ABSTRACT
p53 is the most frequently mutated gene in human cancers, with over half of all tumors harboring mutation at this locus. R248 and R249 (corresponding to porcine R241 and R242), are among the hotspot mutations frequently mutated in liver, lung, breast, and some other cancers. In this study, p53 gene was knocked out or point-edited (R241 and R242 were converted to 241W and 242S) in porcine fetal fibroblast (PFF) cells via CRISPR-Cas9 technique. High throughput sequencing of miRNA and mRNA uncovered a total of 225 differentially expressed miRNAs (DEMs) and 738 differentially expressed genes (DEGs) in the p53 knockout (p53-KO) cells, and a total of 211 DEMs and 722 DEGs in the point-modified (p53-241W242S) cells. Totally 28 annotated DEMs were found to overlap between p53-KO/p53-WT and p53-241W242S/p53-WT miRNAs datasets, of which miR-34 c, miR-218, miR-205, miR-105-1, miR-105-2, miR-206, miR-224 and miR-429 play important roles in p53 regulatory network. Among the top 10 DEGs in p53-KO and p53-241W242S cells, most genes were reported to be involved in tumors, cell proliferation or cell migration. p53-KO and p53-241W242S cells showed a significantly higher (P < 0.01) proliferation rate compared with p53-WT cells. In conclusion, genetic modifications of p53 gene significantly affect the expression levels of a large number of genes and miRNAs in the PFF cells. The p53-edited PFF cells could be used as non-tumor cell models for investigating the p53 signaling network, and as donor cells for somatic nuclear transfer, with the aim to develop porcine models with the corresponding p53 mutations.
Abbreviations: CRISPR-Cas9: Clustered regularly interspaced short palindromic repeats-associated protein 9; PFF: porcine fetal fibroblasts; SCNT: somatic cell nuclear transfer; RNA sequencing: small RNA sequencing and mRNA sequencing; DEGs: differentially expressed mRNAs; DEMs: differentially expressed miRNAs.
KEYWORDS: P53, mutation, CRISPR-Cas9, miRNA, mRNA, RNA sequencing
Introduction
Cancer is the second leading cause of death worldwide, and is estimated to account for 9.6 million deaths in 2018 [1]. In human cancers, p53 mutations were found in more than half of the cases [2]. As a transcription factor, p53 regulates a large number of genes involved in many cellular outcomes such as cell cycle arrest, apoptosis, cellular senescence and DNA repair [3]. Several thousands of p53 mutations were detected in whole-genome sequencing (WGS) and whole-exome sequencing (WES) projects; of those, a few mutations were considered as “hotspot” codons [4,5]. Among these “hotspot” mutations, R248 and R249 are more common in some cancers such as liver and lung carcinoma [5–8].
MicroRNAs (miRNAs) are evolutionarily conserved small noncoding RNAs that serve as important regulators of gene expression at the transcriptional or post-transcriptional level [9]. They play important roles in a variety of biological processes, such as cell proliferation, apoptosis, differentiation and metabolism [10]. Large amounts of experimental findings support that miRNAs interact with the p53 network at various levels [11,12]. Nonetheless, most of the previous studies were carried out in tumor cells or stem cells [11], and rare research works regarding normal somatic cells were reported.
Pigs are considered to be an ideal animal model for human medical research, and pig fetal fibroblasts (PFF) are commonly used as donor cells for somatic cell nuclear transfer (SCNT) in pigs [13]. In this study, we obtained p53 knockout (p53-KO) and modification of R241/R242 (corresponding to R248 and R249 in human) (p53-241W242S) PFF via CRISPR/Cas9 technology, and further analyzed the miRNA-mRNA regulatory networks in these p53 gene-edited cells.
Materials and methods
PFF cell
PFF cells were cultured from Large White fetuses on day 30 of gestation. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, cat. no. 10099141) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 IU/ml penicillin and 100 IU/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Generation of p53-edited cells using CRISPR/Cas9 technology
pSpCas9 (BB)-2A-Puro (PX459) V2.0 was a gift from Feng Zhang (plasmid #62,988, Addgene). Using the sgRNA design tool (http://crispr.mit.edu), 5ʹ-GCATGGGGGGCATGAACCGG-3ʹ (Figure 1) was selected as sgRNA. The sgRNA was ligated into the BbsI site of PX459V2.0 to generate PX459V2.0-p53 sgRNA plasmid, and plasmid DNA was extracted by EndoFree® Plasmid Maxi Kit (Qiagen). The 120 bp ssODN (single-stranded oligodeoxynucleotide) that brings in 241W and 242S mutations was synthesized by Integrated DNA Technology (IDT) Company (USA). These two mutations were located in the middle of the ssODN. To establish p53 gene-edited cell clones, 25 μg PX459V2.0-p53 sgRNA plasmid and 25 μg ssODN were delivered into 3 × 106 cells by BTX ECL 2000 electroporation system under the conditions of 200 V, 1 ms, 3 pulses, 1 repeat. After electroporation, the cell suspension was transferred into a 10 cm petri dish with DMEM culture medium and SCR7 (Xcessbio, USA) to a final concentration of 1 μM. After 30 h of incubation, the culture medium was renewed and puromycin (Sigma, Japan) was supplemented with a final concentration of 3.5 μg/mL. After 48 h of puromycin selection, the cells were seeded into 40 10-cm petri dishes with various cell densities. After 6 to 8 days of culture, single-cell clones on the 10-cm petri dishes were collected and cultured in 24-well plates. After reaching 85% confluence, the cell colonies were subcultured, and about 20% of each clone was used to extract DNA in 10 μL of lysis buffer (0.5% NP40 and 2 μg/μL of Proteinase K). The lysis mixture was incubated at 56°C for 60 min then 95°C for 10 min. The lysate was used as a template for PCR with primer pair (5ʹ-CGGCTTTCTCCTTCTCACTTG-3ʹ and 5ʹ-TGCCTGCTTACCTCGCTTAGT-3ʹ). The touchdown PCR conditions were 94°C for 5 min; 26 cycles of 94°C for 30 s, 68°C (−0.5°C/cycle) for 30 s, 72°C for 40 s; 14 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 40 s; 72°C for 10 min. The PCR products were sequenced on the 3130XL Genetic Analyzer (Applied Biosystem, USA). The experimental design is illustrated in Figure 1(a) as a flowchart.
Figure 1.

Experiment flowchart (a), sequencing results of the region surrounding 241/242 (b) and expression levels of p53 in gene-edited and wild-type PFF cells (c). WT: wild-type; HO: homozygote; KO: knock out, 1, 2, 3, 4, 5 indicate 5 single clones with homozygous deletions. – - – -, base deletion. * P < 0.05.
Detection of p53 expression in gene-edited cells
Total RNA of p53-deletion (p53-KO), p53-241W242S and p53-WT cells (five cell clones per group) with the same passage number (P8) were extracted by RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The isolated total RNA was reverse transcribed into cDNA using Prime ScriptTM RT reagent Kit with gDNA Eraser (Takara, Japan). The thermal cycles were listed as follows: 95°C for 5 min, 40 cycles of 95°C for 15 s and 60°C for 50 s, 95°C for 15 s, 60°C for 15 s and 95°C for 15 s. Porcine GAPDH was used as an internal control. Each reaction was performed in a technical quadruplicate. Primer pairs for qRT-PCR are shown in Table S2.
Detection of potential off-target sites
PCR primers were designed (Table S1) to flank the top 20 off-target sites (OTS) predicted by http://crispr.mit.edu/. The above-mentioned cell lysates were used as PCR templates, and PCR conditions were also the same. The PCR products were sequenced on the 3130XL Genetic Analyzer (Applied Biosystem, USA).
mRNA sequencing analysis
The extracted RNA used for detecting p53 expression was also used to construct cDNA libraries (no technical replicates for each sample) by the TruSeq RNA Sample Preparation Kit (Illumina). Single-end reads were generated on a BGISEQ-500 platform. Adapter contaminated reads, reads of more than 20% of bases with sanger quality smaller than 15, reads with more than 5% of “N”, and reads less than 30 bp were removed. The clean reads were then mapped to the pig reference genome (Sscrofa 11.1; http://asia.ensembl.org/Sus_scrofa/Info/Index) using the HISAT software [14]. Gene expression levels were calculated using the fragments per kilobase of transcript per million mapped fragments (FPKM) method with the RSEM software [15]. Differentially expressed genes (DEGs) were identified with the thresholds of false discovery rate (FDR) < 0.001 and Fold change >10 using the edgeR package [16].
miRNA sequencing analysis
Small RNA was extracted using miRNeasy Micro Kit (Qiagen, Germany) and RNA with a size of 18–30 nt was purified through polyacrylamide gel electrophoresis (PAGE). 5-adenylated and 3-blocked single-stranded DNA adapters were ligated to the small RNA molecules. The ligated RNA was then reverse transcribed into cDNA using Superscript II Reverse Transcriptase (Invitrogen) and further amplified for 15 PCR cycles using primers corresponding to the ends of the adapters. cDNA libraries were subsequently purified by PAGE and then sequenced on the BGISEQ-500 platform. The raw data were filtered by removing low-quality reads, reads without 3ʹ primer and insertions, reads with 5ʹ adaptor contaminants, reads with poly A stretches, and reads smaller than 18 nt. AASRA software (https://doi.org/10.1101/132928) was implemented to map the clean reads to the known porcine miRNA database in the miRBase (http://www.mirbase.org/) library. Regarding unannotated clean reads, the miRDeep2 was employed to predict potential novel miRNA comparing with the Sus scrofa reference genome (Sscrofa 11.1; http://asia.ensembl.org/Sus_scrofa/Info/Index). Differentially expressed miRNAs (DEMs) between the wild-type and gene-edited groups were screened using DEGseq software [17]. MiRNAs with Fold change >10 and Q value<0.001 were considered to be differentially expressed.
Identification of target genes of DEMs
The target genes of DEMs were predicted by three algorithms including TargetScan [18], miRanda [19] and RNAhybrid [20]. Genes that were simultaneously predicted by all three softwares were identified as the target genes of the DEMs. Venn diagram was implemented to intersect the identified DEGs and the target genes of DEMs.
KEGG enrichment analysis
KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of intersected target genes of DEMs and identified DEGs were implemented in ClueGO cytoscape plug-in bioinformatic tool [21]. Adjusted P values (Q values) of each pathway were obtained using Benjamini–Hochberg correction. Q value <0.05 and Kappa coefficient of 0.4 were set as thresholds.
Cell counting kit-8 (CCK-8) assay
CCK8 (Dojindo, Japan) kit was used to assess the proliferation rate of p53-KO, p53-241W242S and p53-WT cells with the same passage number (P8). Cells were seeded in 96-well plates at a density of 5000 cells/well in 100 μL of culture medium. After culturing 12, 24, 48 and 72 h, culture medium was replaced by 100 μL fresh medium containing 10% CCK-8 solution, and absorbance at 450 nm was read 3.5 h later using a fluorescence microplate reader (Tecan Infinite 200 PRO, Switzerland).
Quantitative RT‑PCR validation of mRNA and miRNA
The prepared cDNA used for detecting p53 expression was also used in qRT-PCR validation of DEGs. Small RNA was reverse transcribed into cDNA using Mir-X miRNA First-Strand Synthesis Kit (Takara, Japan). The expression levels of selected DEGs and DEMs were detected using SYBR Advantage qPCR Premix (Takara, Japan) on a 7900 HT Fast Real-Time PCR System (ABI, USA). The thermal cycles for DEGs detection were the same as that for detecting p53 expression. The thermal parameters for DEMs detection were 95°C for 10 s, and 40 cycles of 95°C for 10 s and 60°C for 20 s. Porcine GAPDH and RNU6-1 (U6) were used as internal reference genes for DEGs and DEMs detection, respectively. Each reaction was performed in a technical quadruplicate. The relative expression of DEGs and DEMs was calculated by the 2−ΔΔCt method. Primer pairs for qRT-PCR are listed in Table S2.
Results
Establishment of p53 gene-edited cells
In this study, a total of 146 single-cell clones were obtained after 48 h of puromycin selection (Table 1). Of these 146 clones, 26 clones had the 241W242S mutations (19 homozygotes and 7 heterozygotes), 46 clones contained homozygous deletions, 29 clones had heterozygous deletions, 11 clones had knock-in of a single point mutation, and 34 were wild-type. For the subsequent experiments, 5 homozygous p53-241W242S, 5 p53-KO (homozygous deletion) and 5 WT clones were used (Figure 1(b)).
Table 1.
Number of editing events in monoclonal cells.
| Type | Total | Homozygous modification of 241/242 condons | Heterozygous modification of 241/242 condons | Homozygous deletion | Heterozygous deletion | Point mutation knock-in | Wild type |
|---|---|---|---|---|---|---|---|
| Number of monoclonal cells | 146 | 19 | 7 | 46 | 29 | 11 | 34 |
| Proportion | 100% | 13.01% | 4.79% | 31.51% | 19.86% | 7.53% | 23.29% |
P53 expression level in gene-edited cells
qRT-PCR results showed that p53 expression was not detected in the selected 5 p53-KO clones (Figure 1(c)). P53 expression in the 5 randomly selected p53-241W242S cell clones was significantly higher (P < 0.05) than that in p53-WT cells (Figure 1(c)).
Analysis of the top 20 potential OTS
The top 20 potential OTS were examined in the 5 cell clones with homozygous deletions (p53-KO) and 5 homozygous p53-241W242S clones that used for detecting p53 expression. No OTS was found in all detected clones.
Identification of DEMs and DEGs
In this study, 5 clones each of p53-KO, p53-241W242S and p53-WT cells were used for DEMs and DEGs identification. An average of 109.87 Mb clean reads (out of 117.04 Mb raw reads) per library were obtained from the mRNA sequencing assay, and an average of 26,676,639 clean tags (out of 29,091,120 raw tags) were obtained from the miRNA sequencing data. By comparing with the p53-WT dataset, a total of 225 DEMs (151 down-regulated and 74 up-regulated) were detected in the p53-KO group (Figure 2(a)), and 211 DEMs (172 down-regulated and 39 up-regulated) were detected in the p53-241W242S group (Figure 2(b)). The top 30 DEMs in the p53-KO and the p53-241W242S groups were listed in Table 2, in which 21 DEMs including 5 novel miRNAs and 16 known miRNAs were common to the two lists (overlapping) (Table 2). A total of 28 known DEMs were found common to both the p53-KO and the p53-241W242S cells comparing with the p53-WT cells (Figure 3), of which 16 miRNAs were identified in the top 30 DEMs. In total, 722 DEGs (697 down-regulated and 25 up-regulated) were identified in the p53-KO group (Figure 2(c)), and 738 DEGs (709 down-regulated and 29 up-regulated) were detected in the p53-241W242S group (Figure 2(d)). Next, DEGs were intersected with the target genes of DEMs. A total of 246 DEGs were found to overlap with DEMs targets in the p53-KO group (Figure 4(a)), and 252 DEGs were shown to overlap with DEMs targets in the p53-241W242S group (Figure 4(b)). Totally 121 overlapping DEGs were identified in the two p53-edited groups (Figure 4(c)). The top 30 intersecting genes between DEGs from mRNA sequencing database and target genes of DEMs in the p53-KO and the p53-241W242S groups were listed in Table 3, in which 16 were common in the two lists.
Figure 2.

Volcano-plot distribution map of DEMs in the p53-KO group (a), and the p53-241W242S group (b); volcano-plot distribution maps of DEGs in the p53-KO group (c) and the p53-241W242S group (d). Red dots represent up-regulated mRNA or miRNA, and green dots represent down-regulated mRNA or miRNA.
Table 2.
Lists of the top 30 DEMs in p53-KO and p53-241W242S groups.
| p53-KO vs. p53-WT |
p53-241W242 S vs. p53-WT |
||||
|---|---|---|---|---|---|
| miRNA | LogRatio(KO/WT) | Q-Value | miRNA | LogRatio(241W242 S/WT) | Q-Value |
| novel_mir31 | −2.36 | 0.00E+00 | novel_mir31 | −4.79 | 0.00E+00 |
| novel_mir2 | −2.22 | 0.00E+00 | ssc-miR-429 | −3.12 | 0.00E+00 |
| ssc-miR-9-1 | −2.22 | 0.00E+00 | novel_mir379 | −2.94 | 0.00E+00 |
| ssc-miR-429 | −2.18 | 0.00E+00 | novel_mir2 | −2.76 | 0.00E+00 |
| novel_mir379 | −2.08 | 0.00E+00 | ssc-miR-124a | −2.74 | 0.00E+00 |
| ssc-miR-206 | −2.08 | 0.00E+00 | ssc-miR-206 | −2.39 | 0.00E+00 |
| ssc-miR-205 | −2.08 | 0.00E+00 | ssc-miR-205 | −1.83 | 0.00E+00 |
| ssc-miR-218-5p | −2.07 | 0.00E+00 | ssc-miR-9 | −1.69 | 0.00E+00 |
| ssc-miR-124a | −2.04 | 0.00E+00 | ssc-miR-9-1 | −1.58 | 0.00E+00 |
| ssc-miR-9 | −1.52 | 0.00E+00 | ssc-miR-218 | −1.58 | 0.00E+00 |
| ssc-miR-212 | −1.47 | 0.00E+00 | novel_mir50 | −1.58 | 0.00E+00 |
| ssc-miR-129a-5p | −1.41 | 0.00E+00 | ssc-miR-202-5p | −1.50 | 0.00E+00 |
| ssc-miR-202-5p | −1.35 | 0.00E+00 | ssc-miR-196b-5p | −1.44 | 0.00E+00 |
| ssc-miR-204 | −1.32 | 0.00E+00 | ssc-miR-204 | −1.42 | 0.00E+00 |
| ssc-miR-138 | −1.23 | 0.00E+00 | ssc-miR-196a | −1.40 | 0.00E+00 |
| ssc-miR-183 | −1.19 | 0.00E+00 | ssc-miR-224 | −1.28 | 0.00E+00 |
| ssc-miR-96-5p | −1.19 | 0.00E+00 | ssc-miR-218-5p | −1.05 | 0.00E+00 |
| ssc-miR-182 | −1.13 | 0.00E+00 | ssc-miR-1468 | −2.13 | 4.56E-285 |
| ssc-miR-218 | −1.08 | 0.00E+00 | ssc-miR-2483 | −1.63 | 1.3E-194 |
| ssc-miR-224 | −1.06 | 0.00E+00 | ssc-miR-490-5p | −2.21 | 5.33E-143 |
| ssc-miR-1468 | −1.43 | 2.43E-306 | ssc-miR-551a | −1.15 | 1.61E-137 |
| novel_mir50 | −4.35 | 1.59E-219 | ssc-miR-34 c | −1.28 | 2.07E-133 |
| ssc-miR-184 | −1.31 | 4.53E-178 | novel_mir133 | −4.01 | 3.04E-121 |
| ssc-miR-490-5p | −1.81 | 2.43E-158 | novel_mir573 | −3.92 | 2.68E-102 |
| ssc-miR-34 c | −2.28 | 2.54E-134 | ssc-miR-105-1 | −2.55 | 7.99E-92 |
| ssc-miR-30 c-3p | −1.04 | 1.11E-114 | ssc-miR-490-3p | −2.58 | 4.82E-83 |
| ssc-miR-216 | −1.18 | 3.99E-107 | ssc-miR-142-3p | −1.00 | 9.11E-78 |
| novel_mir573 | −3.92 | 3.45E-105 | novel_mir369 | −1.15 | 4.24E-72 |
| ssc-miR-490-3p | −1.82 | 3.2E-104 | novel_mir677 | −3.70 | 1.3E-69 |
| ssc-miR-105-1 | −2.64 | 7.13E-95 | novel_mir684 | −1.19 | 3.33E-64 |
Q-value: adjusted P-value.
Figure 3.

Overlapping DEMs (annotated) in the p53-KO and the p53-241W242S groups.
Figure 4.

Venn diagrams for the numbers of DEGs from mRNA-seq and DEMs targets in the p53-KO group (a), and in the p53-241W242S group (b). (c) Venn diagrams illustrating the overlapping of DEGs from the two p53-edited groups.
Table 3.
Lists of the top 30 intersecting genes between DEGs from mRNA sequencing and DEMs targets in p53-KO and p53-241W242S groups.
| p53-KO vs. p53-WT |
p53-241W242 S vs. p53-WT |
||||
|---|---|---|---|---|---|
| Gene | LogRatio(KO/WT) | Q-Value | Gene | LogRatio(241W242 S/WT) | Q-Value |
| PGM5 | −3.38 | 0.00E+00 | TNNC1 | −3.59 | 0.00E+00 |
| ACTC1 | −3.16 | 0.00E+00 | ACTC1 | −3.52 | 0.00E+00 |
| CHRNG | −3.02 | 0.00E+00 | CHRNG | −3.06 | 0.00E+00 |
| HSD3B1 | −2.99 | 0.00E+00 | MYLPF | −2.89 | 0.00E+00 |
| SHISA2 | −2.86 | 0.00E+00 | ACTN2 | −2.62 | 0.00E+00 |
| MYLPF | −2.74 | 0.00E+00 | ADAMTS8 | −2.52 | 0.00E+00 |
| ACTN2 | −2.51 | 0.00E+00 | COL11A2 | −2.43 | 0.00E+00 |
| COL11A2 | −2.48 | 0.00E+00 | ACAN | −2.42 | 0.00E+00 |
| IGF2 | −2.39 | 0.00E+00 | SRL | −2.37 | 0.00E+00 |
| RBM24 | −2.26 | 0.00E+00 | IGF2 | −2.31 | 0.00E+00 |
| ACAN | −2.21 | 0.00E+00 | MMP9 | −2.09 | 0.00E+00 |
| CBLN4 | −2.04 | 0.00E+00 | SLC7A3 | −2.03 | 0.00E+00 |
| SRL | −2.04 | 0.00E+00 | RELN | −2.01 | 0.00E+00 |
| SMYD1 | −2.02 | 0.00E+00 | RBM24 | −1.94 | 0.00E+00 |
| ADAMTS8 | −2.01 | 0.00E+00 | PCSK1 | −1.89 | 0.00E+00 |
| SYNPO2 L | −1.91 | 0.00E+00 | SOX10 | −1.85 | 0.00E+00 |
| PDPN | −1.71 | 0.00E+00 | SMYD1 | −1.80 | 0.00E+00 |
| RELN | −1.69 | 0.00E+00 | PEAR1 | −1.78 | 0.00E+00 |
| ALDH1A2 | −1.64 | 0.00E+00 | ACTA1 | −1.77 | 0.00E+00 |
| ST6GAL2 | −1.62 | 0.00E+00 | KRT8 | −1.71 | 0.00E+00 |
| C1QTNF3 | −1.55 | 0.00E+00 | SEMA6A | −1.68 | 1.03E-303 |
| KRT18 | −1.54 | 0.00E+00 | ANOS1 | −1.60 | 6.55E-290 |
| SEMA6A | −1.49 | 1.92E-303 | CLDN4 | −1.57 | 2.23E-286 |
| SLC7A3 | −1.49 | 2.35E-287 | SYNPO2 L | −1.56 | 9.56E-280 |
| NEFL | −1.45 | 5.98E-287 | PRSS23 | −1.54 | 1.15E-261 |
| GFRA1 | −1.41 | 4.71E-256 | ALDH1A2 | −1.44 | 3.01E-255 |
| TMEM88 | −1.39 | 3.79E-244 | AKR1C1 | −1.39 | 1.16E-254 |
| GAS6 | −1.37 | 6.37E-233 | DSG2 | −1.31 | 1.44E-253 |
| LGR5 | −1.26 | 8.54E-232 | EFS | −1.31 | 9.98E-250 |
| UNC45B | −1.20 | 9.84E-231 | SDK2 | −1.30 | 2.54E-249 |
Q-value: adjusted P-value.
KEGG pathway enrichment
KEEG pathway analyses were performed to identify biological functions of intersecting genes between DEGs from mRNA sequencing database and target genes of DEMs in the p53-KO and p53-241W242S groups. Top 20 KEGG pathways for these two pipelines were shown in Figure 5. Eleven of the enriched pathways were common to both the p53-KO and the p53-241W242S groups. These include Neuroactive ligand–receptor interaction, Hippo signaling pathway, Cell adhesion molecules (CAMs), TGF-β signaling pathway, etc. (Figure 5).
Figure 5.

Scatter plot of enriched KEGG pathways in the p53-KO groups (a), and in the p53-241W242S group (b). Rich factor is the ratio of the number of DEGs to the total gene number in that pathway. The size of the dots represents the number of DEGs mapped to the shown pathway and the color denotes – log10 (P value). Top 20 enriched KEGG pathways are shown.
Proliferation rates of P53 gene-edited cells
CCK-8 assay revealed that the proliferation rates of p53-KO and p53-241W242S cells were both significantly higher (P < 0.01) than that of p53-WT cells (Figure 6).
Figure 6.
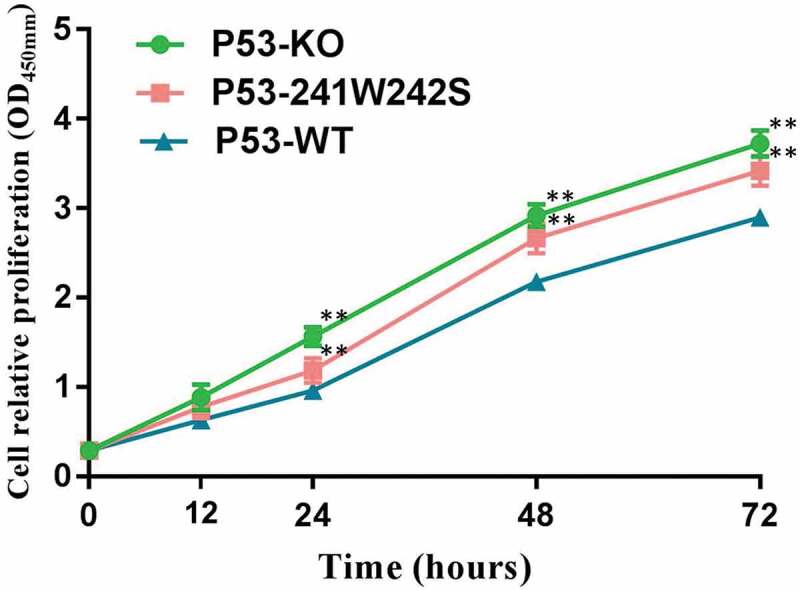
Proliferation profiles of p53-edited cells.
** Highly significantly different from p53-WT (P < 0.01).
Validation of the expression levels of selected DEMs and DEGs
To validate the RNA sequencing results, the expression levels of each nine randomly selected DEMs and DEGs were detected via qRT-PCR. Expression levels of selected miRNAs and genes detected by qRT-PCR were highly consistent with RNA sequencing data (Supplementary Figure 1).
Discussion
In this study, p53-KO and p53-241W242S PFF cells were obtained via ssODN-mediated CRISPR/Cas9 technologies. A total of 146 single-cell clones were isolated, 13.01% and 4.79% of homozygous and heterozygous HDR (homology directed repair) efficiencies were identified, and 31.51% of clones had homozygous deletions (Table 1). qRT-PCR results confirmed that none of the 5 clones with homozygous deletions expressed p53 mRNA (Figure 1(c)), thus they were designated as p53-KO clones. In addition, the top 20 potential OTS were detected and no OTS was found in any of the 10 p53-edited cell clones. Then, these cell clones with the same passage numbers were used for RNA sequencing.
It is well known that p53 gene has an extremely complex network that containing a large number of involving genes, miRNAs and lncRNAs [22,23]. In this study, a total of 225 and 211 DEMs were identified in the p53-KO and the p53-241W242S groups compared with p53-WT cells. Among the DEMs we identified in this study, 186 miRNAs in the p53-KO group and 174 in the p53-241W242S group were novel ones (data not shown). It should be noted that a high overlapping rate (21/30, Table 2) of the top 30 DEMs was found between these two pipelines, signifying the effect of the 241W242S double mutations in the p53 network. Five novel miRNAs were common in the top 10 DEMs in P53-KO and p53-241W242S lists (Table 2). It would be interesting to investigate the roles of these novel miRNAs in the P53 network. A total of 28 overlapping known DEMs were identified in the p53-KO and the p53-241W242S pipelines, of which 16 DEMs appeared in the top 30 lists (Figure 3, Table 2). Of these 16 DEMs, miR-34 c, miR-218, miR-205, miR-105-1, miR-105-2, miR-206, miR-224 and miR-429 have been reported as important effectors in the P53 network [11,24]; miR-9, miR-124a, miR-204, miR-218-5p, miR-490-5p, miR-1468 and, miR-490-3p have been shown to play important roles in cell proliferation and apoptosis in different human tumors such as breast cancer [25,26], lymphoblastic leukemia [27], cervical cancer [28], bladder cancer [29], and HCC [30,31]. Interestingly, the vast majority of the identified DEMs were down-regulated in this study, with 151 down-regulated and 74 up-regulated in the p53-KO group, and 172 down-regulated and 39 up-regulated in the p53-241W242S group. Furthermore, all the top 30 DEMs in the p53-KO or the p53-241W242S group are down-regulated ones (Table 2). Widespread down-regulation of miRNAs is commonly observed in human cancers [32–34], and predominant repression of miRNA expression was reported in cancer cells with mutant p53 [32,35]. Numerous mechanisms have been proposed for the regulation of gene expression by p53 [3,36], we propose that p53 depletion and oncogenic mutations alter it’s transcriptional activity or reduce the maturation of specific miRNAs, resulting in widespread down-regulation of miRNA in PFF cells.
Similar to DEMs, a high overlapping rate (16/30) was also found in the lists of top 30 DEGs of p53-KO and p53-241W242S groups (Table 3). In the top 10 DEGs, 6 genes including ACTC1, CHRNG, MYLPF, ACTN2, COL11A2 and IGF2 were common for these two pipelines (Table 3). A series of studies indicated that loss of imprinting and over-expression of IGF2 gene are associated with the tumorigenesis in different types of cancers [37–40]. ACTC1 was reported as an invasion and prognosis marker in glioma [41]. Both ACTC1 and ACTC2 were reported to be part of the hub genes in prostate cancer containing p53 mutation [42]. COL11A2, PCSK1 and MYLPF genes were also reported to be involved in different tumors [43–45]. In addition, in the top 10 DEGs of p53-KO list, PGM5 has been characterized as a diagnostic and prognostic biomarker for liver and colorectal cancer patients [46,47]. A genetic variation in HSD3B1 was suggested to be a promising predictive biomarker for prostate cancer patients [48,49]. RBM24, also shown in the top 30 DEGs in the p53-241W242S pipeline, was identified as a novel p53 target gene that regulates p21 mRNA stability [50], and was reported to suppress cancer progression by up-regulating miR-25 in nasopharyngeal carcinoma [51]. In the top 10 DEGs of p53-241W242S pipeline, TNNC1 was reported to be one of the hub genes in prostate cancer containing p53 mutation [42], and altered expression of this gene has been shown to be associated with adenocarcinoma in different types of tissues [52]. ADAMTS8, also found in the top 30 DEGs of p53-KO pipeline, displayed both oncogenic and tumor-protective functions in different types of tumors [53,54]. Similar to the identified DEMs, most identified DEGs were also down-regulated, and it would be interesting to further investigate the mechanisms dictating this phenomenon. Among KEGG pathways that were enriched in both pipelines, TGF-beta signaling pathway, Hippo signaling pathway, and cell adhesion molecules (CAMs) were well known to play important roles in cell proliferation, apoptosis, dormancy, autophagy and senescence [55–57]. Furthermore, CCK-8 assay revealed that the cell proliferation capabilities of p53-edited PFF cells were significantly higher (P < 0.01) than that of WT cells (Figure 6). p53-KO and p53-241W242S cells obtained in our study demonstrated significant gene expression modulations, among which some were seen in p53-mutated cell/tissues previously reported, while some hinted novel functions of p53.
In conclusion, we obtained p53-KO and p53-241W242S PFF cells via ssODN-mediated CRISPR/Cas9 technology, and global changes of mRNAs and miRNAs were noted in these p53 gene-edited cells. The obtained p53 gene-edited cells could be useful models for investigating p53 regulatory networks in somatic cells, and can be as donor cells in SCNT for generating pig models with modification of p53 gene.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (31771372).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings will be available in figshare at https://figshare.com/s/877f5513fb15df4584e6.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [2].Yue X, Zhao Y, Xu Y, et al. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017;429(11):1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hollstein M, Hainaut P.. Massively regulated genes: the example of TP53. J Pathol. 2010;220(2):164–173. [DOI] [PubMed] [Google Scholar]
- [4].Hainaut P, Pfeifer GP. Somatic TP53 mutations in the era of genome sequencing. Cold Spring Harb Perspect Med. 2016;6(11):a026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Freed-Pastor WA, PRIVES C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang H, Liao P, Zeng SX, et al. It takes a team: a gain-of-function story of p53-R249S. J Mol Cell Biol. 2019;11(4):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zekri AR, Bahnassy AA, Madbouly MS, et al. p53 mutation in HCV-genotype-4 associated hepatocellular carcinoma in Egyptian patients. J Egypt Natl Canc Inst. 2006;18(1):17–29. [PubMed] [Google Scholar]
- [8].Hagiwara N, Mechanic LE, Trivers GE, et al. Quantitative detection of p53 mutations in plasma DNA from tobacco smokers. Cancer Res. 2006;66(16):8309–8317. [DOI] [PubMed] [Google Scholar]
- [9].O’brien J, Hayder H, Zayed Y, et al. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang W. MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol Biol. 2017;1617:57–67. [DOI] [PubMed] [Google Scholar]
- [11].Liu J, Zhang C, Zhao Y, et al. MicroRNA control of p53. J Cell Biochem. 2017;118(1):7–14. [DOI] [PubMed] [Google Scholar]
- [12].Feng Z, Zhang C, Wu R, et al. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vajta G, Zhang Y, Machaty Z. Somatic cell nuclear transfer in pigs: recent achievements and future possibilities. Reprod Fertil Dev. 2007;19(2):403–423. [DOI] [PubMed] [Google Scholar]
- [14].Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Robinson MD, Mccarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang L, Feng Z, Wang X, et al. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26(1):136–138. [DOI] [PubMed] [Google Scholar]
- [18].Agarwal V, Bell GW, Nam JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:elife05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].John B, Enright AJ, Aravin A, et al. Human MicroRNA targets. PLoS Biol. 2004;2(11):e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:w451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo Q, Beaver JM, Liu Y, et al. Dynamics of p53: a master decider of cell fate. Genes (Basel). 2017;8(2):66. [Google Scholar]
- [23].Lin T, Hou PF, Meng S, et al. Emerging roles of p53 related lncRNAs in cancer progression: a systematic review. Int J Biol Sci. 2019;15(6):1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goeman F, Strano S, Blandino G. MicroRNAs as key effectors in the p53 network. Int Rev Cell Mol Biol. 2017;333:51–90. [DOI] [PubMed] [Google Scholar]
- [25].Selcuklu SD, Donoghue MT, Rehmet K, et al. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J Biol Chem. 2012;287(35):29516–29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shen SQ, Huang LS, Xiao XL, et al. miR-204 regulates the biological behavior of breast cancer MCF-7 cells by directly targeting FOXA1. Oncol Rep. 2017;38(1):368–376. [DOI] [PubMed] [Google Scholar]
- [27].Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res. 2009;69(10):4443–4453.. [DOI] [PubMed] [Google Scholar]
- [28].Xu Y, He Q, Lu Y, et al. MicroRNA-218-5p inhibits cell growth and metastasis in cervical cancer via LYN/NF-kappaB signaling pathway. Cancer Cell Int. 2018;18:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lan G, Yang L, Xie X, et al. MicroRNA-490-5p is a novel tumor suppressor targeting c-FOS in human bladder cancer. Arch Med Sci. 2015;11(3):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu Z, Wang Y, Dou C, et al. MicroRNA-1468 promotes tumor progression by activating PPAR-gamma-mediated AKT signaling in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Zhang LY, Liu M, Li X, et al. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3). J Biol Chem. 2013;288(6):4035–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gurtner A, Falcone E, Garibaldi F, et al. Dysregulation of microRNA biogenesis in cancer: the impact of mutant p53 on Drosha complex activity. J Exp Clin Cancer Res. 2016;35(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Suzuki HI, Yamagata K, Sugimoto K, et al. Modulation of microRNA processing by p53. Nature. 2009;460(7254):529–533. [DOI] [PubMed] [Google Scholar]
- [34].Garibaldi F, Falcone E, Trisciuoglio D, et al. Mutant p53 inhibits miRNA biogenesis by interfering with the microprocessor complex. Oncogene. 2016;35(29):3760–3770. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Y, Hu Y, Fang JY, et al. Gain-of-function miRNA signature by mutant p53 associates with poor cancer outcome. Oncotarget. 2016;7(10):11056–11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9(10):724–737. [DOI] [PubMed] [Google Scholar]
- [37].Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20(6):R321–R339. [DOI] [PubMed] [Google Scholar]
- [38].Schagdarsurengin U, Lammert A, Schunk N, et al. Impairment of IGF2 gene expression in prostate cancer is triggered by epigenetic dysregulation of IGF2-DMR0 and its interaction with KLF4. Cell Commun Signal. 2017;15(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brouwer-Visser J, Huang GS. IGF2 signaling and regulation in cancer. Cytokine Growth Factor Rev. 2015;26(3):371–377. [DOI] [PubMed] [Google Scholar]
- [40].Dong Y, Li J, Han F, et al. High IGF2 expression is associated with poor clinical outcome in human ovarian cancer. Oncol Rep. 2015;34(2):936–942. [DOI] [PubMed] [Google Scholar]
- [41].Ohtaki S, Wanibuchi M, Kataoka-Sasaki Y, et al. ACTC1 as an invasion and prognosis marker in glioma. J Neurosurg. 2017;126(2):467–475. [DOI] [PubMed] [Google Scholar]
- [42].Sun J, Zhang K, Cai Z, et al. Identification of critical pathways and hub genes in TP53 mutation prostate cancer by bioinformatics analysis. Biomark Med. 2019;13(10):831–840. [DOI] [PubMed] [Google Scholar]
- [43].Matsui Y, Chansky HA, Barahmand-Pour F, et al. COL11A2 collagen gene transcription is differentially regulated by EWS/ERG sarcoma fusion protein and wild-type ERG. J Biol Chem. 2003;278(13):11369–11375. [DOI] [PubMed] [Google Scholar]
- [44].Simon EP, Freije CA, Farber BA, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2015;112(44):E5916–E5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mazzoccoli G, Castellana S, Carella M, et al. A primary tumor gene expression signature identifies a crucial role played by tumor stroma myofibroblasts in lymph node involvement in oral squamous cell carcinoma. Oncotarget. 2017;8(62):104913–104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiao Y, Li Y, Jiang P, et al. PGM5: a novel diagnostic and prognostic biomarker for liver cancer. PeerJ. 2019;7:e7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sun Y, Long H, Sun L, et al. PGM5 is a promising biomarker and may predict the prognosis of colorectal cancer patients. Cancer Cell Int. 2019;19:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shiota M, Narita S, Akamatsu S, et al. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Network Open. 2019;2(2):e190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu G, Huang S, Nastiuk KL, et al. Variant allele of HSD3B1 increases progression to castration-resistant prostate cancer. Prostate. 2015;75(7):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jiang Y, Zhang M, Qian Y, et al. Rbm24, an RNA-binding protein and a target of p53, regulates p21 expression via mRNA stability. J Biol Chem. 2014;289(6):3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hua WF, Zhong Q, Xia TL, et al. RBM24 suppresses cancer progression by upregulating miR-25 to target MALAT1 in nasopharyngeal carcinoma. Cell Death Dis. 2016;7(9):e2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Johnston JR, Chase PB, Pinto JR. Troponin through the looking-glass: emerging roles beyond regulation of striated muscle contraction. Oncotarget. 2018;9(1):1461–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Choi GC, Li J, Wang Y, et al. The metalloprotease ADAMTS8 displays antitumor properties through antagonizing EGFR-MEK-ERK signaling and is silenced in carcinomas by CpG methylation. Mol Cancer Res. 2014;12(2):228–238. [DOI] [PubMed] [Google Scholar]
- [54].Cal S, Lopez-Otin C. ADAMTS proteases and cancer. Matrix Biol. 2015;44–46:77–85. [DOI] [PubMed] [Google Scholar]
- [55].Zhang Y, Alexander PB, Wang XF. TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9(4):a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Misra JR, Irvine KD. The hippo signaling network and its biological functions. Annu Rev Genet. 2018;52:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gibson NJ. Cell adhesion molecules in context: CAM function depends on the neighborhood. Cell Adh Migr. 2011;5(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings will be available in figshare at https://figshare.com/s/877f5513fb15df4584e6.


