Abstract
The immunomodulatory potential of mesenchymal stromal cells (MSCs) and regulatory T cells (T‐reg) is well recognized by translational scientists in the field of regenerative medicine and cellular therapies. A wide range of preclinical studies as well as a limited number of human clinical trials of MSC therapies have not only shown promising safety and efficacy profiles but have also revealed changes in T‐reg frequency and function. However, the mechanisms underlying this potentially important observation are not well understood and, consequently, the optimal strategies for harnessing MSC/T‐reg cross‐talk remain elusive. Cell‐to‐cell contact, production of soluble factors, reprogramming of antigen presenting cells to tolerogenic phenotypes, and induction of extracellular vesicles (“exosomes”) have emerged as possible mechanisms by which MSCs produce an immune‐modulatory milieu for T‐reg expansion. Additionally, these two cell types have the potential to complement each other's immunoregulatory functions, and a combinatorial approach may exert synergistic effects for the treatment of immunological diseases. In this review, we critically assess recent translational research related to the outcomes and mechanistic basis of MSC effects on T‐reg and provide a perspective on the potential for this knowledge base to be further exploited for the treatment of autoimmune disorders and transplants.
Keywords: adult stem cells, autoimmune disease, cellular therapy, cytokines, immunotherapy, mesenchymal stem cells, stromal cells, T cells
Mesenchymal stromal cells (MSCs) promote regulatory T cell numbers and immune suppressive functions through mechanisms involving cell‐cell contact, production of soluble mediators, reprogramming of antigen presenting cells, and release of extracellular vesicles that likely represent important components of MSC therapeutic effects in immune/inflammatory diseases.

Significance statement.
Mesenchymal stromal cells (MSCs) and regulatory T cells (T‐reg) are very different cell types. However, they share the common features of actively suppressing or modulating harmful immune responses through diverse molecular mechanisms and of being the focus of extensive translational efforts as cell therapies for immune‐mediated/inflammatory diseases. This review article describes and critically evaluates the current evidence base from fundamental and translational research studies that influences on T‐reg represent an important component of the therapeutic effects of MSC‐based investigational products. The study also examines multiple direct and indirect mechanisms by which MSCs may promote the expansion and immune suppressive potency of T‐reg.
1. INTRODUCTION
The distinctive capacity of mesenchymal stromal cells (MSCs) to differentiate into diverse cell lineages (adipocytes, chondrocytes, and osteoblasts), repair damaged tissues, migrate to sites of injury, and modulate a range of immune/inflammatory effector mechanisms has generated substantial interest among biomedical researchers in the field of regenerative medicine.1, 2 To date, as many as 969 clinical trials have been reported using MSCs as a potential cell therapy for the treatment of diverse immunological and nonimmunological disorders (https://clinicaltrials.gov/). Among the attributes of this versatile cell that make it a suitable candidate for cellular therapy are its ease of isolation from multiple accessible tissues, its amenability to large scale ex vivo culture expansion, its low immunogenicity, and its now well‐documented safety profile. Although the International Society for Cellular Therapy has set minimum criteria for defining MSCs that include expression of CD105, CD73, and CD90 and absence of expression of human leukocyte antigen (HLA)‐DR, CD11b, CD14, CD19, and CD34,1 a general consensus is lacking on how MSCs can be consistently defined at the level of functionality and potency. This difficulty arises, in large part, from heterogeneity in primary MSC phenotypes within different tissues and in ex vivo culture conditions.2, 3, 4
Among their functional properties, MSCs have been consistently shown to suppress adaptive immune responses directly by inhibiting the proliferation of CD4+ (“helper”) and CD8+ (“cytotoxic”) T cells and indirectly by modulating dendritic cell (DC)‐mediated antigen presentation.5 An additional putative mechanism by which MSCs may exert both short‐ and long‐lasting influences on antigen‐specific T‐cell responses is through induction of regulatory T cells (T‐reg).5 In 2008, Di Ianni et al demonstrated increased frequency of T‐reg and prolonged maintenance of T‐reg suppressive activities when human T‐cell subpopulations were cocultured with MSCs.6 As we describe later in this article, a relatively broad range of experimental studies has since been published to confirm this phenomenon and to add mechanistic details,7 and the topic continues garner interest.8, 9, 10 Furthermore, ex vivo‐expanded MSCs and T‐reg have both been shown to display potent immunomodulatory effects in a wide array of animal disease models and have been demonstrated in clinical trials to represent safe, feasible, and potentially effective immunotherapies for human autoimmune diseases and transplants.2, 11, 12 It would seem imperative, therefore, to better understand how the mechanisms underlying MSC‐mediated induction of T‐reg or combined MSC/T‐reg cellular therapy may be successfully translated to the clinical arena. In this review, we reevaluate the existing concepts about the effects of MSCs on T‐reg expansion and the related mechanisms that may lead to T‐reg induction by MSCs under in vitro and in vivo conditions.
2. CURRENT UNDERSTANDING OF T‐REG PHENOTYPES
Based on their developmental origins, T‐reg are classified into two major categories—one that develops de novo in the thymus as “natural” (n)T‐reg and the other that differentiates during activation in the periphery as “induced” (i)T‐reg. While the primary identifying phenotype of T‐reg in humans and rodents is CD4+CD25hiFOXP3+, they have been found to be functionally and phenotypically heterogeneous.12 For humans, but not mice, lower expression of the interleukin (IL)‐7R (CD127) has also been shown to be a useful discriminator of T‐reg from other CD4+ T‐cells.13 Miyara et al further divided T‐reg based on the differential expression of CD45RA and FOXP3 into three main subpopulations: CD45RA+FOXP3lo naive or resting T‐reg; CD45RA−FOXP3+ activated or effector T‐reg; and CD45RA−FOXP3lo “non‐suppressive,” cytokine‐secreting cells.14 Subsequently, a number of other research groups have divided T‐reg into different subpopulations based on the expression of specific surface markers. For example, surface expression of CD39, an ectoenzyme that hydrolyzes adenosine triphosphate into adenosine monophosphate has also been used to define T‐reg subpopulations. Human CD39hi T‐reg have been reported to suppress xenograft versus host disease in a mouse model, while CD39lo T‐reg were nonsuppressive and highly unstable.15 More recently, Mason and colleagues identified more than 22 different human T‐reg subsets by mass cytometry.16 The list of T‐reg subsets as targets for therapeutic exploitation is growing yet there remain significant knowledge gaps regarding the phenotypic identities of T‐reg subpopulations, their relevance to different disease processes, and their potential for immunotherapeutic applications.
3. PRECLINICAL AND CLINICAL EVIDENCE FOR THERAPEUTICALLY RELEVANT EFFECTS OF MSC ON T‐REG
A number of animal model studies have documented increments in T‐reg numbers after MSC administration. For example, in mice with collagen antibody‐induced arthritis (CAIA), Nam et al reported that MSCs induced differentiation of CD4+ T cells to T‐reg in vitro and that FOXP3 expression was upregulated in CAIA mice after MSC infusion.17 Similar findings were reported by Roux et al who observed the induction of functional CD4+FOXP3+ T‐reg when cocultured in vitro with human‐induced pluripotent stem cell‐derived MSC (hu‐iPS‐MSC). These findings were validated in vivo following hu‐iPS‐MSC administration to humanized mice.18 In a rat model of high‐risk corneal allo‐transplantation, Lohan et al reported that pretransplant intravenous administration of third‐party allogeneic MSCs resulted in increased rejection‐free survival associated with higher proportions of T‐reg in the graft draining lymph nodes.19 Bai et al reported increased T‐reg numbers following administration of IL‐17A‐treated MSCs to mice with renal ischemia reperfusion injury that was associated with greater protection from acute kidney injury and was dependent on COX2/prostaglandin E2 (PGE2).20 Groups investigating the in vitro interactions of MSCs with T‐reg also reported T‐reg induction by MSCs derived from different sources.21, 22
As summarized in Table 1, a number of clinical studies have also shown increases in T‐reg numbers or percentages after systemic or localized administration of either autologous or allogeneic MSCs in different human disease and transplantation conditions. Notably, Shi et al reported a significant elevation in the percentage of T‐reg 4 weeks after umbilical cord‐derived MSC infusion in 27 liver allograft recipients.23 Likewise, Li et al reported an increase in T‐reg percentage with a significant decline in the percentage of Th17 cells in systemic lupus erythematosus (SLE) patients with refractory cytopenia after MSC infusion.35 In a phase I/II clinical trial of third‐party MSCs after kidney transplantation, Erpicum et al reported increased frequency of T‐reg 30 days after MSC infusion.25 There are also reports, however, in which no alteration in T‐reg was observed.43 In a long‐term follow‐up study of four kidney transplant patients treated with autologous MSCs, Perico et al reported an increase in T‐reg percentage in two of the patients, while it declined in the third patient and remained stable in the fourth.11 Overall, more randomized, placebo‐controlled clinical studies accompanied by detailed immunological monitoring are needed to determine the conditions under which MSC administration is most likely to consistently induce T‐reg expansion as well as to determine whether changes in circulating T‐reg are accompanied by changes to T‐reg located in disease‐relevant tissues.
Table 1.
Summary of clinical trial reports in which effects of systemic or localized administration of autologous or allogeneic MSC on peripheral blood T‐reg, with or without other immunological effects, were described in patients with medical or surgical conditions involving abnormal immune response or inflammation
| Reference | Source | Disease | Key findings | Route of administration |
|---|---|---|---|---|
| Shi et al23 | Third‐party allogeneic UC‐MSC | Liver transplantation |
|
IV |
| Pers et al24 | Autologous ASC | Severe osteoarthritis |
|
Intra‐articular |
| Erpicum et al25 | Third‐party allogeneic BM‐MSC | Kidney transplantation |
|
IV |
| Ciccocioppo et al26 | Autologous BM‐MSC | Crohn's disease |
|
Intrafistular |
| Karussis et al27 | Autologous BM‐MSC | Multiple sclerosis and amyotrophic lateral sclerosis |
|
Intrathecal and IV |
| Wang et al28 | Allogeneic UC‐MSC | Systemic lupus erythematosus |
|
IV |
| Liang et al29 | Allogeneic BM‐MSC | Systemic lupus erythematosus |
|
IV |
| Zhao et al30 | Third party allogeneic BM‐MSC | Acute GvHD |
|
IV |
| Ghoryani et al31 | Autologous BM‐MSC | Rheumatoid arthritis |
|
IV |
| Gao et al32 | Allogeneic UC‐MSC | Chronic GvHD |
|
IV |
| Kong et al33 | Allogeneic UC‐MSC | Type 2 diabetes mellitus |
|
IV |
| Weng et al34 | Allogeneic BM‐MSC | Dry eyes‐associated chronic GvHD |
|
IV |
| Li et al35 | Allogeneic UC‐MSC and BM‐MSC | Systemic lupus erythematosus |
|
IV |
| Xu et al36 | Autologous BM‐MSC | Liver cirrhosis |
|
Infusion via hepatic artery |
| Fang et al37 | Allogeneic UC‐MSC | Decompensated hepatitis B cirrhosis |
|
Hepatic artery, portal vein and IV |
| Xiao et al38 | Allogeneic BM‐MSC | Refractory aplastic anemia |
|
IV |
| Detry et al39 | Third party unrelated MSC | Liver transplantation |
|
IV |
| Peng et al40 | Donor derived Allogeneic BM‐MSC | Kidney Transplantation |
|
Intra‐arterial and IV |
| Perico et al41 | Autologous BM‐MSC | Kidney transplantation |
|
IV |
| Soeder et al42 | Third‐party allogenic BM‐MAPC | Liver transplantation (single patient) |
|
Intraportal and IV |
| Perico et al11 | Autologous BM‐MSC | Kidney transplantation |
|
IV |
Statements in bold text indicate reported findings that are specific to MSC effects on T‐reg. Abbreviations: ASC, adipose‐derived stromal cells; BM‐MAPC, bone marrow‐derived multipotent adult progenitor; BM‐MSC, bone marrow‐derived mesenchymal stromal cells; GvHD, graft versus host disease; IL‐17, interleukin 17; IV, intravenous; NK cell, natural killer cell; PGE2, prostaglandin E2; TGFβ1, transforming growth factor beta 1; Th17 cells, T helper 17 cells; T‐reg, regulatory T‐cells; UC‐MSC, umbilical cord‐derived mesenchymal stromal cells.
4. POTENTIAL MECHANISMS FOR MSC‐MEDIATED EFFECTS ON T‐REG
As is evident from the literature summarized in the preceding section, a wide range of in vitro and in vivo studies have documented the potential for MSCs to induce, expand, or preferentially support the survival of T‐reg in human and experimental animal species. Nonetheless, the kinetic and mechanistic details related to this phenomenon are incompletely understood and are likely to be complex and context‐dependent. In Figure 1 and in the sections below, we summarize evidence for four basic (and nonmutually exclusive) mechanistic models for MSC effects on T‐reg.
Figure 1.
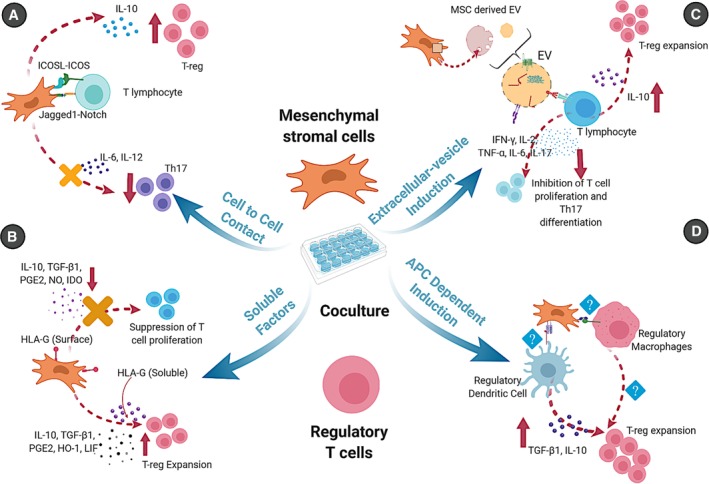
Schematic representation of different mechanisms used by mesenchymal stromal cells (MSCs) for regulatory T cells (T‐reg) induction. A, Cell‐to‐cell contact: Interaction of different molecules such as ICOSL and ICOS, Notch and Notch ligands expressed by MSCs and T lymphocytes upregulates the production of interleukin (IL)‐10 and T‐reg proliferation. B, Secretion of soluble factors: MSCs secrete many soluble factors such as transforming growth factor beta 1, prostaglandin E2, heme oxygenase‐1, human leukocyte antigen G5 and leukemia inhibitory factor that induce T‐reg expansion while suppressing other T cell proliferation. C Antigen presenting cell‐dependent induction: MSC effects on antigen presenting cells (dendritic cells, monocytes, macrophages) induce regulatory phenotypes that promote T‐reg through IL‐10 and TGF‐β1, although the factors responsible for this induction have not been fully elucidated. D, Extracellular vesicle induction: MSC‐derived extracellular vesicles carrying specific RNAs, proteins and other bio‐molecules induce polarization of CD4+ T cells towards T‐reg by increasing production of IL‐10 while decreasing IL‐17, IL‐2, TNF‐α, IFN‐γ, and IL‐6. The figure was created with http://biorender.com
Cell‐to‐cell contact‐dependent mechanisms have been reported to play an important role in the interactions between MSCs and T‐reg under in vitro and in vivo conditions. English et al provided the first in vitro evidence that direct contact between human MSCs and purified CD4+ T cells is important for the induction of T‐reg as elimination of contact by a semipermeable membrane reduced the expression of FOXP3 mRNA to control levels.44 In this study, PGE2 and transforming growth factor beta (TGF‐β) were also mechanistically implicated, suggesting a combined role for contact‐dependent signals and soluble mediators. Subsequently, Lee et al reported that expression of inducible costimulator ligand (ICOSL/CD275) by human MSCs when cocultured with CD4+ T‐cells is essential for T‐reg induction under in vitro conditions as knockdown of ICOSL and use of transwell cultures significantly reduced T‐reg induction and IL‐10 production.45 Mesenchymal stromal cells also express a wide range of other surface adhesion molecules including integrins, vascular cell adhesion molecule (VCAM)‐1, intercellular adhesion molecules (ICAM‐1, ICAM‐2), CD72, and CD58 (LFA‐3), which have been shown to bind to T cells with very high affinity and to play important roles in immune suppression. These molecules help to anchor T cells to MSCs and, in so doing, increase the potency of soluble factors to suppress T‐cell proliferation and proinflammatory effector mechanisms. It is unknown, however, whether these adhesion events specifically promote T‐reg induction and whether inhibiting MSC‐T‐cell adhesion interferes with this aspect of MSC‐mediated immunomodulation. In contrast, signaling through Notch receptors is well documented to play a pivotal role in the development of T‐reg,46 and MSCs express a variety of Notch ligands, including Jagged1, Jagged 2, and Delta‐like (DLL) 1, 3, and 4. Notably, Del Papa et al reported that induction of T‐reg by human MSCs was mediated by Notch1 and, subsequently, Cahill et al demonstrated that the Notch ligand Jagged‐1 was responsible for the expansion of T‐reg by mouse MSCs.47, 48 Finally, Rashedi et al in a study of the influence of toll‐like receptor (TLR) stimulation on MSC immunomodulatory effects showed that indirect contact of MSCs with human CD4+ T cells in a transwell culture system was sufficient for T‐reg induction, but that direct contact resulted in expansion of T‐reg numbers via a Notch‐dependent mechanism.49
Soluble factor‐dependent mechanisms have been identified in a relatively large number of studies as playing a role in the effects of MSCs on T‐reg induction, proliferation, survival or suppressive potency.
TGF‐β1: This cytokine is secreted in an inactive latent form as pro‐TGF‐β1, which is cleaved into two fragments, of which the C‐terminal homodimer represents mature TGF‐β1 and the N‐terminal homodimer is associated with the latency‐associated peptide (LAP) domain forming a small latency complex. Recently, it has also been recognized that glycoprotein A repetitions predominant (GARP) expressed by both MSCs and T‐reg plays a crucial role in the maturation and activation of the LAP/TGF‐β1 complex by interacting with alpha‐beta integrins (αVβ6 and αVβ8) expressed on many lymphocytes.50 Thus, GARP expressed by MSCs may assist in the promotion of T‐reg by directing released TGF‐β1 toward responsive T‐cells. In the study of Cahill et al in a mouse model of allergic airway inflammation, TGF‐β1 neutralization resulted in reduced mRNA and protein levels of FOXP3 and CD25, further confirming that it plays a role in inducing T‐reg differentiation.48 Similarly, Hong et al reported a significant increase in the number of FOXP3+ T‐reg when human CD4+ T cells were cocultured with dental pulp MSCs which was reduced by blockade of TGF‐β1.51
PGE2: Coculture studies of MSCs with human peripheral blood mononuclear cells (PBMC) have indicated that PGE2 is an important mediator of T‐reg promotion.52 Yang et al reported that human MSCs reversed the suppressive deficiency of T‐reg from multiple sclerosis patients by augmenting the production of multiple soluble factors including TGF‐β1 and PGE2.53 Similarly, Tumangelova‐Yuzeir et al reported that coculturing of MSCs derived from glioblastoma multiforme patients with PBMC from healthy donors resulted in secretion of PGE2 along with TGF‐β1 that eventually increased the T‐reg percentage and decreased Th‐17 cell numbers.54 In an in vivo mouse model of colitis, An et al reported that PGE2 secreted by feline adipose tissue‐derived MSCs reduced inflammation by increasing FOXP3+ T‐reg.55
Indoleamine 2,3‐dioxygenase (IDO): The inducible enzyme IDO catalyzes the rate‐limiting step in tryptophan metabolism, leading to accumulation of tryptophan catabolites, including kynurenine, 3‐hydroxyanthranilic acid, and quinolinic acid. These catabolites enhance the effects of TGF‐β1, leading to immune suppression and potentially to induction of T‐reg.56 Li et al reported that human umbilical cord‐derived MSCs cocultured with T cells block cell cycle progression and induce apoptosis through an IDO‐dependent mechanism.57 In the same study, IDO‐lentivirus‐transfected MSCs (IDO‐MSCs) induced a higher percentage of FOXP3+T‐reg in human PBMC. Subsequently, low doses of IDO‐MSCs prolonged graft survival and induced tolerance by increasing antigen‐specific T‐reg in a rabbit kidney transplant model.58
Heme oxygenase‐1 (HO‐1): Another potential contributor to MSC‐mediated induction of T‐reg is the inducible enzyme HO‐1, which catalyzes the rate‐limiting step of heme degradation to bilverdin. Mougiakakos et al reported induction of different subsets of T‐reg in response to HO‐1 produced by human MSCs in vitro.59 In a coculture study between human MSCs and PBMC from asthmatic subjects, Li et al reported that inhibition of HO‐1 resulted in decreased T‐reg proportions.60 Moreover, bone marrow‐derived mesenchymal stromal cells (BM‐MSC) adenovirally transduced to overexpress HO‐1‐induced higher numbers of T‐reg than control BM‐MSCs in a rat model of liver transplantation.61
HLA‐G5: HLA‐G5 is a soluble factor released by MSCs that has potential to increase the number of FOXP3+ cells. Selmani et al reported in vitro induction of T‐reg by human MSCs secreting HLA‐G5. Neutralization of HLA‐G5 partially restored T‐cell proliferation in response to allogeneic stimuli.62 Similarly, Chen et al reported that in vitro expansion of T‐reg in mixed lymphocyte reactions (MLR) by MSCs was abrogated by blockade of HLA‐G5 in SLE patients.63
Leukemia inhibitory factor (LIF): Human MSC constitutively express LIF and it has been reported that LIF is elevated up to sevenfold when MSCs are cocultured with CD3+ lymphocytes.64 Additionally, restoration of human lymphocyte proliferation and a decline in FOXP3+ T‐reg were demonstrated in an MLR assay following addition of a LIF neutralizing antibody.64
It is important to highlight that the soluble molecules that have been thus far recognized to play a role in MSC induction of T‐reg are likely to share signaling pathways and engage in cross‐talk with each other. For example, secretion of PGE2 induces the expression of IDO and kynurenine, whereas TGF‐β and IDO promote each other's gene amplification.65 Similarly, release of TGF‐β1 induces secretion of LIF which, in turn, increases PGE2 production.66 Thus, while experimental studies have often focused on providing evidence for a role for individual mediators, a more plausible mechanistic model is one in which combinations of such factors—produced by MSCs, T cells, and, as described below, secondary cell populations—act within a cascade of codependent events to support T‐reg differentiation, survival and expansion while suppressing the differentiation and proliferation of other T‐cell subsets.44, 67
Antigen presenting cell‐dependent mechanisms: In addition to directly interacting with T‐cells, MSCs also modulate the adaptive immune response through effects on antigen presenting cells (APCs) (DCs, macrophages, and monocytes) that shift them to regulatory phenotypes associated with alternative surface receptor expression profiles and cytokine/chemokine secretion patterns. In 2009, Zhang et al reported that mouse bone marrow‐derived MSCs reprogrammed mature DCs into APCs with distinct jagged‐2‐dependent regulatory properties.68 The findings were corroborated by Zhao et al who documented the generation of FOXP3+ T‐reg from CD4+CD25−FOXP3−T‐cells by bone marrow‐derived MSC‐conditioned regulatory DCs. In this study, regulatory DCs were also characterized by secretion of TGF‐β1 and inhibition of T cell proliferation.69 Further evidence came from Liu et al who demonstrated both in vivo and in vitro induction of IL‐10‐dependent regulatory DC by a mouse embryonic fibroblast‐derived MSCs.70 Cahill et al reported induction of a semi‐mature tolerogenic DC phenotype by MSCs in vitro that induced T‐reg from CD4+CD25−FoxP3− T‐cells. In a mouse model, these authors showed that Jagged‐1 signaling played a key role in MSC expansion of T‐reg.48 It has also been shown by Meleif et al that human MSCs modulate monocyte/macrophages toward alternatively activated (M2) macrophages, which promote T‐reg via secretion of IL‐10 and other soluble factors.71 Consistent with this, Chiossone et al also reported that MSC coculture resulted in polarization of macrophages to an M2‐like phenotype capable of suppressing effector T‐cells and promoting T‐reg.72 Given recent evidence that intravenously administered MSCs become trapped in the lungs and interact there with myeloid lineage immune cells,73 it is quite plausible that mechanisms of APC re‐programming described in in vitro and in vivo experimental studies also operate in clinical settings to indirectly promote induction of T‐reg and suppressive functions.
Extracellular vesicle (EV)‐dependent mechanisms: A more recently‐identified and potentially exciting mechanism whereby MSCs may promote immune‐regulatory effects is through the release of EVs — particularly exosomes.74 Although EV subtypes have complex and overlapping biochemical and physical properties, exosomes are typically defined as nanoparticles of 40 to 100 nm diameter that are generated by internal budding of the lipid membrane of late endosomes to form multi‐vesicular bodies which are subsequently released as exosomes upon fusion with the plasma membrane and contain a “biomolecular cargo” of proteins, glycans, lipids, mRNAs and micro (mi)RNAs which have the potential to regulate immune cell gene transcription, intracellular signaling and effector functions.74 In a 2014 study, Zhang et al reported that MSC‐EVs delayed rejection following allogeneic skin grafts in mice with a concomitant polarization of activated CD4+ T‐cells to CD4+CD25+FOXP3+ T‐reg.75 Subsequently, the same group observed that MSC‐EV induction of T‐reg in in vitro T‐cell stimulation cultures was dependent on the presence of allogenic CD11c+ APC. Furthermore, infusion of MSC‐EVs in a humanized mouse model of graft versus host disease resulted in reduced mortality associated with higher levels of human T‐reg.76 Interestingly, MSC‐EV‐conditioned human DCs have also been shown to have increased secretion of IL‐10 and TGF‐β1 leading to greater T‐reg induction in pancreatic islet antigen‐specific stimulation assays of T‐cells from subjects with type 1 diabetes.77 Finally, Hyvärinen et al recently demonstrated that human MSC‐EVs downregulated the production of IL‐22 and IL‐23 by macrophages and polarized them to regulatory phenotype in a PGE2‐dependent manner.78 Although the potential for MSC‐EV immunotherapy remains high, the significance of their role in T‐reg induction in comparison to other mechanisms of MSC immunomodulation is not yet well understood.
5. CONCLUSION: KNOWLEDGE GAPS AND THE POTENTIAL FOR CLINICAL TRANSLATION OF MSC EFFECTS ON T‐REG
Therapeutic applications of both autologous and allogeneic MSCs have been actively pursued in many experimental human clinical trials during the past decade on the basis of their low immunogenicity, genetic stability, ease of production and diverse immunosuppressive/anti‐inflammatory properties.1, 2, 3, 4, 5, 79, 80 A key concept underlying MSC efficacy in autoimmunity, transplantation and other inflammatory diseases has been their potential to induce or functionally enhance specialized populations of innate and adaptive immune cells with regulatory/suppressive functions.81, 82, 83 In addition to CD4+/FOXP3+ T‐reg, which we have focused on in this review, evidence also exists that MSCs may promote regulatory/tolerogenic populations of CD8+ T cells, DCs and B cells as well as anti‐inflammatory (M2) monocyte/macrophages and Th2‐type CD4+ effector T cells.79 Nonetheless, a substantial number of completed and ongoing clinical trials and in vivo studies of MSCs for immune‐mediated diseases and transplants have been based, at least in part, on the premise that MSCs increase the frequency of T‐reg following systemic or localized administration. As summarized in Table 1, immune profiling studies from clinical trials involving relatively limited numbers of patients with transplants or immune‐mediated disease have provided preliminary evidence for increased T‐reg numbers or proportions following systemic or localized MSC administration.25, 26 The last few years have also witnessed swift progress in the number of clinical trials aimed at assessing the safety, feasibility, and efficacy of ex vivo‐expanded T‐reg in transplantation and efforts have begun to translate this therapy to the clinic.84 The evidence summarized here is encouraging to the extent of clearly establishing the potential for MSCs to induce and/or increase proliferation of T‐reg via a wide range of credible direct and indirect mechanisms. Given that the majority of therapeutically administered MSCs have short in vivo survival, such interactions between MSCs and T‐reg have high theoretical value through sustained “downstream” suppressive effects on proinflammatory T‐helper (Th)‐1 and Th17 responses as well as modulatory effects on innate immune cells such as DCs, monocytes, and macrophages.85 Although clinical application of MSC therapies to immune‐mediated diseases appears promising, it remains unknown whether different types of MSCs have more or less potent effects on T‐reg or serve to promote distinct T‐reg subpopulations. In the years ahead, this and several other unresolved issues such as heterogeneity, stability, plasticity of MSC and T‐reg populations, most effective dose of MSCs for maximum induction of T‐reg, and long‐term safety of MSC‐T‐reg therapy need to be addressed with fresh perspectives from basic scientists and clinician investigators.80, 86
CONFLICT OF INTEREST
M.D.G. declared research funding and in‐kind contributions by Orbsen Therapeutics Ltd., Galway, Ireland for an Irish Research Council‐funded postdoctoral research fellowship. N.N. declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
N.N.: conception and design, manuscript writing, final approval of the manuscript; M.D.G.: conception and design, financial support, manuscript writing, final approval of the manuscript.
ACKNOWLEDGMENTS
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) and is co‐funded under the European Regional Development Fund under Grant Number 13/RC/2073. It has also received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement No 713690 (NN and MDG). MDG is additionally supported by grants from the European Commission [Horizon 2020 Collaborative Health Project NEPHSTROM (grant number 634086) and FP7 Collaborative Health Project VISICORT (grant number 602470)], from Science Foundation Ireland [REMEDI Strategic Research Cluster (grant number 09/SRC/B1794)] and the European Regional Development Fund.
Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells. 2020;38:596–605. 10.1002/stem.3151
Funding information European Regional Development Fund, Grant/Award Number: None; FP7 Health, Grant/Award Number: 602470; H2020 Health, Grant/Award Number: 634086; H2020 Marie Skłodowska‐Curie Actions, Grant/Award Number: 713690; Science Foundation Ireland, Grant/Award Numbers: 09/SRC/B1794, 13/RC/2073
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 2. Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3(2):90‐104. [DOI] [PubMed] [Google Scholar]
- 4. Moll G, Ankrum JA, Kamhieh‐Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149‐163. [DOI] [PubMed] [Google Scholar]
- 5. Lukomska B, Stanaszek L, Zuba‐Surma E, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36(3):309‐318. [DOI] [PubMed] [Google Scholar]
- 7. Engela AU, Baan CC, Dor FJ, et al. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front Immunol. 2012;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai YY, Ni SY, Ma K, et al. Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro. Stem Cell Res Ther. 2019;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kadle RL, Abdou SA, Villarreal‐Ponce AP, et al. Microenvironmental cues enhance mesenchymal stem cell‐mediated immunomodulation and regulatory T‐cell expansion. PLoS One. 2018;13(3):e0193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L, Lai P, Wang Y, et al. Extracellular vesicles from mesenchymal stem cells prevent contact hypersensitivity through the suppression of Tc1 and Th1 cells and expansion of regulatory T cells. Int Immunopharmacol. 2019;74:105663. [DOI] [PubMed] [Google Scholar]
- 11. Perico N, Casiraghi F, Todeschini M, et al. Long‐term clinical and immunological profile of kidney transplant patients given mesenchymal stromal cell immunotherapy. Front Immunol. 2018;9:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharabi A, Tsokos MG, Ding Y, et al. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov. 2018;17(11):823‐844. [DOI] [PubMed] [Google Scholar]
- 13. Liu W, Putnam AL, Xu‐Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899‐911. [DOI] [PubMed] [Google Scholar]
- 15. Gu J, Ni X, Pan X, et al. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol. 2017;14(6):521‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mason GM, Lowe K, Melchiotti R, et al. Phenotypic complexity of the human regulatory T cell compartment revealed by mass cytometry. J Immunol. 2015;195(5):2030‐2037. [DOI] [PubMed] [Google Scholar]
- 17. Nam Y, Jung SM, Rim YA, et al. Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS One. 2018;13(6):e0198740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roux C, Saviane G, Pini J, et al. Immunosuppressive mesenchymal stromal cells derived from human‐induced pluripotent stem cells induce human regulatory T cells in vitro and in vivo. Front Immunol. 2017;8:1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lohan P, Murphy N, Treacy O, et al. Third‐party allogeneic mesenchymal stromal cells prevent rejection in a pre‐sensitized high‐risk model of corneal transplantation. Front Immunol. 2018;9:2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bai M, Zhang L, Fu B, et al. IL‐17A improves the efficacy of mesenchymal stem cells in ischemic‐reperfusion renal injury by increasing Treg percentages by the COX‐2/PGE2 pathway. Kidney Int. 2018;93(4):814‐825. [DOI] [PubMed] [Google Scholar]
- 21. Engela AU, Baan CC, Peeters AM, et al. Interaction between adipose tissue‐derived mesenchymal stem cells and regulatory T‐cells. Cell Transplant. 2013;22(1):41‐54. [DOI] [PubMed] [Google Scholar]
- 22. Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell‐lymphocyte interaction. Haematologica. 2007;92(7):881‐888. [DOI] [PubMed] [Google Scholar]
- 23. Shi M, Liu Z, Wang Y, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Translational Medicine. 2017;6(12):2053‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pers YM, Quentin J, Feirreira R, et al. Injection of adipose‐derived stromal cells in the knee of patients with severe osteoarthritis has a systemic effect and promotes an anti‐inflammatory phenotype of circulating immune cells. Theranostics. 2018;8(20):5519‐5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erpicum P, Weekers L, Detry O, et al. Infusion of third‐party mesenchymal stromal cells after kidney transplantation: a phase I‐II, open‐label, clinical study. Kidney Int. 2019;95(3):693‐707. [DOI] [PubMed] [Google Scholar]
- 26. Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow‐derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60(6):788‐798. [DOI] [PubMed] [Google Scholar]
- 27. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang D, Huang S, Yuan X, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14(5):423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69(8):1423‐1429. [DOI] [PubMed] [Google Scholar]
- 30. Zhao K, Lou R, Huang F, et al. Immunomodulation effects of mesenchymal stromal cells on acute graft‐versus‐host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(1):97‐104. [DOI] [PubMed] [Google Scholar]
- 31. Ghoryani M, Shariati‐Sarabi Z, Tavakkol‐Afshari J, et al. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow‐derived mesenchymal stem cells: a successful clinical trial in Iran. Biomed Pharmacother. 2019;109:1834‐1840. [DOI] [PubMed] [Google Scholar]
- 32. Gao L, Zhang Y, Hu B, et al. Phase II multicenter, randomized, double‐blind controlled study of efficacy and safety of umbilical cord‐derived mesenchymal stromal cells in the prophylaxis of chronic graft‐versus‐host disease after HLA‐haploidentical stem‐cell transplantation. J Clin Oncol. 2016;34(24):2843‐2850. [DOI] [PubMed] [Google Scholar]
- 33. Kong D, Zhuang X, Wang D, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. 2014;60(12):1969‐1976. [DOI] [PubMed] [Google Scholar]
- 34. Weng J, He C, Lai P, et al. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft‐versus‐host disease. Mol Ther. 2012;20(12):2347‐2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X, Wang D, Liang J, et al. Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone Marrow Transplant. 2013;48(4):544‐550. [DOI] [PubMed] [Google Scholar]
- 36. Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29(8):1620‐1628. [DOI] [PubMed] [Google Scholar]
- 37. Fang XQ, Zhang JF, Song HY, et al. Effect of umbilical cord mesenchymal stem cell transplantation on immune function and prognosis of patients with decompensated hepatitis B cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2016;24(12):907‐910. [DOI] [PubMed] [Google Scholar]
- 38. Xiao Y, Jiang ZJ, Pang Y, et al. Efficacy and safety of mesenchymal stromal cell treatment from related donors for patients with refractory aplastic anemia. Cytotherapy. 2013;15(7):760‐766. [DOI] [PubMed] [Google Scholar]
- 39. Detry O, Vandermeulen M, Delbouille MH, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I‐II, open‐label, clinical study. J Hepatol. 2017;67(1):47‐55. [DOI] [PubMed] [Google Scholar]
- 40. Peng Y, Ke M, Xu L, et al. Donor‐derived mesenchymal stem cells combined with low‐dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95(1):161‐168. [DOI] [PubMed] [Google Scholar]
- 41. Perico N, Casiraghi F, Introna M, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soeder Y, Loss M, Johnson CL, et al. First‐in‐human case study: multipotent adult progenitor cells for immunomodulation after liver transplantation. Stem Cells Transl Med. 2015;4(8):899‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keto J, Kaartinen T, Salmenniemi U, et al. Immunomonitoring of MSC‐treated GvHD patients reveals only moderate potential for response prediction but indicates treatment safety. Mol Ther Methods Clin Dev. 2018;9:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non‐redundant roles in human mesenchymal stem cell induction of CD4+CD25(high) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee HJ, Kim SN, Jeon MS, et al. ICOSL expression in human bone marrow‐derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep. 2017;7:44486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mota C, Nunes‐Silva V, Pires AR, et al. Delta‐like 1‐mediated notch signaling enhances the in vitro conversion of human memory CD4 T cells into FOXP3‐expressing regulatory T cells. J Immunol. 2014;193(12):5854‐5862. [DOI] [PubMed] [Google Scholar]
- 47. Del Papa B, Sportoletti P, Cecchini D, et al. Notch1 modulates mesenchymal stem cells mediated regulatory T‐cell induction. Eur J Immunol. 2013;43(1):182‐187. [DOI] [PubMed] [Google Scholar]
- 48. Cahill EF, Tobin LM, Carty F, et al. Jagged‐1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rashedi I, Gomez‐Aristizabal A, Wang XH, et al. TLR3 or TLR4 activation enhances mesenchymal stromal cell‐mediated Treg induction via notch signaling. Stem Cells. 2017;35(1):265‐275. [DOI] [PubMed] [Google Scholar]
- 50. Edwards JP, Thornton AM, Shevach EM. Release of active TGF‐beta1 from the latent TGF‐beta1/GARP complex on T regulatory cells is mediated by integrin beta8. J Immunol. 2014;193(6):2843‐2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hong JW, Lim JH, Chung CJ, et al. Immune tolerance of human dental pulp‐derived mesenchymal stem cells mediated by CD4(+)CD25(+)FoxP3(+) regulatory T‐cells and induced by TGF‐beta1 and IL‐10. Yonsei Med J. 2017;58(5):1031‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu WT, Lin CH, Chiang BL, et al. Prostaglandin E2 potentiates mesenchymal stem cell‐induced IL‐10+IFN‐gamma+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190(5):2372‐2380. [DOI] [PubMed] [Google Scholar]
- 53. Yang H, Sun J, Wang F, et al. Umbilical cord‐derived mesenchymal stem cells reversed the suppressive deficiency of T regulatory cells from peripheral blood of patients with multiple sclerosis in a co‐culture—a preliminary study. Oncotarget. 2016;7(45):72537‐72545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tumangelova‐Yuzeir K, Naydenov E, Ivanova‐Todorova E, et al. Mesenchymal stem cells derived and cultured from glioblastoma multiforme increase Tregs, downregulate Th17, and induce the tolerogenic phenotype of monocyte‐derived cells. Stem Cells Int. 2019;2019:6904638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. An JH, Song WJ, Li Q, et al. Prostaglandin E2 secreted from feline adipose tissue‐derived mesenchymal stem cells alleviate DSS‐induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down‐regulate T cell receptor zeta‐chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752‐6761. [DOI] [PubMed] [Google Scholar]
- 57. Li X, Xu Z, Bai J, et al. Umbilical cord tissue‐derived mesenchymal stem cells induce T lymphocyte apoptosis and cell cycle arrest by expression of indoleamine 2, 3‐dioxygenase. Stem Cells Int. 2016;2016:7495135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. He Y, Zhou S, Liu H, et al. Indoleamine 2, 3‐dioxgenase transfected mesenchymal stem cells induce kidney allograft tolerance by increasing the production and function of regulatory T cells. Transplantation. 2015;99(9):1829‐1838. [DOI] [PubMed] [Google Scholar]
- 59. Mougiakakos D, Jitschin R, Johansson CC, et al. The impact of inflammatory licensing on heme oxygenase‐1‐mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826‐4835. [DOI] [PubMed] [Google Scholar]
- 60. Li JG, Zhuan‐sun YX, Wen B, et al. Human mesenchymal stem cells elevate CD4+CD25+CD127low/− regulatory T cells of asthmatic patients via heme oxygenase‐1. Iran J Allergy Asthma Immunol. 2013;12(3):228‐235. [PubMed] [Google Scholar]
- 61. Shen ZY, Wu B, Liu T, et al. Immunomodulatory effects of bone marrow mesenchymal stem cells overexpressing heme oxygenase‐1: protective effects on acute rejection following reduced‐size liver transplantation in a rat model. Cell Immunol. 2017;313:10‐24. [DOI] [PubMed] [Google Scholar]
- 62. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen‐G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212‐222. [DOI] [PubMed] [Google Scholar]
- 63. Chen C, Liang J, Yao G, et al. Mesenchymal stem cells upregulate Treg cells via sHLA‐G in SLE patients. Int Immunopharmacol. 2017;44:234‐241. [DOI] [PubMed] [Google Scholar]
- 64. Nasef A, Mazurier C, Bouchet S, et al. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253(1–2):16‐22. [DOI] [PubMed] [Google Scholar]
- 65. Chen JY, Li CF, Kuo CC, et al. Cancer/stroma interplay via cyclooxygenase‐2 and indoleamine 2,3‐dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014;16(4):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horita H, Kuroda E, Hachisuga T, et al. Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR‐8/SVneo. Hum Reprod. 2007;22(7):1801‐1809. [DOI] [PubMed] [Google Scholar]
- 67. Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 68. Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged‐2‐dependent regulatory dendritic cell population. Blood. 2009;113(1):46‐57. [DOI] [PubMed] [Google Scholar]
- 69. Zhao ZG, Xu W, Sun L, et al. Immunomodulatory function of regulatory dendritic cells induced by mesenchymal stem cells. Immunol Invest. 2012;41(2):183‐198. [DOI] [PubMed] [Google Scholar]
- 70. Liu X, Qu X, Chen Y, et al. Mesenchymal stem/stromal cells induce the generation of novel IL‐10‐dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189(3):1182‐1192. [DOI] [PubMed] [Google Scholar]
- 71. Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti‐inflammatory macrophages. Stem Cells. 2013;31(9):1980‐1991. [DOI] [PubMed] [Google Scholar]
- 72. Chiossone L, Conte R, Spaggiari GM, et al. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells. 2016;34(7):1909‐1921. [DOI] [PubMed] [Google Scholar]
- 73. Cheung TS, Dazzi F. Mesenchymal‐myeloid interaction in the regulation of immunity. Semin Immunol. 2018;35:59‐68. [DOI] [PubMed] [Google Scholar]
- 74. Rani S, Ryan AE, Griffin MD, et al. Mesenchymal stem cell‐derived extracellular vesicles: toward cell‐free therapeutic applications. Mol Ther. 2015;23(5):812‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang B, Yin Y, Lai RC, et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233‐1244. [DOI] [PubMed] [Google Scholar]
- 76. Zhang B, Yeo RWY, Lai RC, et al. Mesenchymal stromal cell exosome‐enhanced regulatory T‐cell production through an antigen‐presenting cell‐mediated pathway. Cytotherapy. 2018;20(5):687‐696. [DOI] [PubMed] [Google Scholar]
- 77. Favaro E, Carpanetto A, Caorsi C, et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325‐333. [DOI] [PubMed] [Google Scholar]
- 78. Hyvarinen K, Holopainen M, Skirdenko V, et al. Mesenchymal stromal cells and their extracellular vesicles enhance the anti‐inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)‐23 and IL‐22. Front Immunol. 2018;9:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Naji A, Eitoku M, Favier B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323‐3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zheng G, Huang R, Qiu G, et al. Mesenchymal stromal cell‐derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 82. Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71‐76. [DOI] [PubMed] [Google Scholar]
- 83. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383‐396. [DOI] [PubMed] [Google Scholar]
- 84. Romano M, Fanelli G, Albany CJ, et al. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Caplan H, Olson SD, Kumar A, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weiss DJ, English K, Krasnodembskaya A, et al. The necrobiology of mesenchymal stromal cells affects therapeutic efficacy. Front Immunol. 2019;10:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


