Abstract
Understanding fructose metabolism might provide insights to renal pathophysiology. To support systemic glucose concentration, the proximal tubular cells reabsorb fructose as a substrate for gluconeogenesis. However, in instances when fructose intake is excessive, fructose metabolism is costly, resulting in energy depletion, uric acid generation, inflammation, and fibrosis in the kidney. A recent scientific advance is the discovery that fructose can be endogenously produced from glucose under pathologic conditions, not only in kidney diseases, but also in diabetes, in cardiac hypertrophy, and with dehydration. Why humans have such a deleterious mechanism to produce fructose is unknown, but it may relate to an evolutionary benefit in the past. In this article, we aim to illuminate the roles of fructose as it relates to gluconeogenesis and fructoneogenesis in the kidney.
Keywords: fructose, fructolysis, fructoneogenesis, gluconeogenesis, glycolysis
Fructose is a simple sugar in fruits that has a role in storage of fat and glycogen, development of insulin resistance, and an increase in sodium reabsorption, all of which are likely survival processes for animals during time of food shortage.1 The freshwater pacu fish actively feasts on ripe fruits that have fallen into the river and becomes fat in the rainy season.2 To increase their fat stores before beginning their migration, birds that migrate long distances develop a seasonal dietary preference for fruits.3 Hibernating mammals, such as bears and ground squirrels, use their accumulated fat as an energy supply during winter.4,5 Likewise, for early primates, fresh fruits were the main dietary staple.6,7 Fructose remains an important nutrient for humans and wild animals because the kidney, the placenta, and hypoxic tissues use it as a substrate for gluconeogenesis to maintain physiologic concentrations of serum glucose,8 to support fetal organ development,9 and to offer protection from hypoxic insults.10
In modern society, dietary sugars, particularly fructose, have emerged as culprits for the current epidemic of obesity, diabetes, and metabolic syndrome.11 The rise in fructose consumption over the last century has paralleled the rising prevalence of obesity, diabetes, and kidney diseases, leading to the hypothesis that excessive fructose might be a causal factor for the development of kidney disease. One proposed mechanism is via the metabolism of fructose (fructolysis) because it can result in intracellular energy depletion, mitochondrial oxidative stress, and the production of inflammatory mediators such as uric acid.12 Recently, it has emerged that fructose can be endogenously produced in humans13,14 and nonhuman animals,15–21 particularly in diseased kidneys, and that blocking the metabolism of endogenous fructose can slow the development and progression of kidney injury.
Fructose biology is complex because fructose metabolism may be associated with beneficial physiologic responses and pathologic responses. Here, we discuss the various roles of fructose in the kidney.
Clinical Associations of Fructose with Kidney Diseases
Fructose, as a component of high-fructose corn syrup or table sugar, is a major component in most sugar-sweetened soft drinks. The dramatic increase in fructose consumption has stimulated a heated debate over the potential danger of sugar-sweetened beverages.22,23
In 2009, Bomback et al.24 combined cross-sectional and longitudinal analyses to evaluate the association between CKD and sugar-sweetened beverages, but found no association between sugar-sweetened soda and incidence or prevalence of CKD. Although the outcomes of this study should be noted, renal effects are unlikely to be observed at a dosage of only one sugar-sweetened beverage per day.25 Similarly, Lin and Curhan26 identified 3318 women participating in the Nurses’ Health Study with data on soda intake and albuminuria and found no association between daily consumption of one or more serving and incidence of CKD; but, given that only 3% of participants had this level of consumption, this also might account for the negative results.
In contrast, Saldana et al.27 examined the effect of higher doses in a case-control study, and found that an intake of two or more sugar-sweetened beverages per day was associated with an increased incidence of kidney disease. Likewise, Shoham et al.28 found that consumption of two or more sugar-sweetened beverages per day was associated with a higher level of urinary albumin excretion compared with consumption of less than two sugar-sweetened beverages per day. More recently, a 2019 study of a community-based cohort of black Americans also showed that a higher sugar-sweetened beverage consumption was associated with significantly greater odds of incident CKD.29 Moreover, in an intervention study in which a low-fructose diet was administered to participants with CKD, researchers observed a reduction in BP and systemic inflammation.30
Although the possible association of sugar-sweetened beverages with kidney disease may reflect the sugar (and especially fructose) content, sugar-sweetened beverages also contain substantial amount of phosphorus, which could be an alternative risk factor for obesity, vascular injury, systemic hypertension, and kidney disease.31
Fructose Metabolism in Normal Kidney
Fructose Is Physiologically Converted to Glucose in the Kidney
In the mid- to late 20th century, several investigators intensively studied glucose metabolism in the kidney. The first paper documenting renal gluconeogenesis, to our knowledge, was a study in 1937 by Benoy and Elliott32 showing that rat kidney slices were capable of producing glucose in response to pyruvate and lactate. A study using hepatectomized dogs subsequently confirmed this phenomenon by demonstrating that blood sugar declined more rapidly with removal of the kidneys.33 Reinecke and Hauser34 also showed in dogs that serum glucose concentration in the renal vein was higher than in the renal artery. Three decades later, research demonstrated that the rat kidney is capable of producing approximately 26% of serum glucose in the normal fed state, and that renal glucose release increases 46% after starvation35; the human kidney similarly provides approximately 45% of total blood glucose after starvation.8 Interestingly, diabetes also increases renal glucose release by 360% in rats and 300% in humans compared with the nondiabetic condition.35,36 In metabolic acidosis in the rat, gluconeogenesis has also been found to be activated in the proximal tubules, as opposed to the liver.37,38
Renal gluconeogenesis may result from classic substrates such as lactate, glutamine, alanine, and pyruvate, but fructose appears to be the preferred substrate, based on the speed and efficiency of the reaction.39,40 In 1961, by using in situ perfusion in the rat, Salomon et al.41 directly measured the arteriovenous difference of fructose and glucose concentrations after bolus infusion of 25 mg of fructose into the peripheral vessels. They found that decreases in fructose concentration on the passage of blood through the kidney—about 19% on average—were accompanied by equivalent increases in renal venous glucose.41 In 1982, Björkman et al.42 found that intravenous infusion of fructose at 2 mmol/min for 135 minutes into the peripheral vein of humans resulted in a constant rise in glucose concentration in the renal vein (0.17±0.05 mmol/L); they also indicated that 20% of intravenously infused fructose was taken up by the kidney and that the net glucose release from the kidney could be derived from 55% of the net renal uptake of fructose. Interestingly, fructose is metabolized into lactate faster than is glucose. However, this reaction depends on the oxygen level, because it is slower under anaerobic conditions compared with aerobic conditions.43 Gluconeogenesis is driven by the activation of phosphoenolpyruvate carboxykinase, enzymes of the glucose 6-phosphatase system, and fructose bisphosphatase exclusively in the proximal tubular cells,44,45 but not in the distal nephron46,47 (Figure 2).
Figure 2.
Both fructolysis and fructogenesis occuring in the kidney are modulated by uric acid. AR, aldose reductase; FA, fatty acid; FK, fructokinase; Fru, fructose; Fru6P, fructose 6-phosphate; Fru1,6P2, fructose 1,6 bisphosphate; DHAP, dihydroxyacetate phosphate; Glc, glucose; Glc6P, glucose 6-phosphate; G3P; glyeraldehyde 3-phosphate; OXPHOS, mitochondrial oxidative phosphorylation; PEP, phosphoenolpyruvate; PPP, pentose phosphate pathway; SDH, sorbitol dehydrogenase; TCA, tricarboxylic acid cycle.
The proximal straight tubule is a primary site for fructose metabolism. There, glucose transporter 5, expressed on the apical side of the cell membrane, mediates absorption of urinary fructose, which is then metabolized by fructokinase in the cytosol.48 However, fructose metabolism is not restricted to the proximal straight tubule; it has also been documented in the proximal convoluted tubule (Figure 1). In fact, the proximal convoluted tubule expresses fructokinase, as well as aldolase B, which is readily induced.48 A lack of aldolase B in patients with hereditary fructose intolerance causes fructose 1-phosphate accumulation in the proximal convoluted tubule.49
Figure 1.
The S1 and S2 of the proximal tubular cells cooporately metabolize fructose. Both proximal convoluted tubules (PCT) and proximal straight tubules (PST) participate in fructose (Fru) metabolism. AldoB, aldolase B; DHAP, dihydroxyacetate phosphate; FBPase, fructose 1,6-bisphosphatase; FK, fructokinase; Fru1P, fructose 1-phosphate; Fru1,6P2, fructose 1,6 bisphosphate; Glc, glucose; Glc6P, glucose 6-phosphate; G3P; glyeraldehyde 3-phosphate; G6Pase, glucose 6-phosphatase; Glut2/5, glucose transporter 2/5.
Glycolysis Is Less Activated in the Proximal Tubules
In the late 20th century, several researchers demonstrated that the potential for glycolysis is very low in the proximal tubules compared with other parts of the nephron, including from the medullary ascending limb to the medullary collecting tubule. For example, key enzymes for glycolysis—hexokinase, phosphofructokinase, and pyruvate kinase—are minimally expressed in proximal tubular epithelial cells.38,50 Hexokinase, the gateway enzyme of glucose metabolism, phosphorylates glucose to glucose 6-phosphate and is least active in the proximal tubules relative to the entire nephron. In turn, the distal nephron shows higher activity of glycolytic enzymes. Hexokinase activity is 15 times higher in the thick ascending limb of the loop of Henle than in the proximal tubules.51 Likewise, pyruvate kinase, an enzyme catalyzing another irreversible step in the glycolytic pathway, is highly activated in the collecting ducts but not in the proximal tubules.38
Glycolysis contributes to only 4% of the renal ATP produced under aerobic conditions in the proximal tubular cells.38 The glucose reabsorption rate in the proximal tubules is 35 pmol/min per mm, whereas hexokinase activity is approximately 2 pmol/min per mm, suggesting that only 5% of glucose entering the cell is phosphorylated in the proximal convoluted tubules.52 The large portion of glucose is not physiologically used as an energy source,53 but rather is released into the peritubular capillary via glucose transporter 2 in the basolateral membrane of the proximal cells.54 However, Chamberlin et al.55 proposed that glycolysis met the energy needs of distal portions of nephron.
Endogenously Produced Fructose Is a Risk Factor for Renal Injury
A recent scientific advance has been the discovery that the kidney and several other organs are capable of endogenously producing fructose. The key mechanism is the activation of the polyol pathway, in which aldose reductase reduces glucose to sorbitol, which is then oxidized by sorbitol dehydrogenase to fructose (Figure 2). Importantly, studies with mice lacking the fructokinase gene showed that blocking the metabolism (fructolysis) of endogenous fructose in the kidney appears to be beneficial in slowing the development of renal disease (Table 1).
Table 1.
Type of diseases which are ameliorated by fructokinase deficiency
| Type of Disease | Reference |
|---|---|
| Diabetic nephropathy in streptozotocin-induced diabetic mice | 16 |
| AKI in mice with renal ischemia reperfusion | 19 |
| Aging kidney in aged mice | 18 |
| Dehydration-associated kidney injury in mice with heat | 63 |
| Cardiac hypertrophy in mice with 1K1C | 20 |
| Metabolic syndrome in mice with fructose-water | 90 |
| Metabolic syndrome in mice with high salt intake | 15 |
| Intestinal cancer in APC-deficient mice consuming fructose diet | 92 |
1K1C, one-kidney, one-clip; APC, adenomatous polyposis coli.
Diabetic Nephropathy
In diabetes, the polyol pathway may play a key role in the development of diabetic complications.56 Although renal aldose reductase expression is enhanced in diabetes,16,57,58 the functional role of aldose reductase was implicated by the finding that a transgenic mouse line carrying human aldose reductase cDNA developed thrombosis in renal vessels with some collagen deposits in Bowman’s capsule—developments that partially resembled the histologic changes of human diabetic nephropathy.59 We recently found that streptozotocin-induced diabetic mice had higher levels of fructose and urate in the kidney compared with nondiabetic mice, and that diabetic mice lacking fructokinase were protected and exhibited less albuminuria, better renal function, and less tubular injury.16
People with diabetes have higher levels of fructose in the serum and urine,60 which is suggestive of endogenous fructose production. However, the pathologic role of the polyol pathway remains elusive. One practical way to address this issue would be to use aldose reductase inhibitors in patients with diabetes. The Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group61 reported finding that inhibition of aldose reductase with epalrestat could slow the progression of diabetic nephropathy, whereas other studies did not.62 We recommend further trials to determine if aldose reductase inhibitors might provide benefit in diabetic nephropathy.
Other Kidney Diseases
There is evidence that, in addition to diabetes, fructoneogenesis contributes to the development of AKI in mice and humans.19 Investigators reported that seven of 12 pediatric patients developed AKI after undergoing cardiac bypass surgery, and that those with AKI had significantly higher concentrations of fructose in the urine. Given that these patients were in the early postoperative period, fructose in the urine unlikely originated from the hospital diet, but was likely endogenously produced, perhaps in the kidney. The researchers investigated this possibility in mice subjected to ischemia-reperfusion injury of the kidney, finding that wild-type mice with AKI developed severe tubular injury associated with activation of the polyol pathway, as evidenced by high levels of aldose reductase, sorbitol, and endogenous fructose. In contrast, knockout mice lacking fructokinase showed partial protection, with less tubular injury and improved renal function. They also demonstrated a deleterious role for the metabolism of endogenous fructose in mice with radiocontrast-induced nephropathy.19
Recurrent heat stress and dehydration can also cause CKD in mice,63 and this may be relevant to epidemics of CKD that are occurring in Central America, Sri Lanka, and India.64 Chronic, recurrent dehydration also results in aldose reductase activation in both the kidney and hypothalamus, leading to fructoneogenesis at both sites.21,63 Importantly, mice lacking fructokinase were largely protected from CKD induced by repeated heat stress and dehydration.63
Finally, senescence (aging) changes in the kidney may also be mediated by fructokinase activation. Aging wild-type mice are known to exhibit renal dysfunction characterized by an increase in urinary albumin excretion, glomerular collagen IV deposition, and tubulointerstitial injury. However, researchers demonstrated these injuries were ameliorated in frucokinase knockout mice.18
Nonkidney Diseases as a Target of Endogenous Fructose: Cardiac Hypertrophy
In addition to the kidney, other organs likely produce fructose. Recently, Mirtschink et al.20 showed that the heart, particularly the cardiac myocyte, is capable of endogenous fructose production, and that the fructose generated was involved in pathologic cardiac hypertrophy. Specifically, they identified fructokinase as a hypoxia-inducible factor 1α–mediated factor that was activated in mouse models of cardiac hypertrophy induced by hypertension.20 Importantly, fructokinase knockout mice were protected from the cardiac remodeling in these models. The authors also reported finding upregulation of fructokinase in cardiomyocytes obtained from biopsies of patients with hypertrophic cardiomyopathy.
Pathophysiology of Fructose
Potential Role for Uric Acid
Fructokinase (also known as ketohexokinase), which catalyzes the first step of fructose metabolism, phosphorylates fructose to produce fructose 1-phosphate. During this reaction, fructokinase activation requires a phosphate, which reduces intracellular phosphate levels and depletes ATP.12 The rapid reduction of phosphate activates AMP deaminase, which in turn drives adenine nucleotide turnover and stimulates urate production.11,65,66 As such, an excess amount of fructose either from diet or from endogenous production could result in a high amount of intracellular uric acid.11
Findings from studies in cultured cells or in animal models demonstrate multiple potential pathologic roles of uric acid. These include causing endothelial dysfunction, vascular injury, and inflammation that lead to glomerular hypertension, tubulointerstitial injury, and an elevation in systemic BP.11,66–70 Biologic mechanisms include a reduction in endothelial nitric oxide, oxidative stress, and renin-angiotensin activation, all of which may predispose to hypertension.71 Uric acid also modulates metabolic status in response to fructose and increases the risk for animals to develop insulin resistance and metabolic syndrome.66 It can also feed back to amplify both aldose reductase and fructokinase expression.72 Although debate on the pathologic role of uric acid continues, accumulating evidence suggests that it likely has a role in hypertension, insulin resistance, and CKD.73,74
Kidney Disease
One effect of uric acid is to stimulate mitochondrial oxidative stress that causes a decrease in activity of aconitase, an enzyme of the tricarboxylic acid cycle that converts citrate to isocitrate and disconnects fructose metabolism from mitochondrial respiration.65 As a result, triose phosphates, which are metabolites of fructose 1-phosphate catalyzed by aldolase B, enter the glycolytic pathway distal to phosphofructokinase and drive aerobic glycolysis, with the pathologic activation of gluconeogenesis and lipogenesis. This results in the production of glucose, glycogen, lactate, and triglycerides that creates the “Warburg effect” (Figure 2). In 1924, Otto Heinrich Warburg initially described that cancer cells, as opposed to normal cells, exhibit a unique ability to ferment glucose into lactate, even in the presence of sufficient oxygen.75,76
Recently, several studies demonstrated the Warburg effect in the diseased kidney, in which aerobic glycolysis would provide a mechanism for renal fibrosis in several types of kidney diseases (Figure 3).77 In fact, either pharmaceutic blocking of glycolysis or switching aerobic phosphorylation back to oxidative phosphorylation inhibits renal fibrosis from developing in the obstructed kidney.78 Importantly, either fructose or glucose could be an energy source for the Warburg effect and the fructose–uric acid pathway could be a mechanism to drive aerobic glycolysis in the development of renal fibrosis.79,80
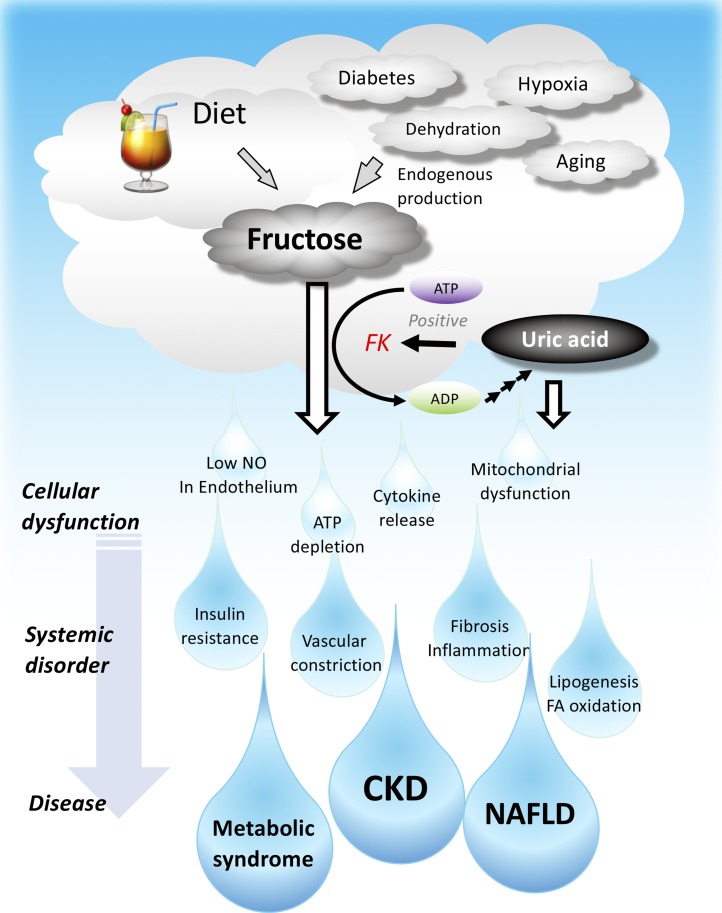
Figure 3.
Both dietary and endogenously produced fructose cause CKD, nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome. FK, fructokinase; NO, nitric oxide.
AKI can be associated with the release of inflammatory cytokines mediated by activation of the transcription factor NF-κB, and one study suggested that endogenously produced fructose and its metabolism via fructokinase can result in such an NF-κB–mediated inflammatory response.19 There is evidence in diabetic mice that tubular injury is also associated with inflammation involving inflammatory cytokines regulated by NF-κB.16 As in the AKI model, studies in a model of diabetic nephropathy found that the expression of inflammatory cytokines (IL-1β, IL-6, and monocyte chemoattractant protein-1) and NF-κB activation were also dependent on endogenous fructose and fructose metabolism in a model of diabetic nephropathy.16,81,82 Fructose also stimulates endothelial cells to induce intercellular adhesion molecule-1.67 The induction of oxidative stress by fructose can also “uncouple” endothelial nitric oxide synthase, resulting in a reduction in nitric oxide availability.83–85 In addition, several transcription factors have been shown to be involved in fructose metabolism, including Sterol regulatory element-binding protein 1 (SREBP1c), Carbohydrate-responsive element-binding protein (ChREBP), and peroxisome proliferators-activated receptor-γ (PPAR-γ) coactivator-1α (PCG-1a), and these factors are currently considered to be involved in fructose-mediated lipogenesis.86 Further studies are needed to clarify their roles in inflammation.
Metabolic Syndrome and Insulin Resistance
By suppressing mitochondrial oxidative phosphorylation, fructose stimulates glycolysis, gluconeogenesis, and lipogenesis. Fructokinase, which is the gatekeeper because it catalyzes the first step of fructose metabolism, is activated in a fructose dose–dependent manner without any negative regulation. Consequently, the production of several metabolites—including glucose, lipid, and glycogen—are enhanced, thereby contributing to the development of obesity, diabetes, and metabolic syndrome. Interestingly, blocking uric acid production partially ameliorated the development of hypertension, hypertriglyceridemia, and insulin resistance in fructose-fed rats,11,66 suggesting that uric acid plays a role in fructose-induced metabolic syndrome (Figure 3).
Insulin resistance is a phenotype exhibiting a decreased uptake of glucose into insulin-dependent tissues, such as skeletal muscle. Common mechanisms include an impairment in insulin signaling associated with lipid alterations, but some insulin resistance results from impaired delivery of glucose that results when endothelian dysfunction causes impaired peripheral perfusion.87,88 Fructose-induced endothelial dysfunction due to endothelial nitric oxide synthase uncoupling is likely involved in insulin resistance in addition to dysregulation of lipid synthesis (Figure 3).67
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease is a hepatic phenotype of metabolic syndrome, and experimental and clinical studies suggest that fructose is one of its strongest risk factors.89 Fructokinase has two isoforms: fructokinase-C and fructokinase-A. Interestingly, mice lacking both isoforms are protected from fructose-induced fatty liver, whereas in mice with genetic deletion of only fructokinase-A fatty liver is more severe than in wild-type mice.90 This finding is likely because fructokinase-C causes rapid ATP degradation, whereas fructokinase-A metabolizes fructose very slowly, with relatively minimal ATP consumption. Hence, blocking fructokinase-A actually increases the amount of fructose available for metabolism by fructokinase-C.
The mechanism by which fructose stimulates de novo lipogenesis and blocks hepatic fatty acid oxidation is shown in Figure 3. With fructose-induced hepatic lipogenesis, uric acid blocks aconitase; this causes citrate to accumulate and move into the cytoplasm to stimulate ATP citrate lyase, activating lipogenesis. There are several studies showing that xanthine oxidase inhibitors could partially reduce fatty liver in both fructose-dependent and fructose-independent models of metabolic syndrome and nonalcoholic fatty liver disease.65,91
Summary
Fructose at physiologic concentrations is predominantly metabolized to produce glucose in the proximal tubular epithelial cells of the kidney. However, sustained exposure to excessive quantities of fructose likely induces fructolysis, resulting in significant ATP depletion and inflammation, leading to tubular injury. Endogenously produced fructose through renal fructoneogenesis, combined with subsequent fructolysis, might be a mechanism for progression of kidney disease. Importantly, uric acid is often a mediator for fructose’s adverse effects. Effects of fructose on other organ systems may also be involved in the development of kidney injury.
Disclosures
Dr. Johnson reports personal fees from Eli Lilly and personal fees from Horizon Pharma, outside the submitted work. In addition, Dr. Johnson has a US Patent number 9,387,245 issued, a US Patent number 8,697,628 issued, a patent number PCT/US2011/046938 pending, and a patent number PCT/US2011/046938 pending, as well as inventorship status on US Patent number 8,557,831 for uric acid and insulin resistance and US Patent number 9,155,740 for uric acid and diabetic nephropathy, and patent applications related to fructose metabolism and metabolic disease. Dr. Johnson and Dr. Lanaspa have patents and patent applications related to their discoveries that they assigned to the University of Colorado. Dr. Johnson, Dr. Lanaspa, and Dr. Tolan are members of and report equity with Colorado Research Partners LLC that is developing inhibitors of fructose metabolism for the treatment of metabolic syndrome and kidney disease. Dr. Johnson and Dr. Nakagawa have several patent applications related to uric acid discovered in the University of Floria and have equity in XORTX Therapeutics that is developing novel xanthine oxidase inhibitors. Dr. Nakagawa reports grants from Fuji Yakuhin Co, grants from Suzuken, and grants from Teijin Pharma, outside the submitted work. In addition, Dr. Nakagawa has two patents; US8557831 and US9155740B2, both were issued on October 13, 2015. Our docket number 11160-006UC2 is pending. Dr. Sanchez-Lozada reports grants from DANONE RESEARCH outside the submitted work. Dr. Tolan reports personal fees from Alnylam Pharmaceuticals, grants and personal fees from Colorado research Partners, outside the submitted work, as well as several contracts and grants related to fructose metabolism and metabolic disease. Dr. Andres-Hernando and Dr. Roncal-Jimenez have nothing to disclose.
Funding
This work was supported in part by National Institutes of Health grants R01DK108408 and R01DK108859.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Johnson RJ, Stenvinkel P, Andrews P, Sanchez-Lozada LG, Nakagawa T, Gaucher E, et al.: Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med 287: 252–262, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Junk WJ: Temporary fat storage, an adaptation of some fish species to the water level fluctuations and related environmental changes of the Amazon river. Amazoniana 9: 315–351, 1985 [Google Scholar]
- 3.Bairlein F: How to get fat: Nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 89: 1–10, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P, Jani AH, Johnson RJ: Hibernating bears (Ursidae): Metabolic magicians of definite interest for the nephrologist. Kidney Int 83: 207–212, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Carey HV, Andrews MT, Martin SL: Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Johnson RJ, Andrews P, Benner SA, Oliver W: Theodore E. Woodward award. The evolution of obesity: Insights from the mid-Miocene [published correction appears in Trans Am Clin Climatol Assoc 124: 294, 2013]. Trans Am Clin Climatol Assoc 121: 295–305; discussion 305–308, 2010 [PMC free article] [PubMed] [Google Scholar]
- 7.Finch CE, Stanford CB: Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol 79: 3–50, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF Jr.: Liver and kidney metabolism during prolonged starvation. J Clin Invest 48: 574–583, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Song G, Wu G, Bazer FW: Functional roles of fructose. Proc Natl Acad Sci U S A 109: E1619–E1628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, et al.: Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356: 307–311, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T, Tuttle KR, Short RA, Johnson RJ: Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 1: 80–86, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Mäenpää PH, Raivio KO, Kekomäki MP: Liver adenine nucleotides: Fructose-induced depletion and its effect on protein synthesis. Science 161: 1253–1254, 1968 [DOI] [PubMed] [Google Scholar]
- 13.Francey C, Cros J, Rosset R, Crézé C, Rey V, Stefanoni N, et al.: The extra-splanchnic fructose escape after ingestion of a fructose-glucose drink: An exploratory study in healthy humans using a dual fructose isotope method. Clin Nutr ESPEN 29: 125–132, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Hwang JJ, Jiang L, Hamza M, Dai F, Belfort-DeAguiar R, Cline G, et al.: The human brain produces fructose from glucose. JCI Insight 2: e90508, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, et al.: High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A 115: 3138–3143, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, et al.: Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25: 2526–2538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, et al.: Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome [published correction appears in Nat Commun 4: 2929, 2013]. Nat Commun 4: 2434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roncal-Jimenez CA, Ishimoto T, Lanaspa MA, Milagres T, Hernando AA, Jensen T, et al.: Aging-associated renal disease in mice is fructokinase dependent. Am J Physiol Renal Physiol 311: F722–F730, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, et al.: Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun 8: 14181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirtschink P, Krishnan J, Grimm F, Sarre A, Hörl M, Kayikci M, et al.: HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 522: 444–449, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song 宋志林 Z, Roncal-Jimenez CA, Lanaspa-Garcia MA, Oppelt SA, Kuwabara M, Jensen T, et al.: Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol 117: 646–654, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al.: Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Neilson EG: The fructose nation. J Am Soc Nephrol 18: 2619–2621, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Bomback AS, Katz R, He K, Shoham DA, Burke GL, Klemmer PJ: Sugar-sweetened beverage consumption and the progression of chronic kidney disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90: 1172–1178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundborn G, Thornley S, Merriman TR, Lang B, King C, Lanaspa MA, et al.: Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity (Silver Spring) 27: 879–887, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Curhan GC: Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol 6: 160–166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saldana TM, Basso O, Darden R, Sandler DP: Carbonated beverages and chronic kidney disease. Epidemiology 18: 501–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, et al.: Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999-2004. PLoS One 3: e3431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebholz CM, Young BA, Katz R, Tucker KL, Carithers TC, Norwood AF, et al.: Patterns of beverages consumed and risk of incident kidney disease. Clin J Am Soc Nephrol 14: 49–56, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brymora A, Flisiński M, Johnson RJ, Goszka G, Stefańska A, Manitius J: Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol Dial Transplant 27: 608–612, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JJ: Potential health concerns of dietary phosphorus: Cancer, obesity, and hypertension. Ann N Y Acad Sci 1301: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Benoy MP, Elliott KA: The metabolism of lactic and pyruvic acids in normal and tumour tissues: Synthesis of carbohydrate. Biochem J 31: 1268–1275, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohn C, Kolinsky M: Effect of blood sugar levels and insulin lack on gluconeogenesis by the kidney of the dog. Am J Physiol 156: 345–348, 1949 [DOI] [PubMed] [Google Scholar]
- 34.Reinecke RM, Hauser PJ: Arterio venous blood sugar studies on the kidney of the eviscerated dog. Fed Proc 7: 99, 1948 [PubMed] [Google Scholar]
- 35.Kida K, Nakajo S, Kamiya F, Toyama Y, Nishio T, Nakagawa H: Renal net glucose release in vivo and its contribution to blood glucose in rats. J Clin Invest 62: 721–726, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J: Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest 102: 619–624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iles RA, Cohen RD, Rist AH, Baron PG: The mechanism of inhibition by acidosis of gluconeogenesis from lactate in rat liver. Biochem J 164: 185–191, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt U, Guder WG: Sites of enzyme activity along the nephron. Kidney Int 9: 233–242, 1976 [DOI] [PubMed] [Google Scholar]
- 39.Krebs HA, Lund P: Formation of glucose from hexoses, pentoses, polyols and related substances in kidney cortex. Biochem J 98: 210–214, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowman RH: Gluconeogenesis in the isolated perfused rat kidney. J Biol Chem 245: 1604–1612, 1970 [PubMed] [Google Scholar]
- 41.Salomon LL, Lanza FL, Smith DE: Renal conversion of fructose to glucose. Am J Physiol 200: 871–877, 1961 [DOI] [PubMed] [Google Scholar]
- 42.Björkman O, Felig P: Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes 31: 516–520, 1982 [DOI] [PubMed] [Google Scholar]
- 43.Hems DA, Gaja G: Carbohydrate metabolism in the isolated perfused rat kidney. Biochem J 128: 421–426, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coffee EM, Tolan DR: Gluconeogenesis. In: Inborn Errors in Metabolism: From Neonatal Screening to Metabolic Pathways, edited by Lee B, Scaglia F, New York, Oxford University Press, 2014, pp 68–91 [Google Scholar]
- 45.Alleyne GA, Scullard GH: Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest 48: 364–370, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guder WG, Schmidt U: The localization of gluconeogenesis in rat nephron. Determination of phosphoenolpyruvate carboxykinase in microdissected tubules. Hoppe Seylers Z Physiol Chem 355: 273–278, 1974 [DOI] [PubMed] [Google Scholar]
- 47.Burch HB, Narins RG, Chu C, Fagioli S, Choi S, McCarthy W, et al.: Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol 235: F246–F253, 1978 [DOI] [PubMed] [Google Scholar]
- 48.Burch HB, Choi S, Dence CN, Alvey TR, Cole BR, Lowry OH: Metabolic effects of large fructose loads in different parts of the rat nephron. J Biol Chem 255: 8239–8244, 1980 [PubMed] [Google Scholar]
- 49.Kranhold JF, Loh D, Morris RC Jr.: Renal fructose-metabolizing enzymes: Significance in hereditary fructose intolerance. Science 165: 402–403, 1969 [DOI] [PubMed] [Google Scholar]
- 50.Guder WG, Ross BD: Enzyme distribution along the nephron. Kidney Int 26: 101–111, 1984 [DOI] [PubMed] [Google Scholar]
- 51.Schmidt U, Marosvari I, Dubach UC: Renal metabolism of glucose: Anatomical sites of hexokinase activity in the rat nephron. FEBS Lett 53: 26–28, 1975 [DOI] [PubMed] [Google Scholar]
- 52.Frohnert PP, Höhmann B, Zwiebel R, Baumann K: Free flow micropuncture studies of glucose transport in the rat nephron. Pflugers Arch 315: 66–85, 1970 [DOI] [PubMed] [Google Scholar]
- 53.Uchida S, Endou H: Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255: F977–F983, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Douard V, Ferraris RP: Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295: E227–E237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamberlin ME, LeFurgey A, Mandel LJ: Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol 247: F955–F964, 1984 [DOI] [PubMed] [Google Scholar]
- 56.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Ludvigson MA, Sorenson RL: Immunohistochemical localization of aldose reductase. II. Rat eye and kidney. Diabetes 29: 450–459, 1980 [DOI] [PubMed] [Google Scholar]
- 58.Terubayashi H, Sato S, Nishimura C, Kador PF, Kinoshita JH: Localization of aldose and aldehyde reductase in the kidney. Kidney Int 36: 843–851, 1989 [DOI] [PubMed] [Google Scholar]
- 59.Yamaoka T, Nishimura C, Yamashita K, Itakura M, Yamada T, Fujimoto J, et al.: Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia 38: 255–261, 1995 [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki T, Akanuma H, Yamanouchi T: Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care 25: 353–357, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Hotta N, Kawamori R, Fukuda M, Shigeta Y; Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group: Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: Multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med 29: 1529–1533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohmura C, Watada H, Azuma K, Shimizu T, Kanazawa A, Ikeda F, et al.: Aldose reductase inhibitor, epalrestat, reduces lipid hydroperoxides in type 2 diabetes. Endocr J 56: 149–156, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, et al.: Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, et al.: Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin J Am Soc Nephrol 11: 1472–1483, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, et al.: Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One 7: e47948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al.: A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290: F625–F631, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, et al.: Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 19: 1712–1720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al.: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al.: Hyperuricemia induces endothelial dysfunction. Kidney Int 67: 1739–1742, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, et al.: Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol 298: F712–F720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al.: Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 40: 355–360, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Lozada LG, Andres-Hernando A, Garcia-Arroyo FE, Cicerchi C, Li N, Kuwabara M, et al.: Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J Biol Chem 294: 4272–4281, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al.: Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis 71: 851–865, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa MA, Ejaz AA, et al.: The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 15: 767–775, 2019 [DOI] [PubMed] [Google Scholar]
- 75.Warburg O: On respiratory impairment in cancer cells. Science 124: 269–270, 1956 [PubMed] [Google Scholar]
- 76.Warburg O: On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 77.Zhang G, Darshi M, Sharma K: The Warburg effect in diabetic kidney disease. Semin Nephrol 38: 111–120, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Q, Su J, Dong G, Zhang M, Huo Y, Dong Z: Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am J Physiol Renal Physiol 316: F1162–F1172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, et al.: Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol 313: F561–F575, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Yin XN, Wang J, Cui LF, Fan WX: Enhanced glycolysis in the process of renal fibrosis aggravated the development of chronic kidney disease. Eur Rev Med Pharmacol Sci 22: 4243–4251, 2018 [DOI] [PubMed] [Google Scholar]
- 81.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, et al.: Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 20: 545–553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, et al.: Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 293: F1256–F1261, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, et al.: Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445, 1999 [DOI] [PubMed] [Google Scholar]
- 84.Nakagawa T: Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: An explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292: F1665–F1672, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, et al.: Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol 7: 36–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herman MA, Samuel VT: The sweet path to metabolic demise: Fructose and lipid synthesis. Trends Endocrinol Metab 27: 719–730, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G: Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baron AD, Zhu JS, Marshall S, Irsula O, Brechtel G, Keech C: Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am J Physiol 269: E709–E715, 1995 [DOI] [PubMed] [Google Scholar]
- 89.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al.: Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol 68: 1063–1075, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, et al.: Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci U S A 109: 4320–4325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al.: Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60: 1258–1269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al.: High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363: 1345–1349, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]