Abstract
Background
Due to their genetic characteristics, their isolation in rainforest areas, and their traditional way of life, Amerindian populations are likely to suffer from a specific spectrum of dermatoses. However, there are few available data on such skin disorders. Our aims were to describe all skin disorders in two Amerindian villages of French Guiana.
Methods
This retrospective study concerned all patients who consulted in the Health Centres of Camopi and Trois‐Sauts between July 1, 2017, and December 31, 2018. We included all patients classified with an ICD code linked to a skin disorder. All medical records were cross‐checked by two dermatologists to correct misclassifications.
Results
A total of 639 patients formed the study population, for 866 different skin disorders. Non‐sexually transmitted infections represented 57.6% of all skin disorders, followed by eczema (11.5%) and bites/envenomations (9.1%). Bacteria were responsible for 238 skin infections, followed by fungi (141 cases) and parasites (69 cases, including 43 scabies, nine cutaneous leishmaniasis, and two tungiasis). We reported a low prevalence of sexually transmitted infections (10 cases) and an absence of skin cancers.
Conclusions
This study revealed the absence of skin cancer in the Amerindian population of the Upper Oyapock and the important burden of infectious and animal‐related diseases. Future studies should assess a possible underestimation of sexually transmitted diseases in this area. Public health policies should target neglected diseases such as cutaneous leishmaniasis, tungiasis, scabies, and envenomations. Atopic dermatitis was a significant and unexpected cause of consultations.
Introduction
French Guiana is an overseas French territory inhabited by communities of different ethnic origins, including Creoles and Maroons of African descent, along with Asian and European populations. Amerindians are the autochthonous inhabitants of French Guiana and live mostly along the rivers of the hinterland, maintaining a traditional way of life based on hunting, fishing, and gathering in the rainforest. They are in frequent contact with illegal Brazilian gold miners working clandestinely in French Guiana and often suffer from harsh economic and hygiene conditions.
There are few available data on skin disorders in Amerindians. Differences in HLA profiles might explain a lower frequency of androgenetic alopecia than in Caucasian populations, 1 while a high frequency of actinic prurigo has been reported among Chimila Indians in Colombia. 2 Only one study in Brazil has screened the full spectrum of dermatologic diseases in such a population, reporting a high prevalence of eczemas and infectious diseases, particularly fungal and parasitic. 3 Conversely, skin cancers seemed to be less frequent than in Caucasian populations. 3 Due to their genetic characteristics, their isolation in remote rainforest areas, and their traditional way of life, 4 one can expect Amerindian populations living in the Amazon to suffer from a different spectrum of dermatoses than Creole and Caucasian people.
Dermatological conditions in the Amerindian villages of French Guiana have never been described. About 12,000 Amerindians live in this territory, divided into six different ethnic groups. Many of them live in cities where ethnic diversity makes their study difficult, and the selection of patients according to their ethnic origin is illegal under French law. However, the study of medical data from the Health Centers for Remote Areas allows an in‐depth analysis of dermatological diseases among two large populations which are almost entirely composed of Amerindians. Indeed, the Upper course of the Oyapock is sparsely populated, the only two settlements being the villages of Camopi and Trois‐Sauts (about 1,700 inhabitants), which are home to the Teko and Wayampi tribes.
Our objective was to describe all skin disorders in the Amerindian villages of the Upper Oyapock over a period of 1‐1/2 years.
Materials and methods
The Health Centres for Remote Areas are primary care centers located in the villages of the French Guiana hinterland. These health centers are administratively managed as departments of the Cayenne General Hospital. Patients belonging to Amerindian traditional communities are followed up in these centers since their birth. Patients with life‐threatening emergencies and complex disorders are referred to the Cayenne General Hospital. Advice from specialists can also be requested through the telemedicine network to a referring doctor in the Cayenne General Hospital. Logistical issues limit the number of available blood tests, as samples must be carried down river to the coast and then by road to the Cayenne Hospital.
Since July 1, 2017, all diagnoses made in these health centers are listed with a code from the ICD 10 (International Classification of Diseases). Clinical data for all patients are saved in a prospective database through the SISv2 software. We extracted from this database all patients classified with an ICD code linked to a skin disorder, seen in consultation with a general practitioner in the Health Centers of Camopi and Trois‐Sauts between July 1, 2017, and December 31, 2018. All medical consultations were on demand. All data from the clinical examinations were cross‐checked by two dermatologists to correct the errors of quotations. This study was conducted in accordance with the Declaration of Helsinki and required no further ethical clearance according to the French Law. All clinical data were routinely used for care, and patients were informed that medical files could be used for retrospective studies.
Results
Population characteristics
In total, files from 661 patients were extracted. Twenty‐two patients were deemed as misclassifications and excluded from this study, as there was no mention of dermatologic lesions in their medical records. The remaining 639 patients formed the study population (Table 1). Among these 639 patients, 866 different skin disorders (Table 2) and 1027 consultations were recorded. Thirty‐six percent of the inhabitants of Camopi consulted at least once for a dermatological condition, with an average of 1.13 consultations/person/year. Sex ratio was almost equal (325 men, 50.9%). A slight predominance of adults over 16 years old (56.3%) was observed. Median age was 21 years (1–77). Concerning nationality, 77.5% of all patients were French, the rest being Brazilian citizens who dwell on the opposite bank. They usually work as illegal gold miners on the French side and visit the Health Centres when in urgent need of health care.
Table 1.
Skin disorders among 639 Amerindian patients of the Upper Oyapock (French Guiana), general characteristics of the study population, 2017–2018
| Characteristics | Number of patients n (%) |
|---|---|
| Consultations | 1,027 (100) |
| Number of patients | 639 (100) |
| Gender | |
| Female | 314 (49.1) |
| Male | 325 (50.9) |
| Median age (years) | 21 |
| Age | |
| Adults | 360 (56.3) |
| Children (<16) | 279 (43.7) |
| Nationality | |
| French | 495 (77.5) |
| Brazilian | 144 (22.5) |
Table 2.
Description of all skin disorders among 639 Amerindians of the Upper Oyapock (French Guiana), 2017–2018
| All disorders n = 866 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wounds, eschars n = 30 (3.5%) | Skin cancers n = 0 | ||||||||||
| Infections, non‐sexually‐transmitted n = 500 (57.6%) |
Sexually transmitted infections n = 10 (1.2%) |
Bites/envenomations n = 79 (9.1%) |
Inflammatory n = 132 (15.2%) |
Others n = 115 (13.4%) |
|||||||
| Bacterial (n = 238) | Parasitic (n = 69) | ||||||||||
|
Impetigo Secondary impetiginization Ecthyma Abscess Furuncle/carbuncle Erysipela Cellulitis Other localized infection Scarlet fever |
67 35 19 44 16 37 12 7 1 |
Scabies Leishmaniasis Hookworm‐related cutaneous larva migrans Myiasis (Dermatobia hominis) Tungiasis Head Lice |
43 9 9 5 2 1 |
Neisseria gonorrhoeae Chlamydia trachomatis Treponema pallidum |
4 3 3 |
Arthropods Scorpions Snakes Bat Human Fish Poisonous plant |
41 21 12 2 1 1 1 |
Atopic dermatitis Urticaria Contact dermatitis Irritative dermatitis Pityriasis rosea Psoriasis Dyshidrosis Seborrheic dermatitis Alopecia areata Acne |
89 17 11 3 3 3 2 2 1 1 |
Other eruption Itching Autosensitization dermatitis Sebaceous cyst Pigmentation disorder Ingrown toenail Acute lymphadenitis Sunburn Beri‐Beri Scars Infectious erythema |
57 29 12 6 3 2 2 1 1 1 1 |
| Viral (n = 52) | Fungal (n = 141) | ||||||||||
|
Hand, foot, and mouth disease Chickenpox Shingles Herpes simplex virus Measles Warts |
20 19 9 2 1 1 |
Dermatophytes Pityriasis versicolor Candida Undetermined cutaneous mycoses |
55 29 27 30 |
||||||||
Infectious diseases represented more than half of all conditions (57.6%). In addition, bite or envenomations (9.1%) and eczema (11.5%) were also of significant importance. There was no case of skin cancer. Teledermatology was used in 52 cases (6%), and the main reason for hospitalization was snake envenomation (n = 9). Some of these clinical presentations are displayed in Figure 1.
Figure 1.
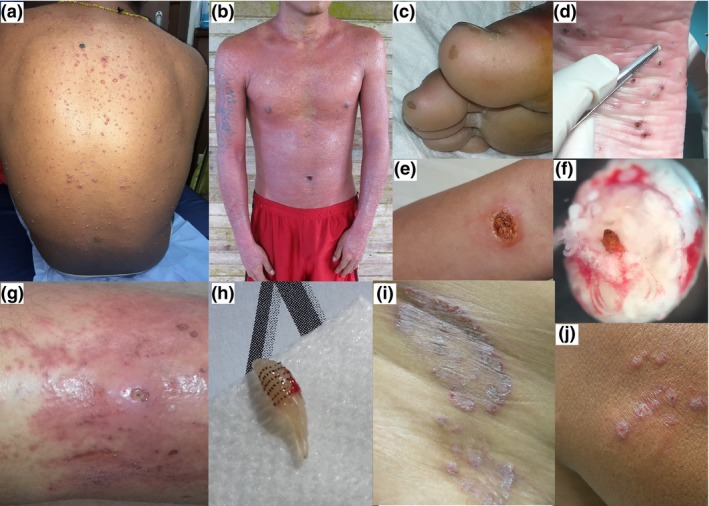
Clinical presentations of several skin disorders among Amerindians of the Upper Oyapock, 2017–2018; chickenpox on a young adult from Camopi (a); diffuse rash after use of traditional Amerindian medicine (b); typical brown scars on the toes following a bat bite by vampire bat (Desmodus rotondus) (c); multiple lesions of Tungiasis, central black dots surrounded by white eggs (d); ulcer caused by Leishmania braziliensis (e); microscopic view of Tunga penetrans after extraction, with the body of the female adult over the abdomen full of eggs (f); furunculoid myiasis caused by Dermatobia hominis (note the central punctum lesion) complicated with erysipela (g); larva of Dermatobia hominis after extraction (h); dermatophytosis on the hip of a young woman from Trois‐Sauts (i); several papules caused by Leishmania guyanensis (j) [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Infectious diseases
Bacterial skin infections were the most frequent disorder (n = 238) and accounted for 27.5% of all dermatologic consultations. The most frequent were impetigo (n = 67), cutaneous abscesses (n = 44), and erysipelas (n = 37). Impetigo was mostly observed in children (83.6%) with locations usually on the face (62.7%). Forty‐four cases of cutaneous abscess were reported, mostly in adults (58.5%) and males (58.5%). Oral antibiotics were used in 75% (n = 33) of cases, and 25% (n = 11) required a primary surgical procedure, which was directly carried out in the Health Centre. Inpatient treatment was never required. Likewise, we reported 37 cases of erysipelas, usually in adults (81%), involving the lower limbs (83.8%). Triggering factors such as wounds, intertrigo, or cutaneous myiasis were mentioned in 59.5% of cases. Inpatient treatment was required in only one case.
Fungal infections were the second most frequent disorder (n = 141). Fifty‐five cases of dermatophytosis were observed. It affected mainly adults (70.9%). Glabrous skin (45.5%) and folds (38.2%) were the most frequent localizations. A teledermatological opinion was requested four times to eliminate leprosy. Local treatment with an imidazole derivative was successful in 94.5% of the cases. Oral treatment was given in only 5.5% of patients. We also reported 29 cases of pityriasis versicolor, mostly in adults (65.5%) and females (62.1%). In 34.5% of cases, pityriasis versicolor was resistant to fungal treatments or recurrent.
We reported 69 cases of parasitic infections (7.9% of all disorders). Scabies was frequently reported (n = 43), with a clear predominance of children (67%). In 74.5% of cases, the family was not treated, and a second administration on the eighth day was missing for 72% of patients. Nine cases of cutaneous leishmaniasis were reported, including four cases of Leishmania guyanensis and two L. braziliensis infections. For three patients, PCR for Leishmania spp was positive, but the precise species could not be identified.
Viral infections were the least common skin infections in our study but remained significant in numbers (n = 51). They mainly affected children, including hand‐foot‐and‐mouth disease (n = 20). Nineteen cases of chickenpox were observed, including 18 in children and one severe case in an adult.
Concerning sexually transmitted diseases, we detected four cases of gonorrhea, three cases of chlamydia infection, and three cases of syphilis.
Bites and envenomations
We reported 79 cases of bites and envenomation. The most common were arthropod bites (n = 41), including ants, wasps, spiders, centipedes, and ticks. These bites were never complicated with bacterial infections or secondary eczema. Five cases of spider bites were recorded, incriminating a Theraphosa blondi in two occasions. There was no complication, and it never required hospitalization. Concerning scorpion bites (n = 21), they mostly affected adults (71.4%), on the hands (11/21) or feet (7/21). The median time before the consultation was 3 hours (1‐26), and the evolution was always favorable (n = 19). The electrocardiogram was performed in eight cases out of 21, and a specialist input was necessary for four occasions. We observed two complications (dysarthria with fasciculation in one patient, vomiting with agitation in another) and one hospitalization (dysarthria). Bites and envenomation by snakes (n = 12) affected mostly males (75%) and adults (91.7%) on the feet (91.7%). Brazilian patients represented one‐half of these consultations. Median time from the bite to the consultation was 3 hours (1‐48), and the tourniquet was used for two patients. Species were identified in 11 cases and were mainly Lachesis muta or Bothrops brazili (8/12). In all patients, a coagulation test was performed, of which eight were pathological. Inpatient treatment was required for nine patients. Complications were diverse (abscess, erysipelas, necrosis, hematoma, or renal failure). Regarding bat bites, the anti‐rabies center was systematically contacted (2/2) and the Zagreb protocol always performed (2/2).
Inflammatory/other diseases
Inflammatory diseases represented 15.2% of all skin disorders (132 cases). Notably, we reported 100 cases of eczema (11.5% of all skin disorders), including 11 cases of contact dermatitis and 89 cases of atopic dermatitis. Among other diseases, we reported no case of androgenetic alopecia or actinic prurigo.
Discussion
This study offers a clear insight of the full spectrum of skin diseases in the Upper Oyapock area. Skin infections were the main reasons for dermatological consultations in this cohort (57.6%). Similar epidemiological features were observed in the Xingu indigenous park in Brazil. 3 These results contrast with those of the European population. In two large British studies of dermatological presentations in general practice, infections represented between 6.1% and 20.3% of skin‐related consultations. 5 , 6 Meanwhile, a low frequency of skin cancers (0.8%) was highlighted in several Brazilian indigenous populations from the Mato Grosso State, 3 which is consistent with our results. Natural skin pigmentation can be protective against basal cell carcinoma, squamous cell carcinoma, and melanoma. The annual incidence of melanoma in Southwestern American Indians is less than one‐tenth of the incidence in Non‐Hispanic Whites of the same areas of Mexico. 7
This study underlines the important role of traditional life habits in the epidemiology of skin disorders in Amerindians. Bacterial infections or wounds were generally related to a post‐traumatic origin and therefore favored by traditional professions and leisure activities. In fact, this high proportion of bacterial skin infections can be explained by several factors such as numerous portals of entry (intertrigo, wound, insect bite); underlying dermatoses (higher ratio of eczema); easier transmission through community living; and warmth and humidity promoting bacterial and fungal growth. Similarly, a high prevalence (11%) of pyoderma has been highlighted in four remote Amerindian villages in Brazil (Amazonas State). 8
In this cohort, the most common fungal infections were dermatophytosis, candidiasis, and pityriasis versicolor. The latter is known to be more common in tropical countries. 9 These infections were responsible for a large number of consultations (n = 29). The persistence of environmental trigger such as heat or humidity might explain frequent recurrences. Conversely, no case of lobomycosis was described in our population, though this fungal infection has already been reported in French Guiana, 10 , 11 as in the Amerindian populations of Colombia 12 and Brazil. 13 The role of dolphins as hosts of Lacazia loboi has been recently discarded, 14 and its parasitic cycle remains unclear.
Concerning parasitic infections, scabies is known to be a common disease in Amerindian populations 3 because of promiscuity and poor socioeconomic conditions. Out of 398 consultations, 36 cases (9%) of scabies were reported in the Xingu Park. 3 Similar figures were observed in our study (5%). We also observed some cases of tungiasis. Infection with Tunga penetrans is a neglected tropical disease 15 and underlines the burden of poor living conditions in Amerindian communities of French Guiana. Cutaneous leishmaniasis was reported on nine occasions, an important incidence compared to the coastal region of French Guiana. 16 , 17
Regarding viral infections, we observed a high proportion of chickenpox. The incidence of varicella is usually lower in tropical areas. 18 However, from the results of our study, an annual incidence of 7.5 cases per 1,000 people per year can be estimated, roughly similar to the figures observed in mainland France. 19 Only one case of viral wart was recorded, while 28 cases out of 398 clinical records were reported in the Xingu Park. 3 Trunk was a common location, probably due to the frequent absence of upper body clothing. 3 The introduction of Western clothes among Amerindians of French Guiana could explain a lower incidence of cutaneous trauma leading to warts.
Another important result of this study is the very small proportion of sexually transmitted infections (STIs). These findings should be interpreted with caution, as a much higher prevalence (4.2–11.6%) was reported in indigenous communities of Argentina, 20 Paraguay, and Peru. 21 STIs might be underdiagnosed in French Guiana due to a lack of awareness in clinicians and patients. Stigma and discrimination in small communities may be an important reason for not getting tested. 22 , 23 Geographical isolation is often cited as a protective factor, limiting contact with other ethnicities and therefore reducing risks of contamination. 24 However, a close monitoring of STIs in these communities should be maintained.
Amerindian populations across South America often share similar living conditions in the rainforest environment. However, cases of bites or envenomation were not described in previous studies, 3 while they represented 9.1% of skin disorders in our patients. Snake bites are now acknowledged as neglected tropical diseases. 25 Many cases of deaths by snake envenomation were recorded in French Guiana between 2007 and 2015. 26 , 27 In the following years, several public health actions 26 , 27 were implemented. Results of these actions can be seen through the well‐codified care offered in patients in our study.
Our results show a high proportion of both eczema (11.5% of all disorders) and parasitic skin diseases (7.9%) in a population which is also highly exposed to soil‐transmitted helminthiasis. 28 These data, in line with previous Brazilian ones, 3 contradict the “hygiene hypothesis” which states that an absence of early exposure to parasitic infection impairs the development of the Th1 pathway and increases Th2 polarization, resulting in an increased incidence of allergic or atopic diseases. 29 , 30
Other studies among native groups across South America reported a lower prevalence of androgenetic alopecia in the Mapuche population than in Caucasians, probably because of different HLA genetic profiles. 1 Similarly, we report no case of androgenetic alopecia. A high prevalence of actinic prurigo has been reported among the Chimila Indians, 2 which was not observed in our patients. Different genetic and environmental factors among indigenous peoples of South America could explain the diverse incidences of skin diseases in these populations.
This retrospective study has several limitations. Diagnoses were made by general practitioners, though we tried to reduce this bias by cross‐checking all diagnoses with dermatologists. Amerindians are known to rely on traditional self‐medication, which might have led us to under‐diagnoses. 31 Despite these limitations, this work provides data on a large population which has been scarcely studied. These findings will be of great help to improve the management of skin diseases in the Amerindian populations of French Guiana and across South America.
Conflict of interest: None.
Funding source: None.
[Correction added on April 02, 2020 after first online publication: the copyright line has been changed.]
References
- 1. Mardones F, Valenzuela Y, Zemelman V. Analysis of androgenetic alopecia in Amerindian people (Mapuche) from southern Chile. Int J Dermatol 2016; 55: e595–e596. [DOI] [PubMed] [Google Scholar]
- 2. Bernal JE, Duran de Rueda MM, Ordonez CP, et al Actinic prurigo among the Chimila Indians in Colombia: HLA studies. J Am Acad Dermatol 1990; 22(6 Pt 1): 1049–1051. [DOI] [PubMed] [Google Scholar]
- 3. Wu JSA, Florian MC, Rodrigues DA, et al Skin diseases in indigenous population: retrospective epidemiological study at Xingu Indigenous Park (XIP) and review of the literature. Int J Dermatol 2017; 56: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 4. Groupe international de travail pour les peuples autochtones− GITPA. 2005. Avalaible at: https://gitpa.org/Peuple%2520GITPA%2520500/gitpa500-2-GUYANEfiche.pdf (last accessed 11 October 2019).
- 5. Kerr OA, Tidman MJ, Walker JJ, et al The profile of dermatological problems in primary care. Clin Exp Dermatol 2010; 35: 380–383. [DOI] [PubMed] [Google Scholar]
- 6. Julian CG. Dermatology in general practice. Br J Dermatol 1999; 141: 518–520. [DOI] [PubMed] [Google Scholar]
- 7. Black WC, Wiggins C. Melanoma among southwestern American Indians. Cancer 1985; 55: 2899–2902. [DOI] [PubMed] [Google Scholar]
- 8. Lawrence DN, Facklam RR, Sottnek FO, et al Epidemiologic studies among Amerindian populations of Amazônia. I. Pyoderma: prevalence and associated pathogens. Am J Trop Med Hyg 1979; 28: 548–558. [PubMed] [Google Scholar]
- 9. Gupta AK, Bluhm R, Summerbell R. Pityriasis versicolor. J Eur Acad Dermatol Venereol 2002; 16: 19–33. [DOI] [PubMed] [Google Scholar]
- 10. Cheuret M, Miossec C, Milley S, et al A 43‐year‐old Brazilian man with a chronic ulcerated lesion. Clin Infect Dis Off Publ Infect Dis Soc Am 2014; 59: 314–315. [DOI] [PubMed] [Google Scholar]
- 11. Heleine M, Blaizot R, Cissé H, et al A case of disseminated paracoccidioidomycosis associated with cutaneous lobomycosis. J Eur Acad Dermatol Venereol 2020; 34: e18–e20. [DOI] [PubMed] [Google Scholar]
- 12. Rodríguez‐Toro G, Tellez N. Lobomycosis in Colombian Amer Indian patients. Mycopathologia 1992; 120: 5–9. [DOI] [PubMed] [Google Scholar]
- 13. Baruzzi RG, Castro RM, D'Andretta C, et al Occurrence of Lobo’s blastomycosis among « Caiabi, » Brazilian Indians. Int J Dermatol 1973; 12: 95–99. [DOI] [PubMed] [Google Scholar]
- 14. Vilela R, Bossart GD, St Leger JA, et al Cutaneous granulomas in dolphins caused by novel uncultivated Paracoccidioides brasiliensis. Emerg Infect Dis 2016; 22: 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldmeier H, Sentongo E, Krantz I. Tungiasis (sand flea disease): a parasitic disease with particular challenges for public health. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2013; 32: 19–26. [DOI] [PubMed] [Google Scholar]
- 16. Simon S, Nacher M, Carme B, et al Cutaneous leishmaniasis in French Guiana: revising epidemiology with PCR‐RFLP. Trop Med Health 2017; 45: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loiseau R, Nabet C, Simon S, et al American cutaneous leishmaniasis in French Guiana: an epidemiological update and study of environmental risk factors. Int J Dermatol 2019; 58: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heininger U, Seward JF. Varicella. Lancet 2006; 368: 1365–1376. [DOI] [PubMed] [Google Scholar]
- 19. Bilan annuel 2017. Réseau Sentinelles, INSERM, UPMC. 2017. Available at: https://www.sentiweb.fr/document/4263 (last accessed 11 October 2019).
- 20. Eirin ME, Delfino CM, Pedrozo WR, et al Health care importance of Treponema pallidum, Chagas’ disease and Human immunodeficiency virus 1 among Amerindians of Argentina: an observational study. Rev Argent Microbiol 2017; 49: 315–319. [DOI] [PubMed] [Google Scholar]
- 21. Russell NK, Nazar K, Del Pino S, et al HIV, syphilis, and viral hepatitis among Latin American indigenous peoples and Afro‐descendants: a systematic review. Rev PanamSalud Publica Pan Am J Public Health 2019; 43: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Melle A, Parriault M‐C, Basurko C, et al Prevalence and predictive factors of stigmatizing attitudes towards people living with HIV in the remote villages on the Maroni River in French Guiana. AIDS Care 2015; 27: 160–167. [DOI] [PubMed] [Google Scholar]
- 23. Van Melle A, Parriault M‐C, Basurko C, et al Knowledge, attitudes, behaviors, and practices differences regarding HIV in populations living along the Maroni river: particularities of operational interest for Amerindian and Maroon populations. AIDS Care 2015; 27: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 24. Mosnier E, Guiraud N, Epelboin L, et al Diagnostic et prise en charge des PVVIH en zones isolées et frontalières en Guyane. 2015. Available at: https://www.hal.inserm.fr/inserm-01422859 (last accessed 11 October 2019).
- 25. Chippaux J‐P. Snakebite envenomation turns again into a neglected tropical disease!. J Venom Anim Toxins Trop Dis 2017; 23: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mutricy R, Egmann G, Marty C, et al Predictors of complications of snake envenomation in Cayenne, French Guiana, 2007–2015. Intensive Care Med 2018; 44: 115–117. [DOI] [PubMed] [Google Scholar]
- 27. Mutricy R, Heckmann X, Douine M, et al High mortality due to snakebites in French Guiana: Time has come to re‐evaluate medical management protocols. PLoSNegl Trop Dis 2018; 12: e0006482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carme B, Motard A, Bau P, et al Intestinal parasitoses among Wayampi Indians from French Guiana. Parasite 2002; 9: 167–174. [DOI] [PubMed] [Google Scholar]
- 29. Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology 2007; 212: 441–452. [DOI] [PubMed] [Google Scholar]
- 30. Fallon PG, Mangan NE. Suppression of TH2‐type allergic reactions by helminth infection. Nat Rev Immunol 2007; 7: 220–230. [DOI] [PubMed] [Google Scholar]
- 31. Odonne G, Berger F, Stien D, et al Treatment of leishmaniasis in the Oyapock basin (French Guiana): a K.A.P. survey and analysis of the evolution of phytotherapy knowledge amongst Wayãpi Indians. J Ethnopharmacol 2011; 137: 1228–1239. [DOI] [PubMed] [Google Scholar]


