Abstract
Introduction
Osteoarthritis (OA), a chronic joint disease, combines with massive inflammation and plays a vital role in cartilage degeneration. The main strategy in clinic is controlling inflammation, thereby treating osteoarthritis. Salvianolic acid A (SAA) is a type of phenolic acid, derived from a traditional chinese herbal medicine Danshen that is extensively used clinically.
Methods and Results
We observed the anti-inflammatory and antiarthritic effects of SAA in IL-1β-stimulated cells. We found that SAA evidently decreased the expression of mainly inflammatory factors, exerted the remarkable effects of anti-inflammation and anti-arthritis. Furthermore, SAA inhibited the expression of Matrix metalloproteinases (MMP1, MMP13), and ADAMTS-5 and raised the synthesis of collagen II and aggrecan. Additionally, the results indicated that SAA gave rise to the effects by down-regulation of NF-κB and p38/MAPK pathways.
Discussion
Our study demonstrates that SAA may be a promising anti-inflammatory for the treatment of OA in clinic.
Keywords: Salvianolic acid A, Osteoarthritis, MMPs, NF-κB pathway
Introduction
Osteoarthritis, a common chronic inflammatory joint disease, deeply influences the life quality of patients.1–3 First, the degenerative changes occur in the cartilage, bone, and other joint structures. Then osteoarthritis leads to swelling, pain, and dysfunction along with cartilage degeneration and producing osteophyte.4 Second, with joint degeneration, many factors including MMP1, MMP13, ADAMTS-5 worsen cartilage degeneration and eventually result in osteoarthritis.5 In present, the main strategies for osteoarthritis are composed of anti-inflammatory treatment, analgesic-based non-surgical treatment and surgical treatment.6 But there are many limitations including the life span of the prosthesis and prosthetic overhaul.7 The current therapeutic drugs of non-steroidal anti-inflammatory drugs (NSAIDs) could treat cartilage degeneration and osteoarthritis in patients.8–10 However, massive use of NSAIDs could give rise to gastric ulcer.11–13 Therefore, developing the effective and safety drug for treating OA is of great value to patients.
SAA is an herbal phenolic acid isolated from the Chinese traditional herb Salvia miltiorrhiza.14,15 It showed versatile pharmacological activities including having protective effect against peroxidative damage to bio membranes and ameliorating focal cerebral ischemia.15–18 It was also indicated to inhibit platelet aggregation and thrombotic diseases.19–21 However, there is no study upon the effect of SAA on alleviating osteoarthritis symptom.
In our study, we aim to observe the effect of SAA, a potential anti-inflammatory, on inflammation in vitro. We focus on investigating the potential effects of SAA on reducing the expression of major inflammatory cytokines and ameliorating osteoarthritis in vitro, which could supply not only useful information for the treatment of such bone disease, but also enhance the understanding of its underlying mechanism. Thus, we used IL-1β to stimulate human chondrocytes to induce OA in vitro and observed whether salvianolic acid A could ameliorate OA. The mechanism of delaying osteoarthritis progression with inhibiting the relative pathways was also investigated.
Materials and Methods
Materials and Reagents
SAA (purity>98%) was purchased from Yuanye Biotechnology (Shanghai, China). Recombinant human IL-1β was purchased from Novoprotein Scientific Inc. (Shanghai, China). The primary antibodies against MMP13, ADAMTS-5, collagen II, and aggrecan were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). For other non-phosphorylation and phosphorylation kinase antibodies, they were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell Culture and Identification
The chondrocytes were obtained from patients with osteoarthritis. We isolated human primary chondrocytes using collagen II digestion. Cartilage was cut into pieces in a sterile environment and digested by trypsin for 30 min at 37°C with a 5% CO2 atmosphere in the incubator. After discarding the trypsin, the cartilage was soaked with culture medium containing 0.4% collagen II. This process lasted for 24 h. After we obtained supernatant from the digested tissue using low-speed centrifugation (800 r/min, 5 min), the sample was centrifuged at 1500 r/min for 5 min, washed with PBS, and centrifuged at 1200 r/min for 5 min again. Finally, we suspended the sediment using hyclone high glucose medium containing 1% penicillin-streptomycin and 10% foetal bovine serum, subsequently, we placed the cell suspension in a 6 cm dish in an incubator at 37°C in a 5% CO2 atmosphere.
Human chondrocytes were seeded on glass slips in 12-well plates and cultured for 72 h. After chondrocytes were washed 3 times with PBS solution, cells were fixed in 4% paraformaldehyde solution for 10 min. Then we treated cells with 1% toluidine blue solution for 30 min at room temperature. Further, glass slips were washed in ethanol. After drying, they were sealed. The chondrocytes were detected with the microscope and photographs were captured. The fixed cells were permeabilized with 0.5% Triton X-100 at room temperature for 20 min. Then cells were sealed with bovine serum albumin. Further, cells were incubated with collagen II antibody overnight at 4 °C, FITC-conjugated secondary antibody was incubated for 1 h at 37 °C in the dark, and the coverslips were then visualized and photos were captured.
All cartilage samples were obtained from the second people’s hospital of Nantong. A written informed consent was obtained from all the patients about the study. This research was approved by the Ethics Committee of the second people’s hospital of Nantong and carried out in accordance with the principles of the Declaration of Helsinki.
Cell Viability Assay
Chondrocytes were cultured in 96-well plates and treated with a series of concentrations of SAA (10, 20, 40, 80 μg/mL) and IL-1β (10 ng/mL) for 24 h at 37 °C. Cell viability was measured by Cell Titer-Lumin Beyotime (Shanghai, China). The experiment was repeated three times. Data were collected with a multimode reader (Tecan Infinite M1000).
Real-Time PCR
Total RNA was extracted from cells using TRIzol reagent and then reverse transcribed to cDNA according to the mRNA concentration. Quantitative real-time PCR was carried out using an Agilent Mx3000P system with the following conditions: pre-denaturation at 95°C for 5 min, then 40 cycles of 95°C for 10 s and 60°C for 30 s, followed by a dissolution curve of 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s. The experiment was repeated three times. The primer sequences used in this study are as follows: MMP-1: forward: 5ʹ-AAATAGTGGCCCAGTGGTTG-3ʹ, reverse: 5ʹ-CACATCAGGCACTCCACATC-3; MMP-13: forward: 5ʹ-GACTTCCCAGGAATTGGTGA-3ʹ, reverse: 5ʹ-TGACGCGAACAATACGGTTA3ʹ; COL2: forward: 5ʹ-GGAGCAGCAAGAGCAAGGAGAAG-3ʹ, reverse: 5ʹ-TGGACAGCAGGCGTAGGAAGG-3ʹ; aggrecan: forward: 5ʹ-ACCCCTGAGGAACAGG-3ʹ, reverse: 5ʹ-GTGCCAGATCATCACCACAC-3ʹ; ADAMTS-5: forward: 5ʹ-CTTGACGTTCGGGCCTGA-3ʹ, reverse: 5ʹ-CACTGTTTCTGGGTGCAG3ʹ.
NF-κB p65 Activity Assay
Nuclear extracts from cells treated SSA were performed using a Nuclear Extract kit (Cayman chemical, 10009277), and DNA-bind activity of NF-κB was examined using an NF-κB (p65) transcription factor assay kit (Cayman chemical, 10007889) following the manufacturer’s instructions.
Western Blot Analysis
The cells were washed twice using PBS, after that the proteins were extracted in a RIPA buffer and PMSF (100:1) mixture, and the protein content was measured after high-speed centrifugation at 4°C (12,000 g/min, 10 min). The protein isolation kit was selected for extracting proteins from cytoplasmic and nuclear fractions, and their concentrations were quantified by using a bicinchoninic acid (BCA) kit (Thermo Scientific, IL, USA). Equal amounts of protein were resolved with 10% SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with blocking buffer for 1 h at room temperature with gentle shaking and then probed with the primary antibodies overnight at 4°C. After washing three times with buffer for 10 min, the membranes were incubated with secondary antibody for 2 h at room temperature. The membranes were visualized with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, USA).
Statistical Analysis
All data are presented as the mean±standard deviation. A one-way analysis of variance (ANOVA) with GraphPad 8.0 (GraphPad Inc, San Diego USA) was used to analyze the differences between groups. p<0.05 indicated statistical significance.
Results
Identification of Human Primary Chondrocytes
We first characterized human primary chondrocytes we obtained using toluidine blue staining and immunohistochemistry staining. Proteoglycan, which exists in the chondrocyte cytoplasm, can be stained by toluidine blue staining. The results showed that human primary chondrocytes were spindle-shaped (Figure 1A). Additionally, collagen II in human primary chondrocytes can also be stained by immunohistochemistry staining (Figure 1B). The staining results indicated that cells isolated from articular cartilage are chondrocytes.
Figure 1.
Identification of human primary chondrocytes. (A) Toluidine blue staining of human primary chondrocytes, and proteoglycans in chondrocytes were stained purple. Scale bar, 100 μm. (B) Collagen II immunohistochemical staining of human primary chondrocytes.
The Effects of Salvianolic Acid A (SAA) on Cell Viability
Firstly, the chemical structure of SAA is shown in Figure 2A. Next, the results of the cell viability assay showed (Figure 2B) no significant cytotoxicity when cells were treated with the different concentrations of SAA and IL-1β for 24 h. However, with the increased dose of SAA (>80 µg/mL), the cell viability may decrease. Thus, the effect of SAA on cells was observed with amounts ranging from 5–80 µg/mL. Additionally, while the cells were treated with IL-1β, SAA showed no significant cytotoxicity (Figure 2C).
Figure 2.
The chemical structure of SAA (A) and the effects of SAA on cell viability of Human primary chondrocytes (B) and (C). The experiment was repeated three times. Data are presented as mean ± standard deviation (SD).
Effects of Salvianolic Acid A on Cytokines Expression in Cells
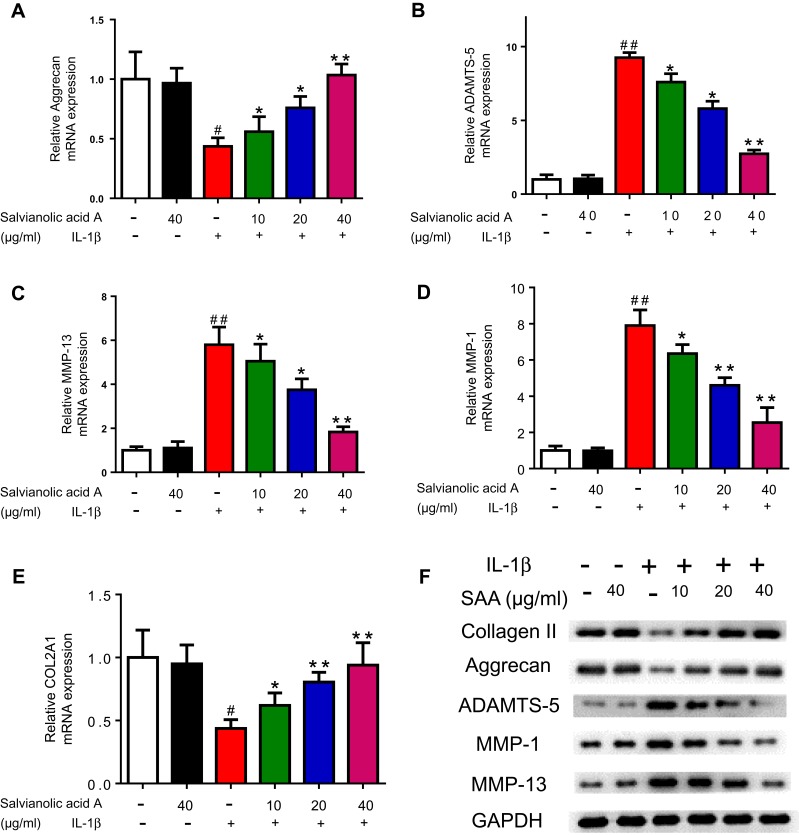
The progress of OA is accompanied with inflammation. Interestingly, large amounts of MMPs are occurring with inflammation and lead to degradation of the extracellular matrix. Further, we tested the effect of SAA on MMP1, MMP13, and ADAMTS-5 in cells. The results indicated that the mRNA expressions following IL-1β stimulation was evidently higher than that in untreated cells (Figure 3A–E). Meanwhile, the results of Western blotting exhibited that the levels of MMP1, MMP13 and ADAMTS-5 were also markedly inhibited after SAA treatment. In terms of Aggrecan and COL2A1, both results showed the inverse trends (Figure 3).
Figure 3.
Effect of SAA on the expression of matrix-degrading proteases and COL2 A1 in IL-1β-induced chondrocytes. The mRNA expression levels of Aggrecan, ADAMTS-5, MMP-13, MMP-1 and COL2 A1 in human primary chondrocytes were measured by RT-qPCR (A–E). The expression of ADAMTS-5, Aggrecan, MMP-1, MMP-13 and COL2 A1 was examined by Western blot analysis (F). *p < 0.05 vs the IL-1β group; **p < 0.01 vs the IL-1β group; #p < 0.05 vs the control group; ##p < 0.01 vs the control group; The experiment was repeated three times. Data are presented as mean ± standard deviation (SD).
Effects of Salvianolic Acid A on Inflammatory Mediators in Cells
The representative inflammatory mediators (contributed to OA progression) including COX-2 and iNOS were also measured by using Western blot and RT-qPCR technologies. Interestingly, after the cells were pre-treated with various concentrations of SAA (SAA) (10, 20, 40 µg/mL) for 2 h, followed by stimulating with or without IL-1β (10 ng/mL) for 24 h, the expression of these inflammatory factors decreased (Figure 4). Therefore, a series of results indicated that SAA alleviates inflammation by regulating the secretion of cytokines.
Figure 4.
The expression of inflammation factors in IL-1β-induced chondrocytes including iNOS and COX-2 in human primary chondrocytes (tested by Western blot (A) and RT-qPCR (B, C)). Data are presented as the mean ± standard deviation (n=3). *p < 0.05 vs the control group; **p < 0.01 vs the control group; ##p < 0.01 vs the IL-1β group.
Effect of Salvianolic Acid A on IL-1β-Induced NF-κB and P38/MAPK Pathway
NF-κB signaling pathway plays a vital role in OA progression. Afterwards, we sought to detect the underlying mechanism of the inhibitory effect of SAA. The results of NF-κB p65 activity assay indicated that SAA treatment led to a marked reduction in NF-κB activity, although il-1β-induced NF-κB activity (Figure 5A). In addition, Western blotting assay indicated that IL-1β stimulation enhanced p65’s phosphorylation, and SAA treatment inhibited IL-1β-induced p65 phosphorylation (Figure 5B, C). The p38/MAPK pathway was also activated in OA chondrocytes, and results in MMPS’ expression and inflammatory mediators’ secretion. Figure 5D, E shows that IL-1β markedly enhanced the phosphorylation of p38, and SAA treatment inhibited IL-1β- triggered p38 phosphorylation. In sum, SAA may exert anti-inflammatory effects and suppress MMPs expression by regulating the NF-κB and p38/MAPK signaling pathway.
Figure 5.
Effect of SAA on IL-1β-induced NF-κB and p-38 signaling pathways (A-E). The expression of phosphorylated NF-κB p65 (p-NF-κB p65), total NF-κB p65, phosphorylated 38 (p-38), total p38 in human primary chondrocytes was tested by Western blot analysis. The experiment was repeated three times. Data are presented as mean ± standard deviation (SD); *p < 0.05 vs the IL-1β group; **p < 0.01 vs the IL-1β group; #p < 0.05 vs the control group; ##p < 0.01 vs the control group.
Discussion
Osteoarthritis is a chronic degenerative disease based on physical changes that can cause synovitis, meniscus injury, various deformity and even disability. At present, non-steroidal anti-inflammatory drugs (NSAIDs) can treat osteoarthritis effectively. Non-steroidal anti-inflammatory drugs can relieve pain, improve joint function, and delay joint structure damage; however, the long-term use of these drugs may cause active peptic ulcers in the gastrointestinal tract and does not effectively relieve the degeneration of cartilage.
Interleukin-1β (IL-1β) is a pro-inflammatory cytokine that is considered the key cytokine in the progression of OA.22,23 Previous studies have demonstrated that IL-1β induced the secretion of neutral metalloproteinases such as MMP1, MMP13, ADAMTS-5 from chondrocytes.24 Furthermore, IL-1β affected the anabolic activity of chondrocytes by inhibiting the synthesis of proteoglycan and collagen II, eventually causing cartilage degeneration. Proteoglycan is a component of articular cartilage that plays an important role in maintaining structural integrity. Recent studies have shown that ADAMTS-5 is a prominent enzyme that promotes proteoglycan degradation in articular cartilage.25 In addition, the stimulation of chondrocytes with IL-1β promoted the expression of ADAMTS-5 and decreased the production of proteoglycan. In this study, SAA inhibited the expression of ADAMTS-5 in IL-1β-stimulated cells, indicated that SAA may be an effective agent in osteoarthritis. Collagen II is one of the major components of the extracellular matrix and plays a vital role in cartilage degradation. Chondrocytes stimulated with IL-1β secreted MMPs that aggravated the destruction of the extracellular matrix. MMPs play a vital role in the transformation of osteoarthritis cartilage and the extracellular matrix. The previous studies have shown that the expression of MMPs was up-regulated in osteoarthritis cartilage,26 which enhanced cartilage degradation by combination with the MMP cleavage site on collagen II. Therefore, inhibiting the expression of MMPs may be a promising treating strategy for OA. Our results show that SAA can significantly suppress IL-1β-stimulated MMP1 and MMP13 expression and increase aggrecan expression, which demonstrated that SAA could inhibit cartilage degradation and OA progressing.
Conclusions
Our results provide solid evidence that SAA inhibited the expression of ADAMTS-5, MMP1, and MMP13 and increased the production of collagen II and aggrecan through regulation of the NF-κB pathway. Overall, we demonstrate that SAA may be of great value to OA treatment. However, lack of the experiments to compare its effects on OA with the drug used in clinic is our paper’s limitation.
Acknowledgment
This research was funded by Research Foundation of the Second People’s Hospital of Nantong.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Cundell DR, Mickle KE. Osteoarthritis: potential for herbal medicines as therapies in the management of chronic inflammatory damage. Curr Immunol Rev. 2018;14(2):68–80. doi: 10.2174/1573395514666180530093702 [DOI] [Google Scholar]
- 2.Kim Y, Oh H-C, Park JW, et al. Diagnosis and treatment of inflammatory joint disease. Hip Pelvis. 2017;29(4):211–222. doi: 10.5371/hp.2017.29.4.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest. 2015;45(4):405–414. doi: 10.1111/eci.2015.45.issue-4 [DOI] [PubMed] [Google Scholar]
- 4.Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16(3):6093–6112. doi: 10.3390/ijms16036093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W-J, Yu X-H, Wang C, et al. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. 2015;448:238–246. doi: 10.1016/j.cca.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 6.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 7.Liang Y-N, Xu B. Factors influencing utilization and satisfaction with external breast prosthesis in patients with mastectomy: a systematic review. Int J Nurs Sci. 2015;2(2):218–224. doi: 10.1016/j.ijnss.2015.04.005 [DOI] [Google Scholar]
- 8.Efstathiou M, Settas L. The effect of non-steroidal anti-inflammatory drugs on matrix metalloproteinases levels in patients with osteoarthritis. Mediterr J Rheumatol. 2017;28(3):133–141. doi: 10.31138/mjr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakata K, Hanai T, Take Y, et al. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthr Cartilage. 2018;26(10):1263–1273. doi: 10.1016/j.joca.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 10.Euppayo T, Punyapornwithaya V, Chomdej S, Ongchai S, Nganvongpanit K. Effects of hyaluronic acid combined with anti-inflammatory drugs compared with hyaluronic acid alone, in clinical trials and experiments in osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):387. doi: 10.1186/s12891-017-1743-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sostres C, Carrera-Lasfuentes P, Benito R, et al. Peptic ulcer bleeding risk. The role of Helicobacter pylori infection in NSAID/low-dose aspirin users. Am J Gastroenterol. 2015;110(5):684. doi: 10.1038/ajg.2015.98 [DOI] [PubMed] [Google Scholar]
- 12.Melcarne L, Garcia-Iglesias P, Calvet X. Management of NSAID-associated peptic ulcer disease. Expert Rev Gastroenterol Hepatol. 2016;10(6):723–733. doi: 10.1586/17474124.2016.1142872 [DOI] [PubMed] [Google Scholar]
- 13.Scheiman JM. NSAID-induced gastrointestinal injury. J Clin Gastroenterol. 2016;50(1):5–10. doi: 10.1097/MCG.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 14.Shi M, Huang F, Deng C, Wang Y, Kai G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit Rev Food Sci Nutr. 2019;59(6):953–964. doi: 10.1080/10408398.2018.1474170 [DOI] [PubMed] [Google Scholar]
- 15.Ding C, Zhao Y, Shi X, et al. New insights into salvianolic acid A action: regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci Rep. 2016;6(1):28734. doi: 10.1038/srep28734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Qi D, Wang X, et al. Protective effect of Salvianolic acid A on ischaemia‐reperfusion acute kidney injury in rats through protecting against peritubular capillary endothelium damages. Phytother Res. 2018;32(1):103–114. doi: 10.1002/ptr.v32.1 [DOI] [PubMed] [Google Scholar]
- 17.Shi X, Sun R, Zhao Y, et al. Promotion of autophagosome–lysosome fusion via salvianolic acid A-mediated SIRT1 up-regulation ameliorates alcoholic liver disease. RSC Adv. 2018;8(36):20411–20422. doi: 10.1039/C8RA00798E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood Q, Wang G-F, Wu G, et al. Salvianolic acid A inhibits calpain activation and eNOS uncoupling during focal cerebral ischemia in mice. Phytomedicine. 2017;25:8–14. doi: 10.1016/j.phymed.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Zhao T, Chang L, Zhang B, et al. Specific combination of salvianolic acids as core active ingredients of Danhong injection for treatment of arterial thrombosis and its derived dry gangrene. Front Pharmacol. 2017;8:361. doi: 10.3389/fphar.2017.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Tan C-N, Wang Y-L, et al. Adsorbed hollow fiber-based biological fingerprinting for the discovery of platelet aggregation inhibitors from Danshen–Honghua decoction. J Sep Sci. 2018;41(12):2651–2660. doi: 10.1002/jssc.v41.12 [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Xiang Y, Zhu N, et al. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp Ther Med. 2017;14(2):961–966. doi: 10.3892/etm.2017.4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil T, More V, Rane D, et al. Pro-inflammatory cytokine Interleukin-1β (IL-1β) controls Leishmania infection. Cytokine. 2018;112:27–31. doi: 10.1016/j.cyto.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 23.Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis–chondrocytes in focus. Cell Signal. 2018. [DOI] [PubMed] [Google Scholar]
- 24.Ra HJ, Lee HJ, Jo HS, et al. Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat. Korean J Physiol Pharmacol. 2017;21(1):19–26. doi: 10.4196/kjpp.2017.21.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat Rev Rheumatol. 2015;11(10):606. doi: 10.1038/nrrheum.2015.95 [DOI] [PubMed] [Google Scholar]
- 26.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. doi: 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]