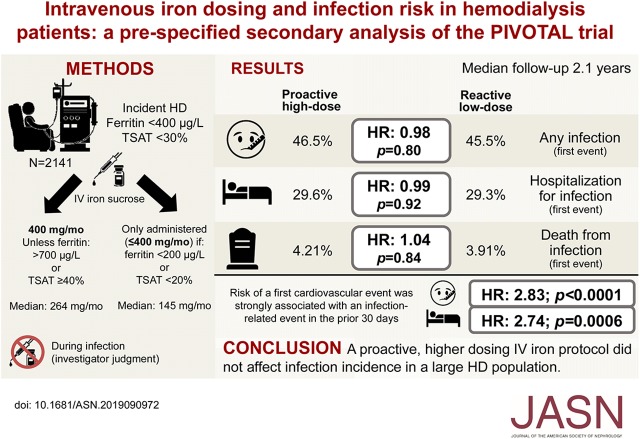
Significance Statement
Experimental and observational data have raised concerns that intravenous (IV) iron might increase the risk of infections. In this analysis from the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial involving 2141 patients on hemodialysis randomly assigned to receive either a high-dose or low-dose IV iron regimen, investigators reported finding no evidence that the two groups differed in incidence of infection, hospitalization for infection, or death from infection. Given the potential cardiovascular benefits of higher-dose IV iron seen in the PIVOTAL trial (due to either a direct effect of the IV iron or a decreased use of erythropoiesis-stimulating agents and thus, less exposure to associated cardiotoxic effects), this analysis provides reassurance for administering higher doses of IV iron than are currently given in many units worldwide.
Keywords: chronic kidney disease, hemodialysis, intravenous iron, infections, randomized controlled trial
Visual Abstract
Abstract
Background
Experimental and observational studies have raised concerns that giving intravenous (IV) iron to patients, such as individuals receiving maintenance hemodialysis, might increase the risk of infections. The Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial randomized 2141 patients undergoing maintenance hemodialysis for ESKD to a high-dose or a low-dose IV iron regimen, with a primary composite outcome of all-cause death, heart attack, stroke, or hospitalization for heart failure. Comparison of infection rates between the two groups was a prespecified secondary analysis.
Methods
Secondary end points included any infection, hospitalization for infection, and death from infection; we calculated cumulative event rates for these end points. We also interrogated the interaction between iron dose and vascular access (fistula versus catheter).
Results
We found no significant difference between the high-dose IV iron group compared with the lose-dose group in event rates for all infections (46.5% versus 45.5%, respectively, which represented incidences of 63.3 versus 69.4 per 100 patient years, respectively); rates of hospitalization for infection (29.6% versus 29.3%, respectively) also did not differ. We did find a significant association between risk of a first cardiovascular event and any infection in the previous 30 days. Compared with patients undergoing dialysis with an arteriovenous fistula, those doing so via a catheter had a higher incidence of having any infection, hospitalization for infection, or fatal infection, but IV iron dosing had no effect on these outcomes.
Conclusions
The high-dose and low-dose IV iron groups exhibited identical infection rates. Risk of a first cardiovascular event strongly associated with a recent infection.
Intravenous iron is widely used to treat iron deficiency, either when oral iron has failed to correct an iron deficit or when oral iron is causing unacceptable side effects. Furthermore, in patients receiving maintenance hemodialysis1 and in those with heart failure,2 it has become incorporated into standard of care. However, there are safety concerns with this treatment.3 Because this mode of administration bypasses the normal physiologic hepcidin-regulated process of iron absorption from the gut,4 there is the potential for iatrogenic iron overload, which is associated with an increased infection risk. There have also been concerns about the potential for intravenous iron to exacerbate infections more acutely (notably gram-negative organisms, mycobacteria, fungi, and Yersinia sp.) both by enhancing bacterial proliferation and by reducing natural defense mechanisms.5 Several studies have shown that, within hours of intravenous administration, there is a reduction in bacterial killing by neutrophils.6 Observational studies examining the relationship between intravenous iron administration and infections have produced conflicting results.7–10 Although some support an increased risk,7,8 others do not.9,10 A meta-analysis of 78 randomized, controlled trials of intravenous iron compared with oral iron or no iron supplementation for treatment of anemia or prevention of blood transfusion suggested that intravenous iron was associated with a significantly higher incidence of infection compared with either oral iron or no iron supplementation among 4400 patients in 24 studies: relative risk, 1.33 (95% confidence interval [95% CI], 1.10 to 1.64).11 A subsequent meta-analysis in patients on dialysis found no association between an increased incidence of infection and intravenous iron, although this included only four studies, all of which were small and of short duration.12
The Proactive IV Iron Therapy in Hemodialysis Patients (PIVOTAL) trial, the largest randomized, controlled trial of iron therapy in any patient population,13 provided an ideal opportunity to examine infection risk with two different treatment strategies with intravenous iron. In this trial, the safety and efficacy of a proactive, high-dose intravenous iron regimen compared with a reactive, low-dose intravenous iron regimen were examined in 2141 patients on hemodialysis followed up for a median of 2.1 years (maximum 4.4 years). Although the primary end point was a composite of all-cause death and nonfatal cardiovascular events, key safety secondary end points focused on infection risk. The statistical analysis plan prespecified analysis of the infection secondary end points.13
Methods
A full description of the study methods, including the study protocol and statistical analysis plan, has previously been reported,13,14 and it is available at NEJM.org.13 In brief, 2141 patients were randomized to a high-dose iron regimen (400 mg monthly, with a cutoff ferritin of 700 µg/L and/or transferrin saturation [TSAT] of 40%) or a low-dose iron regimen (0–400 mg monthly). Importantly, the protocol instructed investigators to withhold iron if the patient developed a new infection deemed sufficient to contraindicate the use of intravenous iron. In such patients, iron therapy was resumed when the investigator judged it to be safe. Patients with active infection at the time of recruitment were also excluded from the trial. Follow-up was for a median of 2.1 years (maximum 4.4 years). The median cumulative iron dose at 1 year was 3.8 g in the high-dose arm and 1.8 g in the low-dose arm. The median monthly iron doses in the groups were 264 versus 145 mg, respectively.13
Safety secondary end points included (1) any infection, (2) hospitalization for infection, and (3) death from infection. Any infection was determined from investigator judgement, and it included patients with mild respiratory, urinary, or catheter infections not considered severe enough to require hospitalization and also, included all infections causing hospitalization or death. Hospitalization for infection was defined as an admission to the hospital caused by an episode of infection that lasted ≥24 hours. Death from infection was determined from the investigator serious adverse event reports, and it was adjudicated by the study end point adjudication committee.
Statistical Methods
Baseline characteristics are summarized as mean (SD) or median (lower quartile, upper quartile) for continuous variables and counts and percentages for categorical data. The data are given for the total group and split on the basis of vascular access status at baseline (catheter versus fistula or graft), with P values for between-group difference on the basis of two-sample t tests or chi-squared tests as appropriate (Table 1).
Table 1.
Characteristics of patients at baseline by vascular access type
| Variable | All Subjects, n=2141 | Catheter at Baseline, n=877 | Fistula/Graft at Baseline, n=1264 | P Value |
|---|---|---|---|---|
| Age, yr | 62.8 (15.01) | 61.2 (15.69) | 63.9 (14.41) | <0.001 |
| Men | 1398 (65.30%) | 556 (63.40%) | 842 (66.61%) | 0.12 |
| Ethnicity | ||||
| White | 1698 (79.31%) | 662 (75.48%) | 1036 (81.96%) | <0.001 |
| Black | 190 (8.87%) | 98 (11.17%) | 92 (7.28%) | |
| Asian | 185 (8.64%) | 79 (9.01%) | 106 (8.39%) | |
| Other | 68 (3.18%) | 38 (4.33%) | 30 (2.37%) | |
| Duration of dialysis treatment, moa | 4.8 (2.83–8.22) | 4.3 (2.66–7.11) | 5.3 (2.98–8.96) | <0.001 |
| AF | 164 (7.66%) | 60 (6.84%) | 104 (8.23%) | 0.24 |
| Heart failure | 86 (4.02%) | 36 (4.10%) | 50 (3.96%) | 0.86 |
| Hypertension | 1557 (72.72%) | 609 (69.44%) | 948 (75.00%) | 0.005 |
| Hyperlipidemia | 535 (24.99%) | 197 (22.46%) | 338 (26.74%) | 0.08 |
| PVD | 187 (8.73%) | 83 (9.46%) | 104 (8.23%) | 0.32 |
| MI | 184 (8.59%) | 64 (7.30%) | 120 (9.49%) | 0.08 |
| Stroke | 176 (8.22%) | 69 (7.87%) | 107 (8.47%) | 0.62 |
| Diabetes | 950 (44.37%) | 403 (45.95%) | 547 (43.28%) | 0.22 |
| Smoking status | ||||
| Current | 249 (11.63%) | 111 (12.66%) | 138 (10.92%) | 0.47 |
| Former | 545 (25.46%) | 220 (25.09%) | 325 (25.71%) | |
| Never | 1347 (62.91%) | 546 (62.26%) | 801 (63.37%) | |
| Weight, kg | 82.1 (20.96) | 80.3 (21.16) | 83.3 (20.73) | 0.001 |
| BMI, kg/m2 | 28.7 (6.91) | 28.2 (6.95) | 29.1 (6.86) | 0.003 |
| SBP, mm Hg | 144.7 (23.68) | 147.6 (24.28) | 142.8 (23.06) | <0.001 |
| DBP, mm Hg | 73.6 (14.80) | 75.8 (15.16) | 72.1 (14.36) | <0.001 |
| Hemoglobin, g/L | 105.6 (13.74) | 104.3 (13.98) | 106.4 (13.50) | <0.001 |
| Ferritin, μg/La | 216.0 (133.00–304.00) | 204.0 (127.00–294.00) | 225.0 (137.00–312.00) | 0.01 |
| TSAT (%)a | 20.0 (16.00–24.00) | 19.0 (15.00–23.00) | 20.0 (16.00–24.00) | <0.001 |
| CRP, mg/La | 6.0 (3.70–14.00) | 6.5 (4.00–14.00) | 6.0 (3.50–14.00) | 0.33 |
| Standardized monthly ESA dosea | 8000.0 (5000.0–12,000) | 8000.0 (6000.0–12,000) | 6000.0 (4000.0–10,000) | 0.006 |
| Primary cause of kidney disease | ||||
| Hypertension | 235 (10.98%) | 88 (10.03%) | 147 (11.63%) | 0.001 |
| Diabetic nephropathy | 712 (33.26%) | 319 (36.37%) | 393 (31.09%) | |
| Glomerular disease | 394 (18.40%) | 171 (19.50%) | 223 (17.64%) | |
| Tubulointerstitial disease | 201 (9.39%) | 83 (9.46%) | 118 (9.34%) | |
| Renovascular disease | 147 (6.87%) | 55 (6.27%) | 92 (7.28%) | |
| Other | 129 (6.03%) | 59 (6.73%) | 70 (5.54%) | |
| Polycystic kidney disease | 117 (5.46%) | 29 (3.31%) | 88 (6.96%) | |
| Unknown | 206 (9.62%) | 73 (8.32%) | 133 (10.52%) | |
| Proactive randomized treatment | 1093 (51.05%) | 449 (51.20%) | 644 (50.95%) | 0.91 |
For categorical variables, number and percentage are reported. For continuous variables, mean and SD are reported. AF, atrial fibrillation; PVD, peripheral vascular disease; MI, myocardial infarction; BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; TSAT, transferrin saturation; CRP, C-reactive protein; ESA, erythropoiesis-stimulating agent.
Median and interquartile range are presented.
Time to first event for any infection, hospitalized infection, and fatal infection were analyzed using Cox proportional hazard models adjusting for randomization stratification variables (vascular access [dialysis catheter versus arteriovenous fistula or graft], diagnosis of diabetes [yes versus no], and duration of hemodialysis treatment [<5 versus ≥5 months]), and hazard ratios (HRs) and 95% CIs were calculated for treatment effects. Time to event curves were calculated as cumulative incidence functions adjusting for the competing risk of deaths not included in the outcome being analyzed. Rates (per 100 patient years) of recurrent infection of any kind and hospitalized infections were compared between treatment groups using the method of Lin et al.15
Because infection rates (usually Staphylococcus sp.) are more common in patients using dialysis catheters compared with those relying on native arteriovenous fistulas,16 the association between type of vascular access and infection rates was also examined. Type of vascular access was recorded monthly on the electronic patient record form during the trial. To simplify the analysis, vascular access was analyzed as “catheter at baseline and for every month of the study follow-up” versus “arteriovenous fistula at baseline and for every month of the study follow-up,” thus excluding any patients who had periods using a catheter and periods using a fistula. Cox models were used to compare the time to first events for all infections and hospitalized infections between these two groups. The models included terms for the access groups, randomized treatment group, the stratification variables for diabetes and duration of hemodialysis treatment, and an interaction term between access group and the randomized treatment group. Cumulative incidence functions split by access group and by access group and randomized treatment group were determined for each end point, adjusting for the competing risk of deaths not included in the outcome being studied.
We created time-varying covariates specifying at a given point in time the most recent iron dose and the current total iron dose. We then determined the association between each of these variables and the outcome of a first infection in time-varying Cox regression models separately in each treatment group adjusting for baseline stratification variables defined by diabetes status, time on dialysis, and vascular access status. These analyses were repeated for the outcome of hospitalized infection. These analyses were also repeated substituting most recent ferritin and TSAT levels for iron dose.
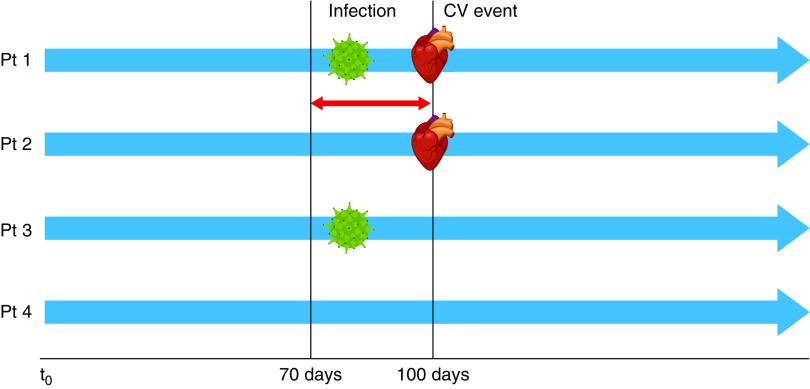
The association between a recent infection and risk of a first cardiovascular event was investigated using infection in the previous 30 days as a time-varying covariate in a Cox regression model adjusted for treatment group and baseline stratification variables (Figure 1). Cardiovascular events were adjudicated by the trial end point adjudication committee blinded to the treatment assignment. The analysis was repeated for infections requiring hospitalization and for any infection. Results reported included HRs, 95% CIs, and P values for the association between presence of a recent infection and a cardiovascular event.
Figure 1.
Schematic representation of the methodology used in the analysis of the association between a recent infection and risk of a first cardiovascular event. Infection in the previous 30 days was used as a time-varying covariate in a Cox regression model adjusted for treatment group and baseline stratification variables (diabetes status, time on dialysis, and vascular access status). Scenarios for four different patients shown. CV, cardiovascular; Pt, patient.
In the vast majority of infections reported, particularly those not requiring hospitalization (but also, for example, in patients hospitalized for pneumonia), no causal infectious agent was identified via culture of fluid, tissue, or blood. Infections were classified according to main organ primarily involved. Where an infectious agent was identified, these were subdivided into gram-positive bacteria, gram-negative bacteria, viruses, and fungi or parasites. Further data on specific organisms are also reported when available.
Results
Rates of Infectious Events
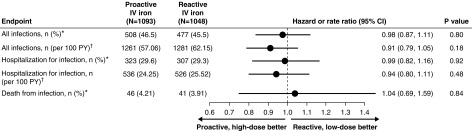
For “all infection episodes,” there were 508 first events (46.5%) in the proactive high-dose arm versus 477 first events (45.5%) in the reactive low-dose arm (HR, 0.98; 95% CI, 0.87 to 1.11; P=0.80) (Figure 2). This represented an incidence of 63.3 per 100 patient years for the high-dose arm versus 69.4 per 100 patient years for the low-dose arm. Corresponding results for “hospitalizations for infections” were 323 first events (29.6%) in the proactive high-dose arm versus 307 first events (29.3%) in the reactive low-dose arm (HR, 0.99; 95% CI, 0.82 to 1.16; P=0.92). For “death from infections,” there were 46 events (4.21%) in the proactive high-dose arm versus 41 first events (3.91%) in the reactive low-dose arm (HR, 1.04; 95% CI, 0.69 to 1.59; P=0.84).
Figure 2.
Comparison of number and percentage of events (expressed as HR) and number of recurrent events per 100 patient years (PY; expressed as rate ratio) for “all infections” and “hospitalization for infection” as well as number and percentage of fatal infections (expressed as HR) between the high-dose intravenous (IV) iron group and the low-dose IV iron group. *Expressed as HR; †expressed as rate ratio. For all endpoints there were near-identical rates of infection for the proactive high-dose and reactive low-dose groups in the study.
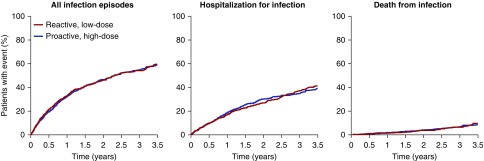
Cumulative event curves for “all infection episodes” were not distinguishable between the high-dose and low-dose treatment assignment arms (Figure 3); 20% of the patients had a first event within the first 6 months, and 40% had a first event by 1.5 years. By 3.5 years, 60% of patients had an infection episode. For “hospitalized infections,” 20% of the patients had a first event within the first year, and 40% had a first event by 3.5 years, with no evidence of a difference between the two groups. For “fatal infections,” the event rate was low with no evidence of a difference between the groups, with most deaths occurring after 1 year of follow-up.
Figure 3.
Comparison of cumulative event curves between the high-dose intravenous iron group and the low-dose intravenous iron group for “all infections,” “hospitalization for infection,” and “death from infection.” For all three endpoints there were near-identical event curves for the proactive high-dose and reactive low-dose groups in the study.
Infection Rates for Patients with Dialysis Catheters versus Patients with Arteriovenous Fistulas
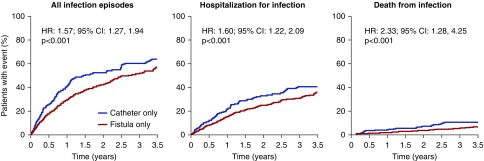
Of the 2141 patients in the study, 260 had a dialysis catheter throughout the entire study period compared with 946 patients who had an arteriovenous fistula throughout the duration of the study. Cumulative event curves for each of the three infection end points are shown in Figure 4. As might be expected, compared with patients with an arteriovenous fistula, patients with a catheter had a higher incidence of having any infection (HR, 1.57; 95% CI, 1.27 to 1.94; P<0.001), a higher incidence of hospitalization for an infection (HR, 1.60; 95% CI, 1.22 to 2.09; P<0.001), and a higher risk of having a fatal infection (HR, 2.33; 95% CI, 1.28 to 4.25; P<0.001).
Figure 4.
Comparison of cumulative event curves between patients dialyzing on a fistula only for the whole study versus those dialyzing on a catheter only for “all infections,” “hospitalization for infection,” and “death from infection.” Patients dialyzing on a catheter had a higher incidence of having any infection, a hospitalization for infection, and a fatal infection compared to those dialyzing on a fistula.
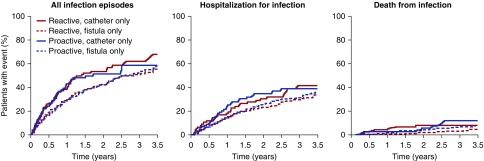
When the risk of infections with “catheter only” versus “fistula only” was compared in relation to the treatment assignment arm, no differences were seen. Thus, patients who were dialyzed on a catheter throughout the entire period of the study had a similar risk of contracting an infection with high-dose iron versus low-dose iron; the same is true for patients dialyzing on an arteriovenous fistula for the entire study (Figures 5 and 6), and this held true for all three infection end points.
Figure 5.
Comparison of cumulative event curves between patients dialyzing on a fistula only for the whole study versus those dialyzing on a catheter only for “all infections,” “hospitalization for infection,” and “death from infection” shown separately for the high-dose group versus the low-dose group. For both groups, patients dialyzing on a catheter had a higher incidence of having any infection, a hospitalization for infection, and a fatal infection compared to those dialyzing on a fistula, but there was no effect of the treatment assignment arm.
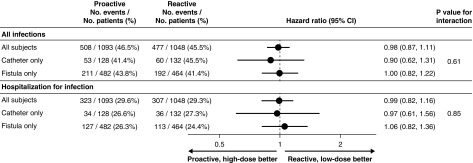
Figure 6.
Forest plot showing HRs and interaction P values for “all infections” and “hospitalization for infection” for all subjects in the trial as well as separated according to patients dialyzing on a fistula only for the whole study versus those dialyzing on a catheter only. Data are adjusted for stratification variables (vascular access, diabetic status, and time on dialysis).
Association between Indices Reflecting Iron Status and Infectious Event/Outcome
There was no evidence of an association between iron status and infection outcomes. The HRs, 95% CIs, and P values are given per 100-unit higher ferritin level and per 5-unit higher TSAT level (Table 2).
Table 2.
Association between iron dose, ferritin, transferrin saturation, and risk of infection
| Association Analyzed | Reactive Low-Dose Iron Group | Proactive High-Dose Iron Group | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Most recent IV iron dose (per 100 mg) and any infection | 1.03 | 0.96 to 1.10 | 0.41 | 1.02 | 0.97 to 1.06 | 0.45 |
| Current total IV iron dose (per 100 mg) and any infection | 1.00 | 0.98 to 1.01 | 0.42 | 1.00 | 0.99 to 1.01 | 0.50 |
| Most recent ferritin (per 100 µg/L) and any infection | 1.04 | 0.98 to 1.10 | 0.16 | 0.97 | 0.93 to 1.01 | 0.12 |
| Most recent TSAT (per 5%) and any infection | 0.90 | 0.85 to 0.96 | <0.001 | 0.97 | 0.92 to 1.01 | 0.15 |
| Most recent IV iron dose (per 100 mg) and hospitalized infection | 1.00 | 0.92 to 1.08 | 0.95 | 1.05 | 0.99 to 1.10 | 0.11 |
| Current total IV iron dose (per 100 mg) and hospitalized infection | 1.00 | 0.99 to 1.02 | 0.45 | 1.00 | 0.99 to 1.01 | 0.53 |
| Most recent ferritin (per 100 µg/L) and hospitalized infection | 1.08 | 1.03 to 1.14 | 0.001 | 0.95 | 0.90 to 1.00 | 0.04 |
| Most recent TSAT (per 5%) and hospitalized infection | 0.88 | 0.82 to 0.95 | <0.001 | 0.92 | 0.87 to 0.98 | 0.006 |
IV, intravenous; TSAT, transferrin saturation.
Association between a Recent Infection and Risk of a First Cardiovascular Event
In the time-updated covariate-adjusted analysis, there were strong associations between the risk of a first cardiovascular event and any infection in the previous 30 days (HR, 2.83; 95% CI, 2.04 to 3.92; P<0.0001); the same was true for hospitalization for infection (HR, 2.74; 95% CI, 1.54 to 4.88; P<0.001).
Characterization of Infectious Agent
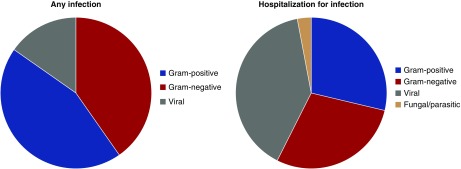
In total, there were 1837 documented infection episodes for any infection. Grouped by organ involvement, 40.2% were of the respiratory tract, 19.4% were unclassified, 20.3% were skin and soft tissue related, and 12.3% were urinary tract related. A total of 144 episodes had an organism identified (64 gram positive, 58 gram negative, and 22 viral). For those infections leading to hospitalization, there was a total of 1130 episodes: 39.4% respiratory, 15.7% sepsis (no specific organ characterized), 8.6% soft tissue or skin, and 11.7% unclassified. In this case, there was a total of 97 events (23 gram positive, 32 gram negative, 39 viral, and 3 fungal/parasitic). Hence, where the infectious agent was identified, similar proportions of gram-positive organisms, gram-negative organisms, and viral agents were seen (Figure 7).
Figure 7.
Proportion of causal infectious organisms for all patients randomized in the PIVOTAL trial where an infectious agent was identified. Similar proportions of gram-positive organisms, gram-negative organisms, and viral agents were seen.
Discussion
The PIVOTAL trial showed no effect of the higher-dosing intravenous iron protocol on infection incidence in a large hemodialysis population. Despite the high-dose iron arm receiving more than double the dose of intravenous iron over the first year and nearly double the median monthly intravenous iron dose overall, no increase in infection incidence was observed compared with the low-dose iron group. Three infection end points were assessed (“all infections,” “hospitalizations for infections,” and “fatal infections”), and the consistency of the findings across all of these provides reassurance that there is no effect of administering higher doses of intravenous iron on incidence of infections.
These findings are at variance with multiple reports in the experimental literature suggesting that iron might enhance bacterial and fungal proliferation5 and also, reduce bacterial defense mechanisms.6 Using the same intravenous iron preparation that was used in the PIVOTAL trial (iron sucrose), Deicher et al.6 found that, within the first 2 hours of dialysis, the percentage of Escherichia coli killed by neutrophils significantly decreased in the group randomized to intravenous iron versus no iron. Observational studies examining the relationship between intravenous iron administration and infections have produced conflicting results, with some for and others against an association.7–10 Several previous reports have indicated that patients dialyzing via a catheter have a greatly enhanced infection risk compared with those using an arteriovenous fistula.16 Similar findings were obtained in the PIVOTAL trial, with significant increases in all three infection end points. However, the trial also allowed us to examine whether there was any effect of iron dosing on the infection risk in relation to vascular access. In both subpopulations of patients (catheter only and fistula only), there was no effect of iron dosing on the incidence of infection across all three end points; this may be reassuring for certain subsets of vulnerable patients, such as the frail elderly dialyzing via long-term catheters.
The design of the study also allowed us to examine whether there was any association between an infection episode and a subsequent cardiovascular event, which has been reported in a number of observational studies both outside the dialysis setting17–19 and in a large United States–based dialysis cohort.20
An analysis of the Atherosclerosis Risk in Community study,17 for example, showed that both inpatient and outpatient infections seemed to be a trigger for a cardiovascular event. In 1312 patients with incident coronary heart disease and 727 patients with incident stroke, the 30-day odds ratio for the event following an inpatient infection was 8.39 (95% CI, 4.92 to 14.41) and the 30-day odds ratio for the event following an outpatient infection was 2.69 (95% CI, 2.14 to 3.37) compared with a control period.
In a nondialysis CKD prospective cohort study (the Canadian Study of Prediction of Risk and Evolution to Dialysis, Death and Interim Cardiovascular Events over Time), Cheikh Hassan et al.18 found that infection (i.e., positive culture, use of antibiotics, or hospitalization for infection) was associated with an increased risk of cardiovascular events, ESKD, and mortality (median follow-up 3.5 years).
In a cohort of 16,874 patients on hemodialysis in the US Renal Data System aged 65–100 years old, Dalrymple et al.20 estimated the relative incidence of a cardiovascular event within 90 days after an infection-related hospitalization compared with other times not within 90 days of such a hospitalization. The authors found that the risk of a cardiovascular event was increased by 25% in the first 30 days after an infection and that it was overall increased 18% in the 90 days after an infection-related hospitalization relative to control periods.20
In the PIVOTAL trial, we confirmed this association in a hemodialysis population randomized to two different intravenous iron regimens. The strengths of our analysis include the facts that data were collected prospectively via an electronic patient record form and that cardiovascular end points were adjudicated by a blinded end point committee. As with all previous studies examining this association, this does not prove causality. An alternative explanation is that patients at risk of a future cardiovascular event are more susceptible to infections, although this has less biologic plausibility.
The lack of any effect of the exploratory analysis of iron dose on infection risk is perhaps no surprise. Because there was no effect overall in the randomized study, it would perhaps have been surprising to have found any significant association in these analyses. The same is true with the analysis of iron markers on infection risk. Because the randomized treatment induced significant differences in ferritin and TSAT between the groups and there was no difference in infections, there is no evidence of a causal relationship between ferritin concentrations or TSAT and infection. Hence, any associations in the analyses can be attributed to reverse causality (during inflammatory states, the serum ferritin increases as an acute-phase protein, and the transferrin saturation is reduced). Infection leads to raised ferritin and reduced TSAT, and hence, one might have expected a recent raised ferritin or lower TSAT observed during an evolving infection to be associated with an increased risk of subsequent infection. Given a possibly longer delay in hospital admission after the initial onset of infection, the association might be stronger in hospitalized infection.
The strengths of this study include the study design (randomized and controlled), the prospective data capture via an electronic database on a monthly basis, and the adjudication of cardiovascular events by an independent committee blinded to the treatment assignment. There are, however, a few limitations to the study. The first is that this is a secondary analysis and that it is not the primary aim of the study, albeit the analysis was prespecified. Although this is the largest randomized trial of iron in any patient population, it was conducted in a cohort of patients receiving hemodialysis. This is a very specific group of patients, with different infection risks and profiles from other patient groups. Given the high incidence of infection in this group of patients, it was a good way to test the hypothesis of iron treatment on the risk of infection, but extrapolating the findings to other patient populations may not be justified. The intravenous iron preparation used in the PIVOTAL trial was iron sucrose. Whether the findings in this study can be extrapolated to other intravenous iron preparations is unknown. In particular, whether the doses of iron sucrose used in the PIVOTAL trial are equivalent to the same doses of other iron preparations is highly questionable, and caution should be exercised in this regard. The follow-up, although adequate, also does not allow extrapolation of results beyond the study period (median follow-up 2.1 years; maximum follow-up 4.4 years). We acknowledge that only 56% of the population could be included in the analysis comparing patients with “fistula only” versus “catheter only,” and therefore, there may potentially be issues with a lack of power to be certain of this finding. Finally, the study does not exclude the possibility that even higher doses of intravenous iron could be harmful in exacerbating infections, which has recently been found in an observational study.8
Nevertheless, the clarity of the findings across all three infection end points as well as the closeness of the HRs to 1.0 provide reassurance that patients recently starting hemodialysis exposed to an intravenous iron regimen of 400 mg iron sucrose monthly, maintaining ferritin concentrations around 600–700 µg/L, were not at increased risk of infections compared with the less-intensive iron strategy. Given the potential cardiovascular benefits seen in the PIVOTAL trial,13 this analysis provides further support for administering higher doses of intravenous iron than are currently given in many units worldwide.
Disclosures
Dr. Anker reports grants and personal fees from Vifor Int, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Servier, grants and personal fees from Abbott Vascular, personal fees from Impulse Dynamics, and personal fees from SJM outside the submitted work. Dr. Bhandari reports personal fees from Pharmacosmos and personal fees from Vifor Pharma during the conduct of the study. Dr. Ford reports grants from Kidney Research UK during the conduct of the study. Dr. Kalra reports grants and personal fees from Vifor during the conduct of the study as well as grants and personal fees from Vifor, personal fees from Pharmacosmos, grants and personal fees from Astellas, personal fees from Bayer, personal fees from MundiPharma, personal fees from Napp, personal fees from AstraZeneca, grants from BergenBio, personal fees from Boehringer Ingelheim, and personal fees from Novonordisk outside the submitted work. Dr. Mark reports personal fees and nonfinancial support from Vifor, personal fees from Astrazeneca, grants from Boehringer Ingelheim, personal fees and nonfinancial support from Pharmacosmos, personal fees from Janssen, personal fees from Novartis, personal fees from Pfizer, personal fees from Bristol Myers Squibb, and personal fees and nonfinancial support from Napp outside the submitted work. Dr. Macdougall reports grants and personal fees from Vifor Pharma outside the submitted work. Dr. McMurray reports nonfinancial support and other from AstraZeneca during the conduct of the study as well as other from Bayer, nonfinancial support and other from Cardiorentis, nonfinancial support and other from Amgen, nonfinancial support and other from Oxford University/Bayer, nonfinancial support and other from Theracos, nonfinancial support and other from Abbvie, other from DalCor, other from Pfizer, other from Merck, nonfinancial support and other from Novartis, nonfinancial support and other from Glaxo Smith Kline, other from Bristol Myers Squibb, nonfinancial support and other from Vifor-Fresenius, nonfinancial support and other from Kidney Research UK, and nonfinancial support and other from Novartis outside the submitted work. Dr. Robertson reports grants from University of Glasgow during the conduct of the study. Dr. Wheeler reports grants and personal fees from Vifor Fresenius during the conduct of the study as well as personal fees from Amgen, personal fees from AstraZeneca, personal fees from Bayer, personal fees from Boehringer Ingehiem, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Napp, personal fees from Mundipharma, and personal fees from Reata outside the submitted work. All remaining authors have nothing to disclose.
Funding
The Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial was funded by Kidney Research UK, which was supported by an unrestricted grant from Vifor Fresenius Medical Care Renal Pharma Ltd.
Acknowledgments
The authors acknowledge the contributions of both Kidney Research UK and the National Institute for Health Research Clinical Research Network (Renal Specialty Group) in the successful delivery of the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial. They also thank the large number of nurses and staff at each of the dialysis centers participating in the trial. Finally, they are grateful to the 2589 patients who participated in and committed themselves to this trial.
This was an academic-led trial, and Vifor Fresenius Medical Care Renal Pharma Ltd. had no input into the study design or delivery of the trial, but they were kept abreast of the progress of the trial via regular study reports and newsletters. Vifor Fresenius Medical Care Renal Pharma Ltd. also provided iron sucrose for the trial free of charge.
The PIVOTAL trial sites and investigators are as follows. England: Basildon and Thurrock Hospital, Basildon: Georgia Winnett; Bradford Teaching Hospital, Bradford: Habib Akbani; Churchill Hospital, Oxford: Christopher Winearls; City General Hospital, Stoke-on-Trent: Julie Wessels; Coventry University Hospital, Coventry: Waqar Ayub; Derriford Hospital, Plymouth: Andrew Connor; Freeman Hospital, Newcastle: Alison Brown; Gloucestershire Royal Hospital, Gloucestershire: Jim Moriarty; Guy’s and St Thomas’ Hospital, London: Paramit Chowdhury; Hammersmith Hospital, London: Megan Griffiths; Heartlands Hospital, Birmingham: Indranil Dasgupta; Hull Royal Infirmary, Hull: Sunil Bhandari; Kent and Canterbury Hospital, Canterbury: Timothy Doulton; King’s College Hospital, London: Iain Macdougall; Leicester General Hospital, Leicester: Jonathan Barratt; Lister Hospital, Stevenage: Enric Vilar; Manchester Royal Infirmary, Manchester: Sandip Mitra; New Cross Hospital, Wolverhampton: Babu Ramakrishna, Johann Nicholas; Norfolk and Norwich Hospital, Norwich: Calum Ross; Northern General Hospital, Sheffield: Arif Khwaja; Nottingham City Hospital, Nottingham: Matt Hall; Queen Alexandra Hospital, Portsmouth: Adam Kirk; Queen Elizabeth Hospital, Birmingham: Stuart Smith, Mark Jesky, Clara Day; Royal Berkshire Hospital, Reading: Bassam Alchi; Royal Cornwall Hospital, Cornwall: Jon Stratton; Royal Devon and Exeter Hospital, Exeter: Helen Clarke; Royal Free Hospital, London: Stephen Walsh; Royal Liverpool Hospital, Liverpool: Rebecca Brown; Royal London Hospital, London: Kieran McCafferty; Royal Preston Hospital, Preston: Laurie Solomon; Royal Shrewsbury Hospital, Shrewsbury: Suresh Ramadoss, Babu Ramakrishna; Royal Sussex Hospital, Brighton: Kolitha Basanyake, Sarah Lawman; Salford Royal Hospital, Manchester: Phil Kalra; Southend University Hospital, Southend: Gowrie Balasubramaniam; Southmead Hospital, Bristol: Albert Power; St George’s Hospital, London: Debasish Banerjee; St Helier Hospital, Carlshalton: Pauline Swift; St James’ Hospital, Leeds: Matt Wellberry-Smith; University Hospital, Aintree: Christopher Goldsmith; and Wirral University Teaching Hospital, Wirral: Thomas Ledson. Wales: Morriston Hospital, Swansea: Ashraf Mikhail; and University Hospital, Cardiff: Ruth Benzimra. Scotland: Ninewells Hospital, Dundee: Samira Bell, Alison Severn; Royal Infirmary of Edinburgh, Edinburgh: John Neary; Victoria Hospital, Kirkcaldy: Arthur Doyle; and Western Infirmary, Glasgow: Peter Thomson. Northern Ireland: Altnagelvin Hospital, Derry: Girish Shivashankar; Antrim Area Hospital, Antrim: Stephanie Bolton, Michael Quinn; Belfast City Hospital, Belfast: Peter Maxwell; and Daisy Hill Hospital, Newry: John Harty. The PIVOTAL committees and coordinating groups are as follows. Steering committee: Dr. Macdougall (chair), Dr. Ford (biostatistician), Dr. Anker, Dr. Bhandari, Dr. Farrington, Dr. Kalra, Dr. McMurray, Dr. Tomson, Dr. Wheeler, and Dr. Winearls. End point adjudication committee: Dr. McMurray (chair), Eugene Connolly, Pardeep Jhund, Michael MacDonald, Dr. Mark, Mark Petrie, and Matthew Walters. Independent data monitoring committee: Alan Jardine (chair), Janet Peacock (biostatistician), Chris Isles, and Donal Reddan. Independent Data and Biostatistical Centre, Robertson Centre for Biostatistics, University of Glasgow: Dr. Ford, Heather Murray, Kirsty Wetherall, Sharon Kean, Claire Kerr, Sarah Boyle, Robbie Wilson, Jane Aziz, Eleanor Dinnett, Amanda Reid, Claire Burton, Ross Clarke, and Neil Hillen. Clinical Coordinating Centre, King’s College Hospital, London: Dr. Macdougall, Ms. White, Ms. Reid, and Sadiq Andani.
Dr. Ford and Ms. Robertson provided biostatistical support. Dr. Macdougall conceived the study, contributed to the study design and statistical analysis plan, and developed the first draft of the manuscript, which was critically reviewed and revised by the other authors. All other authors contributed to the study design and statistical analysis plan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Value of Intravenous Iron: Beyond the Cave of Speculation,” on pages 896–897.
Contributor Information
Collaborators: G Winnett, H Akbani, C Winearls, J Wessels, W Ayub, A Connor, A Brown, J Moriarty, P Chowdury, M Griffiths, I Dasgupta, S Bhandari, T Doulton, I Macdougall, J Barratt, E Vilar, S Mitra, B Ramakrishna, J Nicholas, C Ross, A Khwaja, M Hall, A Kirk, S Smith, M Jesky, C Day, B Alchi, J Stratton, H Clarke, S Walsh, R Brown, K McCafferty, L Solomon, S Ramadoss, B Ramakrishna, K Basanyake, S Lawman, P Kalra, G Balasubramaniam, A Power, D Banerjee, P Swift, M Wellberry-Smith, C Goldsmith, T Ledson, A Mikhail, R Benzimra, S Bell, A Severn, J Neary, A Doyle, P Thomson, G Shivashankar, S Bolton, M Quinn, P Maxwell, J Harty, I Ford, S Anker, K Farrington, J McMurray, C Tomson, D Wheeler, E Connolly, P Jhund, M MacDonald, P Mark, M Petrie, M Walters, A Jardine, J Peacock, C Isles, D Reddan, H Murray, K Wetherall, S Kean, C Kerr, S Boyle, R Wilson, J Aziz, E Dinnett, A Reid, C Burton, R Clarke, N Hillen, C White, C Reid, and S Andani
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group: KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.; ESC Scientific Document Group: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129–2200, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, et al.; Conference Participants: Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Ganz T: Hepcidin and iron regulation, 10 years later. Blood 117: 4425–4433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Vecchio L, Longhi S, Locatelli F: Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J 9: 260–267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deicher R, Ziai F, Cohen G, Müllner M, Hörl WH: High-dose parenteral iron sucrose depresses neutrophil intracellular killing capacity. Kidney Int 64: 728–736, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Cole SR, Kshirsagar AV, Fine JP, Stürmer T, Brookhart MA: Safety of dynamic intravenous iron administration strategies in hemodialysis patients. Clin J Am Soc Nephrol 14: 728–737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, et al.: Variation in intravenous iron use internationally and over time: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 28: 2570–2579, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Tangri N, Miskulin DC, Zhou J, Bandeen-Roche K, Michels WM, Ephraim PL, et al.; DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Investigators: Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 30: 667–675, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litton E, Xiao J, Ho KM: Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: Systematic review and meta-analysis of randomised clinical trials. BMJ 347: f4822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hougen I, Collister D, Bourrier M, Ferguson T, Hochheim L, Komenda P, et al.: Safety of intravenous iron in dialysis: A systematic review and meta-analysis. Clin J Am Soc Nephrol 13: 457–467, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al.; PIVOTAL Investigators and Committees: Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380: 447–458, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al.; on behalf of the PIVOTAL Trial investigators: Randomized trial comparing proactive, high-dose versus reactive, low-dose intravenous iron supplementation in hemodialysis (PIVOTAL): Study design and baseline data. Am J Nephrol 48: 260–268, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ, Yang I, Ying Z: Semiparametric regression for the mean and functions of recurrent events. J R Stat Soc B 62: 711–730, 2000 [Google Scholar]
- 16.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al.: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan LT, Lutsey PL, Pankow JS, Matsushita K, Ishigami J, Lakshminarayan K: Inpatient and outpatient infection as a trigger of cardiovascular disease: The ARIC Study. J Am Heart Assoc 7: e009683, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheikh Hassan HI, Tang M, Djurdjev O, Langsford D, Sood MM, Levin A: Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int 90: 897–904, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Musher DM, Abers MS, Corrales-Medina VF: Acute infection and myocardial infarction. N Engl J Med 380: 171–176, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Dalrymple LS, Mohammed SM, Mu Y, Johansen KL, Chertow GM, Grimes B, et al.: Risk of cardiovascular events after infection-related hospitalizations in older patients on dialysis. Clin J Am Soc Nephrol 6: 1708–1713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]