Abstract
Objectives
The aim of the study was to assess the validity of an easy‐to‐calculate chronic kidney disease (CKD) risk score developed by the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) group in a longitudinal observational study of people living with HIV (PLWH) in the USA.
Methods
PLWH (2002–2016) without prior exposure to potentially nephrotoxic antiretroviral agents and with at least three estimated glomerular filtration rate (eGFR) test results were identified in the Observational Pharmaco‐Epidemiology Research and Analysis (OPERA®) cohort. Three samples were drawn independently using the same eligibility criteria but each using a different eGFR equation, specifically the Cockcroft–Gault (C‐G), Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) eGFR estimation method. Full and short D:A:D risk scores were applied. CKD was defined as a confirmed decrease in eGFR to < 60 mL/min/1.73 m2 (stages 3–5). Poisson models estimated the association between CKD incidence and a one‐point increase in the continuous risk score. The incidence rate ratio (IRR), adjusted IRR (aIRR), and Harrell's discrimination statistic were used to assess validity.
Results
There were 19 444, 22 727 and 22 748 PLWH in the OPERA C‐G, CKD‐EPI and MDRD samples, respectively. The median (minimum–maximum) follow‐up duration was 6.1 (0.3–9.1) years in the D:A:D cohort and ranged from 3.2 to 3.5 (0.2–15.5) years in the OPERA validation samples. The observation time for the majority of PLWH in the D:A:D cohort began prior to 2006, in stark contrast to the OPERA validation samples, where the majority of PLWH were observed after 2011. The CKD incidence ranged from 7.3 per 1000 person‐years [95% confidence interval (CI) 6.8, 7.9 per 1000 person‐years] in OPERA C‐G to 11.0 (95% CI 10.4, 11.6 per 1000 person‐years) in OPERA MDRD. In OPERA samples, IRRs by risk group and adjusted IRRs (full risk score) were similar to those in the D:A:D derivation cohort (adjusted IRR 1.3; 95% CI 1.3, 1.3). Harrell's c‐statistic ranged from 0.87 to 0.92 in the OPERA samples, comparable to that in the derivation cohort (0.92). Results for short scores were similar.
Conclusions
The findings support the validity of the D:A:D risk scoring method for assessing CKD (stages 3–5) probability in an exclusively USA‐based sample regardless of eGFR method.
Keywords: chronic kidney disease, Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) risk score, HIV, renal impairment, validation
Introduction
Several studies have suggested that people living with HIV infection (PLWH) are at increased risk for chronic kidney disease (CKD) with a multifactorial pathogenesis 1, 2, 3, 4, 5. While the use of combination antiretroviral therapy (cART) as the standard of care in HIV infection has resulted in a decreased incidence of HIV‐associated nephropathy, it has also lengthened life expectancy, accompanied by an increased incidence of age‐related diseases, such as CKD 6, 7, 8.
Acute kidney injury (AKI) is also common in this population, and is generally a byproduct of volume depletion, ischaemic renal insult from septicaemia, and nephrotoxic medications 7, 9, 10, 11, 12. The risk of AKI may be exacerbated by noncommunicable comorbidities such as hypertension, diabetes and liver disease. AKI may increase the risk for incident CKD or hasten progression in persons with pre‐existing CKD 12, 13. Studies have linked tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor, and indinavir, a protease inhibitor, to decreased glomerular filtration rates (GFRs) 14, 15. The potential for TDF nephrotoxicity may be increased by the concurrent administration of a select subset of medications, in particular pharmacokinetic enhancers 16, 17, 18. Cohort studies have also found an increased risk of CKD among persons exposed to ritonavir‐boosted atazanavir and lopinavir 17, 18.
A recent systematic review and meta‐analysis estimated the pooled prevalence of CKD [estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2] in PLWH in North America to range from a low of 6.5% [95% confidence interval (CI) 4.9–8.5%] using the Cockcroft–Gault (C‐G) formula to a high of 7.4% (95% CI 6.0–9.1%) using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula 19. Studies based on varying levels of GFR reduction and proteinuria have suggested that CKD prevalence in PLWH may be as high as 33% 7.
The risk of CKD substantially complicates clinical decision‐making. However, the ability of clinicians to assess CKD risk prior to prescribing cART is constrained by the lack of an easy‐to‐implement risk assessment tool. Such risk scores have been proposed previously but were not implemented in routine clinical practice because of concerns about study design 20, 21. In 2015, Mocroft et al. introduced two risk scores for CKD in PLWH using data from the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) study 22. The full risk score included cardiovascular risk factors while a short risk score was designed to estimate risk in patients where only basic HIV information is known. The D:A:D study is a prospective observational study, details of which have been published previously 23. The D:A:D cohorts contribute data on 49 717 persons enrolled at 212 clinics primarily located in Europe and Australia with smaller sites in the USA and Argentina 23. The D:A:D CKD risk score was developed using the C‐G eGFR formula and has been previously validated using comparatively small samples from European and Australian practices as well as data from two international clinical trials 22, 24, 25. To date, however, the algorithm has not been validated in PLWH receiving care in the USA. Given differences in study population and clinical practice, it is unclear if the D:A:D risk score algorithm can be applied in an exclusively USA‐based population. In addition, the majority of laboratories in the USA report eGFR using the Modification of Diet in Renal Disease (MDRD) or CKD‐EPI based estimations, not the C‐G formula used in the development of the D:A:D risk score.
Methods
Study design and population
This study was an observational clinical cohort analysis utilizing electronic health record data from 400 providers, in 85 clinics across 54 cities, obtained from the Observational Pharmaco‐Epidemiology Research and Analysis (OPERA®) database (Fig. 1). The 75 579 HIV‐infected patients in the OPERA database up to 2016 represent approximately 8% of the HIV‐infected patients diagnosed and linked to care in the USA. The OPERA database complies with all Health Insurance Portability and Accountability Act (HIPAA) and Health Information Technology for Economic and Clinical Health Act (HITECH) requirements and received annual institutional review board (IRB) approval by Advarra IRB, including a waiver of informed consent and authorization for use of protected health information.
Figure 1.
People living with HIV in the Observational Pharmaco‐Epidemiology Research and Analysis (OPERA) database and Centers for Disease Control and Prevention (CDC) (2010) state‐by‐state estimates of HIV infection in the USA.
PLWH aged 18 years and older, whose first “normal” eGFR test result (> 60 mL/min/1.73 m2) occurred between 1 January 2002 and 31 December 2016, were observed from the day of the first eligible test result (index) until the first of the following censoring events: last eGFR test result, death, the occurrence of the study outcome (stage 3–5 CKD), loss to follow‐up (i.e. 6 months following the last visit) or study end (31 July 2017). Patients were excluded from the study if they (1) had any pre‐index exposure to TDF, or any boosted protease inhibitor; (2) had fewer than three eGFR measurements on or after the index date; (3) had < 3 months of observation or (4) had a history of kidney transplant, dialysis or diagnosis of moderate (stage 3) or severe (stage 4 or higher) CKD. The 12‐month period preceding index was used to assess patient demographic and clinical characteristics.
Three samples were drawn to validate the D:A:D risk score, each using the same eligibility criteria but each using a different eGFR equation, specifically (1) MDRD, (2) C‐G and (3) CKD‐EPI. The reader should note that differences in eGFR estimation in each of the three formulas may influence entry into the study sample for participants with eGFR values near the entry threshold of 60 mL/min/1.73 m2.
D:A:D CKD risk score
Full and short risk scores were calculated by summing coefficients associated with baseline risk factors (Fig. 2). HIV exposure group (injecting drug user versus all others), gender, hepatitis C virus coinfection, age, nadir CD4 count (≤ 200 cells/μL), baseline eGFR, hypertension, diabetes, and prior CVD at baseline were all significant predictors of CKD and were included in the full risk score model. The shortened version of the risk score estimated CKD risk using only HIV‐specific factors, without the inclusion of traditional cardiovascular risk factors. Patients were categorized as being at low risk of stage 3–5 CKD if the D:A:D risk score was < 0; at medium risk if the score was between 0 and 4, and at high risk if the score was ≥ 5.
Figure 2.
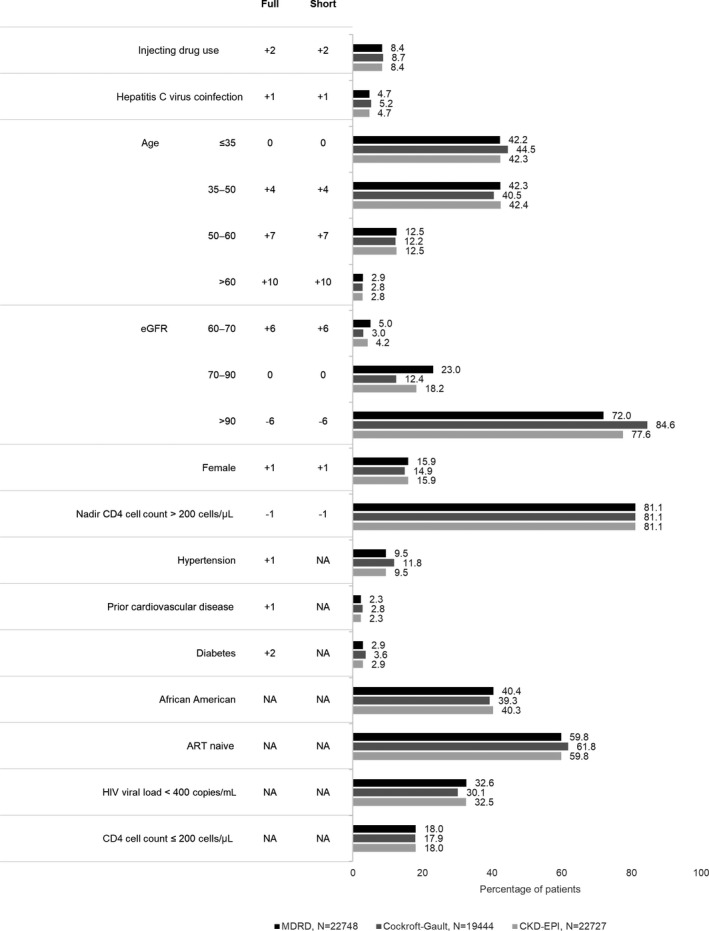
Baseline patient characteristics and Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) full and short risk calculations, by Observational Pharmaco‐Epidemiology Research and Analysis (OPERA) validation cohort. ART, antiretroviral therapy; eGFR, estimated glomerular filtration rate; NA, not applicable.
Study outcome
CKD was defined as a confirmed decrease in eGFR to < 60 mL/min/1.73 m2 (stage 3–5 CKD). A confirmed decrease required that there be at least two consecutive eGFR test results with values < 60 mL/min/1.73 m2, more than 90 days apart. If there were multiple eGFR measurements < 60 mL/min/1.73 m2, each less than 90 days apart, then the total duration of the difference between the dates of the first and last measurements of eGFR < 60 mL/min/1.73 m2 must have exceeded 90 days.
Statistical analysis
The number and proportion of persons developing stage 3–5 CKD, as well as the incidence of CKD per 1000 person‐years (PY) of observation were reported in aggregate and by risk stratum. The validity of each CKD risk score (short and full) was evaluated separately by comparing each of the three OPERA validation samples to the D:A:D derivation cohort using the following metrics: (1) unadjusted CKD incidence rates within risk score stratum, (2) unadjusted incidence rate ratios (IRRs) for the association between CKD and risk stratum (with medium risk as referent), (3) Kaplan–Meier CKD progression rates at 5 years, (4) adjusted IRR for the association between CKD and a one‐point increase in the continuous risk score (Poisson regression) and (5) model discrimination statistics (Harrell's c‐statistic).
Results
Application of study eligibility criteria yielded sample sizes of 22 748, 19 444 and 22 727 in the OPERA MDRD, OPERA C‐G and OPERA CKD‐EPI validation samples, respectively. Demographic and clinical characteristics were similar across all three OPERA samples (Fig. 2). In contrast, there were numerous differences in the underlying populations between the OPERA validation samples and the D:A:D derivation cohort (Table S1). The D:A:D derivation cohort population was slightly older, and more likely to be female, to be coinfected with viral hepatitis B and/or C, to have a history of AIDS, to be ART‐experienced and to have viral loads < 400 HIV‐1 RNA copies/mL. Incomplete data on race in the D:A:D derivation cohort precluded further comparison. Differences between the OPERA validation sample and the D:A:D derivation cohort may reflect the status of HIV care at the time the care was delivered, as 75% of the D:A:D cohort began observation prior to 2007, while 75% of patients in the OPERA validation samples began observation in or after 2007. Accordingly, median (minimum–maximum) follow‐up duration in the D:A:D cohort was notably longer, at 6.1(0.3–9.1) years, than was observed in the OPERA samples, where follow‐up in the three validation samples ranged from 3.2 to 3.5 (0.2–15.5) years.
Median full risk scores were −3 [interquartile range (IQR) −6 to 3] in OPERA MDRD, −3 (IQR −7 to 0) in OPERA C‐G and −3 (IQR −7 to 1) in OPERA CKD‐EPI, each similar to the median full risk score reported in the D:A:D derivation cohort [−2 (IQR −4 to 2)]. Similarly, median short risk scores were −3 (IQR −7 to 2) in OPERA MDRD, −3 (IQR −7 to −1) in OPERA C‐G, −3 (IQR −7 to 0) in OPERA CKD‐EPI, and −2 (IQR −3 to 1) in the D:A:D derivation cohort. Risk scores, both short and full, were notably higher in patients who developed CKD than in those who did not. Median full risk scores among patients developing CKD were 6 (IQR 3 to 10) in OPERA MDRD, 7 (IQR 2 to 12) in OPERA C‐G, 6 (IQR 1 to 10) in OPERA CKD‐EPI, and 10 (IQR 5 to 14) in the D:A:D derivation cohort. Median short risk scores were similar.
The incidence of CKD did vary by eGFR formula, with CKD occurring in 3.4% of patients in OPERA C‐G at a rate of 7.3 (95% CI 6.8, 7.9) per 1000 PY of observation, in 5.2% of patients in the OPERA MDRD sample at a rate of 11.0 (95% CI 10.4, 11.6) per 1000 PY and in 5.2% of patients in the OPERA CKD‐EPI sample at a rate of 10.8 (95% CI 10.2, 11.4) per 1000 PY. The CKD incidence rate observed in the OPERA C‐G sample was slightly higher than that observed in the D:A:D derivation cohort (6.2; 95% CI 5.7, 6.7), which also was predicated on the Cockcroft–Gault equation. Both the OPERA C‐G sample and the D:A:D derivation cohort had notably higher CKD incidence rates than were reported in the original D:A:D validation cohorts: 5.1 (95% CI 4.1, 6.1) in the Royal Free Hospital cohort and 3.8 (95% CI 2.5, 5.1) in the strategies for management of antiretroviral therapy/evaluation of subcutaneous proleukin® in a randomized international trial (SMART/ESPRIT) cohort.
The rate of CKD increased by risk stratum in all three OPERA samples, as it did in the D:A:D derivation cohort itself, using the full risk scores (Fig. 3). Compared to the medium‐risk group, being in the low‐risk group appeared protective (Fig. 4). IRR in the low‐risk group ranged from 0.14 to 0.32 in the OPERA samples as compared to 0.12 in the D:A:D derivation cohort, all with overlapping CIs. In contrast, being in the high‐risk group appeared harmful. The IRR in the high‐risk group ranged from 4.2 to 6.4 in the OPERA samples as compared to 8.1 in the D:A:D derivation cohort, with overlapping CIs only in the OPERA C‐G sample. A similar pattern was observed using the short risk score (data not shown).
Figure 3.
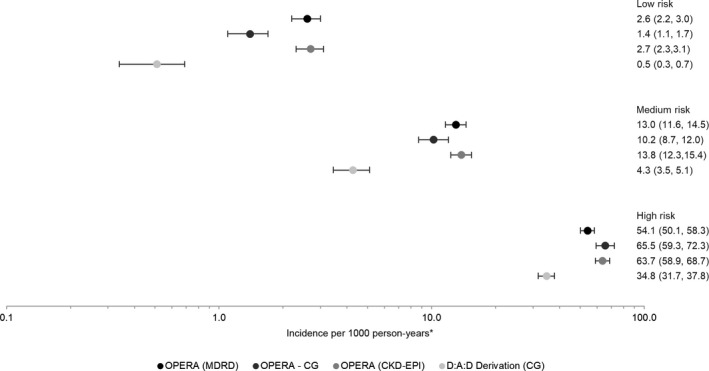
Crude chronic kidney disease (CKD) incidence and 95% confidence intervals, by study population and CKD full risk score. *The scale is logarthmic.
Figure 4.
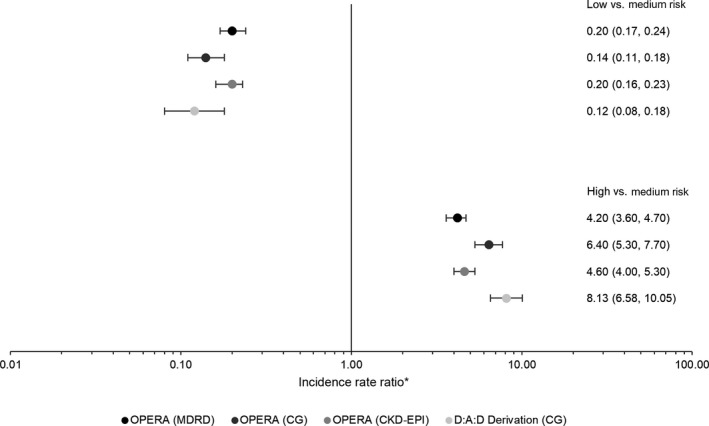
Incidence rate ratios and 95% confidence intervals for the association between chronic kidney disease (CKD) development and CKD full risk group, with medium risk as the referent. *The scale is logarthmic.
Using the full risk score, the probability of developing CKD by year 5 was lowest in the low‐risk group (0.4–0.8%) and highest in the high‐risk group (24.4–27.6%). Five‐year probabilities were lowest in the OPERA C‐G sample for those deemed at low or medium risk and highest in the OPERA MDRD sample for those deemed at high risk. Kaplan–Meier 5‐year progression rates in the D:A:D derivation cohort ranged from 0.2 (95 % CI 0.09, 0.3) in the low‐risk group to 14.5 (95% CI 13.2, 16.1) in the high‐risk group.
The Poisson model generated adjusted IRRs (aIRRs) ranging from 1.25 to 1.29 in the OPERA samples using the full risk score, similar to the 1.32 reported by D:A:D, with overlapping CIs only in the OPERA C‐G cohort (Fig. 5). The aIRRs using the short risk score more closely approximated that reported by the D:A:D (1.33), with aIRRs ranging from 1.25–1.30 and all CIs overlapping. Harrell's c‐statistic in the full risk models ranged from 0.87 to 0.92 in the three OPERA samples, suggesting that the ability to discriminate between low‐, medium‐ and high‐risk groups was similar in all three validation samples. The corresponding c‐statistic in the D:A:D derivation cohort was 0.92. Short risk score results were similar, ranging from 0.87 to 0.91 in the three OPERA samples as compared to 0.91 reported by D:A:D.
Figure 5.
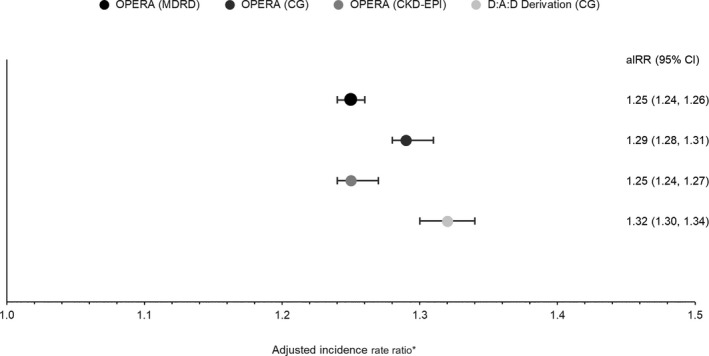
Adjusted incidence rate ratio and 95% confidence intervals for the association between chronic kidney disease (CKD) development and a one‐point increase in the continuous CKD full risk score. *Modelling in the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) derivation cohort was adjusted for baseline injecting drug use, gender, hepatitis C virus coinfection, age, nadir CD4 count, estimated glomerular filtration rate (eGFR), hypertension, prior cardiovascular disease (CVD) and diabetes. Models in all other cohorts were not further adjusted.
Discussion
The D:A:D CKD risk score algorithm was developed in a predominantly non‐US population, using data from PLWH whose observation largely began prior to 2007 and whose CKD risk was predicated on the measurement of renal function as defined by the Cockcroft–Gault formula. The risk score algorithm was validated in two small, international trials using CKD‐EPI to measure eGFR, but the CIs generated for most of the metrics used to assess validity in these small studies were wide, a shortcoming acknowledged by the study authors 22. The risk score was subsequently validated in another study using data from care in Australia, but the sample in that study was also modest in size 25. The current study extends this body of work by validating the D:A:D CKD risk prediction algorithms, both short and full risk equations, in a large, longitudinal cohort of PLWH treated exclusively in the USA and using the three most commonly used eGFR equations.
The reported incidence of kidney function decline in PLWH ranges from 3.3 to 11.2 per 1000 PY, but comparisons of CKD incidence rates across studies is hampered by disparities in the studied populations, time frames and definitions, and differences in eGFR formulas 7. The incidences of stage 3–5 CKD reported in the current study were within this range, although they did vary notably by eGFR formula, ranging from 7.3 to 11.0 per 1000 PY.
Despite notable differences in the demographic and clinical characteristics of the underlying populations, the CKD incidence increased according to risk stratum in all three of the OPERA validation samples and IRRs, both unadjusted and adjusted, were similar between the OPERA samples and the D:A:D validation and derivation cohorts, as were Kaplan–Meier progression rates. The majority of PLWH who developed CKD were classified as being at high risk for CKD by the risk score, while the majority of those who did not develop CKD were classified as being at low risk. Both full and short risk scores were highly predictive of the development of stage 3–5 CKD in all three OPERA samples with excellent concordance statistics (0.87 to 0.92).
Our findings are consistent with those of previous D:A:D CKD validation studies. Mocroft et al. applied the D:A:D short risk score in 2548 individuals from the Royal Free Hospital Clinic Cohort and the full risk score in 2013 PLWH from the SMART/ESPRIT control arms 22. CKD developed in 3.7% and 1.6% of persons, respectively, at a rate of 5.1 (95% CI 4.1–6.1) and 3.8 (95% CI 2.5−5.1) per 1000 PY. The short risk score showed good discrimination in the Royal Free Hospital Clinic Cohort (Harrell's c‐statistic 0.86; 95% CI 0.78–0.90), as did the full risk score in the SMART/ESPRIT cohort (Harrell's c‐statistic 0.87; 95% CI 0.80–0.94). Similarly, Woolnough and colleagues sought to validate both the short D:A:D risk score and the Scherzer risk score in a small (n = 748) retrospective cohort study in Australia 25. CKD developed in 5% of patients. The performance of both risk scores was measured by the area under the receiver operator curve (AUROC). The authors concluded that the short D:A:D risk score (AUROC = 0.85) was superior to the Scherzer score (AUROC = 0.78).
CKD is an important comorbidity in PLWH. The availability of an easy‐to‐calculate CKD risk score has promising implications for clinical practice, enabling clinicians to assess this risk prior to prescribing cART and to engage and educate patients early as to their role in self‐management of CKD risk. Of particular concern for clinicians is the use of the antiretroviral agent TDF. TDF is widely used and generally considered safe and well tolerated, but it is accompanied by the potential for cumulative nephrotoxicity. TDF has also been associated with decreased eGFR or creatinine clearance, as well as with rapid eGFR decline and proteinuria. Co‐administration of TDF with ritonavir‐boosted protease inhibitors may increase this risk 17.
Strengths of this study include the large sample size, the wide representation of patients in care across the USA, and the assessment of CKD risk using the three primary eGFR equations. Sample sizes in all three OPERA validation samples were larger than either the original D:A:D derivation cohort or any of the cohorts subsequently used to validate the algorithm. Accordingly, CIs in those metrics used to assess validity were notably narrower in the current study. Moreover, the inclusion of patients from both small rural clinics and large urban centres, as well as the geographical diversity of the overall population means that this study is highly representative of the real‐world HIV epidemic in the USA. Of note, this study validated the use of the D:A:D CKD risk score for CKD risk prediction in the USA, with a more contemporaneous population. Finally, the study validated the D:A:D short and full risk scores using three different eGFR formulas, an acknowledgement of the importance of the effect of their variability on study findings. The reader should note that all of the eGFR equations continue to be a subject of debate in the literature and, as such, should be used with caution and with in‐depth knowledge of the differences between them and how their performances may vary depending on the purpose for which they are being used 26.
Our study is not without limitations, and results should be interpreted with these in mind. While the OPERA population can provide detailed information on a large subset of the HIV‐infected population in the USA, issues confronting population‐level assessments include such aspects as differential medical care by practice size and specialty, the academic and research orientation of the health care practitioner, race/ethnicity‐based and gender‐based attitudes, and geographical regional health care practices. OPERA clinical data are collected at the point of care and are subject to the record‐keeping practices of each health care provider and the standards of each clinic or organization. Patients may see multiple physician practices for various conditions, which may result in incomplete case ascertainment.
Conclusions
Study findings support the validity of the D:A:D short and full risk scoring method for assessing the probability of stage 3–5 CKD in an exclusively USA‐based sample regardless of eGFR method.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval of the version to be published. AMM, KLS, LB, JSF, RH, KM and GPF had access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. AMM, KLS, LB, JSF, RH, KM, AB, GP, and GPF were involved in the conception and design of the study and data interpretation. AMM, KM and RH were involved in the acquisition of data. KLS, LB and JSF were involved in the data analysis.
Compliance with ethics guidelines
The OPERA database complies with all HIPAA and HITECH requirements and received annual institutional review board (IRB) approval by Advarra IRB including a waiver of informed consent and authorization for use of protected health information.
Supporting information
Table S1. Comparison of demographic and clinical characteristics between the D:A:D derivation and OPERA validation cohorts.
Acknowledgements
This research would not be possible without the patients of the OPERA observational database and the health care providers who care for them. We are also grateful for the contributions of Jeff Briney (SAS programming) as well as Ted Ising and Redemptor Perez (IT Data Management).
Conflicts of interest and sources of funding: APB and GP are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA. KLS, LB, JSF and GPF are employees of Epividian. Epividian has had research funded by ViiV Healthcare and Merck & Co. AMM has received research funding from Gilead Sciences, ViiV Healthcare, Janssen, Merck and Sangamo, and is on advisory boards for Gilead Sciences, ViiV Healthcare, Janssen and Merck. RH has received a research grant from Gilead Sciences; RH has received a research grant from Gilead Sciences; speaker honoraria and fees for advisory board participation from ViiV Healthcare, BMS, Merck, Gilead Sciences, and Janssen; and expenses & honoraria for time and travel for face‐to‐face advisory board meeting participation with Epividian. KM has received research grants from Gilead Sciences, Merck, Janssen, and GSK/ViiV Healthcare KM has received research grants from Gilead Sciences, Merck, Janssen, and GSK/ViiV Healthcare and honoraria for speakers bureau and advisory board participation from Gilead Sciences, Merck, Janssen and GSK/ViiV Healthcare; and fand expenses & honoraria for time and travel for face‐to‐face advisory board meeting participation with Epividian. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
These data were presented in part at the 25th Conference on Retroviruses and Opportunistic Infections, 4–7 March 2018, Boston, MA, USA (Poster 730).
References
- 1. Kooij KW, Vogt L, Wit F et al Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus‐infected middle‐aged individuals compared with human immunodeficiency virus‐uninfected controls. J Infect Dis 2017; 216 (6): 622–631. [DOI] [PubMed] [Google Scholar]
- 2. Abraham AG, Althoff KN, Jing Y et al End‐stage renal disease among HIV‐infected adults in North America. Clin Infect Dis 2015; 60 (6): 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Althoff KN, McGinnis KA, Wyatt CM et al Comparison of risk and age at diagnosis of myocardial infarction, end‐stage renal disease, and non‐AIDS‐defining cancer in HIV‐infected versus uninfected adults. Clin Infect Dis 2015; 60 (4): 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estrella MM, Parekh RS, Astor BC et al Chronic kidney disease and estimates of kidney function in HIV infection: a cross‐sectional study in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2011; 57 (5): 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitahata MM, Drozd DR, Crane HM et al Ascertainment and verification of end‐stage renal disease and end‐stage liver disease in the north American AIDS cohort collaboration on research and design. AIDS Res Treat 2015; 2015: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhatti AB, Usman M, Kandi V. Current Scenario of HIV/AIDS, treatment options, and major challenges with compliance to antiretroviral therapy. Cureus 2016; 8 (3): e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campos P, Ortiz A, Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J 2016; 9 (6): 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wing EJ. HIV and aging. Int J Infect Dis 2016; 53: 61–68. [DOI] [PubMed] [Google Scholar]
- 9. Human Hilton R. Human immunodeficiency virus infection and kidney disease. J R Coll Physicians Edinb 2013; 43 (3): 236–239; quiz 40. [DOI] [PubMed] [Google Scholar]
- 10. Kalim S, Szczech LA, Wyatt CM. Acute kidney injury in HIV‐infected patients. Semin Nephrol 2008; 28 (6): 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallipattu SK, Salem F, Wyatt CM. The changing epidemiology of HIV‐related chronic kidney disease in the era of antiretroviral therapy. Kidney Int 2014; 86 (2): 259–265. [DOI] [PubMed] [Google Scholar]
- 12. Bertoldi A, De Crignis E, Miserocchi A et al HIV and kidney: a dangerous liaison. New Microbiol 2017; 40 (1): 1–10. [PubMed] [Google Scholar]
- 13. Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82 (5): 516–524. [DOI] [PubMed] [Google Scholar]
- 14. Cooper RD, Wiebe, Smith, Keiser P, Naicker S, Tonelli M. Systematic review and meta‐analysis: renal safety of tenofovir disoproxil fumarate in HIV‐infected patients. Clin Infect Dis 2010; 51 (5): 496–505. [DOI] [PubMed] [Google Scholar]
- 15. Mocroft A, Kirk O, Reiss P et al Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV‐positive patients. AIDS 2010; 24 (11): 1667–1678. [DOI] [PubMed] [Google Scholar]
- 16. Diana NE, Naicker S. Update on current management of chronic kidney disease in patients with HIV infection. Int J Nephrol Renovasc Dis 2016; 9: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad 2018; 4 (2): 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mocroft A, Lundgren JD, Ross M et al Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV‐positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 2016; 3 (1): e23–e32. [DOI] [PubMed] [Google Scholar]
- 19. Ekrikpo UE, Kengne AP, Bello AK et al Chronic kidney disease in the global adult HIV‐infected population: a systematic review and meta‐analysis. PLoS ONE 2018; 13 (4): e0195443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ando M, Yanagisawa, Ajisawa A, Tsuchiya K, Nitta K. A simple model for predicting incidence of chronic kidney disease in HIV‐infected patients. Clin Exp Nephrol 2011; 15 (2): 242–247. [DOI] [PubMed] [Google Scholar]
- 21. Scherzer R, Gandhi M, Estrella MM et al A chronic kidney disease risk score to determine tenofovir safety in a prospective cohort of HIV‐positive male veterans. AIDS 2014; 28 (9): 1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mocroft A, Lundgren JD, Ross M et al Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A: D study. PLoS Med 2015; 12 (3): e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friis‐Moller N, Weber R, Reiss P et al Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS 2003; 17 (8): 1179–1193. [DOI] [PubMed] [Google Scholar]
- 24. Flandre P, Pugliese P, Allavena C, Isnard Bagnis C, Cuzin L, Dat'AIDS Study Group . Does first‐line antiretroviral regimen impact risk for chronic kidney disease whatever the risk group? AIDS 2016; 30 (9): 1433–1438. [DOI] [PubMed] [Google Scholar]
- 25. Woolnough EL, Hoy JF, Cheng AC et al Predictors of chronic kidney disease and utility of risk prediction scores in HIV‐positive individuals. AIDS 2018; 32 (13): 1829–1835. [DOI] [PubMed] [Google Scholar]
- 26. Szummer K, Evans M, Carrero JJ et al Comparison of the chronic kidney disease epidemiology collaboration, the modification of diet in renal disease study and the Cockcroft‐Gault equation in patients with heart failure. Open Heart 2017; 4 (2): e000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of demographic and clinical characteristics between the D:A:D derivation and OPERA validation cohorts.


