ABSTRACT
Previous studies show that Long non-coding RNAs (LncRNAs) are involved in the regulation of various human diseases. This study aimed to reveal how LncRNA CRNDE regulated vascular smooth muscle cells (VSMCs) proliferation and apoptosis in abdominal aortic aneurysms (AAA). Here, we found CRNDE was down-regulated in AAA tissues and AngII-stimulated VSMCs. The overexpression of CRNDE promoted VSMCs proliferation and inhibited cell apoptosis. The interaction between CRNDE and Bcl-3 or Bcl-3 and Smad3 was verified. The interference with Bcl-3 or CRNDE reduced Smad3 stability or promoted Smad3 ubiquitination. After pcDNA-CRNDE or pcDNA-CRNDE+si-Bcl-3 was transfected into VSMCs and stimulated with AngII, CRNDE affected VSMCs proliferation and apoptosis via regulating Smad3 via Bcl-3. Vivo experiments showed the overexpression of CRNDE repressed AAA growth. Therefore, we concluded that CRNDE was down-regulated in AAA tissues and AngII-stimulated VSMCs. Furthermore, the overexpression of CRNDE promoted VSMCs proliferation and repressed cell apoptosis in AAA by up-regulating Smad3 via Bcl-3.
KEYWORDS: LncRNA CRNDE, Bcl-3, Smad3, AAA, VSMCs
Introduction
Abdominal aortic aneurysm (AAA) refers to the local expansion of the abdominal aorta and is one of the common aortic aneurysms [1]. Since AAA is usually asymptomatic, the overall mortality caused by AAA is over 80% [2]. Unfortunately, there are currently no drugs available for the treatment of AAA, and treatment options are mainly limited to surgical repair [3,4]. Local structural abnormalities of the arterial wall have been reported to be one of the features of AAA, and the reduction in the number of vascular smooth muscle cells (VSMCs) is an important cause of AAA [5]. Another study found that the apoptosis of VSMCs plays an important regulatory role in accelerating the development of AAA [6]. In addition, there is increasing evidence indicate that promoting VSMCs proliferation or inhibiting cell apoptosis can impede the progression of AAA [7,8]. Therefore, promoting VSMCs proliferation and repressing cell apoptosis is beneficial to alleviate AAA.
Long non-coding RNAs (LncRNAs) are a class of non-coding RNAs that are greater than 200 nucleotides in length [9]. LncRNAs have been shown to be involved in the regulation of a variety of biological processes in cells, including the proliferation, migration, apoptosis, and differentiation of cells [10]. Accumulating evidence indicates that LncRNAs are the core factors that regulate the proliferation and differentiation of VSMCs [11,12]. It has been reported that interference with LncRNA MRAK048635_P1 promotes the proliferation and migration of VSMCs and inhibits cell apoptosis [13]. Studies have shown that the knockdown of LncRNA RNCR3 inhibits VSMCs proliferation and accelerates cell apoptosis [14]. It is worth noting that Zhang et al find that LncRNA PVT1 was significantly up-regulated in the abdominal aortic tissues of AAA patients and further studies find that in Ang II–induced AAA model, the interference with LncRNA PVT1 can inhibit the apoptosis of VSMCs, thereby alleviating the progression of AAA [15]. In addition, a recent study has shown that LncRNA H19 is abnormally overexpressed in AAA tissues, and the knockdown of LncRNA H19 can attenuate AAA via inhibiting the apoptosis of VSMCs [16]. Therefore, exploring more LncRNAs that can regulate VSMCs proliferation or apoptosis is important for mitigating AAA.
Smad3 protein is a member of the Smad protein family [17]. Emerging evidence suggests that Smad3 is involved in the regulation of a variety of cancers, including AAA [18]. In addition, another study has found that in a mouse AAA model, Smad3 plays an important role in protecting vascular wall integrity and slowing down the progression of AAA [19]. Therefore, up-regulating the protein level of Smad3 is important for the relief of AAA. As reported, the overexpression of hCLP46 can up-regulate the expression of Smad3 by inhibiting the ubiquitination and degradation of Smad3 [20]. In addition, another study has indicated that PROTAC can up-regulate the expression of Smad3 by inhibiting the ubiquitination of Smad3, thereby preventing renal fibrosis [21]. Therefore, exploring the strategies to up-regulate the expression of Smad3 through inhibiting the ubiquitination of Smad3 has the potential to alleviate AAA.
In the present study, we found that LncRNA CRNDE was down-regulated in AAA tissues and AngII-stimulated VSMCs. Further studies revealed that the overexpression of LncRNA CRNDE inhibited the ubiquitination and degradation of Smad3 protein by up-regulating Bcl-3, and this up-regulation of Smad3 could promote VSMCs proliferation and inhibit cell apoptosis to ultimately improve AAA.
Materials and methods
Patients and tissue samples
The confirmed AAA patients (aneurysm diameter > 4 cm) were collected from the Henan Provincial People’s Hospital. Full-thickness aortic wall (aortic wall intima, media, and adventitia) tissue specimens were collected from patients. AAA tissues (n = 36) and adjacent tissues (1 cm away from the AAA) were obtained by surgery and quickly stored in liquid nitrogen within 5 min. Our research was approved by the Medical Ethics Committee of Henan Provincial People’s Hospital.
Cell culture
According to methods described in previous reports, vascular smooth muscle cells (VSMCs) were isolated from primary mouse aortic tissue, including mouse abdominal aortic tissues [22]. VSMCs were resuspended in complete Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin and cultured in a humidified incubator with 5% CO2 at 37°C.
Cell transfection
Mouse VSMCs were seeded in a six-well plate at a density of 1 × 105 cells/well. Subsequently, the synthesized si-CRNDE, pcDNA-CRND, and pcDNA-CRNDE+si-Bcl-3 were transfected into VSMCs using the Lipofectamine 2000, respectively. After 8 h of transfection, further analysis was performed.
Real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA was isolated from AAA tissues and differently treated VSMCs by RNAiso Plus (TaKaRa, Dalian, China). Next, reverse transcription of RNA into cDNA using PrimeScrip RT Master Mix. The synthesized cDNA was subjected to Real-time PCR on a Stratagene Mx3005P qPCR instrument by the SYBR Green master mix (Agilent Technologies). GAPDH was used as endogenous control and relative expression of LncRNA CRNDE was calculated by the 2−ΔΔCt method.
MTT assay
The proliferation of differently treated VSMCs was assessed by MTT. Briefly, VSMCs with different treatments were seeded in a six-well plate at a density of 1 × 105 cells/well and cultured overnight. Subsequently, 20 μL of MTT solution was added to each well and incubated at 37°C for 1 h. Next, the medium was removed and 150 μL of DMSO was added to each well to dissolve the blue crystals. Finally, the cell proliferation was identified through measuring the absorbance at 570 nm.
EdU assay
VSMCs with different treatments were seeded into a six-well plate at a density of 1 × 105 cells/well and incubated for 24 h. According to the manufacturer’s instructions, 5-Ethynyl-20-deoxyuridine (EdU) incorporation analysis was performed using the EdU analysis kit. Finally, images were acquired using a laser scanning confocal microscope, and the percentage of EdU-positive cells was calculated.
Flow cytometric analysis
Different treatments of VSMCs were harvested. Double staining of FITC-Annexin V and propidium iodide (PI) was performed by FITC Annexin V Apoptosis Detection Kit (BD Biosciences). The apoptosis of VSMCs was analyzed by using a flow cytometer equipped with CellQuest software (FACScan, BD Biosciences) according to the manufacturer’s instructions.
RNA pull-down assay
VSMCs were quantified and lysed with lysis buffer supplemented with a protease inhibitor cocktail. Purified LncRNA CRNDE was biotinylated by the biotin RNA labeling mix (Ambion Life). The biotinylated LncRNA CRNDE was captured with streptavidin magnetic beads according to the manufacturer’s instructions and then incubated with VSMCs lysate. Finally, the magnetic beads were washed and the eluted proteins were detected by Western blot.
RNA immunoprecipitation (RIP)
RIP experiments were performed according to the instructions of the Magna RIPTM RNA Binding Protein Immunoprecipitation Kit (Millipore). VSMCs were lysed in complete RIP lysis buffer and cell extracts were incubated with RIP buffer containing magnetic beads conjugated to anti-Argonaute 2 (Ago2) antibody (Millipore) or normal mouse IgG (Millipore). Then, the sample was digested with proteinase K, and the RNAs in the precipitated complex were analyzed by qRT-PCR.
Western blot analysis
The different treatments of VSMCs in this experiment were collected. The VSMCs were lysed on ice with RIPA buffer supplemented with protease inhibitors and proteins were extracted. SDS-PAGE was performed to separate the same amount of protein and transfer to PVDF membranes. After blocking the membrane for 1 h in 5% skim milk, the membrane was incubated with specific primary antibody overnight. Subsequently, the membrane was incubated with the secondary antibody for 1 h at room temperature. Finally, the proteins were visualized by enhanced chemiluminescence (ECL) chromogenic substrates and proteins were quantified by Quantity One software.
Co-immunoprecipitation (Co-IP)
In order to examine the interaction between Bcl-3 and Smad3, the anti-Bcl-3 antibody or anti-Smad3 antibody was used for the Co-IP test, respectively. Briefly, VSMCs were lysed in cell lysis buffer, and then the cell lysates were incubated with anti-Bcl-3 antibody or anti-Smad3 antibody and protein G-agarose beads (Thermo Fisher Scientific) overnight. The beads/immune complexes were then washed 3 times with lysis buffer. Finally, the eluted immune complexes were detected by Western blot.
Cycloheximide (CHX)-chase assay
CHX is a protein synthesis inhibitor [23]. After pcDNA-CRNDE and pcDNA-CRNDE+si-Bcl-3 were transfected into mouse VSMCs, respectively, the cells were treated with 20 μg/mL CHX, and the protein level of Smad3 was determined by Western blot at 0, 4, and 8 h.
Ubiquitination assay
To explore the effect of LncRNA CRNDE on the ubiquitination of Smad3, we performed the ubiquitination assay. Specifically, ubiquitination and pcDNA-CRNDE or si-CRNDE plasmids were transfected into mouse VSMCs by jetPRIME, respectively, and the cells were treated with 100 mM proteasome inhibitor MG132 for 6 h [24]. Finally, the protein level of Smad3 was analyzed by Western blot.
Establishment of a mouse model of AAA
Twelve C57BL/6 ApoE−/− mice were anesthetized with 50 mg/kg sodium pentobarbital and 1000 ng/kg/min AngII (Sigma-Aldrich, St. Louis, MO) was injected by subcutaneous osmotic minipump for 4 weeks. The control mice were injected with the same volume of saline. On the 0, 7 and 14 d after induction, Lenti-control (n = 6) or Lenti-CRNDE (n = 6) were injected into AAA mice, respectively. On the 28th day, the mice were sacrificed and aneurysm tissues were taken for the follow-up experiments. Our research was approved by the Medical Ethics Committee of Henan Provincial People’s Hospital.
Ultrasound (US) imaging
The experiment was carried out based on the previous method [25]. Briefly, B-mode US imaging was performed on the 28th day after the induction to assess the diameter of the abdominal aorta diameter.
Statistical analysis
SPSS version 20.0 software was used for all statistical analysis in this experiment. Unless otherwise stated, the data was usually expressed as mean ± standard deviation (SD). Differences between two groups were analyzed by Student’s t-test, and differences among groups were analyzed by one-way ANOVA. P values <0.05 were considered statistically significant.
Results
LncRNA CRNDE is down-regulated in abdominal aortic aneurysm tissues and AngII-stimulated vascular smooth muscle cells
Abdominal aortic aneurysm (AAA) tissues and adjacent normal tissues were obtained from patients with AAA diagnosed by CT scan and abdominal magnetic resonance imaging (n = 36). qRT-PCR was used to screen for LncRNA expressed in AAA, including LncRNA H19 [25], LncRNA PVT1 [15], LncRNA LINC00265 [26], and LncRNA MT1DP [27], LncRNA CRNDE [27]. The results showed that the expression of LncRNA CRNDE was significantly down-regulated in AAA tissues (Figure 1(a)). Mouse vascular smooth muscle cells (VSMCs) were treated with 1 μM AngII for 48 h [25]. According to the results of MTT and EdU assays, it was found that AngII stimulation significantly inhibited the proliferation of mouse VSMCs and reduced the percentage of EdU positive cells (Figure 1(b–d)). From the results of flow cytometry, we found that AngII stimulation significantly promoted the apoptosis of mouse VSMCs (Figure 1(e,f)). In addition, it could be seen from the results of qRT-PCR that the expression of LncRNA CRNDE was significantly down-regulated after AngII stimulation (Figure 1(g)). In summary, the expression of LncRNA CRNDE was down-regulated in AAA tissues and AngII-stimulated VSMCs.
Figure 1.
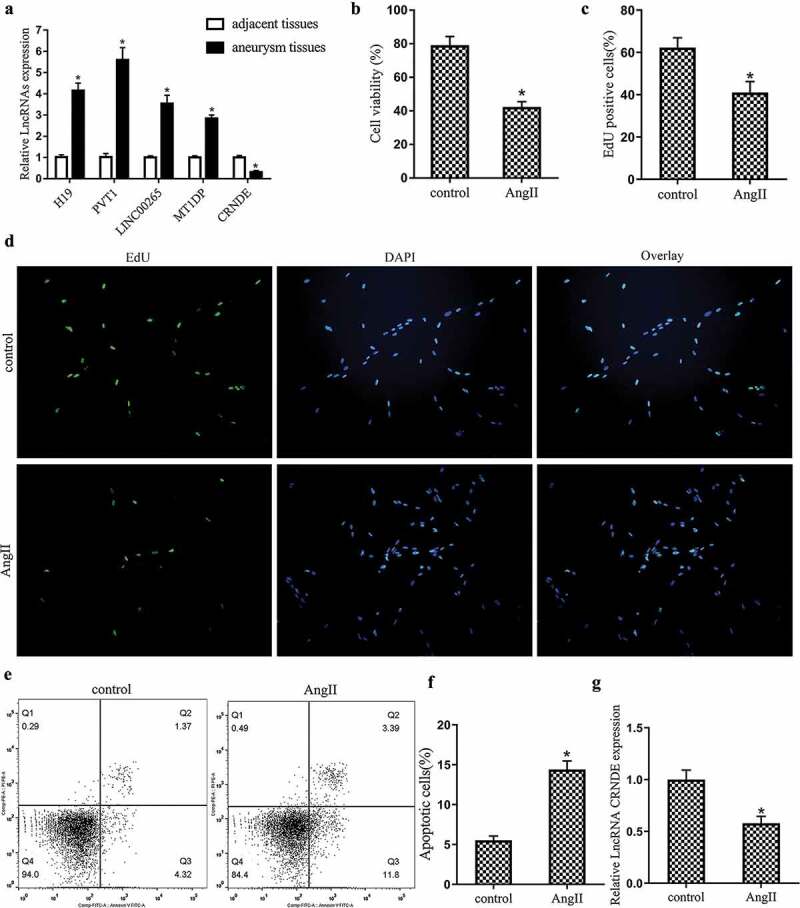
Expression of LncRNA CRNDE in abdominal aortic aneurysm (AAA) tissues and AngII-stimulated vascular smooth muscle cells (VSMCs). AAA tissues and adjacent normal tissues were obtained from patients with AAA diagnosed by CT scan and abdominal magnetic resonance imaging (n = 36). (a) qRT-PCR was used to detect the expressions of LncRNA H19, LncRNA PVT1, LncRNA LINC00265, LncRNA MT1DP, LncRNA CRNDE in AAA tissues and AngII-stimulated VSMCs. Mouse vascular smooth muscle cells (VSMCs) were treated with 1 μM AngII for 48 h. (B&C&D) MTT and EdU were performed to analyze the proliferation of VSMCs and the percentage of EdU positive cells, respectively. (E&F) Flow cytometry was performed to analyze the apoptosis of VSMCs. (g) qRT-PCR was used to detect the expression of LncRNA CRNDE. *P < 0.05 (compared to adjacent tissues group or control group).
Overexpression of LncRNA CRNDE promotes VSMCs proliferation and inhibits cell apoptosis
To investigate the effect of LncRNA CRNDE on the proliferation and apoptosis of VSMCs, we transfected pcDNA or pcDNA-CRNDE into mouse VSMCs and treated the cells with 1 μM AngII for 48 h. From the results of qRT-PCR, it could be seen that the expression of LncRNA CRNDE was markedly down-regulated after the AngII stimulation. Compared with the AngII+pcDNA group, the expression of LncRNA CRNDE was significantly up-regulated in the AngII+pcDNA-CRNDE group (Figure 2(a)). The results of MTT and EdU assays indicated that the AngII stimulation inhibited VSMCs proliferation and reduced the percentage of EdU positive cells. Besides, compared with the AngII+pcDNA group, the proliferation of VSMCs was promoted and the percentage of EdU positive cells was increased in the AngII+pcDNA-CRNDE group (Figure 2(b–d)). From the results of flow cytometry, we found that the AngII stimulation promoted the apoptosis of VSMCs. In addition, compared with the AngII+pcDNA group, the apoptosis of VSMCs was repressed in the AngII+pcDNA-CRNDE group (Figure 2(e,f)). From the above experimental results, the overexpression of LncRNA CRNDE could promote the proliferation of VSMCs and repress the apoptosis of cells.
Figure 2.
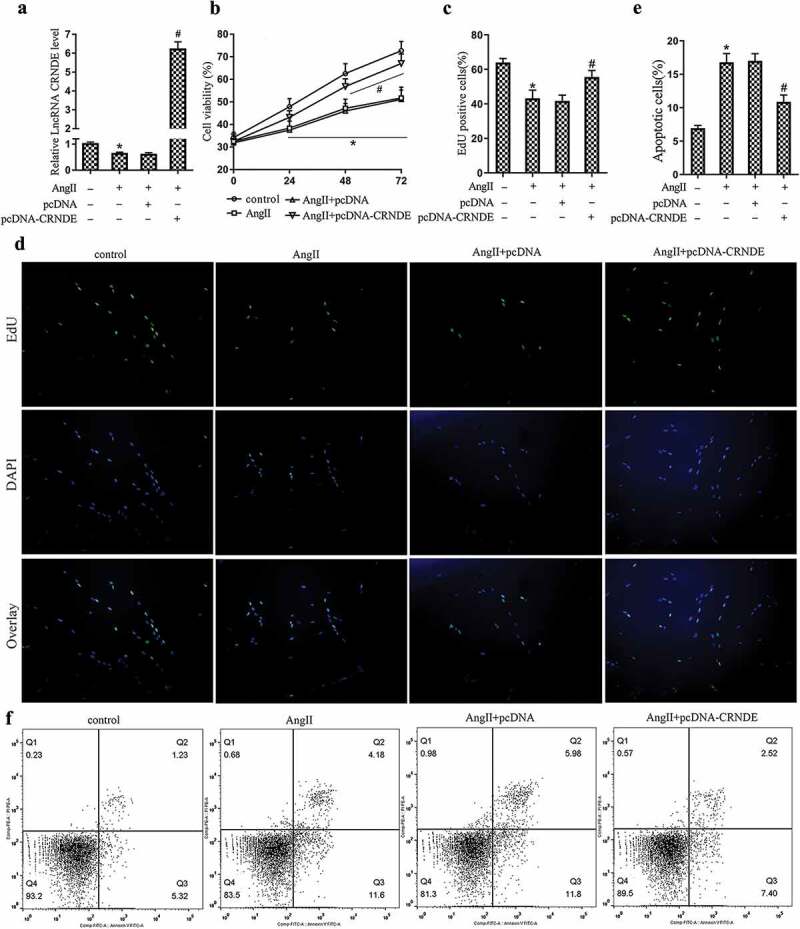
Effect of LncRNA CRNDE on the proliferation and apoptosis of VSMCs. pcDNA or pcDNA-CRNDE was transfected into mouse VSMCs and treated the cells with 1 μM AngII for 48 h. (a) qRT-PCR was used to detect the expression of LncRNA CRNDE. (B–D) MTT and EdU were used to detect the proliferation of VSMCs and the percentage of EdU positive cells. (E,F) Flow cytometry was performed to analyze the apoptosis of VSMCs. *P < 0.05 (compared to control group), #P < 0.05 (compared to AngII+pcDNA group).
LncRNA CRNDE regulates the ubiquitination of Smad3 via Bcl-3
Subsequently, we explored the interaction between LncRNA CRNDE and Bcl-3 by RNA pull-down and RIP experiments. From the RNA pull-down detection data, it was found that the expression of Bcl-3 was detected in the CRNDE pull-down complex (Figure 3(a) and Supplemental Figure 1a). RIP experiments revealed that LncRNA CRNDE was accumulated in Bcl-3 precipitated protein samples (Figure 3(b)). Furthermore, we transfected si-control or si-CRNDE into mouse VSMCs, respectively. Western blot analysis showed that the transfection of si-CRNDE down-regulated the protein level of Bcl-3 (Figure 3(c) and Supplemental Figure 1b,C). Besides, in order to elucidate the interaction between Bcl-3 and Smad3, we performed a Co-immunoprecipitation (Co-IP) experiment. From the results of this experiment, it was found that there is an interaction between Bcl-3 and Smad3 protein (Figure 3(d) and Supplemental Figure 1d,E). Subsequently, we transfected pcDNA-CRNDE or pcDNA-CRNDE+si-Bcl-3 into mouse VSMCs and treated the cells with 20 μg/mL CHX, and then detected the stability of Smad3 protein at 0, 4, and 8 h, respectively. Western blot analysis indicated that Smad3 protein in cells transfected with pcDNA-CRNDE alone was much more stable than cells transfected with pcDNA-CRNDE+si-Bcl-3 (Figure 3(e) and Supplemental Figure 2a,B). Furthermore, we transfected pcDNA-CRNDE or si-CRNDE into mouse VSMCs and treated the cells with 100 mM MG132 for 6 h. From the results of Western blot analysis, the interference with LncRNA CRNDE increased the ubiquitination degradation of Smad3 protein, while the overexpression of LncRNA CRNDE produced the opposite effect (Figure 3(F) and Supplemental Figure 2c–E and Supplemental Figure 3a–C). Taken together, the above experimental results indicated that LncRNA CRNDE bound to Bcl-3 and LncRNA CRNDE could regulate the ubiquitination of Smad3 via Bcl-3.
Figure 3.
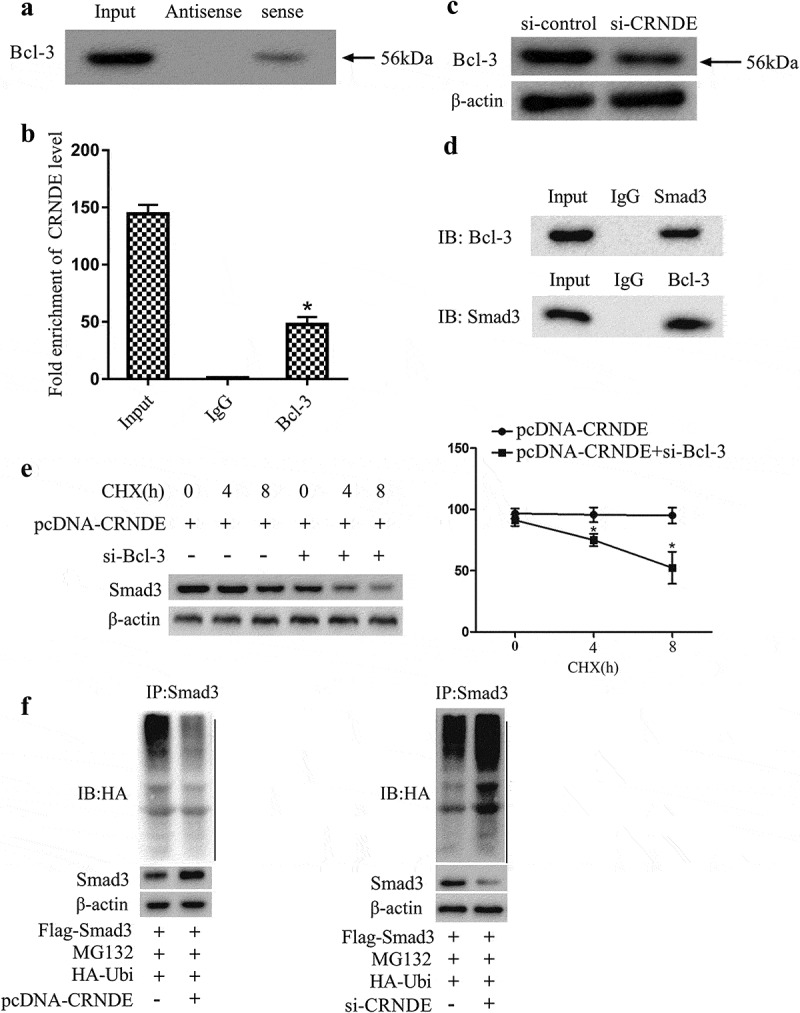
LncRNA CRNDE targets Bcl-3 and regulates the ubiquitination of Smad3 via Bcl-3. (A,B) RNA pull-down and RIP experiments were performed to analyze the interaction between LncRNA CRNDE and Bcl-3. (c) After si-control or si-CRNDE was transfected into mouse VSMCs, Western blot was used to detect the expressions of Bcl-3 and Smad3. (d) Co-immunoprecipitation assay was performed to analyze the interaction between Bcl-3 and Smad3 in mouse VSMCs. (e) pcDNA-CRNDE or pcDNA-CRNDE+si-Bcl-3 was transfected into mouse VSMCs and treated the cells with 20 μg/mL CHX for 0, 4, 8 h. The protein level of Smad3 was analyzed by Western blot. (f) pcDNA-CRNDE or si-CRNDE was transfected into mouse VSMCs and treated the cells with 100 mM MG132 for 6 h. The ubiquitination level of Smad3 protein was detected by Western blot. *P < 0.05 (compared to IgG group or pcDNA-CRNDE group).
LncRNA CRNDE regulates Smad3 through Bcl-3 and affects the proliferation and apoptosis of VSMCs
To further investigate how LncRNA CRNDE affected the proliferation and apoptosis of VSMCs, pcDNA, pcDNA-CRNDE or pcDNA-CRNDE+si-Bcl-3 were transfected into mouse VSMCs and stimulated the cells with AngII. Western blot analysis showed that the overexpression of LncRNA CRNDE up-regulated the expressions of Bcl-3 and Smad3, while this up-regulation was reversed after the transfection of si-Bcl-3 (Figure 4a and Supplemental Figure 3d–F). Moreover, the results of MTT and EdU showed that the overexpression of LncRNA CRNDE promoted VSMCs proliferation and increased the percentage of EdU positive cells, while these effects were reversed after the transfection of si-Bcl-3 (Figure 4(b–d)). According to the results of flow cytometry, we found that the overexpression of LncRNA CRNDE inhibited the apoptosis of VSMCs, while this inhibition was reversed after the transfection of si-Bcl-3 (Figure 4(e,f)). Collectively, our data demonstrated that the overexpression of LncRNA CRNDE could promote VSMCs proliferation and inhibit cell apoptosis via up-regulating Smad3 expression by Bcl-3.
Figure 4.
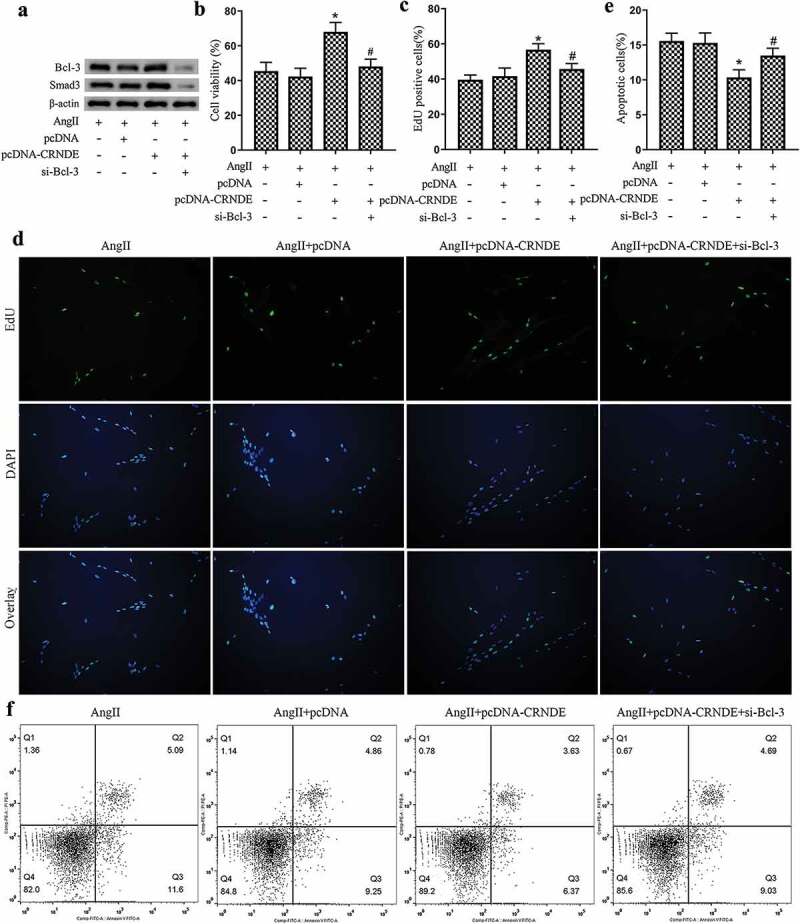
LncRNA CRNDE affects the proliferation and apoptosis of VSMCs by regulating Smad3 through Bcl-3. pcDNA, pcDNA-CRNDE or pcDNA-CRNDE+si-Bcl-3 was transfected into mouse VSMCs and stimulated with AngII. (a) Western blot was used to detect the protein levels of Bcl-3 and Smad3. (B–D) MTT and EdU assays were used to detect the proliferation of VSMCs and the percentage of EdU positive cells, respectively. (E,F) Flow cytometry was performed to analyze the apoptosis of VSMCs. *P < 0.05 (compared to AngII+pcDNA group), #P < 0.05 (compared to AngII+pcDNA-CRNDE group).
Overexpression of LncRNA CRNDE represses mice AAA growth
To further verify the effect of LncRNA CRNDE in vivo, we established a C57BL/6 ApoE−/− mouse model of AAA (n = 12) and injected Lenti-control, Lenti-CRNDE in mice at 0, 7 and 14 d after the establishment. At 28 d after the induction, the mice were sacrificed and the aortic aneurysm diameter was measured by ultrasound (US). Experimental data indicated that the overexpression of LncRNA CRNDE significantly repressed mice AAA growth (Figure 5(a)). From the results of qRT-PCR, it was found that the expression of LncRNA CRNDE was up-regulated in AAA tissues after the injection of Lenti-CRNDE (Figure 5(b)). Western blot analysis showed that the protein levels of Bcl-3 and Smad3 were significantly up-regulated after the injection of Lenti-CRNDE compared with the Lenti-control group (Figure 5(c) and Supplemental Figure 4a–c). Taken together, the above experimental results indicated that the overexpression of LncRNA CRNDE could repress mice AAA growth.
Figure 5.
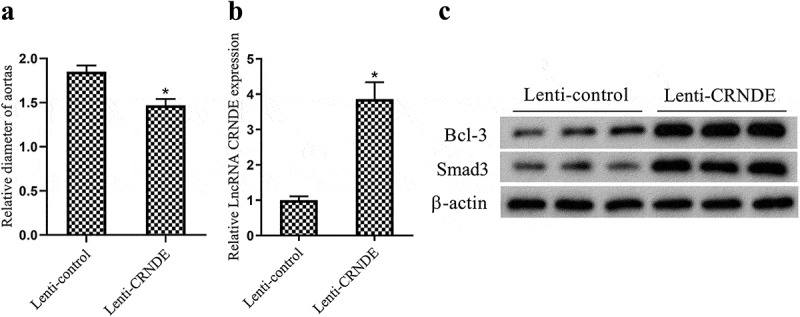
Effect of LncRNA CRNDE on mice AAA growth. After the C57BL/6 ApoE−/− mouse model of AAA (n = 12) was established, Lenti-control, Lenti-CRNDE was injected in mice at 0, 7 and 14 d after the establishment. On the 28th d after the induction, the mice were sacrificed. (a) The aortic aneurysm diameter was measured by ultrasound (US). (b) The expression of LncRNA CRNDE in AAA tissues was detected by qRT-PCR. (c) The protein levels of Bcl-3, Smad3 in AAA tissues were measured by Western blot. *P < 0.05 (compared to the Lenti-control group).
Discussion
Emerging evidence suggests that promoting vascular smooth muscle cells (VSMCs) proliferation and inducing cell apoptosis are beneficial for improving abdominal aortic aneurysm (AAA) [28,29]. Therefore, exploring the methods to promote VSMCs proliferation or inhibit cell apoptosis is of great significance for relieving AAA. In the present study, we found that the overexpression of LncRNA CRNDE could promote VSMCs proliferation and repress cell apoptosis. Further studies revealed that the overexpression of LncRNA CRNDE could inhibit the ubiquitination of Smad3 protein by up-regulating Bcl-3 and ultimately attenuated AAA by up-regulating the expression of Smad3.
Numerous studies have shown that long non-coding RNAs (LncRNAs) are involved in the regulation of biological processes such as proliferation, differentiation, and apoptosis of various cells [30,31]. A recent study has shown that LncRNAs regulate the proliferation and differentiation of VSMCs and are one of the key factors regulating VSMCs [32]. As reported, the overexpression of LncRNA NEAT1 in VSMCs promotes the proliferation and migration of VSMCs, while the interference with LncRNA NEAT1 has the opposite effect [33]. In addition, another study has shown that LncRNA HIF1α antisense RNA 1 (HIF1a-AS1) is overexpressed in the thoracoabdominal aortic aneurysm (TAAA), and the knockdown of LncRNA HIF1α promotes VSMCs proliferation and induces cell apoptosis to improve TAAA [34]. Therefore, regulating the expression of LncRNAs to inhibit VSMCs apoptosis or promote cell proliferation has the potential to improve AAA. In this study, we also found that LncRNA CRNDE was abnormally down-regulated in AAA tissues and AngII-stimulated VSMCs. As expected, the in-depth studies had found that the overexpression of LncRNA CRNDE could promote VSMCs proliferation and inhibit cell apoptosis.
A growing number of studies have shown that Smad3, which is an important member of the Smad protein family, is abnormally expressed in various cancers and contributes to the progression of cancer [35]. It has been reported that the low expression of Smad3 promotes the progression of aortic aneurysms [36]. In addition, another study has indicated that Smad3 is down-regulated in AAA, and the overexpression of Smad3 attenuates AAA by promoting the proliferation of VSMCs and inhibiting cell apoptosis [37]. Therefore, increasing the protein level of Smad3 has the potential to improve AAA. B cell leukemia 3 (Bcl-3) is an essential negative regulator of NF-κB [38]. As reported, Bcl-3 can stabilize NF-κBp50 by inhibiting the ubiquitination of p50 [39]. In addition, it is noteworthy that Bcl-3 can up-regulate the protein level of Smad3 via repressing the ubiquitination of Smad3 protein [40]. Therefore, excavating the methods that can regulate Bcl-3 to up-regulate the expression of Smad3 is important for improving AAA. According to the current research, the overexpression of LncRNA CRNDE up-regulated the protein level of Bcl-3. Further studies had found that this up-regulation of Bcl-3 could attenuate AAA by up-regulating Smad3 expression via inhibiting Smad3 ubiquitination.
In general, in this study, we found that LncRNA CRNDE was abnormally down-regulated in AAA tissues and AngII-stimulated VSMCs. Furthermore, the overexpression of LncRNA CRNDE could repress the ubiquitination of Smad3 protein by up-regulating Bcl-3 and this up-regulation of Bcl-3 could improve AAA by up-regulating Smad3 expression via inhibiting the ubiquitination of Smad3. The limitation of this study was that the number of AAA clinical samples was small, and we would make up for this deficiency by increasing the number of samples in subsequent studies. In addition, the study might provide new insights and strategies for the clinical treatment of AAA, which was of great significance.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 81301328, 2013) and Henan provincial Medical Science and technology research program (grant number 201602216, 2016).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, ad hoc committee on reporting standards, society for vascular surgery and North American chapter, international society for cardiovascular surgery. J Vasc Surg. 1991;13(3):452–458. [DOI] [PubMed] [Google Scholar]
- [2].Chen HZ, Wang F, Gao P, et al. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ Res. 2016;119(10):1076–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baxter BT, Terrin MC, Dalman RL.. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117(14):1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371(22):2101–2108. [DOI] [PubMed] [Google Scholar]
- [5].Lopez-Candales A, Holmes DR, Liao S, et al. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150(3):993–1007. [PMC free article] [PubMed] [Google Scholar]
- [6].Sachdeva J, Mahajan A, Cheng J, et al. Smooth muscle cell-specific Notch1 haploinsufficiency restricts the progression of abdominal aortic aneurysm by modulating CTGF expression. PLoS One. 2017;12(5):e0178538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lyon CA, Williams H, Bianco R, et al. Aneurysm severity is increased by combined Mmp-7 deletion and n-cadherin mimetic (EC4-Fc) over-expression. Sci Rep. 2017;7(1):17342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Milewicz DM. MicroRNAs, fibrotic remodeling, and aortic aneurysms. J Clin Invest. 2012;122(2):490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. [DOI] [PubMed] [Google Scholar]
- [10].Zhao XY, Lin JD. Long noncoding RNAs: a new regulatory code in metabolic control. Trends Biochem Sci. 2015;40(10):586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leung A, Trac C, Jin W, et al. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. 2013;113(3):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bianchessi V, Badi I, Bertolotti M, et al. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in endothelial cells. J Mol Cell Cardiol. 2015;81:62–70. [DOI] [PubMed] [Google Scholar]
- [13].Fang G, Qi J, Huang L, et al. LncRNA MRAK048635_P1 is critical for vascular smooth muscle cell function and phenotypic switching in essential hypertension. Biosci Rep. 2019;39(3): BSR20182229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shan K, Jiang Q, Wang XQ, et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016;7(6):e2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang Z, Zou G, Chen X, et al. Knockdown of lncRNA PVT1 inhibits vascular smooth muscle cell apoptosis and extracellular matrix disruption in a murine abdominal aortic aneurysm model. Mol Cells. 2019;42(3):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun Y, Zhong L, He X, et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J Mol Cell Cardiol. 2019;131:66–81. [DOI] [PubMed] [Google Scholar]
- [17].Datto MB, Frederick JP, Pan L, et al. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19(4):2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moran CS, Seto S-W, Krishna SM, et al. Parenteral administration of factor Xa/IIa inhibitors limits experimental aortic aneurysm and atherosclerosis. Sci Rep. 2017;7:43079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dai X, Shen J, Annam NP, et al. SMAD3 deficiency promotes vessel wall remodeling, collagen fiber reorganization and leukocyte infiltration in an inflammatory abdominal aortic aneurysm mouse model. Sci Rep. 2015;5:10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xing Y, Chu Q, Feng R, et al. hCLP46 increases Smad3 protein stability via inhibiting its ubiquitin-proteasomal degradation. Protein Cell. 2015;6(10):767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Feng S, Fan J, et al. New strategy for renal fibrosis: targeting Smad3 proteins for ubiquitination and degradation. Biochem Pharmacol. 2016;116:200–209. [DOI] [PubMed] [Google Scholar]
- [22].Xu X, Zhang F, Lu Y, et al. Silencing of NONO inhibits abdominal aortic aneurysm in apolipoprotein E-knockout mice via collagen deposition and inflammatory inhibition. J Cell Mol Med. 2019;23(11):7449–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schneider-Poetsch T, Ju J, Eyler DE, et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6(3):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan H, Ma Y-L, Gui Y-Z, et al. MG132, a proteasome inhibitor, enhances LDL uptake in HepG2 cells in vitro by regulating LDLR and PCSK9 expression. Acta Pharmacol Sin. 2014;35(8):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li DY, Busch A, Jin H, et al. H19 induces abdominal aortic aneurysm development and progression. Circulation. 2018;138(15):1551–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Su Y, Wang Y-F, He -D-D, et al. Long non-coding RNA LINC00265 promotes inflammation via sponging miR-let-7a in abdominal aortic aneurysm. Aging (Albany NY). 2019;11(13):4463–4477.31326963 [Google Scholar]
- [27].Yang YG, Li MX, Kou L, et al. Long noncoding RNA expression signatures of abdominal aortic aneurysm revealed by microarray. Biomed Environ Sci. 2016;29(10):713–723. [DOI] [PubMed] [Google Scholar]
- [28].Allaire E, Muscatelli-Groux B, Mandet C, et al. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. J Vasc Surg. 2002;36(5):1018–1026. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Z, Liang K, Zou G, et al. Inhibition of miR-155 attenuates abdominal aortic aneurysm in mice by regulating macrophage-mediated inflammation. Biosci Rep. 2018;38(3):BSR20171432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. [DOI] [PubMed] [Google Scholar]
- [32].Yan B, Yao J, Liu J-Y, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143–1156. [DOI] [PubMed] [Google Scholar]
- [33].Ahmed ASI, Dong K, Liu J, et al. Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc Natl Acad Sci U S A. 2018;115(37):E8660–e8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He Q, Tan J, Yu B, et al. Long noncoding RNA HIF1A-AS1A reduces apoptosis of vascular smooth muscle cells: implications for the pathogenesis of thoracoabdominal aorta aneurysm. Pharmazie. 2015;70(5):310–315. [PubMed] [Google Scholar]
- [35].Tang PM-K, Zhou S, Meng X-M, et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat Commun. 2017;8(1):14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hilhorst-Hofstee Y, Scholte A, Rijlaarsdam M, et al. An unanticipated copy number variant of chromosome 15 disrupting SMAD3 reveals a three-generation family at serious risk for aortic dissection. Clin Genet. 2013;83(4):337–344. [DOI] [PubMed] [Google Scholar]
- [37].Liang B, Che J, Zhao H, et al. MiR-195 promotes abdominal aortic aneurysm media remodeling by targeting Smad3. Cardiovasc Ther. 2017;35(6):e12286. [DOI] [PubMed] [Google Scholar]
- [38].Tassi I, Rikhi N, Claudio E, et al. The NF-kappaB regulator Bcl-3 modulates inflammation during contact hypersensitivity reactions in radioresistant cells. Eur J Immunol. 2015;45(4):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Collins PE, Grassia G, Colleran A, et al. Mapping the interaction of B cell leukemia 3 (BCL-3) and nuclear factor kappaB (NF-kappaB) p50 identifies a BCL-3-mimetic anti-inflammatory peptide. J Biol Chem. 2015;290(25):15687–15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen X, Cao X, Sun X, et al. Bcl-3 regulates TGFbeta signaling by stabilizing Smad3 during breast cancer pulmonary metastasis. Cell Death Dis. 2016;7(12):e2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


