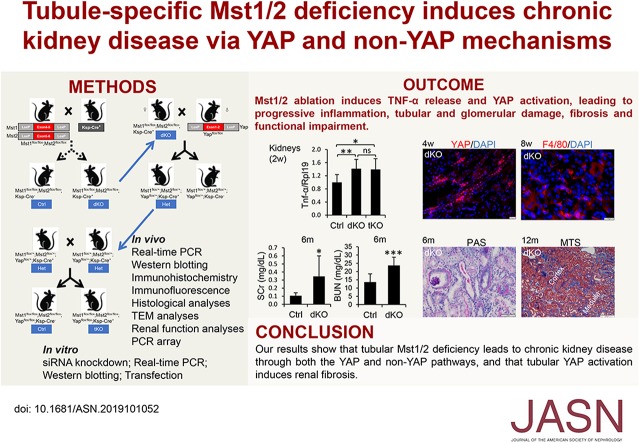
Significance Statement
The serine/threonine kinases MST1 and MST2 are core components of the Hippo pathway, and Yes-associated protein (YAP) is one of the pathway’s main effectors. However, the biologic functions of the Hippo/YAP pathway in adult kidneys are not well understood, and the role of MST1 and MST2 in the kidney has not been studied. In studies using knockout mice (with tubule-specific deletion of both Mst1 and Mst2) and mouse inner medullary collecting duct cells, the authors demonstrate that tubular deletion of Mst1 and Mst2 activates YAP, which induces inflammation, tubular lesions, fibrosis, and functional impairment; they also show that pathogenesis involves reciprocal stimulation of TNF-α and YAP signaling activities. Their findings indicate that tubular YAP activation induces renal fibrosis and CKD, thus revealing a novel and critical mechanism underlying this condition.
Keywords: Hippo, MST1, MST2, YAP, renal fibrosis, CKD
Visual Abstract
Abstract
Background
The serine/threonine kinases MST1 and MST2 are core components of the Hippo pathway, which has been found to be critically involved in embryonic kidney development. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are the pathway’s main effectors. However, the biologic functions of the Hippo/YAP pathway in adult kidneys are not well understood, and the functional role of MST1 and MST2 in the kidney has not been studied.
Methods
We used immunohistochemistry to examine expression in mouse kidneys of MST1 and MST2, homologs of Hippo in Drosophila. We generated mice with tubule-specific double knockout of Mst1 and Mst2 or triple knockout of Mst1, Mst2, and Yap. PCR array and mouse inner medullary collecting duct cells were used to identify the primary target of Mst1/Mst2 deficiency.
Results
MST1 and MST2 were predominantly expressed in the tubular epithelial cells of adult kidneys. Deletion of Mst1/Mst2 in renal tubules increased activity of YAP but not TAZ. The kidneys of mutant mice showed progressive inflammation, tubular and glomerular damage, fibrosis, and functional impairment; these phenotypes were largely rescued by deletion of Yap in renal tubules. TNF-α expression was induced via both YAP-dependent and YAP-independent mechanisms, and TNF-α and YAP amplified the signaling activities of each other in the tubules of kidneys with double knockout of Mst1/Mst2.
Conclusions
Our findings show that tubular Mst1/Mst2 deficiency leads to CKD through both the YAP and non-YAP pathways and that tubular YAP activation induces renal fibrosis. The pathogenesis seems to involve the reciprocal stimulation of TNF-α and YAP signaling activities.
CKD is a major health problem, with an overall prevalence of 11% in China and in the world.1,2 Over the last 20 years, no new drugs have been approved to specifically prevent CKD due to our poor understanding of the mechanism underlying CKD.3
Serine/threonine kinases MST1 and MST2 are the core components of the Hippo pathway, which were originally identified as regulators of organ size in Drosophila. Core components of this pathway, such as Warts, Hippo, and Salvador (Sav) restrict cell proliferation and promote apoptosis by repressing the downstream effector Yokie in Drosophila. The Hippo pathway is highly conserved in mammals. MST1/2 and LATS1/2 in mammals are homologs of Hippo and Warts in Drosophila, respectively. MST1/2 phosphorylates the scaffold protein Sav1, and together, they phosphorylate LATS1/2 and the adaptor protein MOB1. LATS1/2 and MOB1 together in turn phosphorylate the main effectors of the Hippo pathway, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), resulting in their cytoplasmic sequestration and degradation. When Hippo signaling is inactivated, unphosphorylated YAP and TAZ translocate into the nucleus and form a complex with TEA-domain family members, thereby activating cell proliferation.4–7
Hippo/YAP signaling plays a critical role in early embryonic kidney development. For instance, deletion of Yap from the ureteric bud lineage using Hoxb7-Cre mice led to reduced and abnormal branching and defective ureter-bladder junction.8 Deletion of Yap in the condensing mesenchyme using Six2-Cre mice reduced nephron formation.9
The functions of the Hippo/YAP pathway in the adult kidney are less well understood. Mice with deletion of Sav1 in renal tubules by Ksp-Cre did not show overt renal histologic or functional abnormalities by 8 weeks of age.10 However, these mice developed a number of renal abnormalities, including tubular cell hyperproliferation, tubular lesions, and inflammation at 12 months of age.11 The mice were more susceptible to fibrogenesis after kidney injury.10,12 Inactivation of Yap in fibroblasts decreased UUO-induced renal fibrogenesis.13–15 These results suggest a role for YAP activation in renal fibrogenesis, but it remains to be determined whether tubular YAP activation plays a role in renal fibrosis. In addition, YAP was also found to be involved in autosomal dominant polycystic kidney disease,16 and diabetic kidney diseases.17
MST1 and MST2 play important roles in early embryonic development and organ size control. Mice with Mst1 null alleles, Mst2 null alleles, or one copy of either Mst1 or Mst2 were viable and morphologically normal.18,19 However, depletion of both Mst1 and Mst2 resulted in embryonic lethality at embryonic day 8.5, suggesting redundant roles of Mst1 and Mst2.18 Removal or inhibition of Mst1/2 in newborn or even adult mice resulted in liver enlargement and hepatocellular carcinoma.18,20–26 Likewise, in mouse intestines and pancreas, inactivation of Mst1/2 leads to intestinal stem cell overproliferation, colonic tumorigenesis, and pancreas overgrowth.27–29
MST1 and MST2 can function independently of the YAP pathway. For example, MST1 can interact with PHLPPs, FoxO1, and FoxO3 to induce apoptosis.30–32 Furthermore, MST1 interacted with RASSF proteins and Raf1 to coordinate the crosstalk between the AKT and ERK pathways.33 Beyond the roles in apoptosis and proliferation, MST1/2 maintained bioenergetic activities and mitochondrial dynamics independently of canonical Hippo signaling in CD8α+ dendritic cells.34
The functional role of MST1 and MST2 in the kidney has not been studied. In this study, we found that Ksp-Cre–mediated Mst1/2 deletion in tubular cells did not lead to any defects in embryonic kidney development, but it did result in tubular cell hyperproliferation, inflammation, tubular injury, renal fibrosis, and renal dysfunction in adult kidneys. These phenotypes were largely dependent on YAP, but non-YAP mechanisms were also involved. Our results also show for the first time that tubular YAP activation induces renal fibrosis.
Methods
Generation of Renal Tubule–Specific Mst1/2 Double-Knockout, Mst1/Mst2/Yap Triple-Knockout, and Yap Knockout Mice
Mst1 knockout mice, Mst2 knockout mice, and Mst1flox/flox;Mst2flox/flox mouse lines were provided as described,18,35 and Yapflox/flox mice were purchased from Jackson Laboratory (stock no. 027929). To generate Mst1/2 double-knockout (dKO) mice, we crossed the Mst1flox/flox;Mst2flox/flox mice with Ksp-Cre transgenic mice. We also crossed Mst1flox/flox;Mst2flox/flox;Ksp-Cre mice with Yapflox/flox to generate Mst1/Mst2/Yap triple-knockout (tKO) mice (Mst1flox/flox;Mst2flox/flox;Yapflox/flox;Ksp-Cre). Yap conditional knockout (cKO) mice were generated by breeding Yapflox/flox mice with Ksp-Cre mice. The three mutant mice were maintained in a mixed inbred C57BL/6 and 129/Sv background. Male mice were used for analysis unless otherwise indicated. Mst1flox/flox;Mst2flox/flox or Mst1flox/flox;Mst2flox/flox;Yapflox/flox mice were used as controls for Mst1/2 dKO or Mst1/Mst2/Yap tKO mice, respectively.
All animal studies were approved by The Chinese University of Hong Kong Animal Experimentation Ethics Committee. Animals are housed in the Laboratory Animal Services Centre in The Chinese University of Hong Kong with a 12‐hour day‐night cycle.
We did not choose to use Hoxb7-Cre or Six2-Cre mice because these two lines express Cre at much earlier embryonic stages than Ksp-Cre. In the Hoxb7-Cre line, Cre is expressed in the Wolffian duct as early as E9 and in all epithelial structures derived from the ureteric bud,36 and in the Six2-Cre line, Cre-mediated recombination is restricted to the cap mesenchyme from the onset of metanephric development at E10.5. Conditional deletion of Yap/Taz, Lats1/2, or Nf2 using Hoxb7-Cre or Six2-Cre led to severe defects in embryonic kidney development and early postnatal death.8,9,37,38 In contract to these two lines, Ksp-Cre is not expressed in embryonic kidneys at E10.5 and E11.5. In E15.5 kidneys Ksp-Cre is expressed in the stalks of the ureteric bud and developing tubules after the S-shaped body stage of nephrogenesis.
TNF-α Antibody Treatment
Male Mst1/2 dKO mice at 4 weeks of age received an intraperitoneal injection of TNF-α antibody (AB-410-NA; R&D Systems) at a dose of 5 μg/g body wt. Control and other Mst1/2 dKO mice received an injection of nonspecific goat IgG (AB-108-C; R&D Systems) at the same dose. Sixteen to 18 hours later, the mice received another injection of the antibodies or nonspecific IgG, and 4 hours after the second injection, kidneys were harvested.
Histology and Immunohistochemistry
Paraffin kidney sections were used for periodic acid–Schiff, Sirius red, and Masson trichrome staining. Paraffin sections from control, Mst1/2 dKO kidneys, Mst1 global knockout (gKO), and Mst2 gKO mice were also used for immunohistochemistry for MST1, MST2, TNF-α, and CCL20. Antigen retrieval was performed in a 0.01 M citrate buffer (pH 6.0). Tissue sections were incubated overnight with rabbit anti-MST1 (22245–1-AP; Proteintech), rabbit anti-MST2 (ab52641; Abcam), rabbit anti–TNF-α (A11534; ABclonal Technology), or rabbit anti-CCL20 (ab9829; Abcam) antibodies. The signals were developed using the Histostain Plus LAB-SA Detection System (859243; Invitrogen, Waltham, MA) or the UltraSensitive S-P kit (KIT-9710; MXB Biotechnologies, Fuzhou, China).
Immunofluorescence
Cryostat sections were treated with 1% SDS for 4 minutes. The sections were incubated with anti-YAP (#14074; Cell Signaling), anti-Ki67 (NB110–89717; Novus Biologicals), anti-F4/80 (14–4801; eBioscience), anti-Tamm–Horsfall Protein (THP)/Uromodulin (MAB5175; R&D Systems), anti–E-cadherin (3195; Cell Signaling), anti-Megalin (sc-16478; Santa Cruz Biotechnology), or anti-AQP2 (sc-9882; Santa Cruz Biotechnology) antibodies followed by Alexa Fluor 488-, 546-, or 555-labeled secondary antibodies (Invitrogen). Images were captured with a regular or confocal fluorescent microscope (FV1000; Olympus, Mannheim, Baden-Württemberg, Germany). Ten fields per section and three sections per kidney were examined for each animal to determine the positively stained cells per field using ImageJ.
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Assay
Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay was performed using the ApopTag Plus Fluorescein In Situ Apoptosis Detection kit (S7111; Chemicon International, Temecula, CA) as we previously described.39–41 Ten fields per section and three sections per kidney were examined for each animal to determine the apoptotic tubular epithelial cells per field using ImageJ.
Transmission Electron Microscopy
Kidneys were trimmed and fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) overnight at 4°C, postfixed in 2% osmium tetroxide for 1 hour, dehydrated in ascending graded ethanol, and embedded in EPON 812 at 60°C for 24 hours. Ultrathin 60-nm-thick sections were stained with uranyl acetate and lead citrate and examined in a Hitachi H-7700 transmission electron microscope.
Serum Creatinine and BUN Assays
Serum creatinine levels were measured using the Creatinine Liquicolor kit (#0430–120; Stanbio Lab). Briefly, 3 μl of serum samples, water (as blank), and standards were added into a 96-well plate in duplicates; 135 μl of Reagent 1 was immediately added into each well followed by incubation at 37°C for 5 minutes. Absorbance A1 was measured at 550 nm; 45 μl of Reagent 2 then was added into each well and incubated at 37°C for 5 minutes. Absorbance A2 was measured at 550 nm. Serum creatinine concentrations were calculated according to the manufacturer’s instructions.
Serum BUN levels were measured using the Stanbio Enzymatic Urea Nitrogen kit (#2050–450). Briefly, 1μl serum samples and standards were added into a 96-well plate in duplicates; 100 μl Enzyme Reagent was immediately added into each well followed by incubation at 37°C for 5 minutes, and 100 μl Color Reagent 2 was then added into each well and incubated at 37°C for 5 minutes. Absorbance was measured at 600 nm. Serum BUN concentrations were calculated using the formula provided by the manufacturer.
Cell Culture, Transfection, and siRNA Targeting
Mouse IMCD3 cells (ATCC CRL-2123) were cultured in DMEM (Invitrogen) supplemented with 10% FBS. Cells were transfected with pcDNA3.1, wild-type (wt) YAP, or constitutively active (ca) YAP (Flag-YAP S127A) plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. For Mst1 and Mst2 targeting, IMCD3 cells were transfected with scrambled siRNA control (AM4613; Ambion) or a mixture of mouse Mst1 and Mst2 siRNA sequences (Shanghai GenePharma Co., Ltd., Shanghai, China). Two Mst1 siRNA sequences were used (sense): 5′-GCCAGAUUGUUGCAAUCAATT-3′ and 5′-CCAAAGGAGUGUCAAUAUUTT-3′. One Mst2 sequence was used (sense): 5′-GCUUGGAGAAGGGUCUUAUTT-3′. Twenty-four hours after transfection, cells were incubated in FBS-free DMEM containing 0.1% BSA for 16 hours before cells were harvested.
Nuclear Protein Extraction
Kidney tissues were homogenized in hypotonic buffer (10 mM Hepes, pH 7.1, 50 mM NaCl, 0.3 M sucrose, 0.1% Triton X-100, 0.1 mM EDTA, and 1 mM dithiothreitol) and incubated for 10 minutes on ice. Cell nuclei were pelleted by centrifugation at 1500×g and washed with hypotonic buffer without Triton X-100. Nuclear pellets were resuspended in nuclear extraction buffer (10 mM Hepes, pH 7.1, 500 mM NaCl, 0.5% NP-40, 0.1 mM EDTA, and 1 mM dithiothreitol) and sonicated. Nuclear extracts were obtained after centrifugation at 10,000×g.
Western Blotting
Kidney tissues and cells were lysed and sonicated. Western blotting was performed using anti-MST1 (#3682; Cell Signaling), anti-MST2 (ab52641), antiphospho-LATS1 (#8654; Cell Signaling), anti-LATS1 (#3477; Cell Signaling), antiphospho-MOB1 (#8699; Cell Signaling), anti-MOB1 (#3863; Cell Signaling), antiphospho-YAP (#4911; Cell Signaling), anti-YAP (#4912; Cell Signaling), anti-TAZ (A15806; ABclonal Technology), anti-Lamin B1 (ab16048; Abcam), anti-Ngal (AF1857; R&D Systems), anti–α-smooth muscle actin (ab5694; Abcam), anti-Fibronectin (AB2033; Merk Millipore), anti-Col1α1 (ab292; Abcam; bs-10423R; Bioss), or anti–E-cadherin (BD-610182; BD Biosciences) primary antibodies. The MST1 and MST2 antibodies recognize both the full-length and cleaved forms.
RNA Isolation and Real-Time PCR Analyses
Total RNA was extracted using illustra RNAspin Mini (#25–0500–71; GE Healthcare) according to the manufacture’s instruction. Reverse transcription was performed using the PrimeScript RT reagent kit (TAKARA). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on the ABI Prism 7900 Sequence Detection System (PE Biosystems).
PCR Array
Kidneys from WT and Mst1/2 dKO mice at 2 weeks of age were used for PCR array analysis for inflammatory factors. The RT2 Profiler PCR Array Mouse Cytokines & Chemokines kit was purchased from Qiagen (PAMM-150Z). Five WT and eight Mst1/2 dKO RNA samples were each reverse transcribed, and the WT and dKO cDNA samples were combined to be one WT sample and one dKO sample, respectively. The assay and analysis were performed according to the manufacture’s instruction.
Luciferase Assay
IMCD3 cells were transiently transfected with an NF-κB–responsive luciferase reporter containing three copies of NF-κB response elements in combination with pTK-RL (containing a thymidine kinase promoter upstream of Renilla) in a ratio of 10:1 to control for transfection efficiency with or without cotransfection with caYAP plasmid. Approximately 24 hours after transfection, the medium was replaced with serum-free medium containing 0.1% BSA. Sixteen hours later, cells were lysed, and luciferase activity was determined with the Dual Reporter Assay kits (E1910; Promega). Experiments were performed in quadruplicate wells. Relative light units were calculated as ratios of firefly and Renilla luciferase values.
Statistical Analyses
All data are represented as mean±SD of three or more independent replicates. The t test was used. A P value of 0.05 was considered statistically significant.
Results
Kidney Overgrowth in Tubular Cell–Specific Mst1/2 Knockout Mice
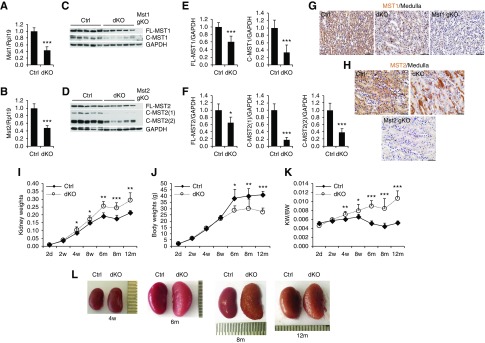
Our initial studies show that MST1 and MST2 play redundant roles in the kidney. Therefore, we generated tubular cell Mst1/2 dKO mice by crossing Mst1f/f;Mst2f/f mice with Ksp-Cre transgenic mice. Mst1/2 mRNA and protein (both full-length and cleaved forms) were dramatically reduced in dKO kidneys compared with control kidneys (Figure 1, A–F). Immunohistochemical staining showed that MST1 and MST2 were expressed in all renal tubules in control kidneys (Figure 1, G and H, Supplemental Figure 1). MST1 and MST2 were not found in glomeruli (Supplemental Figure 1). MST1 staining was dramatically decreased in the collecting ducts and distal nephrons of Mst1/2 dKO kidneys (Figure 1G). MST2 staining in Mst1/2 dKO kidneys was mosaic in the collecting ducts and distal nephrons, with abolished expression in the majority of tubular cells and compensatory enhanced expression in some other tubular cells (Figure 1H). MST1 and MST2 signals were absent in some but not all of the proximal tubular cells (Supplemental Figure 1). As negative controls, the kidneys of global Mst1 knockout (Mst1 gKO) or global Mst2 knockout (Mst2 gKO) mice did not show any MST1 or MST2 staining, respectively.
Figure 1.
MST1 and MST2 were expressed in renal tubular cells and deletion of Mst1/2 in tubular cells caused kidney overgrowth. (A–F) Mst1 and Mst2 mRNA and protein levels in the kidneys of control (Ctrl; Mst1flox/flox;Mst2flox/flox) and Mst1/2 dKO (Mst1flox/flox;Mst2flox/flox;Ksp-Cre) mice. Kidneys collected from 4-week-old male Ctrl and Mst1/2 dKO mice were analyzed for (A and B) Mst1 and Mst2 mRNA levels by real-time PCR or (C and D) MST1 and MST2 protein levels by western blotting. Quantitative analysis of full-length (FL-) and cleaved (C-; E) MST1 and (F) MST2 protein levels was performed by densitometry. Rpl19 was used as internal Ctrl for real-time PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading Ctrl for western blotting. (G and H) Immunohistochemistry for MST1 and MST2 in Ctrl and Mst1/2 dKO kidneys. Paraffin kidney sections from 8-week-old mice were used for immunohistochemistry with 3,3′-Diaminobenzidine (DAB) staining (brown). Mst1 gKO and Mst2 gKO kidneys were used as respective negative Ctrl. Scale bar: 50 µm. (I–L) Kidney size and appearance in Mst1/2 dKO mice. (I) Kidney weights, (J) body weights, and (K) ratios of kidney weights (KW) to body weights (BW) are presented for Ctrl and Mst1/2 dKO male mice at different ages. (L) Representative macroscopic images of kidneys of the indicated genotypes at different ages are presented. (A–F) n=5. n=4/5/5/5/6/7/6 (Ctrl) and 4/9/4/5/6/7/6 (dKO) for the different ages in (I–L). In (L), 1 unit=0.5 mm. Comparisons were made between Ctrl and dKO in (I–K). *P<0.05; **P<0.01; ***P<0.001.
Kidney weights of dKO mice were similar to those of control mice at 2 days and 2 weeks of age but became greater from 4 weeks onward (Figure 1I). Body weights were similar between dKO and control mice by 8 weeks of age and decreased in dKO mice at 6 months and later stages (Figure 1J). Increases in ratios of kidney weight to body weight were observed beginning at 4 weeks, and they were aggravated at 6, 8, and 12 months of age (Figure 1K). dKO kidneys were larger in size than control kidneys and showed corrugated surfaces at 8 and 12 months of age (Figure 1L). These results suggest that deletion of Mst1/2 in tubular cells caused kidney overgrowth.
Age-Dependent YAP Activation and Hyperproliferation in Tubular Cells of the Mst1/2 dKO Mice
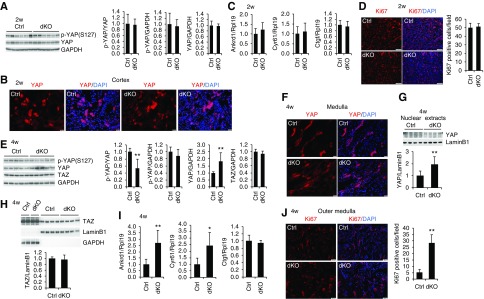
We examined YAP activity and cell proliferation in dKO mice at 2 and 4 weeks of age. Surprisingly, phosphorylated YAP and total YAP levels in the kidney were similar between control and dKO mice at 2 weeks of age (Figure 2A). YAP was more highly expressed in the tubular cells of the cortex than in those of the medulla (Supplemental Figure 2). YAP protein was restricted to cytoplasm in both control and dKO kidneys (Figure 2B, Supplemental Figure 2). The YAP target genes Ankrd1, Ctgf, and Cyr61 were not altered by Mst1/2 deletion (Figure 2C). Consistently, the numbers of tubular cells positive for Ki67, a marker for proliferating cells, were also similar between the two genotypes (Figure 2D).
Figure 2.
Deletion of Mst1/2 in renal tubular cells activated YAP in mice at 4 weeks but not at 2 weeks of age. (A) Phosphorylated YAP (p-YAP) and total YAP levels in the kidneys of control (Ctrl) and Mst1/2 dKO mice at 2 weeks of age. Kidneys collected from male mice were analyzed for p-YAP and YAP levels by western blotting. Quantitative analysis of p-YAP relative to YAP, p-YAP relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and YAP relative to GAPDH was performed by densitometry. (B) Immunofluorescence for YAP in the kidneys of Mst1/2 dKO at 2 weeks of age. Frozen kidney sections from male Ctrl and Mst1/2 dKO mice were used for immunofluorescence for YAP (red). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar: 20 µm. (C) Expression of the YAP target genes Ankrd1, Cyr61, and Ctgf in Ctrl and Mst1/2 dKO kidneys at 2 weeks of age. Kidneys collected from male mice were analyzed for Ankrd1, Cyr61, and Ctgf mRNA levels by real-time PCR. (D) Immunofluorescence for Ki67 in the kidneys of Mst1/2 dKO at 2 weeks of age. Frozen kidney sections from Ctrl and Mst1/2 dKO mice were used for immunofluorescence for Ki67 (red). Sections were counterstained with DAPI (blue). Ki67-positive tubular cells were counted in ten different fields of each section from each of three Ctrl or Mst1/2 dKO mice (right panel). Scale bar: 100 µm. (E) p-YAP, YAP, and TAZ levels in the kidneys of Ctrl and Mst1/2 dKO mice at 4 weeks of age. Kidneys collected from male mice were analyzed for p-YAP, YAP, and TAZ levels by western blotting. Quantitative analysis of p-YAP relative to YAP, p-YAP relative to GAPDH, YAP relative to GAPDH, and TAZ relative to GAPDH was performed by densitometry. (F and G) Nuclear localization of YAP in Mst1/2 dKO kidneys at 4 weeks of age. Frozen sections from Ctrl and Mst1/2 dKO kidneys were used for immunofluorescence for YAP (red). Nuclei were stained with DAPI (blue). Scale bar: 20 µm. Nuclear extracts from Ctrl and Mst1/2 dKO kidneys were subjected to western blotting for YAP (G, upper panel). Quantitative analysis of nuclear YAP protein levels was performed by densitometry (G, lower panel). (H) Nuclear TAZ levels in Mst1/2 dKO kidneys at 4 weeks of age. Cytosolic and nuclear extracts from Ctrl and Mst1/2 dKO kidneys were subjected to western blotting for TAZ (upper panel). Quantitative analysis of nuclear TAZ protein levels was performed by densitometry (lower panel). (I) Expression of the YAP target genes Ankrd1, Cyr61, and Ctgf in Ctrl and Mst1/2 dKO kidneys at 4 weeks of age. Kidneys collected from male mice were analyzed for Ankrd1, Cyr61, and Ctgf mRNA levels by real-time PCR. (J) Immunofluorescence for Ki67 in the kidneys of Mst1/2 dKO mice at 4 weeks of age. Frozen kidney sections from male Ctrl and Mst1/2 dKO mice were used for immunofluorescence for Ki67 (red). Nuclei were stained with DAPI (blue). Ki67-positive tubular cells in the outer medulla were counted in ten different fields of each section from each of three Ctrl or Mst1/2 dKO mice (right panel). Scale bar: 50 µm. Rpl19 was used as internal Ctrl for real-time PCR. GAPDH was used as loading Ctrl for whole lysates, and LaminB1 was used as loading Ctrl for nuclear extracts. n=4 for (C); n=3 for (D, F, and J); and n=4 for (I). *P<0.05; **P<0.01.
At 4 weeks of age, phospho-YAP levels were decreased, and total YAP levels were increased in Mst1/2 dKO kidneys, whereas TAZ protein levels did not change (Figure 2E). In contrast to the cytosolic expression in control mice, YAP was localized in the nuclei of tubular cells in the kidneys of dKO mice (Figure 2F, Supplemental Figure 3). Of note, YAP was not expressed in proximal tubules, and no nuclear YAP staining was found in the proximal tubules of dKO kidneys (Supplemental Figure 3). Cell fractionations showed that more YAP protein was detected in nuclear extracts of Mst1/2 dKO kidneys than in those of control kidneys (Figure 2G). Nuclear TAZ levels were not altered by deletion of Mst1/2 (Figure 2H). Ankrd1 and Cyr61 mRNA levels were higher in dKO kidneys from both male (Figure 2I) and female mice (Supplemental Figure 4). Ki67-positive tubular cells were mostly found in the outer medulla, and they were much increased in Mst1/2 dKO kidneys compared with control kidneys (Figure 2J). These results indicate that ablation of Mst1/2 in tubular cells exhibited age-dependent effects on YAP activation, which may explain the unaltered kidney sizes at 2 weeks and the increased kidney sizes at 4 weeks of age in dKO mice.
We also examined phosphorylated LATS1 and MOB1 levels in Mst1/2 dKO kidneys. Phosphorylated and total LATS1 and MOB1 levels were similar between WT and dKO mice at 2 weeks of age (Supplemental Figure 5). Interestingly, both total and phospho-LATS1 protein levels were increased in dKO kidneys compared with control kidneys at 4 weeks of age (Supplemental Figure 6). However, phospho-MOB1 was much reduced in dKO kidneys compared with control kidneys despite the increased total MOB1 expression. Because LATS1/2 and MOB1 need to function together to control YAP phosphorylation, the decreased MOB1 phosphorylation may explain the reduced phospho-YAP levels in dKO kidneys at 4 weeks of age. The increased LATS1 and MOB1 levels are consistent with previous findings showing that YAP induced its upstream negative regulators to provide a negative feedback onto itself and control the total output of YAP activity.42
Progressive Tubular Damage in Mst1/2 dKO Mice
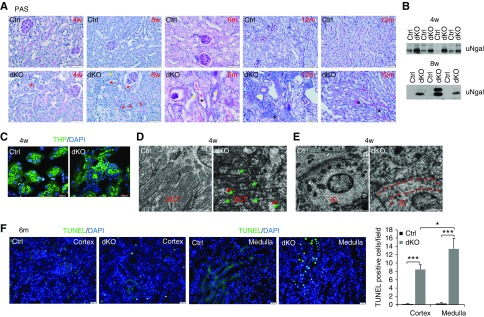
We then performed periodic acid–Schiff staining to study the tubular structure in the mutant mice. No noticeable changes in kidney structure and urinary Ngal (a marker for tubular injury) were found in Mst1/2 dKO mice at 2 weeks of age (Supplemental Figure 7). However, increased cellularity was observed in the tubular epithelia as well as interstitium of dKO kidneys compared with control kidneys from 4 weeks onward (Figure 3A). Multiple layering of the epithelium or tubular cell sloughing was also observed in dKO kidneys (Figure 3A, yellow asterisks). Thickening of tubular basement membrane was seen in Mst1/2 dKO kidneys at 4 and 8 weeks of age (Figure 3A, red asterisks), and it became more apparent in older kidneys, suggesting that the epithelial cells acquired a secretory phenotype. Bowman’s capsule basement membrane thickening and glomerular atrophy were also found in Mst1/2 dKO kidneys at 6 and 12 months of age. Protein casts were formed in 6-month-old dKO kidneys and became more severe in 12-month-old dKO kidneys. Urinary Ngal levels were much higher in dKO kidneys than in control kidneys at 4 and 8 weeks of age (Figure 3B).
Figure 3.
Deletion of Mst1/2 in renal tubular cells induced tubular damage in the kidney. (A) Representative images of periodic acid–Schiff (PAS) staining. Paraffin sections from control (Ctrl) and Mst1/2 dKO kidneys at 4 and 8 weeks and 6 and 12 months of age were used for PAS staining. Red asterisks indicate tubules with thickening of tubular basement membrane, yellow asterisks indicate multiple layering of the epithelium and tubular cell sloughing, and black asterisks indicate tubular casts. Scale bars: 50 µm for 4 and 8 weeks and 6 months of age; 100 µm for 12 months of age. (B) Ngal levels in urine. Spot urine samples from Ctrl and Mst1/2 dKO mice at 4 and 8 weeks of age were subjected to western blotting for Ngal. (C) Immunofluorescence for THP. Frozen kidney sections from Ctrl and Mst1/2 dKO mice at 4 weeks of age were used for immunofluorescence for THP (green). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar: 20 µm. (D and E) Representative transmission electron microscopy photographs of mitochondrial morphology in tubular cells of Ctrl and Mst1/2 dKO kidneys at 4 weeks of age. (D) Red arrows indicate swelling, and green arrows indicate loss of cristae. (E) The stripe between the two dotted red lines indicates the area that is supposed to have many mitochondria but actually lacks mitochondria in the interacted cells of Mst1/2 dkO kidneys. DCT, distal convoluted tubule; IC, intercalated cell. Scale bar: 2.0 µm. (F) TUNEL-positive tubular cell numbers in Mst1/2 dKO kidneys. Paraffin kidney sections from Ctrl and Mst1/2 dKO mice at 6 months of age were used for TUNEL staining. Nuclear localization of TUNEL signal was demonstrated by cyan-colored nuclei after merging with DAPI. TUNEL-positive tubular cells in the cortex and medulla were counted in ten different fields of each section from each of three Ctrl or Mst1/2 dKO mice (right panel). Scale bar: 20 µm. *P<0.05; ***P<0.001.
We also examined THP as an additional marker for tubular structure and function. As expected,43 THP was localized to the apical pole of TAL cells in control kidneys, but it was also found on basal and lateral membranes and even in cytoplasm in addition to the apical signal in dKO kidneys (Figure 3C).
Mitochondrial damage has been considered as a crucial contributor to tubular cell injury.44–47 As shown by transmission electron microscopy, elongated large mitochondria with aligned cristae were seen lying in basal infoldings of distal convoluted tubules of control kidneys. In sharp contrast, many small and round mitochondria were observed in tubular cells of Mst1/2 dKO kidneys, and these mitochondria were severely damaged, showing swelling and loss of cristae (Figure 3D). Mitochondria were barely seen in the cytoplasm of intercalated cells of Mst1/2 dKO (Figure 3E).
Many more TUNEL-positive tubular cells were found in dKO kidneys than in control kidneys at 6 months of age, and there were more TUNEL-positive tubular cells in the medulla than in the cortex in dKO kidneys (Figure 3F). The levels and pattern of E-cadherin expression in renal tubules of Mst1/2 dKO mice did not differ from those of control mice at 4 weeks of age, indicating normal cell polarity (Supplemental Figure 8). Taken together, tubular cell–specific knockout of Mst1/2 led to progressive tubular damage and cell death without alterations in cell polarity.
Inflammation in Tubular Cell–Specific Mst1/2 dKO Mice
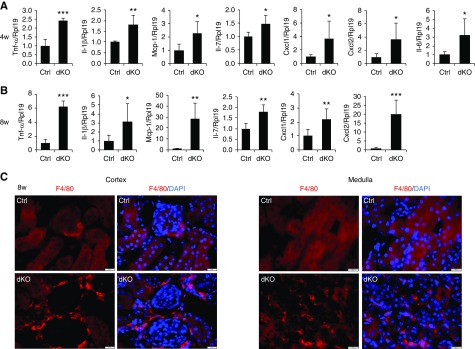
Histologic examination showed more nuclear staining in the interstitium of dKO kidneys (Figure 3A), indicating immune cell infiltration. We further measured several inflammatory factors in the kidneys of male dKO mice. mRNA levels of Tnf-α, Il-1β, Mcp-1, Il-7, Cxcl1, Cxcl2, and Il-6 were all increased in dKO mice at 4 weeks (Figure 4A), and they further increased at 8 weeks of age (Figure 4B) compared with respective controls. Increases in these factors except Il-7 were also found in female dKO mice at 4 weeks of age (Supplemental Figure 9). Furthermore, more positive cells for F4/80, a marker for macrophages, were found in the cortex and medulla of dKO mice than in control mice at 8 weeks of age (Figure 4C). These results indicate that tubular cell–specific knockout of Mst1/2 resulted in progressive renal inflammation.
Figure 4.
Deletion of Mst1/2 in renal tubular cells induced renal inflammation. (A and B) Expression of inflammatory factors in Mst1/2 dKO kidneys. Kidneys collected from male control (Ctrl) and Mst1/2 dKO mice at (A) 4 and (B) 8 weeks of age were analyzed for Tnf-α, Il-1β, Mcp-1, Il-7, Cxcl1, Cxcl2, and Il-6 mRNA levels by real-time PCR. (C) Infiltration of macrophages in the cortex and medulla of Mst1/2 dKO kidneys. Frozen kidney sections from Ctrl and Mst1/2 dKO mice at 8 weeks of age were used for immunofluorescence staining for F4/80 (red). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Rpl19 was used as internal Ctrl for real-time PCR. n=4/5 (4/8 weeks). Scale bar: 20 µm. *P<0.05; **P<0.01; ***P<0.001.
Renal Fibrosis in Tubular Cell–Specific Mst1/2 dKO Mice
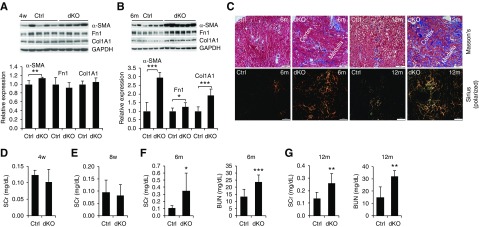
Mst1/2 dKO kidneys showed a corrugated appearance, an indication of fibrosis (Figure 1L); thus, we examined whether Mst1/2 dKO kidneys developed renal fibrosis. At 4 weeks of age, the levels of α-smooth muscle actin already showed a slight but significant increase in Mst1/2 dKO kidneys compared with control kidneys, whereas fibronectin 1 and type 1 collagen α1 did not change (Figure 5A). At 6 months of age, all three fibrotic markers were increased in male dKO mice (Figure 5B). As shown by Masson trichrome and Sirius red staining, increases in collagen deposition were seen in the interstitium of both cortex and medulla at 6 months, and they were much aggravated at 12 months of age in dKO kidneys (Figure 5C). Of note, female dKO mice exhibited reduced fibrotic responses compared with male dKO mice (Supplemental Figure 10). Together, progressive interstitial fibrosis was developed in Mst1/2-deficient kidneys.
Figure 5.
Deletion of Mst1/Mst2 in renal tubule cells induced kidney fibrosis and functional impairment in mice. (A and B) Expression of α-smooth muscle actin (α-SMA), fibronectin 1 (Fn1), and type 1 collagen α1 (Col1A1). Kidneys from control (Ctrl) and Mst1/2 dKO mice at (A) 4 weeks and (B) 6 months of age were subjected to western blotting for α-SMA, Fn1, and Col1A1 (upper panels). Quantitative analysis of α-SMA, Fn1, and Col1A1 protein levels was performed by densitometry (lower panels). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading Ctrl. (C) Representative photographs of Masson trichrome and Sirius red staining. Paraffin sections from Ctrl and Mst1/2 dKO kidneys at 6 and 12 months of age were used for Masson trichrome and Sirius red staining to assess fibrosis. Photographs for Sirius staining were taken under polarized light with a polarizing microscope. Scale bar: 200 µm. (D–G) Serum creatinine (SCr) and BUN levels. Serum samples from Ctrl and Mst1/2 dKO mice at (D) 4 and (E) 8 weeks and (F) 6 and (G) 12 months of age were measured for creatinine and BUN levels. n=5/4 (Ctrl/dKO) for (D), n=5 for (E), and n=6 for (F and G). *P<0.05; **P<0.01; ***P<0.001.
Impaired Kidney Function in Tubular Cell–Specific Mst1/2 dKO Mice
Serum creatinine levels did not change between control and dKO mice at 4 and 8 weeks (Figure 5, D and E), but serum creatinine and BUN levels were much higher in dKO mice than in control mice at 6 and 12 months of age (Figure 5, F and G). Consistently, dKO mice had a decreased survival time compared with that of control mice (Supplemental Figure 11). These results suggest that ablation of Mst1/2 in renal tubules resulted in progressive impairment in kidney function.
Rescue of Kidney Overgrowth, Structure, Inflammation, and Fibrosis by Yap Deletion in Mst1/2 dKO Mice
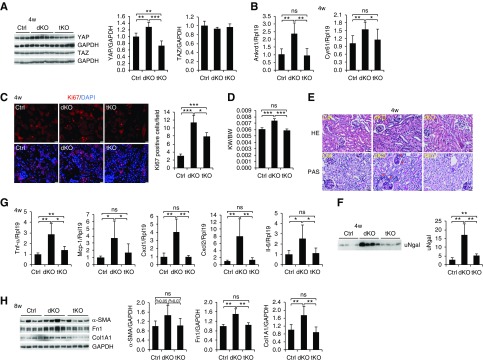
Because YAP but not TAZ was activated by deletion of Mst1/2 (Figure 2, E–H), we generated tubular cell–specific Mst1/Mst2/Yap tKO mice to determine whether the phenotypes observed in Mst1/2 dKO mice were mediated by YAP. YAP mRNA and protein levels were reduced in the tKO kidneys compared with control kidneys, whereas TAZ protein levels did not change (Figure 6A, Supplemental Figure 12A). The increased expression of Ankrd1 and Cyr61 in Mst1/2 dKO kidneys was completely rescued by Yap deletion in mice at 4 weeks of age (Figure 6B). Ki67-positive tubular cell numbers in Mst1/Mst2/Yap tKO mice were lower than the numbers in Mst1/2 dKO mice, but they were still higher than the numbers in control mice (Figure 6C). The increased ratios of kidney weight to body weight in Mst1/2 dKO mice were restored to the control levels by deletion of Yap at 4 weeks of age in both males (Figure 6D) and females (Supplemental Figure 13).
Figure 6.
The phenotypes observed in Mst1/2 dKO mice were largely mediated by YAP. (A) YAP and TAZ expression in the kidneys of control (Ctrl), Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice. Kidney lysates from Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO at 4 weeks of age were used for western blotting for YAP and TAZ (left panel). Quantitative analysis of YAP and TAZ protein levels relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels was performed (right panels). (B) mRNA levels of the YAP target genes Ankrd1 and Cyr61 in the kidney. Kidneys were used for real-time PCR analysis for Ankrd1 and Cyr61 mRNA levels. (C) Immunofluorescence for Ki67 (red) in Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO kidneys (left panels). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar: 20 µm. Ki67-positive tubular cells were counted in ten different fields of each section from each mouse (right panel). (D) Ratios of kidney weight to body weight in Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice. Male mice at 4 weeks of age were used to calculate the ratios of kidney weight (KW) to body weight (BW). (E) Representative photographs of hematoxylin and eosin (HE) and periodic acid–Schiff (PAS) staining of the kidneys of Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice. Paraffin sections from the mice at 4 weeks of age were used for HE and PAS staining. Red asterisks indicate tubules with thickening of tubular basement membrane. Scale bar: 25 µm. (F) Urinary Ngal levels. Spot urine samples from Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice at 4 weeks of age were subjected to western blotting for Ngal. Quantitative analysis was performed by densitometry (left panel). (G) mRNA levels of Tnf-α, Mcp-1, Cxcl1, Cxcl2, and Il-6 in the kidneys of Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice. Kidneys of male mice at 4 weeks of age were used for real-time PCR analysis. (H) Expression of α-smooth muscle actin (α-SMA), fibronectin 1 (Fn1), and type 1 collagen α1 (Col1A1) in the kidneys of Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice at 8 weeks of age. Quantitative analysis of α-SMA, Fn1, and Col1A1 protein levels was performed by densitometry (left panels). GAPDH was used as loading Ctrl for western blotting. Rpl19 was used as internal Ctrl for real-time PCR. n=5 for (B and G), n=3 for (C), and n=5/4/4 (Ctrl/dKO/tKO) for (D). ns, no significance. *P<0.05; **P<0.01; ***P<0.001.
Mst1/2 dKO kidneys exhibited aberrant renal tubules with much more nuclei and thickening of tubular basement membrane. By contrast, the structure of tKO kidneys was not apparently different from that of control kidneys (Figure 6E). Urinary Ngal levels in tKO were much lower than the levels in dKO mice, but they were still higher than the levels in control mice (Figure 6F).
The elevated expression of Mcp-1, Cxcl1, Cxcl2, and Il-6 mRNA in Mst1/2 dKO kidneys was returned to physiologic levels in Mst1/Mst2/Yap tKO kidneys (Figure 6G). As far as Tnf-α was concerned, its expression in tKO kidneys was only reduced by 50% compared with Mst1/2 dKO kidneys, and it was still higher than the expression in control kidneys (Figure 6G).
α-Smooth muscle actin, fibronectin 1, and type 1 collagen α1 levels in dKO kidneys in Mst1/2 dKO mice were completely rescued by deletion of Yap at 8 weeks of age (Figure 6H). Taken together, these results indicate that the effects of ablation of Mst1 and Mst2 on renal overgrowth, tubular damage, inflammation, and fibrosis were largely mediated by YAP. Because Tnf-α levels were still higher in tKO kidneys than in control kidneys (Figure 6G), Tnf-α induction in Mst1/2 dKO kidneys may have been achieved through both YAP-dependent and -independent pathways.
YAP-Independent Induction of Tnf-α in Tubular Cells by Deletion of Mst1/2 in 2-Week-Old Mice
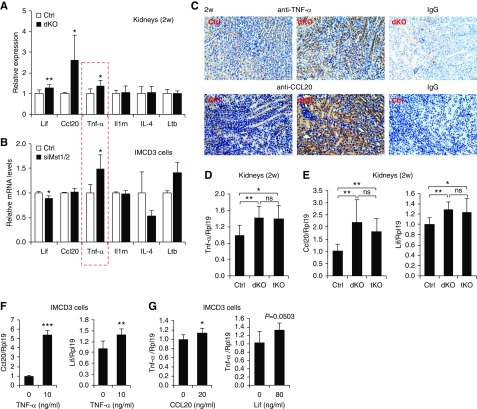
YAP activation, kidney overgrowth, and tubular injury were observed in Mst1/2 dKO mice at 4 weeks but not at 2 weeks of age (Figures 1, I and L, 2, B and F, and 3A, Supplemental Figure 7). Renal inflammation was also found in 4-week-old dKO mice (Figure 4, Supplemental Figure 9). To determine whether there was any inflammation in the kidneys of Mst1/2 dKO mice at 2 weeks of age when YAP was not activated (Figure 2, A–C) and tubular injury was not induced yet (Supplemental Figure 7), we performed PCR array analysis for 84 key secreted proteins central to the immune response. Forty-two genes showed a 1.5-fold or greater expression change in Mst1/2 dKO kidneys compared with controls, with 14 genes being upregulated (Supplemental Figure 14, A and B). Eight of the upregulated genes were at barely detectable levels, and only six genes were readily detectable (Supplemental Figure 14B). We performed real-time PCR to validate the upregulation of the six genes and found that only Ccl20, Tnf-α, and Lif were higher in dKO than in control kidneys, whereas Il1rn, Il-4, and Ltb did not change (Figure 7A). Immunohistochemistry showed that TNF-α was predominantly expressed in the tubular cells of 2-week-old control kidneys, and this expression was increased in Mst1/2 dKO kidneys (Figure 7C). In IMCD3 cells, TNF-α expression was increased by Mst1/2 knockdown, whereas expression of Lif, Ccl20, Il1rn, Il-4, and Ltb was not altered (Figure 7B, Supplemental Figure 15). These results suggest that TNF-α is a primary target of Mst1/2 deficiency in renal tubular cells in 2-week-old mice. Our results also indicate that inflammatory response occurred before tubular injury.
Figure 7.
Mst1/2 deficiency increased Tnf-α expression in tubular cells independently of YAP in 2-week-old mice. (A) mRNA levels of Lif, Ccl20, Tnf-α, Il1rn, Il-4, and Ltb in the kidneys of control (Ctrl) and Mst1/2 dKO mice. Kidneys of male mice at 2 weeks of age were used for real-time PCR analysis. (B) mRNA levels of Lif, Ccl20, Tnf-α, Il1rn, Il-4, and Ltb in IMCD3 cells. Cells were transfected with Ctrl or Mst1/2 siRNAs (siMst1/2). The red box indicates that only Tnf-α was increased in both the kidneys and IMCD3 cells. (C) Immunohistochemistry for TNF-α and CCL20 in Ctrl and Mst1/2 dKO kidneys. Paraffin kidney sections from 2-week-old mice were used for immunohistochemistry with 3,3′-Diaminobenzidine (DAB) staining (brown). Normal rabbit IgG was used negative Ctrl. Scale bar: 20 µm. (D and E) mRNA levels of (D) Tnf-α as well as (E) Ccl20 and Lif in the kidneys of Ctrl, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice. Kidneys of male mice at 2 weeks of age were used for real-time PCR analysis. (F) Effects of TNF-α on Ccl20 and Lif mRNA levels in IMCD3 cells. Cells were serum starved overnight before they were treated with or without 10 ng/ml TNF-α protein for 6 hours. Cells were collected for analysis for Ccl20 and Lif mRNA levels. (G) Effects of CCL20 and Lif on Tnf-α mRNA levels in IMCD3 cells. Cells were serum starved overnight before they were treated with or without 20 ng/ml CCL20 or 80 ng/ml Lif for 6 hours. Cells were collected for analysis for Tnf-α mRNA levels. n=4/8 (Ctrl/dKO) for (A); n=3 for (B, F, and G); and n=7/9/7 (Ctrl/dKO/tKO) for (D). ns, no significance. *P<0.05; **P<0.01; ***P<0.001.
Because YAP in the kidney was not activated in mice at 2 weeks, this indicates that TNF-α can be induced independent of YAP. This notion was supported by the higher TNF-α levels in Mst1/2/Yap tKO kidneys than in control kidneys in 4-week-old mice (Figure 6G), and it was further validated by the failure of Yap deletion to reduce the TNF-α levels in Mst1/2 dKO mice at 2 weeks of age (Figure 7D).
Ccl20 and Lif were also increased in Mst1/2 dKO kidneys but not altered by Mst1/2 knockdown in IMCD3 cells; thus, the increases in Ccl20 and Lif levels were likely to be indirect effects of Mst1/2 deficiency, and they were most likely to be upregulated by TNF-α. Indeed, TNF-α treatment stimulated Ccl20 and Lif mRNA expression in IMCD3 cells (Figure 7F), whereas CCL20 and Lif had minor effects on TNF-α expression (Figure 7G). CCL20 was also predominantly expressed in the tubular cells of 2-week-old kidneys, and this expression was increased in Mst1/2 dKO kidneys (Figure 7C). As with Tnf-α, the increases in Ccl20 and Lif levels in dKO kidneys were not altered by deletion of Yap (Figure 7E).
Reciprocal Stimulation of TNF-α and YAP
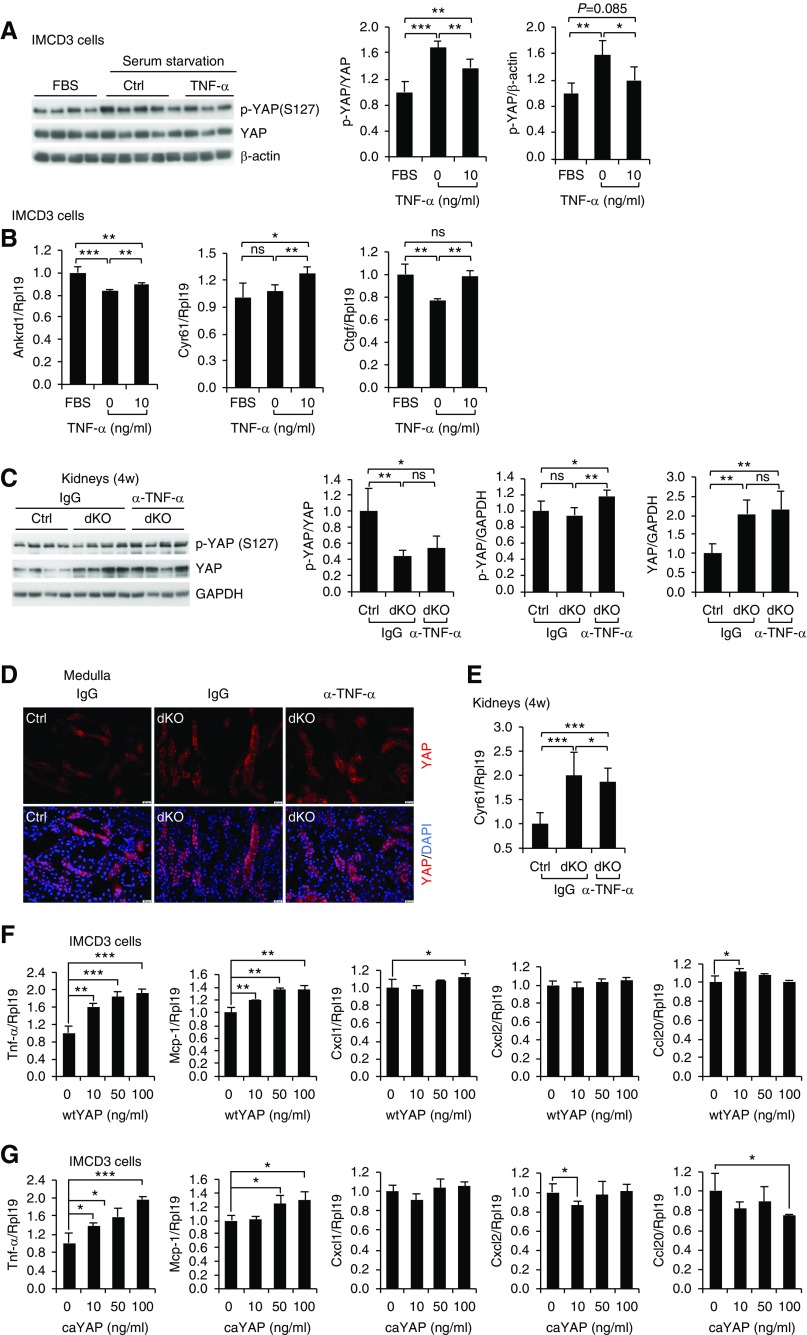
As an attempt to determine whether the elevated TNF-α levels in 2-week-old dKO kidneys may play a role in triggering YAP activation in older dKO kidneys, we treated IMCD3 cells with TNF-α. TNF-α at 2 ng/ml did not alter YAP phosphorylation (Supplemental Figure 16), but TNF-α at 10 ng/ml inhibited YAP phosphorylation (Figure 8A). TNF-α at 10 ng/ml also promoted the expression of the YAP target genes Ankrd1, Ctgf, and Cyr61 (Figure 8B). Conversely, immunoneutralization of TNF-α in Mst1/2 dKO mice at 4 weeks of age inhibited YAP activity as indicated by the increased phospho-YAP levels (Figure 8C) and decreased nuclear YAP levels (Figure 8D) and Cyr61 expression (Figure 8E). These results indicate that TNF-α stimulated YAP activity.
Figure 8.
TNF-α and YAP formed a positive feedback loop. (A) Effects of TNF-α treatment on YAP phosphorylation. IMCD3 cells were serum starved overnight before they were treated with or without 10 ng/ml TNF-α protein or treated with complete medium (FBS) for 2 hours. Cell lysates were used for western blotting for phosphorylated YAP (p-YAP; S217) and total YAP. Quantitative analysis of p-YAP relative to YAP and p-YAP relative to β-actin levels was performed by densitometry. (B) Effects of TNF-α treatment on Ankrd1, Cyr61, and Ctgf mRNA levels. IMCD3 cells were serum starved overnight before they were treated with or without 10 ng/ml TNF-α protein or treated with complete medium (FBS) for 6 hours. (C–E) Effects of immunoneutralization of TNF-α on YAP signaling in Mst1/2 dKO mice. Mice at 4 weeks of age were injected with normal IgG or anti–TNF-α antibody (α-TNF-α). Sixteen to 18 hours later, the mice received another injection of the antibodies or normal IgG. Four hours after the second injection, kidneys were harvested (C) for western blotting for phospho-YAP and YAP, (D) for immunofluorescence for YAP (red), or (E) for real-time PCR analysis for Cyr61 mRNA. (C) Quantitative analysis of p-YAP relative to YAP, p-YAP relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and YAP relative to GAPDH was performed by densitometry. (D) Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar: 20 µm. (F and G) Effects of wtYAP or caYAP on mRNA levels for Tnf-α, Mcp-1, Cxcl1, Cxcl2, and Ccl20. IMCD3 cells were transfected with increasing amounts of (F) wtYAP or (G) caYAP plasmids. The cells were harvested for real-time PCR analysis for Tnf-α, Mcp-1, Cxcl1, Cxcl2, and Ccl20 mRNA levels. n=4 for (B), n=3 for (F and G), and n=7 for (E). ns, no significance. *P<0.05; **P<0.01; ***P<0.001.
We then examined the effects of YAP on expression of Tnf-α, Mcp-1, Cxcl1, Cxcl2, and Ccl20 in IMCD3 cells (Figure 8, F and G). Among those factors, Tnf-α is the one that was more highly and dose-dependently stimulated by both wtYAP (Figure 8F) and caYAP (YAP S127A) (Figure 8G). Interestingly, caYAP also stimulated NF-κB–responsive luciferase activity (Supplemental Figure 17). Therefore, YAP stimulated Tnf-α expression in IMCD3 cells.
Discussion
Despite considerable progress in understanding the biologic functions of the Hippo/YAP pathway, the role of MST1 and MST2 in adult kidney remained unknown. In this study, we found that MST1 and MST2 were predominantly expressed in renal tubules. These observations prompted us to generate tubular cell–specific Mst1/2 dKO mice using the Ksp-Cre mouse line. We did not choose to use Hoxb7-Cre or Six2-Cre mice because these two lines express Cre at much earlier embryonic stages than Ksp-Cre.48,49 The delayed Cre expression may explain why kidney development was not affected by Ksp-Cre–mediated deletion of Sav1.10,11 Consistently, our study showed that deletion of Mst1/2 by Ksp-Cre did not alter kidney weight and structure in newborn mice. These results suggest that Hippo signaling is not critical for late embryonic and neonatal kidney development.
The Hippo pathway is well known for its role in organ size control. A previous study removed Mst1 and Mst2 in all organs in newborn pups and found that the liver, stomach, heart, and spleen were enlarged, but the kidney and lung sizes were not altered.18 Contrary to this observation, our Mst1/2 dKO mice developed renomegaly. Interestingly, a recent study administered tamoxifen into Sav1flox/flox, Ksp-CreER mice from 8 to 9 weeks of age, and 6 weeks later, the kidney size was found to be increased.12 These results are consistent with our results, and they collectively indicate that Hippo signaling also controls kidney size.
It is puzzling to us that Mst1/2 deletion did not increase YAP activity at 2 weeks of age. It would be interesting in future studies to find out the non-MST1/2 mechanisms that retain YAP in the cytoplasm. Nevertheless, our results indicate that Hippo/YAP signaling does not seem to be essential for postnatal kidney growth.
YAP and TAZ are the two effectors of the Hippo signaling pathway, but their individual activation mechanisms have not been clearly demonstrated. In mice at 4 weeks of age, tubular YAP activity was increased by Mst1/2 deletion, but TAZ activity was not changed. This observation is different from the liver-specific Mst1/2 knockout,22,23 and embryonic kidney condensing mesenchyme–specific Lats1/2 knockout,38 in which both YAP and TAZ were activated. In many other studies using cell-specific deletion of Nf2, Sav1, Mst1/2, or Lats1/2, only YAP activity was examined; thus, it is unknown whether YAP and TAZ were differentially regulated in those cells.
We demonstrated that Tnf-α elevation is a primary effect of Mst1/2 deficiency and that it is independent of YAP in 2-week-old mice. It is unknown by this study how Mst1/2 regulates Tnf-α expression. A previous study showed that cleaved MST1 phosphorylated H2B at Ser 14, thus immobilizing RCC1 (a guanine nucleotide exchange factor) on the chromosomes and reducing nuclear RanGTP levels, which led to inactivation of nuclear transport machinery. As a result, nuclear localization signal–containing proteins, including NF-κB-p65, were restricted to the cytoplasm. Knockdown of Mst1 allowed resumption of nuclear entry and accumulation of NF-κB-p65.50 Whether this mechanism was responsible for the increased tubular Tnf-α expression in Mst1/2 dKO kidneys at 2 weeks of age warrants further investigation. Interestingly, we did observe increased NF-κB-p65 levels in the nuclei of tubular cells in a few of Mst1/2 dKO kidneys at 2 weeks of age that did not even have increased Tnf-α levels yet (data not shown), indicating that increased NF-κB signaling occurred before Tnf-α elevation and may be responsible for the initial YAP-independent increases in Tnf-α expression.
YAP was not activated at 2 weeks of age but became activated at 4 weeks of age in Mst1/2 dKO kidneys. We found in IMCD3 cells that TNF-α at 10 ng/ml increased YAP activity, although at a lower level (2 ng/ml), it had no effect. Neutralization of TNF-α reduced YAP activity in Mst1/2 dKO kidneys at 4 weeks of age. Therefore, our in vitro and in vivo experiments both show that TNF-α activates YAP. It is possible that the initial YAP-independent TNF-α elevation may be at least partially responsible for the YAP activation in older mice when TNF-α reached certain levels.
We also found that YAP stimulated Tnf-α expression in IMCD3 cells, suggesting that there is a positive feedback loop between TNF-α and YAP. Consistently, reciprocal activation of the YAP and NF-κB pathways was observed in human colon cancer cells.51 YAP and TAZ were found to promote expression of proinflammatory factors in endothelial cells by facilitating JNK actions.52 In another study, however, it was shown that YAP inhibited LPS-induced endothelial inflammation, whereas TNF-α activated YAP.53 In chondrocytes, TNF-α inhibited YAP/TAZ activity, and YAP attenuated TNF-α– or IL-1β–induced NF-κB signaling in chondrocytes.54 YAP and TAZ also inhibited NF-κB signaling in alveolar epithelial type 2 cells.55 Therefore, how TNF-α/NF-κB signaling and YAP signaling act on each other seems to be cell type and context dependent.
In summary, we found that deletion of Mst1/2 in renal tubular cells led to progressive inflammation, tubular damage, fibrosis, and functional impairment, and these phenotypes were largely mediated by YAP. Tubular TNF-α expression was induced via both YAP-dependent and -independent mechanisms. The positive feedback between TNF-α and YAP may contribute at least in part to the progression of the disease.
Disclosures
None.
Funding
This work was supported by Health and Medical Research Fund 05161376, Hong Kong Food and Health Bureau (to Dr. Xia), and General Research Fund 14164817, Collaborative Research Fund C4024-16W, the Hong Kong Research Grants Council, and Ministry of Science and Technology of China Research Fund grant 2018YFC1312704 (to Dr. Huang).
Supplementary Material
Acknowledgments
We thank Josie Lai and Jean Kung (The Chinese University of Hong Kong) for technical assistance with the electronic microscopy and Corinna Au (The Chinese University of Hong Kong) for assistance with histology. We also thank Dr. Fang Wang (Massachusetts General Hospital) for the NF-κB responsive luciferase reporter.
Dr. Xia and Dr. Xu designed the study; Dr. Xu, Dr. L. Wang, Dr. Zhang, Dr. W. Li, Ms. Y. Wang, Dr. Meng carried out experiments; Dr. Huang, Dr. Lan, Dr. J. Li, Dr. Mak, Dr. Qin, and Dr. Zheng provided key reagents and revised the paper; and all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tubular MST1/2 Deletion and Renal Fibrosis,” on pages 893–894.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101052/-/DCSupplemental.
Supplemental Figure 1. Immunohistochemistry for MST1 and MST2 in control and Mst1/2 dKO kidneys.
Supplemental Figure 2. YAP localization in control Mst1/2 dKO kidneys at 2 weeks of age.
Supplemental Figure 3. YAP localization in control Mst1/2 dKO kidneys at 4 weeks of age.
Supplemental Figure 4. Expression of the YAP target genes Ankrd1, Cyr61, and Ctgf in female control and Mst1/2 dKO kidneys at 4 weeks of age.
Supplemental Figure 5. Expression of phosphorylated and total LATS1 and MOB1 in the kidneys of control and Mst1/2 dKO mice at 2 weeks of age.
Supplemental Figure 6. Expression of phosphorylated and total LATS1 and MOB1 in the kidneys of control, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice at 4 weeks of age.
Supplemental Figure 7. Kidney structure and urinary Ngal levels in control and Mst1/2 dKO mice at 2 weeks of age.
Supplemental Figure 8. E-cadherin localization in control, Mst1/2 dKO, and Mst1/2/Yap tKO kidneys.
Supplemental Figure 9. Inflammation in Mst1/2 dKO kidneys in female mice.
Supplemental Figure 10. Comparison in fibrotic responses in the kidney between male and female Mst1/2 dKO mice.
Supplemental Figure 11. Survival curves comparing control and Mst1/2 dKO mice.
Supplemental Figure 12. Yap expression in Mst1/Mst2/Yap tKO mice and kidney sizes and inflammatory factors in tubule-specific Yap conditional knockout mice at 4 weeks of age.
Supplemental Figure 13. Ratios of kidney weight over body weight in female control, Mst1/2 dKO, and Mst1/Mst2/Yap tKO mice.
Supplemental Figure 14. PCR array analysis for inflammatory cytokines and chemokines in the kidneys of Mst1/2 dKO mice at 2 weeks of age.
Supplemental Figure 15. Effects of Mst1 and Mst2 siRNAs on Mst1 and Mst2 expression in IMCD3 cells.
Supplemental Figure 16. Effects of a low dose of TNF-α on YAP activity in IMCD3 cells.
Supplemental Figure 17. Effects of caYAP on NF-κB–responsive luciferase activity.
References
- 1.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al.: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Levin A, Tonelli M, Bonventre J, Coresh J, Donner J-A, Fogo AB, et al.; ISN Global Kidney Health Summit participants: Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Susztak K: The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 15: 568–588, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D: The hippo signaling pathway in development and cancer. Dev Cell 19: 491–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu FX, Zhao B, Guan KL: Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163: 811–828, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo JH, Guan KL: Interplay between YAP/TAZ and metabolism. Cell Metab 28: 196–206, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Misra JR, Irvine KD: The hippo signaling network and its biological functions. Annu Rev Genet 52: 65–87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reginensi A, Hoshi M, Boualia SK, Bouchard M, Jain S, McNeill H: Yap and Taz are required for Ret-dependent urinary tract morphogenesis. Development 142: 2696–2703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, et al.: Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, et al.: The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci Rep 6: 31931, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kai T, Tsukamoto Y, Hijiya N, Tokunaga A, Nakada C, Uchida T, et al.: Kidney-specific knockout of Sav1 in the mouse promotes hyperproliferation of renal tubular epithelium through suppression of the Hippo pathway. J Pathol 239: 97–108, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Leung JY, Wilson HL, Voltzke KJ, Williams LA, Lee HJ, Wobker SE, et al.: Sav1 loss induces senescence and Stat3 activation coinciding with tubulointerstitial fibrosis. Mol Cell Biol 37: e00565–e00616, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gui Y, Li J, Lu Q, Feng Y, Wang M, He W, et al.: Yap/Taz mediates mTORC2-stimulated fibroblast activation and kidney fibrosis. J Biol Chem 293: 16364–16375, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang M, Yu M, Xia R, Song K, Wang J, Luo J, et al.: Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. J Am Soc Nephrol 28: 3278–3290, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, et al.: YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol 27: 3117–3128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai J, Song X, Wang W, Watnick T, Pei Y, Qian F, et al.: A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev 32: 781–793, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Harris RC: Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol 27: 1689–1700, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al.: Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A 107: 1431–1436, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Medoff BD, Chen L, Li L, Zhang XF, Praskova M, et al.: The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci U S A 105: 20321–20326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, et al.: Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med 8: 352ra108, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Hagenbeek TJ, Webster JD, Kljavin NM, Chang MT, Pham T, Lee HJ, et al. : The Hippo pathway effector TAZ induces TEAD-dependent liver inflammation and tumors. Sci Signal 11: eaaj1757, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, et al.: Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest 127: 137–152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim W, Khan SK, Liu Y, Xu R, Park O, He Y, et al.: Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut 67: 1692–1703, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loforese G, Malinka T, Keogh A, Baier F, Simillion C, Montani M, et al.: Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol Med 9: 46–60, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Wei L, Fan F, Ji S, Zhang S, Geng J, et al.: Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun 6: 6239, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al.: Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16: 425–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D: The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE: Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol 32: 5116–5128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, et al.: Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A 108: E1312–E1320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, et al.: A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125: 987–1001, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W, et al.: Mst1 is an interacting protein that mediates PHLPPs’ induced apoptosis. Mol Cell 38: 512–523, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A: Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 284: 11285–11292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano D, Nguyen LK, Matallanas D, Halasz M, Doherty C, Kholodenko BN, et al.: Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat Cell Biol 16: 673–684, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Du X, Wen J, Wang Y, Karmaus PWF, Khatamian A, Tan H, et al.: Hippo/Mst signalling couples metabolic state and immune function of CD8α+ dendritic cells. Nature 558: 141–145, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Deng Y, Feng B, Mak KK: Mst1/2 kinases modulate glucose uptake for osteoblast differentiation and bone formation. J Bone Miner Res 33: 1183–1195, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, et al.: Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reginensi A, Enderle L, Gregorieff A, Johnson RL, Wrana JL, McNeill H: A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat Commun 7: 12309, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeill H, Reginensi A: Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J Am Soc Nephrol 28: 852–861, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Chen B, Wang Y, Meng C, Huang H, Huang XR, et al.: RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc Natl Acad Sci U S A 115: E1475–E1484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Xu C, Wang Y, Meng C, Liu W, Zhao Y, et al.: Lethal (3) malignant brain tumor-like 2 (L3MBTL2) protein protects against kidney injury by inhibiting the DNA damage-p53-apoptosis pathway in renal tubular cells. Kidney Int 93: 855–870, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Li X, Zhao Y, Meng XM, Wan C, Yang B, et al.: Dragon (repulsive guidance molecule RGMb) inhibits E-cadherin expression and induces apoptosis in renal tubular epithelial cells. J Biol Chem 288: 31528–31539, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, et al.: A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev 29: 1271–1284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O: The rediscovery of uromodulin (Tamm-Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z, et al.: PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14: 880–897, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han SJ, Jang HS, Noh MR, Kim J, Kong MJ, Kim JI, et al.: Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency exacerbates mitochondrial and cell damage after kidney ischemia-reperfusion injury. J Am Soc Nephrol 28: 1200–1215, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, et al.: Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715–726, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao X, Somlo S, Igarashi P: Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, et al.: Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol 277: F599–F610, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Wong CH, Chan H, Ho CY, Lai SK, Chan KS, Koh CG, et al.: Apoptotic histone modification inhibits nuclear transport by regulating RCC1. Nat Cell Biol 11: 36–45, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Wang Q, Gao X, Yu T, Yuan L, Dai J, Wang W, et al.: REGγ controls hippo signaling and reciprocal NF-κB-YAP regulation to promote colon cancer. Clin Cancer Res 24: 2015–2025, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, et al.: Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540: 579–582, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao YY, et al.: YAP controls endothelial activation and vascular inflammation through TRAF6. Circ Res 123: 43–56, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, et al.: Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun 9: 4564, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, et al.: Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest 129: 2107–2122, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.