. Exposure to internal muscle tissue loads under the ischial tuberosities during sitting is elevated at abnormally high or low body mass indices. J Biomech. 2010;43(2):280‐286. [DOI] [PubMed] [Google Scholar]
- 33. Centers for Disease Control (CDC) . National Health and Nutrition Examination Survey: Healthy Weight, Overweight, and Obesity Among US Adults. 2003. Report #03–0260.
- 34. Stekelenburg A, Gawlitta D, Bader DL, Oomens CWJ. Deep tissue injury: How deep is our understanding? Arch Phys Med Rehabil. 2008;89(7):1410‐1413. [DOI] [PubMed] [Google Scholar]
- 35. Linder‐Ganz E, Gefen A. Stress analyses coupled with damage laws to determine biomechanical risk factors for deep tissue injury during sitting. J Biomech Eng. 2009;131(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 36. Agam L, Gefen A. Pressure ulcers and deep tissue injury: a bioengineering perspective. J Wound Care. 2007;16(8):336‐342. [DOI] [PubMed] [Google Scholar]
- 37. Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound Manag. 2008;54(10):26‐35. [PubMed] [Google Scholar]
- 38. Schwartz D, Gefen A. The biomechanical protective effects of a treatment dressing on the soft tissues surrounding a non‐offloaded sacral pressure ulcer. Int Wound J. 2019;16(3):684‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gefen A, Alves P, Creehan S, Call E, Santamaria N. Computer modeling of prophylactic dressings: an indispensable guide for healthcare professionals. Adv Skin Wound Care. 2019;32(7S Suppl 1):S4‐S13. [DOI] [PubMed] [Google Scholar]
- 40. Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94(3):281‐288. [DOI] [PubMed] [Google Scholar]
- 41. Keeley L. Reducing the risk of ventilator‐acquired pneumonia through head of bed elevation. Nurs Crit Care. 2007;12(6):287‐294. [DOI] [PubMed] [Google Scholar]
- 42. Pender LR, Frazier SK. The relationship between dermal pressure ulcers, oxygenation and perfusion in mechanically ventilated patients. Intensive Crit Care Nurs. 2005;21(1):29‐38. [DOI] [PubMed] [Google Scholar]
- 43. Peterson M, Schwab W, McCutcheon K, van Oostrom JH, Gravenstein N, Caruso L. Effects of elevating the head of bed on interface pressure in volunteers. Crit Care Med. 2008;36(11):3038‐3042. [DOI] [PubMed] [Google Scholar]
- 44. Gefen A, Clark M. Saving lives through pressure ulcer research: revisiting our decade‐old perspective of aetiology. Wounds Int. 2019;10(2):5‐9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 in the Supplementary MaterialsThe finite element simulations in this video file demonstrate considerable soft tissue stress concentrations near the sacral bone which intensify and spread as migration in bed occurs.


