Abstract
Background
Exopolysaccharides (EPSs) secreted from lactic acid bacteria are carbohydrate polymers with reported biological activities. In this study, we extracted and characterized the composition as well as antioxidant and biofilm-inhibitory properties of EPS from Lactobacillus coryniformis NA-3 isolated from northeast Chinese sauerkraut (Suan Cai).
Methods
Lactobacillus coryniformis NA-3 was identified with 16S rDNA amplification and Neighbor Joining (NJ) phylogenetic analysis. EPS derived from Lactobacillus coryniformis NA-3 (EPS-NA3) was analyzed, including compositions by high-performance liquid chromatography (HPLC), functional groups by Fourier-transform infrared spectroscopy (FT-IR) and glycosidic bond configuration by Hydrogen-1 Nuclear Magnetic Resonance (1H NMR). Antioxidant activity of EPS was evaluated with hydroxyl and superoxide radical-scavenging. Anti-biofilm activities of EPS-NA3 were checked through inhibition and dispersion.
Results
The monosaccharide composition of EPS included α-rhamnose, α-mannose, α-galactose, and α-glucose in a ratio of 2.6:1.0:5.0:3.3. The free radical-scavenging abilities of EPS-NA3 were 37.77% ± 1.56% and 78.87% ± 3.07% on hydroxyl and superoxide reactive oxygen species respectively. Moreover, EPS-NA3 attenuated the formation of Bacillus cereus and Salmonella typhimurium biofilms by inhibition ratios of approximately 80% and 40% respectively. Additionally, treatment with EPS-NA3 dispersed established biofilms of B. cereus and S. typhimurium by approximately 90% and 20% respectively.
Conclusion
These results suggest that EPS-NA3 may be developed as antioxidant and anti-biofilm agents for industrial and clinical applications due to its capacity of scavenging free radicals, inhibition of bacterial biofilm formation, and dispersion of established biofilms.
Keywords: Lactobacillus coryniformis NA-3, exopolysaccharide, antioxidant, anti-biofilm, inhibition, dispersion
Popular scientific summary
The first report characterizing a novel exopolysaccharide (EPS) from Lactobacillus coryniformis NA-3, isolated from Northeast Chinese sauerkraut (Suan Cai).
EPS derived from Lactobacillus coryniformis NA-3 is a heteropolysaccharide exhibited antioxidant activity.
EPS derived from Lactobacillus coryniformis NA-3 inhibits pathogen biofilm formation or disperses biofilm.
Lactic acid bacteria (LAB) can produce exopolysaccharides (EPSs) on bacterial cell wall to form a capsule, or secreted in the environment as a viscous slime (1–3) for retaining moisture to protect bacteria or for scavenging external nutrients. EPSs are long-chain carbohydrate polymers linked by repeating monosaccharide units and classified into homopolysaccharides (HoPSs) and heteropolysaccharides (HePSs) according to monosaccharide composition (4). Homopolysaccharides mainly include glucans and fructans, occasionally galactans (5). HePSs are produced by a large variety of mesophilic (Lactobacillus casei, Lb. rhamnosus, etc.) and thermophilic (Lb. acidophilus, Lb. helveticus, etc.) LAB strains (6). Interest in EPSs produced by Lactobacillus spp. has increased due to potential economic reasons of replacing natural gums (7). Bacterial EPSs have shown improvements, compared with eukaryotic polysaccharides, in rheological characteristics and stability while also reducing the requirement for arable land necessary for their production (8).
Over the past decades, several studies have examined the physical properties of LAB-derived EPSs, such as their individual characteristics as biological agents for thickening, gelatinization, stabilization, and emulsification in food industry applications (9). In addition, EPSs from LAB are reported to provide health benefits, such as anti-tumor, immune-stimulating, and antioxidant activities as well as inhibition of bacterial growth and biofilm production (10–12). In particular, antioxidant and anti-biofilm activities have attracted increasing research attention as desirable properties to explore for medical, pharmaceutical, and industrial applications. An increase in oxidative stress is one factor that underlies the pathology of many diseases, such as diabetes, cancer, liver diseases, and several chronic degenerative diseases (13).
LAB-derived EPSs have been shown to enhance cellular defense mechanisms through antioxidant activity that reduces the oxidative damage caused by reactive oxygen species and free radicals (14, 15). Similarly, EPSs from LAB have been tested for biofilm inhibition to aid in the prevention of some clinical diseases caused by pathogenic bacteria, such as infections caused by Staphylococcus epidermidis, Pseudomonas aeruginosa, Vibrio cholerae, and Yersinia (16). Many EPS-producing Lactobacillus species, such as Lactobacillus helveticus, L. plantarum (17), L. fermentum (18), and L. acidophilus (19) have also shown anti-biofilm activity. Interference with biofilm formation by pathogenic bacteria may comprise a large part of the antimicrobial effects of EPSs (12) which is important for preventing chronic and recurrent infections.
EPS-producing LAB have been isolated from diverse sources, most often from fermented foods (3, 20). Northeastern Chinese sauerkraut (or Suan Cai) is a traditional fermented food with a complex microbial community. Lactobacillus spp., including L. plantarum, L. casei (21), and L. coryniformis (22) are relatively abundant. Among Lactobacilli, L. coryniformis has been studied less than other species, although it is typically found in fermented vegetable products (23). A safety assessment of L. coryniformis CECT 5711 revealed no deleterious enzymatic activities for this species, although it exhibits an intrinsic antibiotic resistance profile (24). L. coryniformis CECT 5711 has also been reported to display antimicrobial activity (24) and is used as a probiotic strain to enhance intestinal function in healthy adults (25). However, the physical properties and biological activities of EPSs isolated from L. coryniformis have not been studied yet; in light of its uses as a probiotic, this species may prove to be a valuable source as an EPS-producing strain, safe for consumption and medical applications.
In the present work, we describe the isolation of EPS-producing strain L. coryniformis NA-3, extraction (by ethanol precipitation) and characterization of the EPS composition (with Fourier-transform infrared spectroscopy [FT-IR] and high-performance liquid chromatography [HPLC] analysis), and subsequent evaluation of its antioxidant and anti-biofilm activities. Our findings indicate that EPS produced by this LAB strain could serve as a substantial source of food production and pharmaceuticals, and has the potential of developing as a nutraceutical.
Materials and methods
Screening and identification of EPS-producing LAB
For isolation of LAB, (Suan Cai) brine was serially diluted in phosphate buffer solution (PBS; pH = 7.4) and plated on De Man, Rogosa, and Sharpe (MRS) (Solarbio, Beijing, China) agar medium with 0.3% CaCO3 (Xilong, Guangdong, China). The plates were incubated for 24–48 h at 37°C under anaerobic conditions. EPS-producing LAB strains were first screened by transparent zones or haloes showing calcium solubilization, and then viscid, ‘ropy’ colonies were isolated from calcium solubilizing colonies (26, 27). A single colony from each strain was inoculated into 10 mL of fresh MRS medium, and incubated at 37°C without shaking. Bacterial suspensions were centrifuged and the cell pellets were used for total DNA extraction with TIANamp Bacteria DNA Kit (Tiangen Biotech, Beijing, China). The 16S rDNA amplification was carried out with primers 27F (5’-AGTTTGATCMTGGCTCAG-3’) and 1492R (5’-TACGGYTACCTTGTTACGACTT-3’) using the following polymerized chain reaction (PCR) program: denaturation at 94°C for 3 min, 35 cycles at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 2 min, followed by a final extension cycle at 72°C/10 min. The PCR products were sent to Sango Biotech (Shanghai, China) for sequencing, and the sequences were identified using the NCBI non-redundant nucleotide BLAST (http://blast.ncbi.nlm.nih.gov). Subsequently, multiple sequence alignment (GenBank: EU626008.1, EU626013.1, MF629001.1, CP044506.1, NR_113175.1, FJ429977.1, NR_109004.1, EU626020.1, NR_117812.1, EU626019.1, EU331258.1, NR_118877.1, KX503225.1, FJ749472.1, and NR_040783.1) and Neighbor Joining (NJ) phylogenetic analysis were conducted using Mega 5.1. All isolates were stored at −80°C in medium with 30% glycerol (final concentration).
Growth conditions of bacterial strains
The pathogenic bacterial strains used for biofilm inhibition and dispersion assays were Bacillus cereus (CICC 21261) (Gram-positive) and Salmonella typhimurium (CICC 22956/ATCC 14028) (Gram-negative), purchased from the China Center of Industrial Culture Collection.
L. coryniformis NA-3 was activated anaerobically in MRS broth at 37°C. S. typhimurium was grown in Tryptic Soy Broth (TSB) medium containing 1.7% tryptone (OXOID, Basingstoke, England), 0.3% peptone (Aoboxing, Beijing, China), 0.5% NaCl (Xilong, Guangdong, China), 0.25% K2HPO4 (Xilong, Guangdong, China), and 0.25% glucose (Xilong, Guangdong, China). B. cereus was cultivated in Nutrient Broth (Aoboxing, Beijing, China).
EPS extraction from L. coryniformis NA-3
As described by Waśko et al. (28) and Yang et al. (29), the hot water extraction method was used to extract EPS from L. coryniformis NA-3 with minor modifications. L. coryniformis NA-3 was grown in MRS agar medium at 37°C for 24 h under anaerobic conditions. Then colony of L. coryniformis NA-3 was transferred to fresh MRS agar medium and incubated without shaking for 24 h. L. coryniformis NA-3 suspension was then inoculated at a 1/100 (v/v) dilution to 100 mL and 2,250 mL (750 mL × 3) of MRS medium successively and cultured at 37°C for 48 h under anaerobic conditions.
Following incubation, 2,250 mL of L. coryniformis NA-3 culture suspension was centrifuged at 12,000×g for 10 min and cells were washed twice with 0.9% NaCl. Then cells were treated with 80°C distilled water for about 20 h. After centrifugation (12,000×g for 20 min at 4°C), sediments were removed and the supernatant was retained as EPS solution. The EPS was precipitated by adding a three-fold volume of chilled ethanol, and the suspension was left to stand at 4°C for 4 days. Crude EPS was obtained after centrifugation at 12,000×g for 20 min, then resuspended in distilled water with 14% trichloroacetic acid (TCA) (Fuchen, Tianjin, China) and kept at 4°C for 24 h. Soluble proteins were removed after centrifugation at 12,000×g for 20 min at 4°C; supernatant was dialyzed for 3 days against distilled water (MWCO 7,000 Da, DL BioChem, USA), then concentrated and lyophilized, thus resulting in isolated polysaccharide.
Molecular weight (Mw) determination of EPS
The uniformity of EPS was determined with a Waters 2695 HPLC system (Milford, MA, USA) equipped with a TSK gel GMPW XL column (300 mm × 7.8 mm, Tosoh Corp., Tokyo, Japan) and a refractive index detector. EPS solution (5 mg/mL), 10 μL, was injected and eluted with re-distilled water at a flow rate of 1 mL/min. The linear regression of dextran standards (Sigma-Aldrich, St. Louis, MO, USA) was calibrated to calculate the molecular weight of EPS.
Determination of total sugar content
The phenol–sulfuric acid method was used to determine total carbohydrate using a 96-well plate and glucose as the standard (30). In brief, 20-μL EPS (1 mg/mL) or glucose solution and 20-μL phenol (5% water solution) were mixed in a well, 100-μL sulfuric acid was added to the mixture, and the mixture was measured at a wavelength of 480 nm using a microplate reader (BioTek Instruments, Inc. Winosky, VT, USA) after incubation for 30 min at 25°C.
Determination of EPS monosaccharide composition
Monosaccharide composition of EPS was determined by hydrolyzing the sample, which was then neutralized with BaCO3 and concentrated by rotary evaporation after hydrolysis in 1-M sulfuric acid in 100°C water bath for 3 h. The monosaccharide solution was eluted by acetonitrile and water (9:1, v:v ) with a Shodex HILICpak VG-50 4E (250 mm × 4.6 mm, Shodex, Japan) using HPLC (Waters 2695, Milford, MA, USA) equipped with an evaporative light scattering detector (6100 Chromachem, ESA lnc.). Rhamnose, arabinose, mannose, galactose, and glucose were used as sugar standards (Tokyo Chemical Industry, Japan).
Infrared (FT-IR) spectroscopic analysis of EPS
The potassium bromide (KBr) pellet pressing method was used in FT-IR to analyze the chemical composition of EPS over a wavelength range of 4,000 cm−1 to 400 cm−1.
Nuclear magnetic resonance (NMR) spectroscopy analysis of the EPS
NMR spectra of EPS were measured on a Bruker Avance-500 spectrometer (Bruker Corporation, Karlsruhe, Germany). The sample was dissolved in D2O (>99.0%) and the internal calibration standard was MeOH-d4 (δH 3.31 and 4.87).
Antioxidant activity of EPS
Hydroxyl radical-scavenging activity
The hydroxyl radical-scavenging activity of EPS was determined according to the method described by Wu et al. (31), with few modifications. In brief, PBS (20 mM, pH 7.4), 50 μL; 12.5-mM 1,10-phenanthroline solution, 25 μL; 2.5-mM FeSO4 solution, 25 μL; and 20-mM H2O2, 25 μL were added successively to each well of 96-well plates and mixed thoroughly. Then 100-μL EPS aliquots at various concentrations were added to the mixture, incubated at 37°C for 1 h, and immediately measured at 536 nm. Ascorbic acid was used as a positive control. Each experiment was performed in triplicate.
Hydroxyl radical-scavenging activity was expressed as the following formula:
Scavenging activity (%) = (As – Ac)/(Ao – Ac) × 100
where ‘As’ is the absorbance of the sample in the presence of different concentrations of EPS, ‘Ac’ is the absorbance of the sample in the absence of EPS, and ‘Ao’ is the absorbance of the sample without both EPS and H2O2.
Superoxide radical-scavenging activity
The superoxide radical-scavenging activity of EPS was determined following the protocols of Zhang (32) with minor modifications. An aliquot of 50 μL of Tris-HCl buffer (pH 8.0, 150 mM) was mixed with 25 μL of pyrogallol (1.50 mM, dissolved in 10-mM HCl) and 100-μL EPS aliquots at various concentrations; ascorbic acid was used as a positive control. The mixture was incubated at 25°C for 30 min after thorough mixing, and the absorbance of the mixture was measured at 325 nm. Each experiment was performed in triplicate.
Scavenging of superoxide radicals generated by pyrogallol autoxidation was calculated as follows:
Scavenging activity (%) = [1 – (A11 – A10)/(A01 – A00)]
where ‘A00’ is the absorbance of the sample in the absence of EPS and pyrogallol, ‘A01’ is the absorbance of the sample containing pyrogallol but no EPS, ‘A10’ is the absorbance of the sample containing EPS but no pyrogallol, and ‘A11’ is the absorbance of the sample containing EPS and pyrogallol.
Anti-biofilm activities of EPS
Inhibition activity
The biofilm inhibition assay was performed as follows: B. cereus and S. typhimurium suspensions were inoculated in a 24-well plate (Costar, Corning, USA) with sterile EPS, at a final concentration of 500 μg/mL. Wells without EPS were simultaneously used as blank controls. A rectangular microscope cover glass (1 cm in diameter) was inserted into every well and the 24-well plate was incubated at 37°C for 24 h. The glass slides were carefully washed with PBS (pH 7.4) and biofilms that adhered to the slides were observed using a fluorescence microscope (Carl Zeiss Axio Vert.A1, Jena, Germany) after staining with 0.01% acridine orange solution for 5 min.
After qualitative analysis with fluorescence microscopy, quantitative measurement was done using a 96-well plate (Costar, Corning, USA) according to previously published methods (17, 33). B. cereus and S. typhimurium were added individually to the 96-well plate with different concentrations of EPS. The final concentrations of the EPS were 500, 250, 125, 62.50, and 31.25 μg/mL in sterile PBS (pH = 7.4). Negative controls lacked EPS and blank controls were only with PBS without any bacterial strains. The plates with B. cereus and S. typhimurium were incubated at 37°C for 24 h. The suspension in each well was removed and the microtiter wells were washed for four times with PBS to remove non-adherent cells. Biofilms were stained with MTT solution (prepared with the corresponding medium) and incubated in dark for 3 h at 37°C (34, 35). Dimethyl sulfoxide (DMSO) was used to rinse after removal of MTT solution. Optical density of each well was measured at 490 nm (OD490) using a Microplate Reader (BioTek, USA). The inhibition by EPS was inversely proportional to the OD490 value and the results were expressed using inhibition ratios.
Dispersion activity
The general procedures of biofilm dispersion experiments were conducted as follows: Biofilms of B. cereus and S. typhimurium were first inoculated in a 24-well plate (Costar, Corning, USA), as described above for inhibition assay, with a rectangular microscope cover glass added to each well, and incubated at 37°C for 24 h. The glass slides with biofilms were carefully washed with PBS (pH 7.4) and placed into another 24-well plate filled with fresh culture medium with or without EPS (dissolved with PBS, final concentration of 500 μg/mL), and incubated at 37°C for 24 h. The glass slides were observed under a fluorescence microscope (Carl Zeiss Axio Vert.A1, Jena, Germany) after careful washing and then staining with 0.01% acridine orange for 5 min.
However, for this assay, the efficacy of EPS in biofilm dispersion was determined after biofilm formation. As mentioned in the section ‘Inhibition activity’, after formation of biofilms, the bacterial suspensions were removed and the microtiter wells were washed for four times with PBS to remove non-adherent cells. Then fresh medium with different concentrations of EPS was added to each well (final concentrations were 500, 250, 125, 62.50, and 31.25 μg/mL). Negative and blank controls were as mentioned above. After incubation at 37°C for 24 h, the subsequent procedures were the same as described in the section ‘Inhibition activity’.
Statistical analysis
All results were shown as mean ± standard deviation (SD) and data were analyzed by one-way ANOVA and Duncan’s test using SPSS software, version 17.0; P < 0.05 was used to identify statistically significant differences. All experiments included three biological replicates.
Results
Screening and identification of EPS-producing L. coryniformis
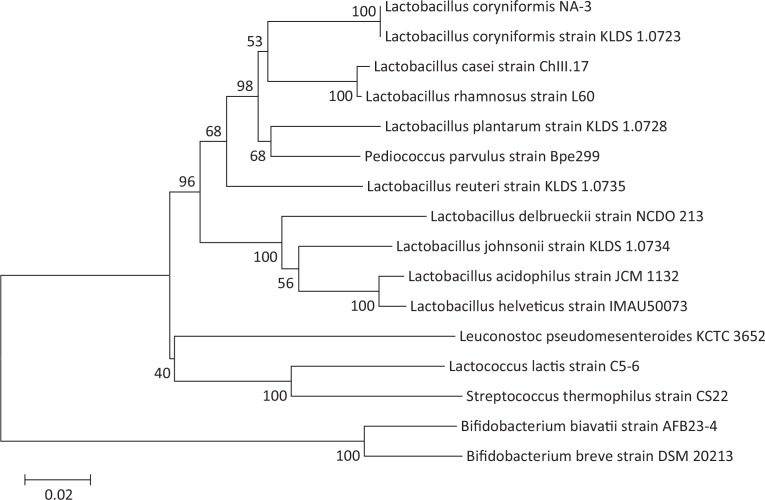
Initially, 247 LAB strains were screened from Suan Cai fermentation brine based on colony morphology and the presence of haloes in the medium indicating calcium solubilization. A total of 39 Gram-positive and catalase negative strains with viscid, mucoid colonies were streaked to isolation and identified by Sanger sequencing to determine genus and species. Among these 39 strains, BLAST searches of 16S rRNA sequence showed that the collections comprised the following: 2 Pediococcus ethanolidurans, 1 Pediococcus parvulus, 14 L. coryniformis, 16 L. plantarum, 3 L. brevis, 1 L. sake sub sp. sakei, 1 L. paracasei, and 1 L. harbinensis. One particularly viscid or ropy strain, designated L. coryniformis NA-3, was used for further experiments. To confirm the phylogenetic relationship of this strain with other LAB, we constructed a phylogenetic tree using an alignment and distance matrix of 16S sequences from NA-3 with those of LAB and bifido bacteria available in public databases (Fig. 1). Branching patterns showed that NA-3 was closely related to L. coryniformis strain KLDS 1.0723 (>99%), with which it clustered apart from other species of this genus, thus confirming the membership of NA-3 in the L. coryniformis species.
Fig. 1.
Neighbor joining-dendrogram showing the phylogenetic relationship between16S rDNA nucleotide sequences of Lactobacillus spp., obtained from the GenBank database, and Lactobacillus coryniformis NA-3 16S sequence, isolated in this study.
EPS-NA3 is a heteropolysaccharide composed of four monomers
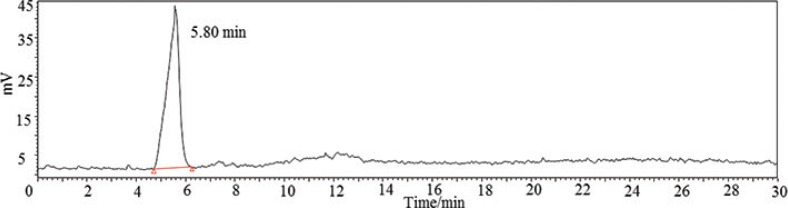
A chromatogram showing the uniformity of EPS-NA3 is shown in Fig. 2. The molecular weight was 8.6×106 Da, calculated by linear regression with dextran standards (Log Mw = −0.7574 x + 11.328, R² = 0.9966, where Mw: the molecular weight, and x: retention time). Previous studies have found that the molecular mass of most HePSs is between 1×104 Da and 6×106 Da (4, 36, 37). For example, Cerning (9) has indicated that S. salivarius sp. thermophilus grown on skimmed milk could produce two fractions of EPS, one was close to 2×106Da and another was 3.5×104 Da. In this work, we found that the molecular weight of EPS-NA3 was basically comparable with previous reports, but was a bit more than the maximum molecular weight found in the literature.
Fig. 2.
HPLC chromatogram showed the molecular weight of the EPS produced by Lactobacillus coryniformis NA-3, calculated by linear regression with dextran standards.
The composition of EPS-NA3, extracted from batch cultures of NA-3 and subjected to HPLC analysis was determined. The yield of EPS was 6.9 mg/L. The phenol–sulfuric acid method revealed that the total sugar content of EPS was 61%. Acid hydrolysis of EPS followed by HPLC analysis (Supplementary Figs. S1 and S2) showed that the monosaccharide composition of EPS included rhamnose, mannose, galactose, and glucose. The molar ratio of four monomers Rha:Man:Gal:Glc was 2.6:1.0:5.0:3.3. The presence of different monomers indicated that the EPS was a HePS.
FT-IR shows characteristic peaks for polysaccharide functional groups
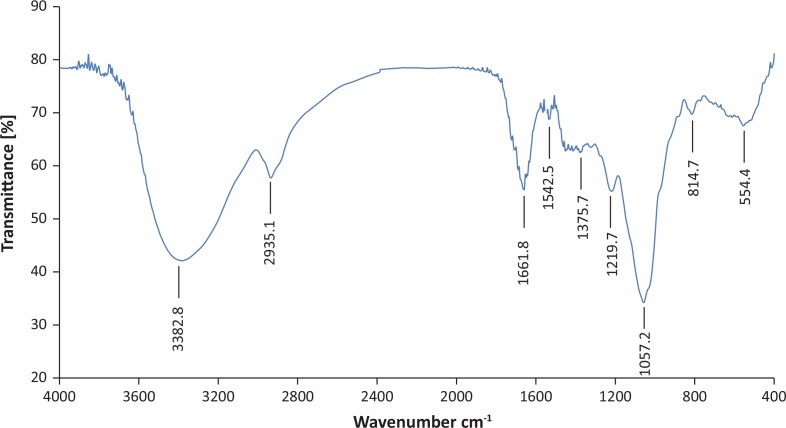
In order to determine the characteristic structure of EPS, FT-IR spectroscopy of EPS-NA3 was conducted. The FT-IR spectrum of EPS-NA3 showed a complex pattern of peaks from 3000 cm−1 to 1000 cm−1 (Fig. 3). Specifically, several characteristic functional groups were revealed, such as a broad-stretching hydroxyl group at 3382.8 cm−1; a weak C-H stretching peak of methyl group at 2935.1 cm−1; a distinct peak corresponding to an amide I>C=O stretching and C-N bending of protein and peptide amines at 1661.8 cm−1; and a peak at 1375.7 cm−1 assigned to >C=O stretching of COO− as well as a C-O band from the same COO− group (27). The presence of characteristic hydroxyl groups suggested that EPS-NA3 was a polysaccharide. Furthermore, in the fingerprint region of polysaccharide, the main absorption bands were a sharp peak at 1057.2 cm−1 and a weak peak at 1219.7 cm1, which are typical of C-O (alcoholic hydroxyl group) and C-O-C groups (carbon–oxygen absorption peak on the ring) (38). Thus, FT-IR spectroscopy revealed that EPS-NA3 we extracted from NA-3 culture medium contained most of the characteristic absorption peaks associated with polysaccharides.
Fig. 3.
The FT-IR spectrum of exopolysaccharide (EPS) produced by Lactobacillus coryniformis NA-3 with the KBr pellet pressing method; x-axis is wave number (cm−1) and y-axis is transmittance (%).
NMR spectrum of EPS-NA3
Hydrogen-1 Nuclear Magnetic Resonance (1H NMR) was always used to analyze the glycosidic bond configuration of polysaccharides (39). The EPS-NA3 was further characterized by one-dimensional 1H-NMR as shown in Fig. 4. It was the same as polysaccharide, the 1H NMR spectrum of EPS-NA3 also consisted of mainly three regions. The first region is anomeric proton region (δH 4.5–5.5 ppm), which is often used to differentiate the anomeric protons of sugar residues in polysaccharides; the second one is ring proton region (δH 3.1–4.5 ppm), a typical region for polysaccharides, including many crowded signals due to the presence of many sugar residues; and the last is the alkyl region (δH 1.2–2.3 ppm) (40, 41). Four major chemical shift signals (δ4.956, δ5.040, δ5.110, and δ5.179) in δ4.5–5.5 ppm were obtained in 1H NMR, suggesting that EPS-NA3 mainly contained four monosaccharide residues, corresponding to the existence of four monomers. Chemical shifts between 4.9 ppm and 5.3 ppm are typical of the anomeric protons of these α-linked residues, whereas β-anomeric protons usually resonate between 4.9 ppm and 5.3 ppm (39), certifying that all sugar residues in EPS-NA3 were linked by α-glycosidic bond. The signals of EPS-NA3 detected in the spectrum in δ3.2–4.4 were resolved with difficulty due to the protons attached to C2 and C6, resulting in the overlapping of chemical shifts (42). Results of 1H NMR spectrum were corresponding with the analysis of EPS-NA3 by HPLC and FT-IR, drawing a conclusion that EPS-NA3 was completely confirmed as polysaccharide. EPS-NA3 comprised α-mannose, α-glucose, α-galactose, and α-rhamnose residues.
Fig. 4.
The1H NMR spectrum of EPS-NA3.
Antioxidant properties of EPS-NA3
Hydroxyl radical-scavenging property
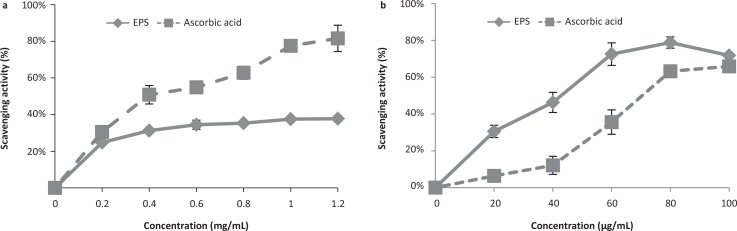
Hydroxyl radicals are powerful oxidants that react with almost all biological molecules, including nucleic acids, proteins, lipids, and carbohydrates (11). The in vitro antioxidant activity of EPS-NA3 was evaluated by assaying its ability to scavenge hydroxyl radicals. The EPS exhibited concentration-dependent scavenging activity against hydroxyl radicals from 0.2 mg/mL to 1.2 mg/mL (Fig. 5a). The antioxidant activity of 0.2 mg/mL EPS (24.71% ± 0.83%) was similar to that of ascorbic acid (30.52% ± 3.16%) in the same concentration. In addition, scavenging increased with concentration, although ascorbic acid activity increased more rapidly than that of EPS. Eventually, the scavenging activity of EPS-NA3 (37.77% ± 1.56%) plateaued at 1.2 mg/mL, about half that of ascorbic acid (81.59 ± 7.18%), indicating that EPS-NA3 may serve as a good alternative to ascorbic acid, with a low antioxidant effect.
Fig. 5.
Free radical-scavenging activities of EPS derived from Lactobacillus coryniformis NA-3 and ascorbic acid (positive control): hydroxyl radicals (a) and superoxide radicals (b). Data are represented as mean ± standard deviation (SD) of three replicates per experiment. Three independent experiments were conducted for each assay.
Superoxide radical-scavenging property
Superoxide radicals, singlet oxygen atoms, are active oxygen-free radicals produced in the human body that can trigger lipid peroxidation in vivo (43). In addition to hydroxyl radicals, we tested the ability of EPS-NA3 to neutralize superoxide in order to determine whether there were differences in its specificity for scavenging free radicals. The scavenging activity of EPS-NA3 was also concentration-dependent against superoxide radicals (Fig. 5b) generated by pyrogallol autoxidation. The scavenging capability of EPS was higher than that of ascorbic acid, with 20–60 μg/mL (30.55% ± 3.37%–72.59% ± 6.14%) of EPS-NA3 exhibiting roughly twice the antioxidant activity of ascorbic acid (6.38% ± 1.45%–35.61% ± 6.63%) at the same concentration. The activities of both EPS-NA3 and ascorbic acid gradually plateaued and the gap was reduced at concentrations higher than 80 μg/mL. However, the maximum superoxide scavenging activity of EPS (78.87% ± 3.07%) was higher than that of ascorbic acid (63.23% ± 1.54%) at the same concentration (80 μg/mL), thus indicating that this EPS is a more potent antioxidant against O2−than ascorbic acid.
In vitro anti-biofilm activities of EPS-NA3
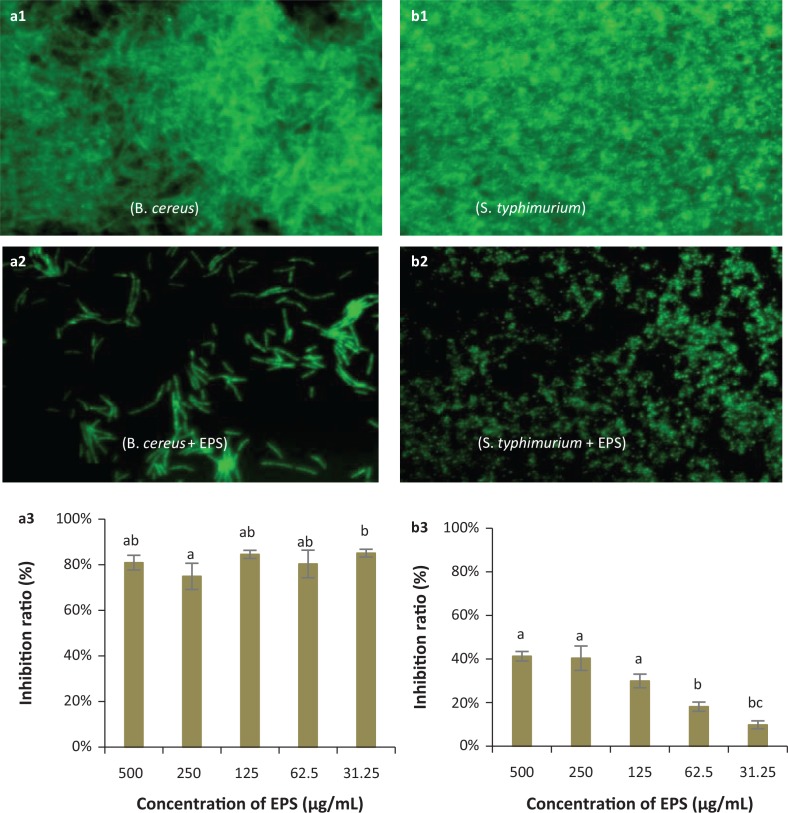
Given its capacity for antioxidant activity, we next decided to test whether EPS-NA3 also exhibited the ability to inhibit biofilm formation (31), given the role of EPS in preventing colonization by competing bacteria. Observation by fluorescence microscopy of B. cereus (Figs. 6a1 and 6a2) and S. typhimurium (Figs. 6b1 and 6b2) biofilms that developed in the absence or presence of EPS-NA3, respectively, revealed that the density of biofilms was lower when the pathogens were cultured with EPS-NA3 compared with the control group, which was not exposed to EPS. The EPS-NA3 was, therefore, able to reduce biofilm formation by B. cereus and S. typhimurium. Inhibition ratios determined by measuring with UV spectrophotometry indicated that against B. cereus (Fig. 6a3) and S. typhimurium (Fig. 6b3) biofilms, EPS-NA3 had a strong ability for inhibition of B. cereus biofilm formation, although less so for S. typhimurium. The inhibition ratio reached 80%, and the effect was concentration-independent in B. cereus assays when the concentrations of EPS-NA3 were between 31.25 μg/mL and 500 μg/mL. Inhibition of S. typhimurium biofilm formation was also concentration-dependent, with inhibition ratios ranging from 9.71% ± 1.80% to 40.87% ± 2.2% with EPS concentrations between 31.25 μg/mL and 500 μg/mL respectively. Although EPS-NA3 successfully reduced biofilm formation by both B. cereus and S. typhimurium, it was more effective against B. cereus than S. typhimurium.
Fig. 6.
Representative micrographs (40×) showing the inhibitory effects of EPS on biofilm formation by pathogenic bacteria: Bacillus cereus (a1, a2, a3) and Salmonella typhimurium (b1, b2, b3) and spectrophotometric analyses of EPS biofilm inhibition. (a1, b1): Negative controls (pathogen only, no EPS) for biofilm formation by fluorescence microscopy; (a2, b2): Biofilm formation in the presence of EPS observed by fluorescence microscopy; (a3, b3): Inhibition ratios of EPS against B. cereus and S. typhimurium biofilms respectively. a,b,cDifferent letters indicate significant differences between concentrations (P < 0.05).
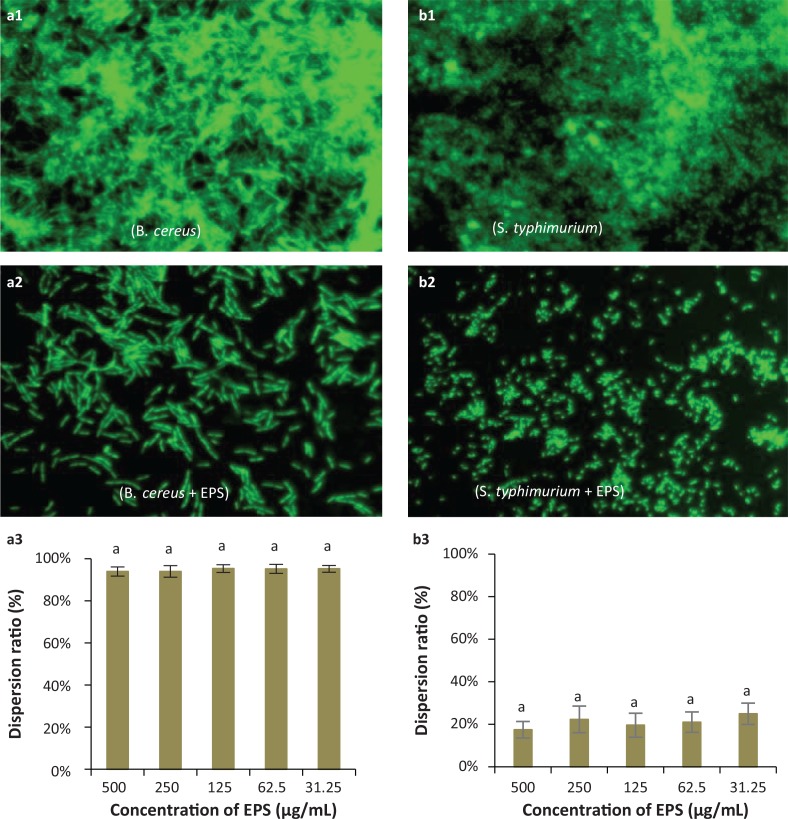
To explore further the anti-biofilm activity of EPS-NA3, we also exposed fully formed biofilms to EPS to observe any potential dispersant activity. Fluorescence microscopy showed that EPS-NA3 exerts a dispersive effect on B. cereus (Figs. 7a1 and 7a2) and S. typhimurium (Figs. 7b1 and 7b2) biofilms. Dispersion ratios determined with spectrophotometry (Figs. 7a3 and 7b3) indicated that as much as 90% of B. cereus biofilm was dispersed by EPS-NA3, independent of concentration (range from 31.25 μg/mL to 500 μg/mL), while only approximately 20% of the S. typhimurium biofilm dispersed in the presence of EPS-NA3 (also independent of concentration). These data show that EPS-NA3 is effective for inhibiting the formation of biofilms as well as for removing mature biofilms, although more effectively against B. cereus than against S. typhimurium.
Fig. 7.
Representative micrographs (40×) showing the dispersion activity by EPS against established biofilms of the pathogenic bacteria Bacillus cereus (a1, a2, a3) and Salmonella typhimurium (b1, b2, b3), and spectrophotometric analyses of EPS-mediated dispersion of biofilms produced by these species. (a1, b1): Observation by fluorescence microscopy of negative controls (pathogens only, no EPS) for biofilm dispersion; (a2, b2): Observation by fluorescence microscopy of biofilm dispersion by Lactobacillus coryniformis NA-3-derived EPS; (a3, b3): Dispersion ratios of EPS on different bacterial biofilms. a,b,cDifferent letters indicate significant differences between treatments (P < 0.05).
Discussion
EPS-producing Lactobacillus spp. have long been established as an integral component in the production of many foods worldwide, and in that capacity they have been classified as ‘generally recognized as safe (GRAS)’. LAB-derived EPSs are high molecular weight, long-chain, linear or branched biopolymers secreted to the external environment or adhered to the bacterial cell surface (44). Owing to their reported probiotic characteristics, such as anti-tumor, immune-stimulating, antioxidant, and anti-biofilm activities, EPSs produced by LAB have become the focus of research across several disciplines, spanning pharmaceutical development, cancer biology, food science, and industrial engineering.
In this study, we isolated the EPS-producing Lactobacillus sp. L. coryniformis NA-3 from northeastern Chinese sauerkraut (Suan Cai), which is rich in a variety of potentially probiotic microorganisms. Many EPS-producing LAB have been previously screened from a variety of fermented foods such as milk and fermented cabbage (3, 20). However, to our knowledge, this work represents the first report characterizing EPS from L. coryniformis. As significant components of the cell surface, exopolysaccharides play a critical role in mediating cell-to-cell interactions (45). LAB-derived EPSs produced in vitro can exhibit antimicrobial properties that have been proposed to act by binding to biofilm-related signal molecules or glycocalyx receptors on the surfaces of pathogen cells. This receptor binding subsequently disrupts communication between cells, thereby interfering with the formation of biofilms, and eventually leading to the inhibition of pathogen growth and proliferation (12). We extracted EPS from L. coryniformis NA-3 for analysis of its compositions and physico-chemical characteristics, and subsequently found evidence of its antioxidant ability and biofilm-inhibiting activity.
Polysaccharides are soluble in water but not in alcohol, so we used a hot water-based extraction followed by alcohol precipitation to isolate EPS, and then added TCA for deproteinization. In this work, we observed that EPS yields were very low (6.9 mg/mL), possibly due to culture of NA-3 in MRS broth. Although there are no reports about EPS yield from L. coryniformis, previous works have shown that EPS production from other strains is affected by many factors such as carbon source (46), composition of medium, temperature, and pH (4). Studies have also suggested that carbon source is a significant factor that promoted EPS production and high EPS could be produced in the presence of sucrose (39, 47). Actually, it has been proved that the EPS production could be improved when strain was grown in the optimal incubation conditions compared to incubating in a control grown on commercial MRS medium (48). Therefore, L. coryniformis NA-3 might produce higher levels of EPS after optimization of culture conditions in the future.
FT-IR spectroscopy and 1H NMR spectrum analysis of the extracted EPS showed absorption peaks characteristic of sugar, and acid hydrolysis followed by HPLC revealed that EPS-NA3 was a HePS composed of α-rhamnose, α-mannose, α-galactose, and α-glucose. Previous studies have shown that EPS isolated from L. casei LC2W is composed of glucose, rhamnose, and galactose (20). Similarly, the EPS from L. plantarum was found to contain three monosaccharides: mannose, glucose, and galactose (49). Recent researches have also reported EPS extracted from another L. plantarum composed of glucose, galactose, and fructose (39), and a new EPS from Lactobacillus fermentum YL-11, including four different monosaccharides (galactose, glucose, mannose, and arabinose). Although glucose, mannose, and galactose are always present in most of EPSs, composition of EPS monomers varies from strain to strain.
The free radical theory of aging proposes that essential biological processes become disrupted with increasing age, eventually leading to serious illnesses such as diabetes and Alzheimer’s disease (50). Therefore, studies exploring free radical-scavenging of natural antioxidants, such as those produced by LAB, can lead to safe and effective medicines that inhibit the progression of chronic diseases (32). Gomaa and Yousef (47) have suggested that the EPS from Virgibacillus salarius BM02 was able to scavenge hydroxyl radicals and the scavenging activity reached 60.00% ± 0.06% at 10 mg/mL. However, studies of EPS from Pseudomonas aeruginosa exhibited better scavenging activity on both hydroxyl and superoxide radicals: it could scavenge both hydroxyl radicals up to 50% at a concentration of 60 μg/mL and superoxide radicals up to 70% at a concentration of 60 μg/mL (31). In our examination of the antioxidant properties of L. coryniformis EPS-NA3, we found that this EPS could scavenge hydroxyl radicals and superoxide radicals. In comparison, the maximum scavenging activity of L. coryniformis EPS-NA3 on hydroxyl radicals is lower than the EPS from Virgibacillus salarius BM02, but the former (around 35%) is better than the latter (less than 10%) when they worked at the same concentration of 1 mg/mL. On the other hand, the scavenging activity of L. coryniformis EPS-NA3 on hydroxyl radicals is less than the EPS isolated from Pseudomonas aeruginosa; however, our EPS showed better ability on superoxide radicals (72%) at a concentration of 60 μg/mL. Interestingly, the scavenging activity of L. coryniformis EPS-NA3 on hydroxyl and superoxide radicals varies greatly with concentrations, showing a better ability to scavenge superoxide radicals than hydroxyl radicals. Prior studies have indicated that EPS can reduce levels of cell-damaging free radicals and oxidizing agents (15) but is affected by molecular weight, composition, degree of polymerization, and number of side chains of EPS (33). Hence, the structural differences are most likely to be responsible for different scavenging activities.
In addition to antioxidant ability, we also focused on the ability of EPS-NA3 to mitigate the formation of biofilms and to reduce established biofilms. Biofilms are surface-attached extracellular matrices composed of a complex of nucleic acids, proteins, polysaccharides, and lipids. The polysaccharide components play significant roles as virulence factors that mediate pathogenesis or as essential signals in host-pathogen interactions (46, 49). The formation of biofilms by pathogenic bacteria can pose a serious public health risk by decreasing bacterial susceptibility to antimicrobial agents and thus improving their defense mechanisms (51). Previous research has demonstrated that many EPSs from Lactobacillus spp. can decrease biofilm formation by pathogens or disperse established biofilms, thereby exhibiting antibacterial activities. EPS isolated from L. plantarum showed anti-biofilm activity on the biofilm of P. aeruginosa, S. typhimurium, Staphylococcus aureus, and Listeria monocytogenes at a concentration of 256 μg/mL and found that dispersion effects are better than inhibition (17). Moreover, EPS from Lactobacillus fermentum S1 had a favorable anti-biofilm activity against Escherichia coli and Staphylococcus aureus and the highest inhibition ratios are 32% and 43% respectively (52). Previous studies have demonstrated that EPS from L. plantarum YW32 and L. acidophilus A4 were found to possess anti-biofilm activity on both Gram-negative and Gram-positive bacteria (19, 53). In this study, we observed that pathogen-produced biofilms decreased when cultured in the presence of EPS-NA3 solution, indicating that EPS played a critical role in attenuating biofilm formation. EPS-NA3 showed a high inhibition and dispersion ratios on B. cereus (Gram-positive) biofilm, reaching 80% and 90% respectively. However, no significant effect was observed between 31.25 and 500 μg/mL of EPS-NA3 (P > 0.05). Obviously, both inhibition and dispersion ratios on B. cereus in presence of EPS-NA3 were much higher than the above-reported ratios, suggesting that EPS-NA3 is a highly effective biofilm inhibitor on B. cereus. In contrast, biofilm inhibition against S. typhimurium was concentration-dependent; higher concentration led to higher inhibitory efficacy, with a maximum inhibition of 40% at an EPS concentration of 500 μg/mL. The dispersion ratio was about 20% for S. typhimurium and the level of activity was independent of dosage. These results indicated that EPS-NA3 also worked on some biofilms formed by Gram-negative bacteria in agreement with the previous experimental results. Overall, EPS-NA3 had a stronger effect against the biofilm of B. cereus than against that of S. typhimurium.
Conclusion
The EPS extracted from L. coryniformis NA-3, screened from northeastern Chinese sauerkraut (Suan Cai), is a HePS comprising α-rhamnose, α-mannose, α-galactose, and α-glucose, with antioxidant and anti-biofilm properties. Based on the results of this study, we conclude that EPS-NA3 can scavenge free radicals, especially superoxide radicals, inhibit biofilm formation, and disperse the biofilms of B. cereus and S. typhimurium. Additional research on EPS-NA3 could further characterize the mechanisms underlying its activity and optimize its production and composition for potential applications as an antioxidant and anti-biofilm agent in food and pharmaceutical industries.
Supplementary Material
Acknowledgments
This study was supported by the National Key R&D Program of China (2018YFD0500600) and the National Natural Science Foundation of China (31572440).
Conflict of interests
The authors declare no potential conflicts of interest.
References
- 1.Fontana C, Li S, Yang Z, Widmalm G. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohyd Res 2015; 402: 87–94. doi: 10.1016/j.carres.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Jia K, Tao X, Liu Z, Zhan H, He W, Zhang Z, et al. Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties. Int J Biol Macromol 2019; 128: 710–17. doi: 10.1016/j.ijbiomac.2018.12.245 [DOI] [PubMed] [Google Scholar]
- 3.Ye G, Chen Y, Wang C, Yang R, Bin X. Purification and characterization of exopolysaccharide produced by Weissellacibaria YB-1 from pickle Chinese cabbage. Int J Biol Macromol 2018; 120: 1315–21. doi: 10.1016/j.ijbiomac.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 4.Behare PV, Singh R, Kumar M, Prajapati JB, Singh RP. Exopolysaccharides of lactic acid bacteria: a review. J Food Sci Tech Mys 2009; 46(1): 1–11. doi: 10.1111/j.1750-3841.2008.01020.x [DOI] [Google Scholar]
- 5.Xu Y, Cui Y, Yue F, Liu L, Shan Y, Liu B, et al. Exopolysaccharides produced by lactic acid bacteria and bifido bacteria: structures, physiochemical functions and applications in the food industry. Food Hydrocolloid 2019; 94: 475–499. doi: 10.1016/j.foodhyd.2019.03.032 [DOI] [Google Scholar]
- 6.Rahbar Saadat Y, Yari Khosroushahi A, Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohyd Polym 2019; 217: 79–89. doi: 10.1016/j.carbpol.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 7.Kumar AS, Mody K, Jha B. Bacterial exopolysaccharides – a perception. J Basic Microbiol 2007; 47(2): 103–17. doi: 10.1002/jobm.200610203 [DOI] [PubMed] [Google Scholar]
- 8.Donot F, Fontana A, Baccou JC, Schorr-Galindo S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohyd Polym 2012; 87(2): 951–62. doi: 10.1016/j.carbpol.2011.08.083 [DOI] [Google Scholar]
- 9.Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. Fems Microbiol Rev 1990; 87(1): 113–30. doi: 10.1016/0378-1097(90)90701-q [DOI] [PubMed] [Google Scholar]
- 10.Chabot S, Yu HL, Léséleuc LD, Cloutier D, Calsteren MRV, Lessard M, et al. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF, IL-6 and IL-12 in human and mouse cultured immunocompetent cells, and IFN-$\gamma$ in mouse splenocytes. Le Lait 2001; 81(6): 683–97. doi: 10.1051/lait:2001157 [DOI] [Google Scholar]
- 11.Li S, Huang R, Shah NP, Tao X, Xiong Y, Wei H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacteriumbifidum WBIN03 and Lactobacillus plantarum R315. J Dairy Sci 2014; 97(12): 7334–43. doi: 10.3168/jds.2014-7912 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: structure, bioactivity and associations: a review. Carbohyd Polym 2019; 207: 317–32. doi: 10.1016/j.carbpol.2018.11.093 [DOI] [PubMed] [Google Scholar]
- 13.Xing J, Wang G, Zhang Q, Liu X, Yin B, Fang D, et al. Determining antioxidant activities of lactobacilli by cellular antioxidant assay in mammal cells. J Funct Foods 2015; 19: 554–62. doi: 10.1016/j.jff.2015.09.017 [DOI] [Google Scholar]
- 14.Aruoma OI. Characterization of drugs as antioxidant prophylactics. Free Radical Biol Med 1996; 20(5): 675. doi: 10.1016/0891-5849(95)02110-8 [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 2000; 66(8): 725–35. doi: 10.1016/s0024-3205(99)00643-8 [DOI] [PubMed] [Google Scholar]
- 16.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284: 1318–22. doi: 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 17.Mahdhi A, Leban N, Chakroun I, Chaouch MA, Hafsa J, Fdhila K, et al. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog 2017; 109: 214–220. doi: 10.1016/j.micpath.2017.05.046 [DOI] [PubMed] [Google Scholar]
- 18.Sarikaya H, Aslim B, Yuksekdag Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int J Food Prop 2017; 20(2): 362. doi: 10.1080/10942912.2016.1160923 [DOI] [Google Scholar]
- 19.Kim Y, Oh S, Kim SH. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochem Bioph Res Co 2009; 379(2): 324–9. doi: 10.1016/j.bbrc.2008.12.053 [DOI] [PubMed] [Google Scholar]
- 20.Ai L, Zhang H, Guo B, Chen Wu Y. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W. Carbohyd Polym 2008; 74(3): 353–7. doi: 10.1016/j.carbpol.2008.03.004 [DOI] [Google Scholar]
- 21.Xiong T, Guan Q, Song S, Hao M, Xie M. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 2012; 26(1): 178–81. doi: 10.1016/j.foodcont.2012.01.027 [DOI] [Google Scholar]
- 22.Fang F, Feng T, Du G, Chen J. Evaluation of the impact on food safety of a Lactobacillus coryniformis strain from pickled vegetables with degradation activity against nitrite and other undesirable compounds. Food Addit ContamPart A 2016; 33(4): 623–30. doi: 10.1080/19440049.2016.1156774 [DOI] [PubMed] [Google Scholar]
- 23.Martín R, Olivares M, Marín ML, Xaus J, Fernández L, Rodríguez JM. Characterization of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int J Food Microbiol 2005; 104(3): 267–77. doi: 10.1016/j.ijfoodmicro.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Lara-Villoslada F, Sierra S, Martin R, Delgado S, Roiguez JM, Olivares M, et al. Safety assessment of two probiotic strains, Lactobacillus coryniformis CECT5711 and Lactobacillus Gasseri CECT5714. J Appl Microbiol 2007; 103(1): 175–84. doi: 10.1111/j.1365-2672.2006.03225.x [DOI] [PubMed] [Google Scholar]
- 25.Olivares M, Díaz-Ropero MP, Gómez N, Lara-Villoslada F, Sierra S, Maldonado JA, et al. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol 2006; 107(2): 104–11. doi: 10.1016/j.ijfoodmicro.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 26.Ruas-Madiedo P, de Los Reyes-Gavilán CG. Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Dairy Sci 2005; 88(3): 843–56. doi: 10.3168/jds.S0022-0302(05)72750-8 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Ahmed Z, Feng W, Li C, Song S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int J Biol Macromol 2008; 43(3): 283–8. doi: 10.1016/j.ijbiomac.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 28.Waśko A, Polak-Berecka M, Skrzypek H, Kreft A. Production of exopolysaccharides by a probiotic strain of Lactobacillus rhamnosus: biosynthesis and purification methods. Acta Aliment Hung 2013; 42(2): 220–8. doi: 10.1556/AAlim.42.2013.2.9 [DOI] [Google Scholar]
- 29.Yang C, He N, Ling X, Ye M, Zhang C, Shao W, et al. The isolation and characterization of polysaccharides from longan pulp. Sep Purif Technol 2008; 63(1): 226–30. doi: 10.1016/j.seppur.2008.05.004 [DOI] [Google Scholar]
- 30.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956; 28(3): 350–6. doi: 10.1021/ac60111a017 [DOI] [Google Scholar]
- 31.Wu S, Liu G, Jin W, Xiu P, Sun C. Antibiofilm and anti-Infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front Microbiol 2016; 7: 102. doi: 10.3389/fmicb.2016.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S. Antioxidative activity of lactic acid bacteria in yogurt. Afr J Microbiol Res 2011; 5(29): 5194–520. doi: 10.5897/AJMR11.997 [DOI] [Google Scholar]
- 33.Li W, Ji J, Rui X, Yu J, Tang W, Chen X, et al. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. Food Sci Technol 2014; 59(2): 732–9. doi: 10.1016/j.lwt.2014.06.063 [DOI] [Google Scholar]
- 34.Cao X, Liu D, Xia Y, Cai T, He Y, Liu J. A novel polysaccharide from Lentinus edodes mycelia protects MIN6 cells against high glucose-induced damage via the MAPKs and Nrf2 pathways. Food Nutr Res 2019; 63(1): 1598. doi: 10.29219/fnr.v63.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Wu Q, Pan W, Hussain S, Mehmood S, Chen Y. A novel polysaccharide from the sarcodonaspratus triggers apoptosis in hela cells via induction of mitochondrial dysfunction. Food Nutr Res 2018; 62(1): 1285. doi: 10.29219/fnr.v62.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vuyst L, Degeest B. Heteropolysaccharides from lactic acid bacteria. Fems Microbiol Rev 1999; 23(2): 153–77. doi: 10.1111/j.1574-6976.1999.tb00395.x [DOI] [PubMed] [Google Scholar]
- 37.Sanalibaba P, Cakmak GA. Exopolysaccharides production by lactic acid bacteria. Appl Microbiol 2016; 2(2): 1000115. doi: 10.4172/2471-9315.1000115 [DOI] [Google Scholar]
- 38.Nataraj S, Schomäcker R, Kraume M, Mishra IM, Drews A. Analyses of polysaccharide fouling mechanisms during crossflow membrane filtration. J Membrane Sci 2008; 308(1–2): 152–61. doi: 10.1016/j.memsci.2007.09.060 [DOI] [Google Scholar]
- 39.Zehir Şentürk D, Dertli E, Erten H, Şimşek Ö. Structural and technological characterization of ropy exopolysaccharides produced by Lactobacillus plantarum strains isolated from Tarhana. Food Sci Biotechnol 2020; 29(1): 121–29. doi: 10.1007/s10068-019-00641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elnahas M, Amin M, Hussein M, Shanbhag V, Ali A, Wall J. Isolation, characterization and bioactivities of an extracellular polysaccharide produced from streptomyces sp. MOE6. Molecules 2017; 22(9): 1396. doi: 10.3390/molecules22091396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail B, Nampoothiri KM. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 2010; 192(12): 1049–57. doi: 10.1007/s00203-010-0636-y [DOI] [PubMed] [Google Scholar]
- 42.Gan L, Li X, Wang H, Peng B, Tian Y. Structural characterization and functional evaluation of a novel exopolysaccharide from the moderate halophile Gracilibacillus sp. SCU50. Int J Biol Macromol 2019; doi: 10.1016/j.ijbiomac.2019.11.143 [DOI] [PubMed] [Google Scholar]
- 43.Yin JY, Nie SP, Chao Z, Yin W, Xie MY. Chemical characteristics and antioxidant activities of polysaccharide purified from the seeds of Plantagoasiatica L. J Sci Food Agricult 2010; 90(2): 210–17. doi: 10.1002/jsfa.3793 [DOI] [PubMed] [Google Scholar]
- 44.Oleksy M, Klewicka E. Exopolysaccharides produced by Lactobacillus sp.: biosynthesis and applications. Crit Rev Food Sci 2018; 58(3): 450–62. doi: 10.1080/10408398.2016.1187112 [DOI] [PubMed] [Google Scholar]
- 45.Olaya R, Kaplan JB, Jean-Marc G. Antibiofilm polysaccharides. Environ Microbiol 2013; 15(2): 334–46. doi: 10.1111/j.1462-2920.2012.02810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polak-Berecka M, Choma A, Waśko A, Górska S, Gamian A, Cybulska J. Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohyd Polym 2015; 117: 501–9. doi: 10.1016/j.carbpol.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 47.Gomaa M, Yousef N. Optimization of production and intrinsic viscosity of an exopolysaccharide from a high yielding Virgibacillus salarius BM02: study of its potential antioxidant, emulsifying properties and application in the mixotrophic cultivation of Spirulina platensis. Int J Biol Macromol 2020; 149: 552–61. doi: 10.1016/j.ijbiomac.2020.01.289 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Magdalena PB, Adam WK, Agnieszka KK. Optimization of culture conditions for exopolysaccharide production by a probiotic strain of Lactobacillus rhamnosus E/N. Pol J Microbiol 2014; 63(2): 253–7. [PubMed] [Google Scholar]
- 49.Wang Y, Li C, Liu P, Ahmed Z, Xiao P, Bai X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohyd Polym 2010; 82(3): 895–903. doi: 10.1016/j.carbpol.2010.06.013 [DOI] [Google Scholar]
- 50.Muller FL, Lustgarten MS, Youngmok J, Arlan R, Holly VR. Trends in oxidative aging theories. Free Radic Biol Med 2007; 43(4): 477–503. doi: 10.1016/j.freeradbiomed.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 51.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001; 33(8): 1387–92. doi: 10.1086/322972 [DOI] [PubMed] [Google Scholar]
- 52.Wang K, Niu M, Song D, Song X, Zhao J, Wu Y, et al. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J Biosci Bioeng 2020; 129(2): 206–14. doi: 10.1016/j.jbiosc.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Zhao X, Yang Y, Zhao A, Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol 2015; 74: 119–26. doi: 10.1016/j.ijbiomac.2014.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.