Abstract
Purpose
The purpose of this work is to assess the clinical outcome of pediatric patients diagnosed with pheochromocytoma and paraganglioma (PPGL) detected in France since 2000.
Methods
A retrospective multicenter study was conducted that included all patients younger than 18 years with PPGL diagnosed in France between 2000 and 2016. Patients were identified from 4 different sources: the National Registry of Childhood Solid Tumors, the French Pediatric Rare Tumors Database, the French registry of succinate dehydrogenase (SDH)-related hereditary paraganglioma, and the nationwide TenGen network.
Results
Among 113 eligible patients, 81 children with available data were enrolled (41 with adrenal and 40 with extra-adrenal PPGL). At diagnosis, 11 had synchronous metastases. After a median follow-up of 53 months, 27 patients experienced a new event (n = 7 second PPGL, n = 1 second paraganglioma [PGL], n = 8 local recurrences, n = 10 metastatic relapses, n = 1 new tumor) and 2 patients died of their disease. The 3- and 10-year event-free survival rates were 80% (71%-90%) and 39% (20%-57%),respectively, whereas the overall survival rate was 97% (93%-100%)at 3 and 10 years. A germline mutation in one PPGL-susceptibility gene was identified in 53 of the 68 (77%) patients who underwent genetic testing (SDHB [n = 25], VHL [n = 21], RET [n = 2], HIF2A [n = 2], SDHC [n = 1], SDHD [n = 1], NF1 [n = 1]). Incomplete resection and synchronous metastases were associated with higher risk of events (P = .011, P = .004), but presence of a germline mutation was not (P = .11).
Conclusions
Most pediatric PPGLs are associated with germline mutations and require specific follow-up because of the high risk of tumor recurrence.
Keywords: pheochromocytoma, paraganglioma, pediatric, genetic, SDHB
Pheochromocytoma and paraganglioma (PPGL) are rare neuroendocrine tumors derived from chromaffin cells of the adrenal medulla for pheochromocytoma (PHEO) or of extra-adrenal paraganglia for paraganglioma (PGL). Only 10% to 20% of tumors are detected before age 18 years, with an incidence of 0.2 to 0.5 cases per million [1, 2].
Unlike other malignant diseases, up to recently no specific histological or molecular markers existed to evaluate PPGL malignancy at first surgery [3]. The revised World Health Organization recommendations have defined malignant PPGL as metastatic disease that is histologically diagnosed by the presence of chromaffin tissues in organs that usually lack chromaffin cells [4]. Metastases mostly occur in lymph nodes, bones, lungs, and the liver. PPGLs are considered neuroendocrine tumors with the highest frequency of genetically determined cases. In pediatric patients, 50% to 70% of PPGLs are driven by a germline mutation in a PPGL-susceptibility gene [5]. Many PPGL-susceptibility genes have been identified and their list grows every year. Transcriptomic studies, using different large cohorts, have shown that genetically determined PPGLs are separated into 2 clusters. Tumors in cluster 1 carry mutations in the VHL, SDHA, SDHB, SDHC, SDHD, SDHAF2, FH, MDH2, GOT2, SLC25A11 or HIF2A gene, and are characterized by induction of a pseudo-hypoxic tumorigenic pathway. Tumors in cluster 2 harbor mutations in the RET, NF1, TMEM127 or MAX gene, and are characterized by activation of the mitogen-activated protein kinase and mTOR signaling pathways [6, 7]. The clinical outcome of patients with sporadic PPGL seems more favorable than that of patients with genetic forms, which are characterized by an early onset and high risk of multiple PPGLs or other syndromic lesions. It has been reported that the incidence of metastases is higher in PPGLs with an SDHB, SLC25A11, and FH mutation than in sporadic PPGLs, or PPGLs associated with other germline mutations [6, 7, 8]. The objective of this study was to determine the clinical outcome of children and adolescents with PPGL diagnosed in France between 2000 and 2016.
1. Methods
A. Patients
All patients with a PPGL diagnosed in France between 2000 and 2016 before age 18 years were eligible. Pediatric patients were identified in the National Registry of Childhood Solid Tumors (RNTSE). In addition, all centers of the French Society of Pediatric Oncology (SFCE), the French registry of SDH-related hereditary paraganglioma (PGL.R), the TenGen network, and the French Rare Pediatric Tumor database (FRACTURE) [9], as well as all pediatric pathology and surgery units in Paris, were contacted to identify additional cases. There were no specific guidelines in France for treatment and follow-up of pediatric PPGL.
B. Clinical Information
A case report form was used to collect demographic, clinical, and biochemical data as well as the tumor histological characteristics, detailed description of the clinical management, and outcome. The family clinical history and the genetic analysis results, when PPGL genetic testing was performed, were also recorded. In accordance with French law, the parents of each child signed an informed consent before genetic testing. Measurements of tumor largest diameter were based on computed tomography imaging. The French FRACTURE database has obtained legal and ethical approval from the French Data Protection Authority (CNIL) registration number DR-2011–408, and date October 14, 2011.
C. Statistical Analyses
Statistical analyses were carried out using GraphPad Prism software. Results were reported as medians with ranges for quantitative data, and as numbers and percentages for qualitative data. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Event-free survival (EFS) was defined as the time from diagnosis to disease progression, second malignancy, death whatever the cause, or last follow-up. Several prognostic factors (tumor type [PHEO or PGL], tumor size [≥ 5 cm or < 5 cm], quality of surgical resection [complete or incomplete], presence of metastases at diagnosis or not, and identification of a mutation in a PPGL-susceptibility gene vs no germline mutation identified) were evaluated using Peto’s test rather than unweighted log-rank tests because the relatively short follow-up time required a specific analysis of late events with very few patients at risk. All tests were 2-sided with a confidence level of 5%.
2. Results
A. Demographic and Tumor Characteristics
Overall, 77 patients with a PPGL that was diagnosed between 2000 and 2016 were registered in the RNTSE and treated in the 18 SFCE centers participating in this study. In addition, 36 patients with PPGL who were not registered in the RNTSE were identified in the archives of the participating SFCE centers for the 2000 to 2016 period. Among these 113 patients, 81 had available clinical data and were included in the analysis.
The tumor characteristics are detailed in Table 1. The tumor site was adrenal in 41 patients and extra-adrenal in 40. The median age at diagnosis was 13.3 years (range, 2.2-18 years). In 75 patients (93%), clinical symptoms, such as abdominal pain or high blood pressure, led to the diagnosis. The tumor was detected during a systematic clinical screening in 6 patients identified as mutation carriers in families with PPGL predisposition. Five patients (6%) had a previous or a synchronous malignancy: medullary thyroid carcinoma (n = 1 patient with multiple endocrine neoplasia type 2), Wilms tumor (n = 1 patient with Beckwith-Wiedemann syndrome), ependymoma (n = 1), Poppema nodular paraganglioma (n = 1), and gastrointestinal stromal tumor associated with PGL and pulmonary chondroma, as part of the Carney triad (n = 1).
Table 1.
Clinical, biochemical, and treatment characteristics of patients with pheochromocytoma and paraganglioma: baseline data
| Pheochromocytoma | Paraganglioma | ||
|---|---|---|---|
| No. of patients | 41 (50%) | 40 (50%) | |
| Abdomen Pelvis | 25 (62%) 1 | ||
| Thorax | 5 (12.5%) | ||
| Neck | 7 (17.5%) | ||
| Unilateral Bilateral | 37 (91%) 4 (9%) | Head | 2 (5%) |
| Sex: male | 28 (68%) | 20 (50%) | |
| Age at diagnosis (median, range), y | 11.5 (2.2-18) | 13.3 (4, 6-18) | |
| Detected by clinical screening after positive presymptomatic genetic test (number, percentage) | 5 (12%) | 1 (2.5%) | |
| Synchronous metastases | 5 (12%) | 6 (15%) | |
| Tumor largest diameter | |||
| < 5 cm | 20 (50%) | 22 (61%) | |
| 5-10 cm | 20 (50%) | 11 (30.5%) | |
| > 10 cm | 0 | 3 (8.3%) | |
| Missing data | 1 | 4 | |
| Fractionated metanephrines/urinary creatinine | |||
| > 3 N | 31 (86%) | 25 (69%) | |
| N | 5 (13.8%) | 11 (30.5%) | |
| Not available | 5 | 4 | |
| Treatment | |||
| Primary resection | 40 (97%) | 38 (74%) | |
| Complete excision | 33 (82%) | 28 (73%) | |
| Microscopically incomplete | 5 (12.5%) | 7 (18.4%) |
Measurements of tumor largest diameter were based on computed tomography imaging.
Abbreviation: N, laboratory normal values.
At diagnosis, PHEO (total n = 41) were unilateral in 37 (90%) patients, and bilateral in 4 patients. Among the 40 PGLs, 33 were unique and 7 (17%) multiple, and 25 (62%) were located in the abdomen. In 11 patients, PPGL was metastatic at diagnosis with histologically proven synchronous lymph node metastases (n = 7), and/or a metastatic spreading in bones (n = 3) and lungs (n = 3) (multiple sites possible).
B. Treatment Characteristics and Clinical Outcome
Up-front tumor resection was performed in 78 (96%) patients. Primary tumor resection was complete in 61 patients, microscopically incomplete in 12, and macroscopically incomplete in 5. Seven patients received additional therapies: chemotherapy (n = 4), external radiotherapy on the tumor bed (n = 4), and metabolic radiotherapy with 131I-mIBG (131I-metaiodobenzylguanidine) (n = 1).
The median follow-up was 53 months (range, 2-173 months). Six patients were lost to follow-up 1 to 9 months after surgery. During this period, 27 patients (n = 14/41 with PHEO and 13/40 with PGL) experienced an event. The median time between the initial diagnosis and the first event was 48 months (range, 1-133.2 months), but late events after 5 years were reported in 8 of 30 patients (n = 4 with PHEO and n = 4 with PGL). Specifically, one patient with von Hippel Lindau disease had a non-PPGL second malignancy (clear cell carcinoma) 38 months after the initial PHEO diagnosis. A new PPGL was detected for 8 patients, among whom 7 patients had a new PHEO in the contralateral adrenal gland after a median follow-up of 45.6 months (range, 1-82.1 months), and 1 patient had multiple new cervical PGLs. Local relapse occurred in 8 patients (n = 2 with PHEO and n = 6 with PGL), after a median time of 38.5 months (range, 3-134 months). Primary tumor resection was complete in 4 patients, incomplete in 3 patients, and 1 could not undergo surgery but received external radiotherapy on the tumor bed. Metastatic relapse was recorded for 10 patients (n = 4 with PHEO and n = 6 with PGL), within a median time of 44.5 months (range, 1-96 months]. The 3-year and 5-year EFS rates were 55% (23%-78%) and 33% (9%-61%) respectively in patients with metastatic disease.
Among the 27 patients with an event after the first PPGL diagnosis, 9 (33%) had multiple metastatic relapses leading to the death of 2 patients, 9 and 10 months after the initial diagnosis. No other death was reported among the patients included in this series.
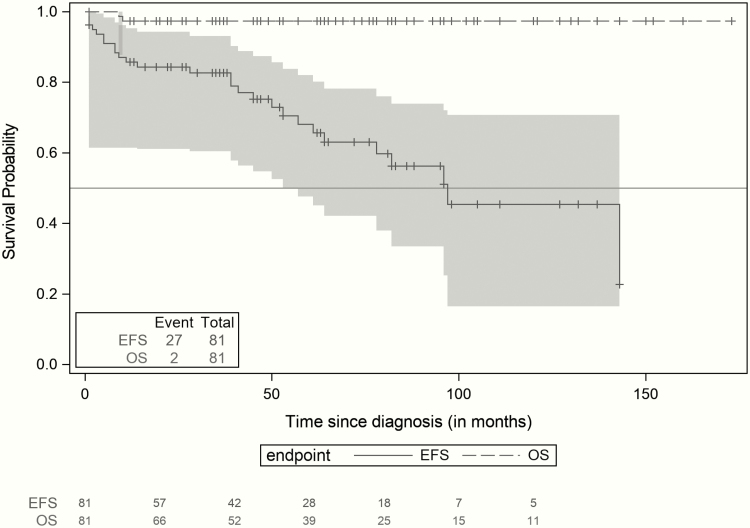
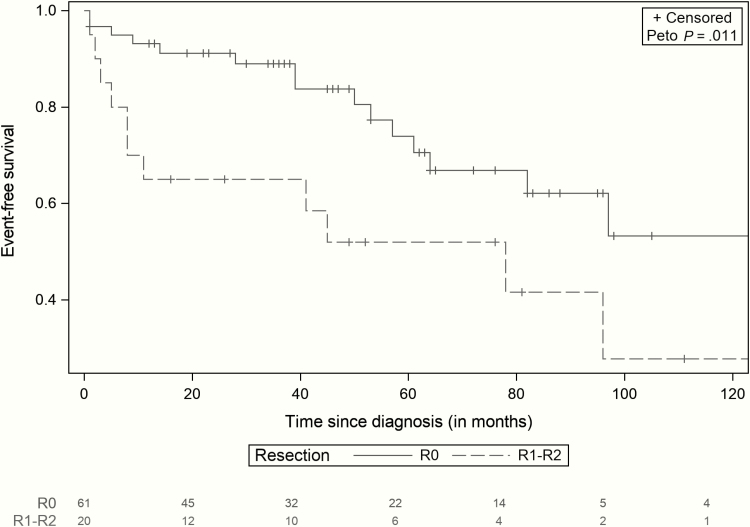
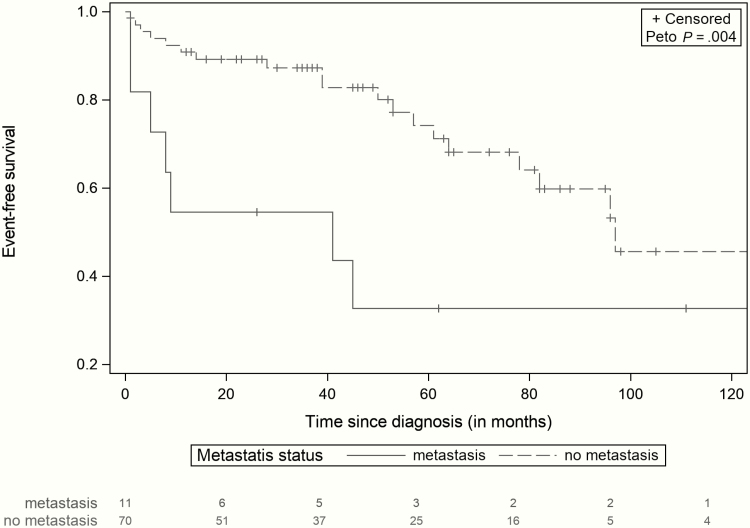
Overall, the 3-year, 5-year, and 10-year EFS rates were 80% (71%-90%), 61% (47%-75%), and 39% (20%-57%), respectively, and the OS rate was 97% (93%-100%) at 3, 5, and 10 years after diagnosis (Fig. 1). In this cohort, incomplete surgical resection of the initial PPGL and presence of a metastatic disease at the initial diagnosis were associated with a significant higher risk of local and metastatic relapses (P = .011, and P = .004), respectively (Figs. 2 and 3).
Figure 1.
Event-free survival and overall survival of patients with pheochromocytoma and paraganglioma (95% CI). Kaplan-Meier survival curves and 95% confidence band: overall survival (OS) and event-free survival (EFS) curves of pediatric patients diagnosed with pheochromocytoma and paraganglioma. Two patients died, and 27 presented with a new event.
Figure 2.
Event-free survival of patients with complete resection (R0) and with incomplete resection (R1-R2) (95% CI). Comparison of event-free survival (EFFS) between patients with primary complete tumor resection (N = 61), and patients with incomplete tumor resection (N = 20). Incomplete resection was associated with a higher risk of events (P = .011).
Figure 3.
Event-free survival of patients with and without metastases (95% CI). Comparison of event-free survival (EFS) between patients without metastasis disease at diagnosis (N = 70), and patients with metastasis disease at diagnosis (N = 11). Presence of synchronous metastases at diagnosis was associated with a higher risk of events (P = .004).
C. Genetic Testing
A pathogenic germline mutation in a PPGL-susceptibility gene (SDHB [n = 25], VHL [n = 21], RET [n = 2], HIF2A [n = 2], SDHC [n = 1], SDHD [n = 1], and NF1 [n = 1]) was identified in 53 out of the 68 (77%) patients who underwent genetic testing (Table 2). The genotype-phenotype correlations are reported in Table 2. The mutation rate was 77% in patients with PHEO (27/35) and 78% in patients with PGL (26/33). Among the patients with genetic testing results, 24 were in the group of 27 patients who had an event during follow-up, and 22/24 (92%) carried a mutation: SDHB (n = 9), VHL (n = 9), SDHC (n = 1), RET (n = 1), HIF2A (n = 1) and NF1 (n = 1). Among the 68 children who underwent genetic testing, neither the identification of a germline mutation (P = .11) nor the SDHB mutation status (P = .83) had a significant impact on outcome.
Table 2.
Clinical characteristics and relationship with local and distant metastases
| Mutated gene | SDHB | VHL | SDHC | SDHD | HIF2A | NF1 | RET |
|---|---|---|---|---|---|---|---|
| No. of patients | 25 | 21 | 1 | 1 | 2 | 1 | 2 |
| Median age (range), y | 13.3 (6.6-18) | 11 (2-18) | 16 | 11 | 10.1 (9.1-10.3) | 12 | 17 (14.6-18) |
| Sex: male | 13 | 14 | 0 | 0 | 1 | 1 | 2 |
| PHEO PGL | 7 18 | 17 4 | 0 1 | 0 1 | 1 1 | 0 1 | 2 0 |
| Metastasis at diagnosis | 1 | 2 | 1 | 0 | 1 | 1 | 0 |
| Event | 9 | 9 | 1 | 0 | 1 | 1 | 1 |
| PHEO PGL | 1 8 | 9 0 | 0 1 | 1 0 | 0 1 | 1 0 | |
| Median time to event, mo | 45 | 39 | 45 | 0 | 8 | 20 | 72 |
| Second non-PPGL tumor | 1 | ||||||
| Metachronous PPGL | – | 7 | 1 | – | – | – | |
| Local relapse | 7 | – | – | – | – | 1 | |
| Metastatic relapse | 2 | 1 | 0 | 1 | 1 | – |
Abbreviations: PHEO, pheochromocytoma; PGL, paraganglioma; PPGL, pheochromocytoma and paraganglioma.
3. Discussion
Over the last decade, many reports have described PPGL clinical and genetic characteristics in large series of adult patients. These insights have helped to reduce treatment-related morbidity and mortality, with efficient and gene-specific preventive strategies. However, the subgroup of pediatric PPGL is still poorly studied because previous reports were mostly based on few cases [2, 10, 11-16]. The present study described 81 patients with PPGL diagnosed before age 18 years, one of the largest series of pediatric patients [15, 6, 17-22] (Table 3).
Table 3:
Review of literature data
| Authors and date of publication | Patients, No. | Age at diagnosis, y | PHEO/PGL ratio | PGL localization: abdomen thorax neck other | PHEO: bilateral at diagnosis, No. | New PPGL, No., % | Relapse (N) and % | Time to relapse | Metastatic at diagnosis No., % | Death No. | Percentage of mutation carriers in tested patients, % | Mutated gene: cluster 1 (SDHB, SDHC, SDHC, VHL) cluster 2 (RET, NF1, HIF2A) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argentina, Barontini et al, 2006 [1] | 58 | 11.0 | 4.2 | 7/1/–/1 | 5 | 16 | 7 (12%) | 12–60 mo | 0 | 4 | 39 | Cluster 1 VHL 16; SDHB 1 Cluster 2 NF1 1; RET 2 |
| USA, Pham et al, 2006 [22] | 30 | 14.7 | 0.66 | 14/1/3/– | 0 | 0 | 9 (30%) | 0–103 mo | 14 (46%) | 0 | 100 | Cluster 1 SDHB and SDHD 2 Cluster 2 RET 3 |
| USA, King et al, 2011 [23] | 49 | 11.6 | 0.26 | 22/–/3/– | 0 | 0 | 32 (65%) | 0–29 y | 0 | 5 | 82 | Cluster 1 VHL 6; SDHB 27; SDHD 4 Cluster 2 NF1 2 |
| Mutlinational, Bausch et al, 2013 [5] | 177 | 13 | 15.6 | 36/9/8/– | 43 | 49 | 68 (38%) | 0–25 y | 10 (5%) | 8 | 80 | Cluster 1 VHL 80; SDHB 25; SDHD 17; SDHA 1 SDHC 1 Cluster 2 NF1 6; RET 1 |
| Spanish, Cascon et al, 2013 [19] | 36 | 13.8 | 0.62 | 17/5 | 7 | – | 4 (11%) | 1 | 69 | Cluster 1 VHL 8; SDHB 11; SDHD 4 Cluster 2 RET 1; MAX 1 | ||
| India, Mishra et al, 2014 [20] | 24 | 14–16 | 3 | – | 10 | 2 | 2 (8%) | 3 | 0 | |||
| Multinational, Pamporaki et al, 2017 [18] | 95 | 13.3 | 0.54 | – | 11 | 20 | 57 (60%) | 31 (32%) | – | 80.4 | Cluster 1 VHL 25; SDHB 36; SDHD 9 Cluster 2 NF1 1; RET 3 | |
| USA, Babic et al, 2017[21] | 55 | 13.4 | 1.75 | 12/2/6/– | 8 | 11 | 22 (40%) | 6 mo–16 y | 9 (16%) | – | 80 | Cluster 1 VHL 21; SDHB 13; SDHD 4; SDHA 1 Cluster 2 NF1 1; RET 3; MAX 1 |
| Current study, 2019 | 81 | 13.3 | 1.02 | 25/5/7/3 | 7 | 7 | 27 (33%) | 1–133.2 mo | 11 (13.5%) | 2 | 77 | Cluster 1 VHL 21; SDHB 25; SDHC 1; SDHD 1; HIF2A 2 Cluster 2 NF1 2; RET 2 |
| Total | 605 | 13.2 | 3.07 | 133/23/27/4 | 91 | 105 | 222 (36%) | 1 mo–29 y | 81 (13.3%) | 23 | 67.4 | Cluster 1 VHL 177; SDHB 138; SDHC 2; SDHD 40; SDHA 2; HIF2A 2 Cluster 2 NF1 12; RET 13; MAX 2 |
Abbreviations: PHEO, pheochromocytoma; PGL, paraganglioma; PPGL, pheochromocytoma and paraganglioma; USA, United States of America.
The patients’ recruitment from the RNTSE supports the representativeness of this cohort. Although patients with tumors classified as benign are not systematically included in the registry, patients who were identified in the archives of the pathology and surgery departments in Paris were also addressed at SFCE centers. In our cohort, despite a relatively short follow-up time, 27 (33%) patients presented with an event: Twenty-six patients had tumor relapses, including 10 (38%) metastatic relapses and 1 patient with a non-PPGL second malignancy. In addition, among the 68 (83%) patients who underwent genetic testing for mutations in PPGL-susceptibility genes, 53 (77%) had a pathogenic mutation, including 25 (47% of mutation carriers) with an SDHB mutation. These mutation rates are in line with previous reports (see Table 3).
Five patients (6%) had a previous malignancy. Tumors of the multiple endocrine neoplasia type 2, von Hippel Lindau disease, and neurofibromatosis type 1 spectra can be associated with PPGL [7, 23-26]. Conversely, PPGL association with nephroblastoma and ependymoma is rare. However, few cases of PHEO in patients with Beckwith-Wiedemann syndrome have been described [27], and a recent genetic analysis reported a D631Y RET mutation in a patient with recurrent anaplastic ependymoma associated with a familial history of PHEO [28]. In our cohort, the patient with PHEO after an ependymoma (n = 1) did not undergo genetic testing, but the clinical and familial features did not suggest a multiple endocrine neoplasia type 2.
The proportion of patients with metastatic PPGL at diagnosis was high: Fourteen percent of patients had synchronous metastases, and 38% of patients who relapsed had metachronous metastases, including 4 patients with localized PPGL at diagnosis. In our analysis, the presence of metastases at diagnosis was associated with a higher risk of recurrence and a 5-year EFS rate of 33%. Our results are in line with previous reports, in which the 5-year survival rate of pediatric patients with nonmetastatic PPGL was 90% [29] and ranged from 30% to 60% in patients with metastatic disease for whom the 10-year survival rate was 31% [30-32]. The prognostic impact of incomplete surgical resection has to be stressed. In our study, incomplete resection at first surgery was significantly associated with a higher risk of an event. Indeed, surgery is the only curative option for localized PPGL. In our study, the 10-year OS rate was 97%, despite the high rate of events during the follow-up (27/81, mostly tumor recurrences). However, we must acknowledge that late events may have been underestimated in our series because the median follow-up was rather short. Indeed, in our series, 27% of patients with more than 5 years of follow-up experienced a late relapse. This high incidence of late relapses in pediatric PPGL has already been reported in adult patients with PPGL diagnosed before age 20 years in whom the median interval between diagnosis and the first event was 9.1 years [23].
Since 2000, many PPGL predisposition genes have been identified [13, 25, 26, 33-36]. Recent studies showed that 50% to 70% of pediatric patients with PPGL carry a causal mutation [20, 29] (see Table 3). Indeed, mutations in SDHB, SDHC, SDHD, VHL, RET, and NF1 are frequently associated with PPGL [18, 23, 25, 26, 35], whereas mutations in SDHA, SDHAF2, TMEM127, HIF2A, FH, SLC25A11, and MAX have been described only in limited series of patients [33, 34, 36, 37, 38]. Because this retrospective study covers a long period, genetic screening was not proposed as a systematic procedure for all patients, especially those with a PPGL diagnosed more than 10 years ago. Before 2006, only patients whose phenotype or family history suggested a predisposition syndrome were advised to undergo genetic counseling. In our cohort, among the 68 patients who underwent genetic testing, 53 (77%) were carriers of a pathogenic mutation in a PPGL-susceptibility gene. This proportion is probably underestimated because a large proportion of patients in whom no germline mutation was identified was tested by Sanger targeted sequencing before 2010, at a period when only a subset of the genes now known to be associated with a predisposition to PPGL were routinely analyzed. Therefore, a new analysis by next-generation sequencing with a panel including all identified PPGL-susceptibility genes might also identify a pathogenic mutation in patients with negative genetic testing results at the time of the diagnosis. This may increase the mutation detection rate in our cohort [39]. Nowadays, genetic counseling is recommended for all patients with PPGL [3, 29] to identify genetically determined PPGL and to propose predictive genetic testing to mutations carriers’ relatives. The proportion of patients with an SDHB mutation was as expected in our series, but only one patient carried an SDHD mutation, which is rather low because this mutation is described in 7% to 11% of previous pediatric series of PPGL [5, 18, 19, 21, 22, 23]. This may be due to a bias in patient recruitment and may be because SDHD mutations are mostly associated with cervical PGL treated in ears, nose, and throat departments and less likely to be declared in the RNTSE, nor referred to pediatric oncology departments.
Among the 24 of 27 patients who presented with an event during the follow-up and underwent genetic testing, 22 (92%) carried a pathogenic genetic mutation in a PPGL-susceptibility gene. In our series, carrying a mutation in one of these genes was not shown to be associated with a significant increased risk of recurrence, despite a trend to higher risk of relapse after 40 months of follow-up. This result may be explained by the relatively short follow-up of SDHB-mutations carriers leading to a poor estimation of the risk of late event. Considering the high incidence of germline mutations in this population, careful and lifelong follow-up aiming at earlier detection and treatment of novel lesions is now recommended for all pediatric patients with PPGL.
In this study spanning more than 2 decades, the lack of guidelines for treatment and follow-up of pediatric PPGL led to suboptimal care because 7% of patients were lost to follow-up after surgery, 16% did not benefit from genetic testing, and most patients with negative genetic testing did not benefit from a new next-generation sequencing analysis of all PPGL-susceptibility genes. These results stress the need for guidelines for pediatric PPGL patients. In a large study comparing pediatric vs adult patients with PPGL, Pamporaki et al showed that despite the higher prevalence of metastatic disease in children, metastatic recurrences were mostly diagnosed in patients with PPGL during adulthood [18]. No evidence suggests that surveillance should be different in children compared with adults; however, pediatric oncologists should be aware of the importance of prolonging the follow-up of children with PPGL into adulthood. Considering the high risk of genetic predisposition in pediatric patients with PPGL, the current protocols recommend surveillance through regular examinations for early detection of recurrence or of new tumors for all pediatric patients with PPGL, as is done for high-risk adult patients (ie, patients with genetically determined disease, tumors greater than 50 mm, and extra-adrenal location) for whom lifetime follow-up is indicated [29]. The European practice guidelines for long-time follow-up recommend monitoring blood pressure and 24-hour urinary fractionated metanephrines and optionally chromogranin A once a year, together with whole-body magnetic resonance imaging every 1 to 2 years, for the early detection of recurrence or of new tumors, while minimalizing radiation exposure [29, 40, 41]. Neck magnetic resonance angiography may be discussed with specialists because PPGL detection may be difficult in the neck [40], regardless of genetic background.
4. Conclusions
This retrospective analysis underlines the need for improvement of pediatric care for patients with PPGL after surgery. Because of the high incidence of hereditary PPGL, genetic counseling and screening for germline mutations are mandatory to improve clinical follow-up guided by genetic background and for the detection of asymptomatic mutation carriers among relatives. Moreover, as suggested in international guidelines, all pediatric PPGL patients, regardless of the identification of a germline mutation, require careful and lifelong follow-up because of the high risk of late events.
Acknowledgments
We are very grateful to all clinical research associates, genetic counselors, and junior and senior medical staff for their help collecting data and ongoing support for this work.
Financial Support: The French FRACTURE database is supported by the Enfants, Cancer et Santé association, which did not take part in any of the analyses in this manuscript.
Glossary
Abbreviations
- EFS
event-free survival
- OS
overall survival
- PGL
paraganglioma
- PHEO
pheochromocytoma
- PPGL
pheochromocytoma and paraganglioma
- RNTSE
National Registry of Childhood Solid Tumors
- SFCE
French Society of Pediatric Oncology
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Ann N Y Acad Sci. 2006;1073:30-37. [DOI] [PubMed] [Google Scholar]
- 2. Ciftci AO, Tanyel FC, Senocak ME, Büyükpamukçu N. Pheochromocytoma in children. J Pediatr Surg. 2001;36(3):447-452. [DOI] [PubMed] [Google Scholar]
- 3. Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. [DOI] [PubMed] [Google Scholar]
- 4. Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol. 1990;21(11):1168-1180. [DOI] [PubMed] [Google Scholar]
- 5. Bausch B, Wellner U, Bausch D, et al. Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer. 2014;21(1):17-25. [DOI] [PubMed] [Google Scholar]
- 6. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. [DOI] [PubMed] [Google Scholar]
- 7. Björklund P, Pacak K, Crona J. Precision medicine in pheochromocytoma and paraganglioma: current and future concepts. J Intern Med. 2016;280(6):559-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gimenez-Roqueplo AP, Favier J, Rustin P, et al. ; COMETE Network Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63(17):5615-5621. [PubMed] [Google Scholar]
- 9. Bisogno G, Ferrari A, Bien E, et al. Rare cancers in children—the EXPeRT Initiative: a report from the European Cooperative Study Group on Pediatric Rare Tumors. Klin Padiatr. 2012;224(6):416-420. [DOI] [PubMed] [Google Scholar]
- 10. Stackpole RH, Melicow MM, Uson AC. Pheochromocytoma in children. Report of 9 case and review of the first 100 published cases with follow-up studies. J Pediatr. 1963;63:314-330. [DOI] [PubMed] [Google Scholar]
- 11. Kaufman BH, Telander RL, van Heerden JA, Zimmerman D, Sheps SG, Dawson B. Pheochromocytoma in the pediatric age group: current status. J Pediatr Surg. 1983;18(6):879-884. [DOI] [PubMed] [Google Scholar]
- 12. Ein SH, Shandling B, Wesson D, Filler RM. Recurrent pheochromocytomas in children. J Pediatr Surg. 1990;25(10):1063-1065. [DOI] [PubMed] [Google Scholar]
- 13. De Krijger RR, Petri BJ, Van Nederveen FH, et al. Frequent genetic changes in childhood pheochromocytomas. Ann N Y Acad Sci. 2006;1073:166-176. [DOI] [PubMed] [Google Scholar]
- 14. Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr (Phila). 2005;44(8):715-719. [DOI] [PubMed] [Google Scholar]
- 15. Bhansali A, Rajput R, Behra A, Rao KL, Khandelwal N, Radotra BD. Childhood sporadic pheochromocytoma: clinical profile and outcome in 19 patients. J Pediatr Endocrinol Metab. 2006;19(5):749-756. [DOI] [PubMed] [Google Scholar]
- 16. Reddy VS, O’Neill JA Jr, Holcomb GW 3rd, et al. Twenty-five-year surgical experience with pheochromocytoma in children. Am Surg. 2000;66(12):1085-1091; discussion 1092. [PubMed] [Google Scholar]
- 17. Ganesh HK, Acharya SV, Goerge J, Bandgar TR, Menon PS, Shah NS. Pheochromocytoma in children and adolescents. Indian J Pediatr. 2009;76(11):1151-1153. [DOI] [PubMed] [Google Scholar]
- 18. Pamporaki C, Hamplova B, Peitzsch M, et al. Characteristics of pediatric vs adult pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2017;102(4):1122-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cascón A, Inglada-Pérez L, Comino-Méndez I, et al. Genetics of pheochromocytoma and paraganglioma in Spanish pediatric patients. Endocr Relat Cancer. 2013;20(3):L1-L6. [DOI] [PubMed] [Google Scholar]
- 20. Mishra A, Mehrotra PK, Agarwal G, Agarwal A, Mishra SK. Pediatric and adolescent pheochromocytoma: clinical presentation and outcome of surgery. Indian Pediatr. 2014;51(4):299-302. [DOI] [PubMed] [Google Scholar]
- 21. Babic B, Patel D, Aufforth R, et al. Pediatric patients with pheochromocytoma and paraganglioma should have routine preoperative genetic testing for common susceptibility genes in addition to imaging to detect extra-adrenal and metastatic tumors. Surgery. 2017;161(1):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pham TH, Moir C, Thompson GB, et al. Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics. 2006;118(3):1109–1117. [DOI] [PubMed] [Google Scholar]
- 23. King KS, Prodanov T, Kantorovich V, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plouin PF, Fitzgerald P, Rich T, et al. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res. 2012;44(5):390-399. [DOI] [PubMed] [Google Scholar]
- 25. Neumann HP, Bausch B, McWhinney SR, et al. ; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459-1466. [DOI] [PubMed] [Google Scholar]
- 26. Mannelli M, Castellano M, Schiavi F, et al. ; Italian Pheochromocytoma/Paraganglioma Network Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94(5):1541-1547. [DOI] [PubMed] [Google Scholar]
- 27. Caza T, Manwaring J, Riddell J. Recurrent, bilateral, and metastatic pheochromocytoma in a young patient with Beckwith-Wiedemann syndrome: a genetic link? Can Urol Assoc J. 2017;11(5):E240-E243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ospina NS, Maraka S, Donegan D, Morris JC. Clinical features of a family with multiple endocrine neoplasia type 2A caused by the D631Y RET mutation. Thyroid. 2017;27(10):1332-1334. [DOI] [PubMed] [Google Scholar]
- 29. Plouin PF, Amar L, Dekkers OM, et al. ; Guideline Working Group European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174(5):G1-G10. [DOI] [PubMed] [Google Scholar]
- 30. Pacak K, Eisenhofer G, Ahlman H, et al. ; International Symposium on Pheochromocytoma Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3(2):92-102. [DOI] [PubMed] [Google Scholar]
- 31. Turkova H, Prodanov T, Maly M, et al. Characteristics and outcomes of metastatic SDHB and sporadic pheochromocytoma/paraganglioma: an National Institutes of Health study. Endocr Pract. 2016;22(3):302-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parenti G, Zampetti B, Rapizzi E, Ercolino T, Giachè V, Mannelli M. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J Oncol. 2012;2012:872713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burnichon N, Brière JJ, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19(15):3011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bayley JP, Kunst HP, Cascón A, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11(4):366-372. [DOI] [PubMed] [Google Scholar]
- 35. Neumann HP, Pawlu C, Peczkowska M, et al. ; European-American Paraganglioma Study Group Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292(8):943-951. [DOI] [PubMed] [Google Scholar]
- 36. Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42(3):229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fishbein L, Nathanson KL. Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet. 2012;205(1-2):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108-119. [DOI] [PubMed] [Google Scholar]
- 39. Crona J, Verdugo AD, Granberg D, et al. Next-generation sequencing in the clinical genetic screening of patients with pheochromocytoma and paraganglioma. Endocr Connect. 2013;2(2):104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rednam SP, Erez A, Druker H, et al. Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e68-e75. [DOI] [PubMed] [Google Scholar]
- 41. Tufton N, Shapiro L, Srirangalingam U, et al. Outcomes of annual surveillance imaging in an adult and paediatric cohort of succinate dehydrogenase B mutation carriers. Clin Endocrinol (Oxf). 2017;86(2):286-296. [DOI] [PubMed] [Google Scholar]