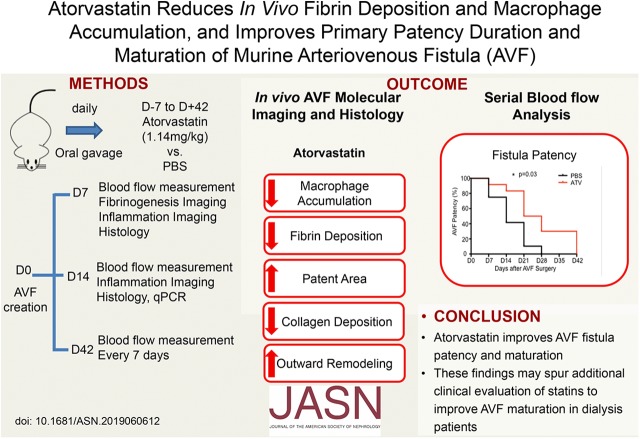
Significance Statement
Experimental studies have established that inflammatory and thrombogenic responses play critical roles in patency and maturation of arteriovenous fistulas placed surgically for dialysis vascular access. In this study of mice given atorvastatin or PBS starting 7 days before creation of an arteriovenous fistula, use of atorvastatin was associated with favorable outward remodeling, preserved arteriovenous blood flow, and longer duration of primary arteriovenous fistula patency. These statin-mediated benefits occurred following reductions in the thrombogenic and inflammatory macrophage response detected within 2 weeks after arteriovenous fistula creation. These findings provide insights into in vivo molecular mechanisms that underlie primary arteriovenous fistula failure, provide a foundation to test novel pharmacotherapeutics that aim to improve arteriovenous fistula maturation, and support further clinical evaluation of statin therapy to improve maturation and patency.
Keywords: arteriovenous fistula, arteriovenous access, dialysis access, thrombosis, chronic inflammation
Visual Abstract
Abstract
Background
Arteriovenous fistulas placed surgically for dialysis vascular access have a high primary failure rate resulting from excessive inward remodeling, medial fibrosis, and thrombosis. No clinically established pharmacologic or perisurgical therapies currently address this unmet need. Statins’ induction of multiple anti-inflammatory and antithrombotic effects suggests that these drugs might reduce arteriovenous fistula failure. Yet, the in vivo physiologic and molecular effects of statins on fistula patency and maturation remain poorly understood.
Methods
We randomized 108 C57Bl/6J mice to receive daily atorvastatin 1.14 mg/kg or PBS (control) starting 7 days before end-to-side carotid artery–jugular vein fistula creation and for up to 42 days after fistula creation. We then assessed longitudinally the effects of statin therapy on primary murine fistula patency and maturation. We concomitantly analyzed the in vivo arteriovenous fistula thrombogenic and inflammatory macrophage response to statin therapy, using the fibrin-targeted, near-infrared fluorescence molecular imaging agent FTP11-CyAm7 and dextranated, macrophage-avid nanoparticles CLIO-VT680.
Results
In vivo molecular-structural imaging demonstrated that atorvastatin significantly reduced fibrin deposition at day 7 and macrophage accumulation at days 7 and 14, findings supported by histopathologic and gene-expression analyses. Structurally, atorvastatin promoted favorable venous limb outward remodeling, preserved arteriovenous fistula blood flow, and prolonged primary arteriovenous fistula patency through day 42 (P<0.05 versus control for all measures).
Conclusions
These findings provide new in vivo evidence that statins improve experimental arteriovenous fistula patency and maturation, indicating that additional clinical evaluation of statin therapy in patients on dialysis undergoing arteriovenous fistula placement is warranted.
Compared with prosthetic grafts or indwelling catheters, the arteriovenous fistula (AVF) furnishes the preferred surgical dialysis access for patients with advanced renal disease due to its longer patency rate, lower infection rate, and lower associated mortality rate.1 Disappointingly, AVFs have a primary unassisted patency rate of <60%, increasing patient morbidity and mortality due to reliance on non-AVF dialysis access.2 In addition, primary AVF failure requires recourse to endovascular or surgical procedures to attempt to salvage the AVF, which beyond the risk and burden for patients, entails up to fourfold higher healthcare costs.3 Despite the widespread recognition of a need for higher primary AVF patency rates, no clinically established pharmacologic or perisurgical therapies currently address this unmet need.4 Therefore, further understanding of the mechanisms of AVF failure and development of new clinical strategies to reduce AVF failure hold vital interest for the dialysis community.
AVF failure involves exaggerated constrictive remodeling, medial fibrosis, venous stenosis and thrombosis after the vascular injury, and altered flow dynamics produced by surgical AVF creation.5–7 Although recent clinical studies show that pre-existing or postoperative neointimal hyperplasia does not associate with AVF failure,8,9 impaired outward remodeling and excessive postoperative medial fibrosis both associate with AVF failure.7,10 Beyond venous stenosis as a cause of AVF failure, fistula thrombosis is common and occurs in approximately one in five patients within 6 weeks after fistula creation.11 Studies further show that patients with hypercoagulable states experience greater rates of access thrombosis, suggesting the potential for antithrombotic treatment to improve fistula patency and maturation.12,13 However, antiplatelet-agent administration with either clopidogrel or ticlopidine, while improving AVF patency, does not increase the proportion of fistulas that are suitable for dialysis.11,14 In addition, although experimental studies demonstrate the importance of the inflammatory response in the pathogenesis of AVF failure,15–21 to date clinical anti-inflammatory therapies have not yet increased primary AVF maturation rates.11,22,23 Therefore, therapies likely to provide durable AVF maturation will likely need to promote both favorable outward remodeling and reduced thrombosis rates.
Statins—widely prescribed, effective therapeutic agents approved by the Food and Drug Administration—have pleiotropic anti-inflammatory and antithrombotic properties,24 including the ability to inhibit thrombus formation and macrophage infiltration in vivo in vascular disease.25 Statins thus appear attractive as agents to improve AVF maturation and prolong AVF patency, particularly given their demonstrated tolerability in patients on dialysis.26 Clinically, several observational studies suggest that statins might improve AVF outcomes,26–28 although a retrospective study and meta-analysis showed that statin therapy had a neutral effect on the primary fistula failure rate,29,30 albeit acknowledging significant methodologic limitations due to heterogeneity in statin studies. In particular, a clinical study found a distinct effect of different statins on AVF, with only a high dose of atorvastatin (ATV) associating with a lower risk of one-stage AVF primary failure. In addition, the effects of statins on AVF outcome depended on the type of the fistulas.26 Without a definitive randomized trial of statin therapy, it remains unclear whether statins can improve AVF usability rates. Therefore, studies to probe the mechanisms of statins on AVF maturation could shed light on whether statins merit further clinical exploration to improve AVF maturation rates.
To address several knowledge gaps regarding statins and their effects on in vivo AVF patency and pathobiology, we investigated the ability of statin administration to improve AVF maturation and prolong primary AVF patency in mice. In a jugular vein-to-carotid artery AVF model, mice randomly received ATV or saline control and then underwent investigation using in vivo molecular imaging of fibrin deposition and inflammatory macrophage accumulation, as well as concomitant assessment of AVF patency by serial measurements of AVF blood flow. Following in vivo studies, resected AVF underwent gene-expression analysis and histopathologic analyses of AVF structure, collagen content, wall thickness, and remodeling to assess putative statin benefits on AVF healing.
Methods
Murine Jugular Vein-to-Carotid Artery AVF Model
All animal procedures were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital (MGH) and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH). C57BL/6 mice used in this study were 12–16 weeks old (n=113; 108 male; five female; Jackson Laboratory, Bar Harbor, ME). In a pilot study, five female mice that underwent AVF surgery did not develop durable AVF patency, with all AVFs occluded by 7–14 days (Supplemental Figure 1). Therefore, the rest of the study used male mice. The average weight of male mice at the time of AVF surgery was 28.9±1.2 g (n=20).
Statin Therapy
At 7 days before AVF creation, mice were randomized to an ATV or PBS group (Figure 1). ATV (McKesson Medical-Surgical, Jacksonville, FL) or PBS was administered via daily oral gavage using a straight 22-gauge feeding needle. The ATV group received a daily dose of 1.14 mg/kg dissolved in 0.1 ml of PBS.25 We used an ATV dose of 1.14 mg/kg, which corresponded to an 80-mg dose administered to a 70-kg patient25; ATV was chosen given its high potency and established clinical effectiveness.31 The PBS control group received daily oral gavage of 0.1 ml of PBS.
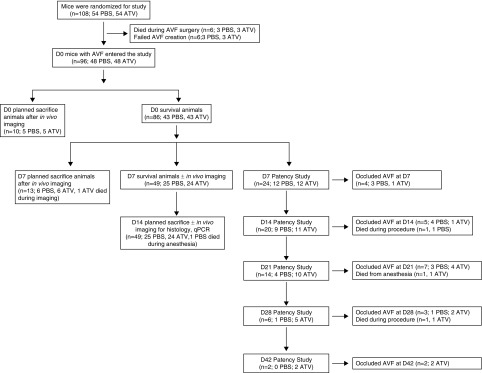
Figure 1.
Study flowchart. C57Bl/6J mice (n=108) were randomized to receive daily atorvastatin 1.14mg/kg or PBS, starting 7 days before end-to-side carotid artery-jugular vein fistula creation. A total of 12 mice died during AVF surgery or failed AVF creation; thus 96 mice entered the study. Mice were then divided into different groups as shown in the study flowchart.
AVF Surgery
AVFs were created via end-to-side anastomosis of the ipsilateral internal jugular vein and carotid artery as previously established by Wong et al.32 Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (12 mg/kg). After blunt tissue dissection, the distal end of the jugular vein was ligated and the vein was transected just proximal to the ligation, and then mobilized. An arteriotomy (approximate diameter of 1.0 mm) was next made in the carotid artery. The carotid arterial and jugular vein were flushed with heparinized saline (100 U/ml) before surgical creation of the anastomosis. An end-to-side external jugular vein-to-carotid artery anastomosis was then performed using 10-0 Ethilon sutures (Figure 2A). Sham-surgery controls were performed using blunt dissection of the contralateral left carotid artery and jugular vein, without anastomosis creation. Both surgical incisions were closed using 7-0 Ethicon sutures. Each mouse was recovered and returned to the animal facility. After surgery, all animals received daily 300 μl heparinized saline intraperitoneally for 3 days. The AVF surgery survival rate was >80%.
Figure 2.
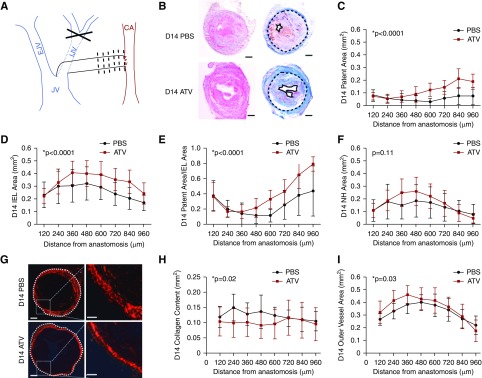
ATV treatment increases day 14 fistula patent area and decreases collagen deposition in the vessel wall. (A) AVF creation using end-to side internal jugular vein-to-carotid artery anastomosis. Black dotted lines depict imaging and histologic analyses performed at 120-μm intervals from the anastomosis. (B) Representative hematoxylin and eosin and Carstairs staining showing an increase in patent area and IEL area in ATV-treated animals compared with PBS-treated animals. Black solid line, patent area; black dotted line, IEL. Scale bar, 100 μm. (C) Fistula patent area measured every 120 μm, revealing an increase in patent area in ATV-treated animals (P<0.001 versus PBS group). (D) IEL area at day 14 in ATV-treated mice was higher (P<0.001 versus PBS group), indicating greater positive AVF remodeling. (E) The ratio of the patent area to IEL area, a measure of AVF remodeling, was significantly higher in ATV-treated animals (P<0.001). (F) Neointimal hyperplasia (NH) area at day 14 was comparable between groups (P=0.11). (G) Representative polarized micrographs of PSR-stained sections from AVFs on day 14 shows vein wall collagen (red) from ATV- and PBS-treated animals. White dotted line, collagen-defined outer AVF border. (H) The total vein wall collagen content was significantly reduced in ATV-treated animals at day 14 (P=0.02 versus PBS). (I) On day 14, the collagen-enclosed area, a measure of AVF positive remodeling, was significantly larger in ATV-treated animals (P=0.03). Error bars display the SD. The two-way ANOVA test was used to assess for statistically significant differences in anatomic AVF parameters between ATV and PBS groups as a function of distance from the anastomosis. P<0.05 was considered statistically significant. CA, carotid artery; D14, day 14; EJV, external jugular vein; IJV, internal jugular vein; JV, jugular vein.
AVF Blood Flow Measurement and Primary Study End Point
Blood flow in AVFs was measured intraoperatively 15 minutes after AVF creation using a Doppler flow probe (0.5PSB476; Transonic System Inc., Ithaca, NY). While mice were anesthetized using isoflurane (SomnoSuite; Kent Scientific Corporation, Torrington, CT), the Doppler probe was placed directly under the fistula venous outflow to measure blood flow rates. In animals undergoing euthanasia at day 7 or 14 (n=24), the AVF blood flow rate measured before euthanasia was compared with the degree of venous stenosis on histopathologic images obtained at 480 μm away from the anastomosis. The percentage stenosis was defined as the (intima area)/(intima area+patent area)×100%.32 The AVF blood flow rate was then measured serially in vivo weekly until AVF blood flow was occluded (n=24, defined as blood flow rates <0.1 ml/min), and served as the primary study end point.
Intravital Microscopy
Fibrin deposition and inflammatory macrophage responses post-AVF creation were evaluated in vivo using epifluorescence intravital microscopy (IVM; Eclipse 90i; Nikon, Tokyo, Japan) with targeted molecular imaging agents, as previously performed in our laboratory.25,33–36 Before in vivo imaging, anesthetized mice received intravenous (i.v.) FITC-conjugated dextran (FITC-dextran; mol wt, 2,000,000; excitation [ex]/emission [em], 490/520 nm; 0.5 mg in 100 μl PBS; Sigma) to obtain angiograms for anatomic coregistration.37
In Vivo Molecular Imaging of Fibrin Deposition
A subgroup of mice underwent assessment of fibrin deposition at day 0 (n=5 in each group; Supplemental Figure 2) or day 7 (n=9 in each group) post-AVF creation. Fibrin deposition in the AVF was evaluated using the fibrin-targeted near-infrared fluorescence (NIRF) molecular imaging agent FTP11-CyAm7 (150 nmol/kg; ex/em, 750/770 nm; Center for Systems Biology Chemistry Core at MGH).34,35,38 For acute day 0 fibrin imaging, FTP11-CyAm7 was i.v. injected 75 minutes after AVF creation, followed by IVM 45 minutes later. On day 7 post-AVF surgery, FTP11-CyAm7 was injected retro-orbitally 45 minutes before in vivo imaging. The in vivo FTP11-CyAm7 fluorescence signal was collected using an epifluorescence microscope (Eclipse 90i) equipped with filters for CyAm7 (ex, 710/75 nm; em, 810/90 nm).
In vivo Molecular Imaging of Macrophage Accumulation
A subgroup of AVF mice underwent in vivo assessment of the subacute AVF macrophage inflammatory response at day 7 (n=10 in each group) or day 14 (n=5 in each group) post-AVF surgery. Macrophages in vivo were visualized via dextranated macrophage-avid nanoparticles (CLIO-VT680; 10 mg/kg i.v.; ex/em, 670/688 nm; Center for Systems Biology Chemistry Core at MGH).25,36 On day 6 post-AVF surgery, CLIO-VT680 was injected i.v. In vivo fluorescence imaging performed on day 7 used epifluorescence IVM. FTIC-dextran and CLIO-VT680 fluorescence signals were collected using an ex/em 460–500/510–560 nm and ex/em 650/710 nm bandpass filter. All IVM image settings (exposure time: FITC channel, 10 milliseconds; CLIO-VT680 channel, 50 milliseconds; FTP-CyAm7 channel, 50 milliseconds; gain, 1.0 for all channels) were kept constant for all time points and samples.
IVM Image Analysis
IVM image analysis used ImageJ Software (64-bit, 1.47 g; NIH, Bethesda, MD). The mean and maximum signal intensities of FTP11-CyAm7 and CLIO-VT680 were measured in all images obtained by in vivo IVM. The signal intensities were measured at 120-μm intervals from the anastomosis to 960 μm distally down the venous outflow limb. Control fluorescence signals were measured in the contralateral sham-operated jugular vein. The venous outflow target-to-background ratio for FTP11-CyAm7 or CLIO-VT680 was defined as the venous outflow NIRF signal divided by the contralateral sham-surgery vein NIRF signal.
Histology and Immunohistochemistry
After euthanasia, mice were perfused with PBS and AVFs were resected. AVF tissue was immersed in optimal cutting temperature medium and 6-µm axial sections were obtained. Fluorescence microscopy used an epifluorescence microscope with filters for CLIO-VT680, FTP11-CyAm7, as well as FITC channel fluorescence. The percentage of CLIO-positive pixels in the vein wall at day 7 was calculated as the number pixels of positive CLIO divided by the total pixels in the vessel wall.
Adjacent sections were stained with hematoxylin and eosin to assess general morphology and Carstairs stain to identify collagen, fibrin, and neointimal formation (Supplemental Figure 3). Patent area, internal elastin lamina (IEL) area, and neointimal area were measured based on Carstairs staining (n=8 in each group; Supplemental Figure 4). To visualize macrophages on immunofluorescence staining, sections were incubated with a primary antibody to cluster of differentiation 68 (CD68; 1:1000, rat anti-mouse; BD Biosciences), and then the secondary goat anti-rat antibody (1:100; BD Bioscience) or Cy3-donkey anti-rat antibody (1:1000; Jackson ImmunoResearch Laboratories, Inc.). Confocal fluorescence microscopy (SP8 confocal; Leica) was used to visualize the colocalization of CLIO-VT680 with CD68. The percentage of immunofluorescence-labeled, CD68-positive cells in the vein wall cells were counted in cells with 4′,6-diamidino-2-phenylindole (DAPI)-defined nuclei per 20× high power fields. The percentage of CD68-positive cells was calculated as the number of positive CD68 cells divided by the total DAPI-positive cells.
Polarization Microscopy of Picrosirius Red-Stained Vein Wall Collagen
AVF collagen in the vein wall was assessed using Picrosirius Red (PSR) staining.25 AVF sections from each group on day 7 and 14 (n=6 per group) were stained with PSR solution (24901-500; Polysciences). The polarization micrographs were obtained using an Eclipse 90i microscope. The total collagen content was measured as total positive pixels in vein wall area. The total collagen-enclosed area was defined as the area within the outside border of the surface area.
Quantitative Real-Time PCR
On day 14, the venous limb of the AVF underwent total RNA extraction using TRIZOL reagent (Invitrogen) with digested DNase I. Reverse transcription was performed using 200 ng of RNA from each sample via GoScript Reverse transcription (Promega). Real-time PCR was performed using the GoTaq 2-Step RT-qPCR System (A6010; Promega). The mRNA levels of matrix metalloproteinase-2 (MMP2), MMP9, monocyte-chemoattractant protein-1, CD68, C-C chemokine receptor type 2, procollagen I, and procollagen III were measured. The primers we used are listed in Table 1. All samples were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase mRNA to calculate the relative expression levels.
Table 1.
Primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| MMP2 | 5′-GATAACCTGGATGCTGTCG-3′ | 5′-CCAAACTTCACGCTCTTG-3′ |
| MMP9 | 5′-AGCACAACAGCTGCTACGATAAG-3′ | 5′-GCGCTTCCGGCACGCTGGAATGATCTAA-3′ |
| CCR2 | 5′-ACCTGTAAATGCCATGCAAGT-3′ | 5′-TGTCTTCCATTTCCTTTGATTTG-3′ |
| MCP-1 | 5′-TTAAAAACCTGGATCGGAACCAA-3′ | 5′-GCATTAGCTTCAGATTTACGGGT-3′ |
| CD68 | 5′-ACCTGTAAATGCCATGCAAGT-3′ | 5′-TFTCTTCCATTTCCTTTGATTTG-3′ |
| Col1 | 5′-GAGTACTGGATCGACCCTAACCA-3′ | 5′-GACGGCTGAGTAGGGAACACA-3′ |
| ColIII | 5′-AAGGCTGCAAGATGGATACT-3′ | 5′-GTGCTTACGTGGGACAGTCA-3′ |
| GAPDH | 5′-CCTGGAGAAACCTGCCAAGTATGA-3′ | 5′-TTGAAGTCACAGGAGACAACCTGG-3′ |
CCR2, C-C chemokine receptor type 2; Col1, collagen I; ColIII, collagen III; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical Analysis
All results are reported as the mean±SEM. Statistical analysis was performed using Prism (version 5.0c; GraphPad). Data sets were tested for normality using the Shapiro–Wilk test. If the data were normally distributed, two-group comparisons of parametric data were performed using a two-tailed t test, whereas nonparametric data were analyzed with the Mann–Whitney U test. Significant differences among multiple groups were assessed using ANOVA to compare all groups with the control group. Pearson correlation coefficients were calculated. In all analyses, P values <0.05 were considered statistically significant.
Results
ATV Therapy Favorably Increases AVF Venous Limb Outward Remodeling, and Reduces Collagen Deposition in the Vein Wall
Vein wall remodeling with luminal diameter expansion and venous wall thickening during AVF maturation permits safe-access cannulation during dialysis. However, excessive vein wall fibrosis or rigidity can restrict fistula outward remodeling and impair fistula maturation.7,39,40 This study used experimental AVF constructed using vein mobilization and creation of an end-to-side anastomosis to an artery (Figure 2A).32 Experiments then explored the effects of statin treatment on multiple in vivo measures of AVF remodeling and in vivo pathobiology and primary patency. To investigate the effects of ATV at day 14 on AVF neointimal hyperplasia formation and venous limb remodeling, histopathologic assays assessed multiple AVF morphologic parameters (n=8 mice per group; eight sections per mouse; Figure 2B, Supplemental Figures 4 and 5). The ATV-treated group exhibited significantly higher AVF patent area than did the PBS controls at day 14 (P<0.001; Figure 2C). The ATV-treated group also displayed a significantly larger IEL area, a measure of favorable AVF positive remodeling (P<0.001 versus control; Figure 2D). Further, consistent with favorable remodeling, ATV-treated mice showed a greater patent area/IEL area ratio (P<0.001; Figure 2E), driven by greater patent areas rather than a reduced neointimal area, because the neointima area was comparable between groups (P>0.05; Figure 2F). Concordantly, the venous limb outflow circumference was also significantly larger in the ATV-treated group at both day 7 and day 14 (P=0.01 and P=0.001; Supplemental Figure 5, A and B).
Excessive vein wall fibrosis can limit AVF remodeling and maturation. To investigate the effects of ATV on vein wall fibrosis, venous limb fistula sections were analyzed for collagen deposition by PSR staining (n=6 mice per group; eight sections analyzed per mouse; Figure 2G). At day 7, the AVF collagen-positive area trended lower in ATV-treated mice (P=0.10; Supplemental Figure 5C). By day 14, ATV significantly reduced collagen deposition in the vein wall (P=0.02 versus PBS; Figure 2H). The collagen-enclosed area, defined as the AVF area within the outer border defined by PSR staining, was also larger in the ATV-treated group on both day 7 (P=0.02; Supplemental Figure 5D) and day 14 (P=0.03; Figure 2I), indicating favorable outward remodeling in the AVF group. The ratio of collagen-enclosed area to collagen content, another measure of favorable AVF outward remodeling, was similar at day 7 (P=0.26) but significantly higher in the ATV group by day 14 (P=0.03; Supplemental Figure 5F).
ATV Reduces In Vivo Fibrinogenesis in AVF by Day 7 as Assessed by Molecular Imaging
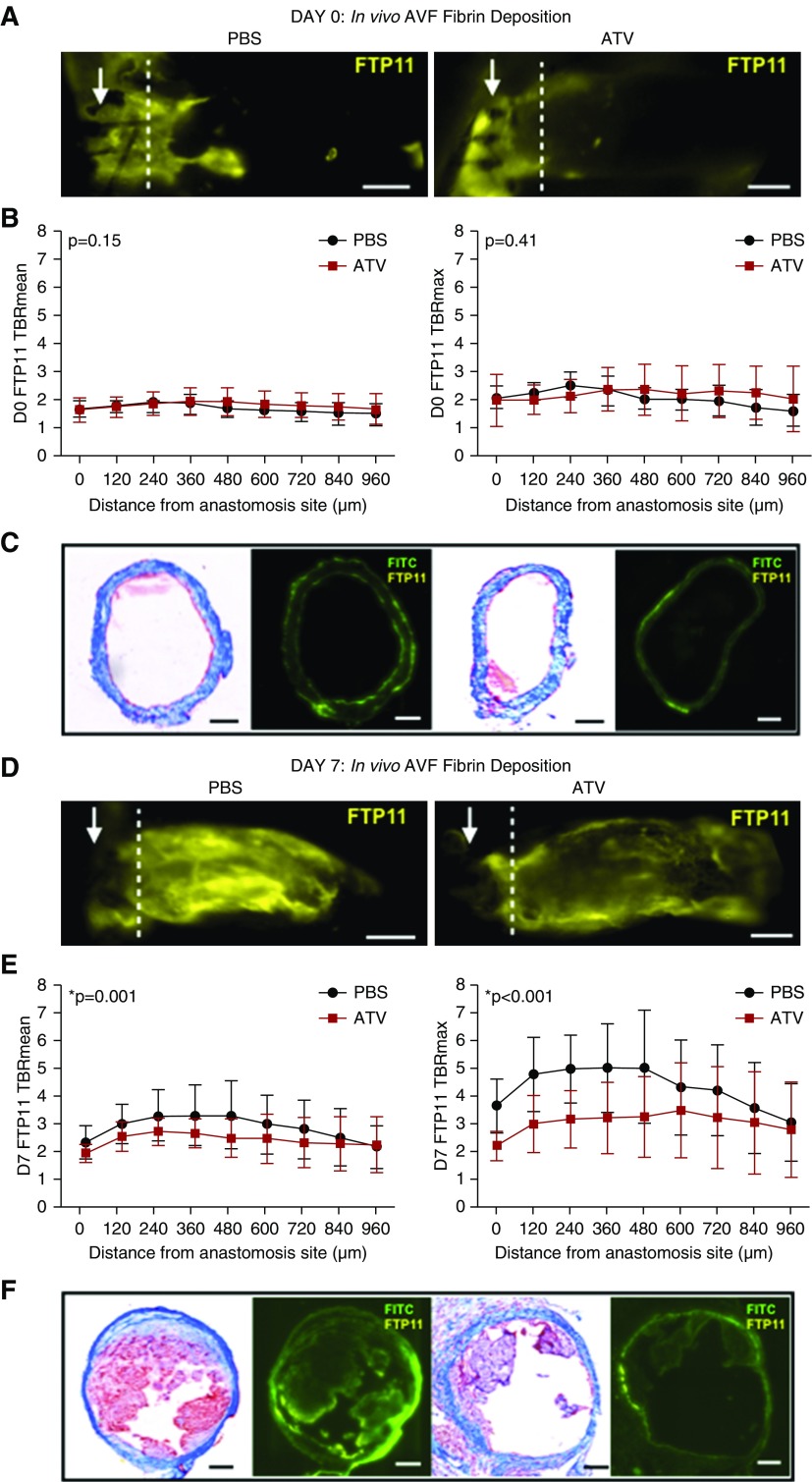
Endothelial denudation and altered shear stress after AVF creation may induce platelet activation and a thrombogenic endothelial surface, which can augment fibrin generation and promote AVF occlusion.41–46 To explore in vivo aspects of thrombosis associated with AVF remodeling in ATV-treated mice, we investigated fibrin deposition immediately post-AVF creation and subacutely using IVM.47,48 Fibrin deposition was analyzed across the venous limb after i.v. injection of the fibrin-specific molecular imaging agent FTP11-CyAm7 (Figure 3).34,35,38 The contralateral sham-surgery jugular vein exhibited negligible fibrin signal (Supplemental Figure 6A), and served as the background area for the calculation of the fibrin target-to-background ratios. Measurement of the fibrin signal across the AVF at 120-μm intervals 2 hours after AVF creation showed similar fibrin deposition between groups (P>0.05, n=5 in each group; Figure 3, A–C). In contrast, by day 7, ATV-treated mice exhibited significantly lower AVF fibrin accumulation in vivo (P<0.005, n=9 in each group; Figure 3, D–F). Histologic assessment using Carstairs staining confirmed fibrin deposition in areas demarcated by IVM fibrin molecular imaging (Figure 3, C and F).
Figure 3.
ATV reduces fibrin deposition in AVFs. (A) Fibrin deposition was visualized on IVM using the fibrin-specific NIRF molecular imaging agent FTP11-CyAm7 (yellow) at day 0. Arrow represents anastomosis. Scale bar, 250 μm. (B) FTP11-CyAm7 fibrin signal intensity was measured at 120-μm intervals from the anastomosis site. On day 0 immediately after AVF creation, the mean and maximum FTP11-CyAm7 fibrin signal intensity was comparable between ATV- and PBS-treated mice (P>0.05). (C) Representative Carstairs staining and fluorescence microscopy showing comparable fibrin deposition at day 0 in ATV- and PBS-treated animals. Scale bar, 100 μm. (D) At day 7, fibrin deposition was visualized on IVM using the fibrin-specific imaging agent FTP11-CyAm7 (yellow). Scale bar, 250 μm. (E) At day 7, the mean and maximum FTP11-CyAm7 signal intensities were much lower in ATV-treated animals than those in PBS-treated animals (P≤0.001). Error bars display the SD. (F) Representative Carstairs staining and fluorescence microscopy showing decreased fibrin deposition at day 7 in ATV-treated animals compared with PBS-treated animals. Scale bar, 100 μm. The two-way ANOVA test was used to assess for statistically significant differences in fibrin deposition between ATV and PBS groups as a function of distance from the anastomosis. P<0.05 was considered statistically significant. D0, day 0. TBR, target-to-background ratio.
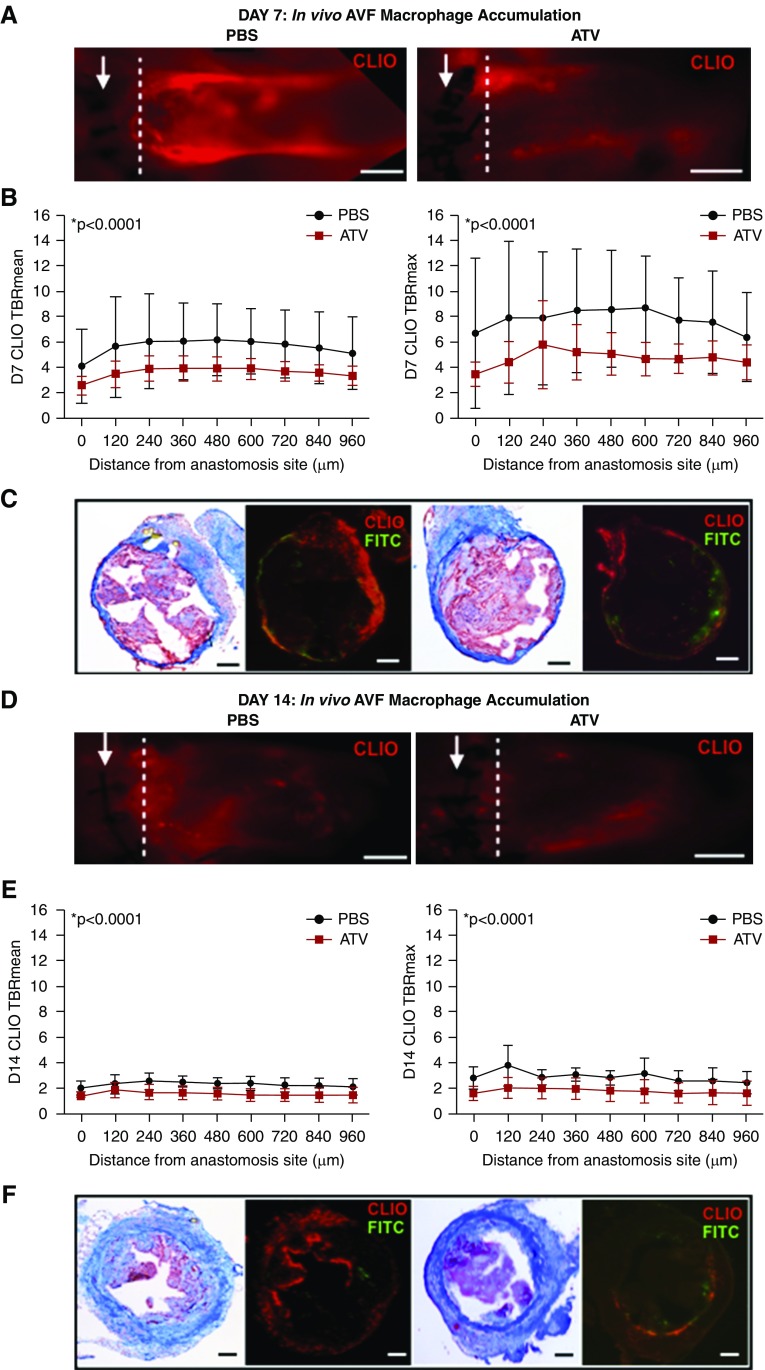
ATV Attenuates the AVF Inflammatory Macrophage Response In Vivo as Assessed by Nanoparticle-Enhanced Molecular Imaging
Increased inflammation associates with lower AVF patency rates.18,27,49 To explore anti-inflammatory mechanisms underlying prolonged AVF patency in statin-treated mice, we investigated the macrophage response on day 7 post-AVF creation. The macrophage-sensitive nanoreporter CLIO-VT680 coupled with IVM-evaluated AVF macrophages in vivo (Figure 4).33,50 ATV treatment significantly reduced in vivo macrophage accumulation in AVF by day 7 (n=10 mice per group, P<0.001; Figure 4, A–C) and by day 14 (n=5 mice per group, P<0.001; Figure 4, D–F), fluorescence microscopy corroborated the in vivo findings (Figure 4, C and F).
Figure 4.
ATV decreases the in vivo macrophage inflammatory response in AVFs. (A) The inflammatory response was visualized by IVM using the macrophage-sensitive nanoparticle CLIO-VT680 (red) at day 7. CLIO-VT680 signal intensity was measured at 120-μm intervals distal to the anastomosis. Arrow represents anastomosis. Scale bar, 250 μm. (B) At day 7, mean and maximum CLIO-VT680 signal intensity was significantly reduced in ATV-treated animals compared with PBS-treated animals (P<0.001). (C) Ex vivo Carstairs and fluorescence microscopy imaging were obtained and AVF structure was identified using autofluorescence (green). CLIO-VT680 signals (red) were also visualized in ex vivo fluorescence microscope. Scale bar, 100 μm. (D) Inflammatory response was visualized on IVM via a nanoreporter CLIO-VT680 (red) at day 14. Scale bar, 250 μm. (E) At day 14, mean and maximum CLIO-VT680 signal intensities were much lower in ATV-treated animals versus PBS (P<0.001). Error bars display the SD. (F) Representative ex vivo Carstairs and fluorescence microscope images. Scale bar, 100 μm. The two-way ANOVA test was used to assess for statistically significant differences in macrophage presence between ATV and PBS groups as a function of distance from the anastomosis. P<0.05 was considered statistically significant. D7, day 7. TBR, target-to-background ratio.
Histopathologic Analyses of ATV on Venous Outflow Limb Inflammation and Collagen Deposition
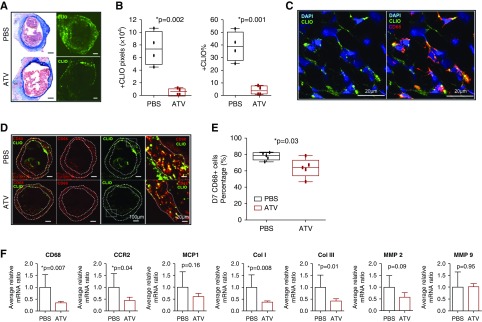
In mice with AVF, in vivo molecular imaging of macrophage accumulation by CLIO-VT680+ uptake was analyzed in the venous limb at a location of 360 μm away, distal to the anastomosis (Figure 5A). The total number of CLIO+ pixels (indicating macrophage content) was 6515±2701 in the ATV-treated group versus 74,833±13,270 in the saline control group (P=0.002, 16 sections per group; n=4 mice per group with four sections analyzed per mouse). The percentage of CLIO+ pixels was also markedly lower in the ATV-treated group (4.1%±1.7% versus 39%±5.8% in the control group, P=0.001; Figure 5B).
Figure 5.
ATV decreases macrophage accumulation in the venous outflow limb of AVF assessed by fluorescence microscopy, immunofluorescence, and real-time PCR. (A) Representative fluorescence microscopy images of PBS- and ATV-treated AVF at day 7. CLIO (green) signals were identified in the venous outflow limb. Scale bar, 100 μm. (B) Fluorescence microscopy analysis showed the total number of CLIO+ pixels and the percentage of CLIO+ pixels in PBS-treated mice were markedly higher than ATV-treated group (P<0.05). (C) Confocal immunofluorescence microscope images (20×) showed CLIO colocalized with CD68+ macrophages. Blue, DAPI; green, CLIO; red, CD68. (D) Immunofluorescence microscopy images of CD68+ cells (red) in AVFs at day 7 showed CD68+ macrophages were identified in the venous outflow limb of ATV- and PBS-treated mice. Scale bar, 100 μm (left three panels) and 20 μm (right panel). (E) The percentage of DAPI+ cells that were CD68+ macrophages in the vein wall was significantly lower in ATV-treated animals than in PBS-treated animals on day 7 (P=0.03). (F) Real-time PCR analysis of the fistula wall show that CD68 and C-C chemokine receptor type 2 (CCR2) RNA expression were significantly lower in ATV-treated animal (P<0.05). There were no significant differences in monocyte-chemoattractant protein-1 (MCP-1), MMP2, or MMP9 RNA expression between ATV- and PBS-treated animals (P>0.05). However, ATV reduced both collagen I and III (Col I and Col III) RNA expression (P<0.05). Bars represent the mean±SD.
Immunofluorescence images showed that CLIO colocalized with CD68+ macrophages that localized primarily in the vein wall adventitia (Figure 5, C and D). The percentage of CD68+ macrophages in the vein wall was next analyzed at multiple locations from the anastomosis site in both ATV- and PBS-treated mice. The percentage of DAPI+ cells that were CD68+ macrophages in the adventitia was 63.6%±11.5% in the ATV group versus 77.9%±4.7% in the PBS control group (P=0.03; Figure 5E).
Statin effects on molecular mediators of venous outflow inflammation and venous outflow remodeling was next assessed via quantitative real-time PCR of inflammation and procollagen mRNA transcripts (Figure 5F). At day 14, CD68 mRNA expression decreased by 65.2% in the venous limb of ATV-treated mice compared with PBS-treated mice (P=0.007). ATV also decreased C-C chemokine receptor type 2 expression in day 14 AVFs by 55.9% (P=0.04). Monocyte-chemoattractant protein-1 expression trended lower in the ATV group at day 14 (P=0.16). The expression of genes that encode components of both collagen I and III was significantly lower in the ATV-treated group at day 14 (P=0.008 and P=0.01, respectively). However, the expression of MMP2 and -9 expression did not differ between groups (P>0.05).
ATV Increases AVF Patency in Concert with Preserving AVF Blood Flow Rates
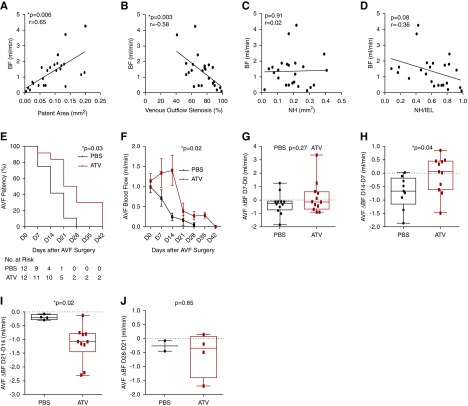
ATV or PBS was initiated 7 days before planned AVF surgery. At day 0, mice underwent AVF creation. In animals undergoing euthanasia at day 7 or 14 (n=24), the AVF blood flow rate measured before euthanasia was compared with the degree of venous stenosis on histopathologic images obtained at 480 μm distal to the anastomosis. AVF blood flow rate and patent area correlated strongly and positively (r=0.65, P=0.006; Figure 6A). The percentage of venous outflow stenosis correlated negatively with AVF blood flow (r=−0.58, P=0.003; Figure 6B). However, neointimal hyperplasia area and AVF blood flow did not correlate (P=0.91; Figure 6C). There was a nonsignificant trend of the ratio of neointimal hyperplasia area to IEL area, a measure of remodeling, with lower AVF blood flow rates (P=0.08; Figure 6D).
Figure 6.
Atorvastatin preserves AVF blood flow and patency. Assessment of AVF histopathologic parameters and AVF blood flow. (A) AVF blood flow (BF) significantly correlated with AVF patent area (r=0.65, P=0.006). (B) AVF blood flow also correlated negatively with the percentage of AVF venous outflow stenosis (r=−0.58, P=0.003). (C) AVF blood flow did not correlate with neointimal hyperplasia (NH) area (P=0.91). (D) AVF blood flow trended lower as a function of the ratio of neointimal hyperplasia to IEL area (r=−0.36, P=0.08). (E) Kaplan–Meier survival analysis demonstrating that ATV treatment significantly prolonged AVF patency (P=0.03 versus PBS). (F) The preservation of AVF blood flow was significantly higher in the ATV-treated groups (P=0.02 versus PBS). (G) AVF blood flow changes from day 7 to day 0 and day 28 to day 21 were similar between the ATV- and PBS-treated groups. (H) However, between day 14 and day 7, ATV-treated animals exhibited a reduced blood flow rate decrease compared with PBS-treated animals. (I) In contrast between day 21 and day 14, ATV-treated animals exhibited a greater decrease in blood flow decrease compared with PBS-treated animals. (J) ATV and PBS animals exhibited similar reductions in AVF blood flow rates between day 28 (D28) and D21. Line represents median blood flow.
To investigate the effects of ATV on AVF patency, animals were randomized to ATV or PBS group (n=24). The AVF blood flow rate was measured every 7 days after fistula creation using an ultrasound flow probe until AVF occlusion occurred, defined as an AVF blood flow rate <0.10 ml/min. ATV significantly increased the AVF patency rate over the entire study period (P=0.03; Figure 6E). The median AVF patency time in the ATV group was 28 days, compared with 14 days in the PBS group.
Kaplan–Meier survival analysis demonstrated the well improved patency rate for ATV therapy was robust over a range of AVF blood flow thresholds used to define AVF occlusion (0.05–0.20 ml/min; Supplemental Figure 7, D–G). Analysis of weekly AVF blood flow rate changes demonstrated that decreases in AVF blood flow rates fell in ATV-treated animals compared with PBS controls (P=0.02; Figure 6F). AVF blood flow rate changes from immediately post-AVF creation to day 7 did not differ between groups (P=0.27; Figure 6G). From day 7 to day 14, however, ATV preserved AVF blood flow rates (blood flow changes between day 7 to day 14: ATV of +0.05±0.2 ml/min versus PBS of −0.71±0.20 ml/min, P=0.04; Figure 6H). In contrast, AVF blood flow decreased more at later time points in ATV-treated animals than in PBS-treated animals between day 21 and day 14 (−0.2±0.04 versus −1.2±0.2 ml/min, P=0.02; Figure 6I). Both groups exhibited comparable AVF blood flow changes from day 28 to day 21 (0.27±0.19 ml/min versus 0.57±0.4 ml/min, P=0.65; Figure 6J).
Discussion
This in vivo investigation demonstrates that ATV therapy improves murine AVF outward remodeling and primary patency in concert with a number of salutary molecular and physiologic effects including reduced accumulation of fibrin accumulation and inflammatory macrophages, greater outward remodeling while reducing AVF wall collagen content, and greater preservation of AVF blood flow. The overall results provide new insights into the favorable effects of statins on AVF maturation, and provide support to the clinical evaluation of statins for improving AVF patency rates in patients on hemodialysis.
After fistula surgery, platelets, fibrin, and red blood cells accumulate near the anastomosis, spurring monocyte and lymphocyte recruitment and propagating an inflammatory response. Fibrin is the primary proteinaceous component of the initial thrombus scaffold and provides a surface for thrombus propagation and eventual vessel occlusion. Statins can decrease thrombin generation, impair procoagulant reactions catalyzed by thrombin, enhance endothelial thrombomodulin expression, and reduce the production of the inhibitor of fibrinolysis plasminogen activator inhibitor-1.48,51 This study showed that ATV reduced in vivo AVF fibrin deposition by day 7, which preceded the observed higher patent luminal areas at day 14 (Figures 2 and 3). Strategies to prevent fibrin deposition may therefore contribute to improved fistula patency.
Recognition of how inflammation participates critically in the pathogenesis of AVF failure has increased. Inflammatory mediators and macrophages regulate pathologic AVF remodeling and associate clinically with AVF failure.33,52,53 In addition, a clinical study showed that statin therapy associates with a lower risk of AVF primary failure in patients receiving hemodialysis treatment, but not in the hemodialysis-naive cohort, which suggests improvement of human AVF primary failure rates by ATV might be related to its anti-inflammatory actions, rather than lipid-lowering properties.54 This study investigated the AVF macrophage inflammatory response by employing in vivo fluorescence molecular imaging of the validated macrophage-avid nanoparticle reporter (CLIO-VT680),25,36,50 as well as by immunohistochemical and mRNA detection. Macrophages accumulated abundantly in the AVF venous limb at day 7, followed with a reduction observed at day 14. Statin administration substantially reduced the macrophage content in healing AVF in vivo as well by histopathologic and mRNA analysis (Figures 4 and 5), demonstrating a new statin-mediated, anti-inflammatory effect in AVF.55 In contrast to alternative anti-inflammatory AVF therapies such as liposomal prednisolone,20 statins have ready clinical access and possess a favorable safety profile. These attributes render statins readily attractive for reducing clinical AVF inflammation and primary AVF failure. Given the findings that patients with atherosclerosis and elevated blood levels of inflammatory C-reactive protein benefit preferentially from statin therapy,56 future clinical AVF patency studies might test whether patients on dialysis who have higher systemic inflammation may similarly benefit from statin therapy.
Endothelial cell injury occurs during the technically demanding construction of the AVF anastomosis. The intima responds to injury by subendothelial fibroproliferation and neointima formation, as part of the reparative process after vascular injury.57 Neointimal hyperplasia develops when the venous endothelial cells encounter increased BP and flow, conditions that triggers structural remodeling of the vein wall. Increased vascular wall shear stress also triggers endothelial cell production and release of nitric oxide, a potent vasodilator that relaxes smooth muscle.58 This salutary endothelial cell function promotes AVF dilation and maturation. For example, extensive de-endothelialization of human vein grafts during coronary artery bypass grafting associates with poorer patency of vein grafts.59 In a diabetic rat with aortocaval fistulas, rosuvastatin improved fistula blood flow, increased the number of circulating endothelial progenitor cells, and improved endothelium-dependent relaxation.60 In a murine AVF study, simvastatin treatment reduced AVF expression of mRNAs encoding vascular endothelial growth factor and MMP9, and decreased cellular proliferation and number of cells positive for α-smooth muscle actin. Simvastatin-treated fibroblasts under hypoxia further displayed decreased α-smooth muscle actin expression, migration, and proliferation.55 Our study demonstrated that ATV-treated AVFs exhibit decreased inflammatory responses, specifically with reduced macrophage accumulation and MMP expressions. These data suggest that statins exert multiple mechanisms for improving AVF patency and maturation, among them anti-inflammatory, anti-proliferative, and proendothelial function effects.
The generation of adequate, but not excessive, blood flow via outward remodeling is paramount in developing an AVF that can support long-term hemodialysis. In this murine study, statins preserved AVF blood flow rates particularly in the first 14 days after AVF creation, and this enhanced blood flow rate preceded the observed improvement in AVF patency rates out to day 42. Notably, statin-based increases in outward remodeling, reductions in fibrin generation and macrophage infiltration, and reductions in collagen deposition in the AVF wall also manifested by day 7–14 (Figures 2–4). The subsequent reductions in blood flow observed after day 14 despite statin therapy suggest a need for additional anti-inflammatory, antithrombotic, and/or antifibrosis therapies to extend AVF patency.
This study demonstrates that ATV treatment induced outward remodeling, a critical feature underlying usable AVF access,7 as shown by increased AVF patent area and IEL area. Statins furthermore reduced collagen content in the AVF wall (Figure 2, Supplemental Figure 5). In addition, ATV reduced AVF fibrin-rich thrombi, macrophage inflammation, and intimal hyperplasia. These combined features likely underlie the higher AVF blood flow rate and the improved AVF patency rates observed in the ATV group. In addition to achieving outward remodeling, maintaining fistula patency may also be important because it offers the opportunity for more durable endovascular interventions (e.g., percutaneous transluminal angioplasty) to promote AVF maturation. Clinical studies have shown that endovascular treatment of immature AVFs can increase AVF usability rates to as high as 90%.61 In addition, endovascular intervention for thrombosed AVFs have poorer outcomes than dysfunctional but patent fistulas.62,63
Benefits of statins on both AVF patency and AVF outward remodeling emerged as early as 7 days after AVF surgery, a relatively short time point after AVF creation. Statin therapy began 7 days before surgery, a feasible schedule in the clinic given the elective nature of AVF construction. A prior murine study of short-term statin administration (duration 4–10 days) improved venous thrombosis thrombus resolution and vein wall scarring.25 In addition, a clinical study demonstrated that a single dose of statin could reduce periprocedural myocardial infarction in patients undergoing elective percutaneous coronary intervention.64
This study has certain limitations. First, statins were not evaluated upon a CKD milieu given the observed higher mortality rate of combining surgical AVF creation with induction of CKD by 5/6 nephrectomy (data not shown). Second, this study tested only one statin. Although statins generally share mechanisms of benefit,25 one clinical study suggests the superiority of ATV over other statins in reducing AVF primary failure.26 Third, this study did not investigate female mice with AVFs due to their inability to sustain extended AVF patency in a pilot study, potentially related to smaller vessels and body size (Supplemental Figure 1). Further assessment in larger vessels or animals could assess the effects of statins on AVF patency in female subjects. Fourth, murine AVF creation in mice used the internal jugular vein and carotid artery, which do not mimic peripheral vessel AVF creation in humans; however, multiple prior studies demonstrated that this procedure in mice recapitulates many aspects of human AVF pathology.32 Fifth, although murine fistula were not cannulated, fistula development and maturation were assessed using blood flow measurements and histologic assessments that reflect human AVF maturation evaluation by ultrasound criteria.65 Further randomized trials are needed in patients with AVFs to confirm these experimental findings. Sixth, central fistulas (jugular vein-to-carotid artery, required in mice due to their smaller size) may have different physiology and hemodynamics compared with peripheral AVF circuits, and may require further study in larger animal models. Lastly, we did not evaluate other time points of statin initiation besides 7 days before AVF creation.
In conclusion, ATV decreases in vivo AVF fibrinogenesis and macrophage inflammation, favorably affects AVF outward remodeling, and prolongs AVF patency in vivo. These findings provide a pathophysiologic and molecular evidence rationale for the clinical use of statins to improve AVF patency and maturation in patients on dialysis. This proposition would require formal clinical evaluation, given the limitations of animal experiments.
Disclosures
Dr. Jaffer reports personal fees from Abbott Vascular, Acrostak, Biotronik, Boston Scientific, and Philips, grants from Canon, and grants and personal fees from Siemens, outside the submitted work. Dr. Jaffer also reports equity interest in Intravascular Imaging Incorporated, grants from National Institutes of Health, during the conduct of the study, and sponsored research from Shockwave. In addition, Dr. Jaffer has issued patents related to intravascular imaging and molecular imaging technology. MGH has a patent licensing arrangement with Canon, and Dr. Jaffer has the right to receive royalties. Dr. Libby reports grants from National Heart, Lung, and Blood Institute (NHLBI), during the conduct of the study, being an unpaid consultant to—or involved in clinical trials for—Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Merck, Novartis, Pfizer, and Sanofi-Regeneron, and being a member of a scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Medimmune, Novartis, Olatec Therapeutics, and XBiotech, Inc. Dr. Libby receives laboratory funding from Novartis and is on the board of directors for XBiotech, Inc.
Funding
Dr. Jaffer was supported by NIH/NHLBI grant R01 HL137913. Dr. Misra was supported by NIH/NHLBI grants HL098967 and DK107870. The authors received funding from NIDDK.
Supplementary Material
Acknowledgments
We thank Sophia Zhao for assistance with statistical analyses.
Dr. Cui and Dr. Jaffer designed the study. Dr. Cui, Mr. Jhajj, and Ms. Grau carried out experiments. Dr. Cui, Dr. Kessinger, Mr. Jhajj, and Ms. Grau analyzed the data. Dr. Cui, Mr. Jhajj, Ms. Grau, Dr. Kessinger, and Dr. Jaffer made the figures. Dr. McCarthy synthesized the agents used in this study. Dr. Cui, Dr.Jaffer, Dr. Misra, and Dr. Libby drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060612/-/DCSupplemental.
Supplemental Figure 1. Female AVF blood flow rate and body weight compared with age-matched male animals.
Supplemental Figure 2. In vivo imaging and blood flow measurement study protocol.
Supplemental Figure 3. Representative histological images of AVF outflow using H&E and Carstairs’ staining.
Supplemental Figure 4. Morphological assessments of AVF using Carstairs’ and Picrosirius red staining.
Supplemental Figure 5. Histological analyses of AVF outflow and collagen content.
Supplemental Figure 6. Representative intravital microscopy (IVM) imaging of fibrin (FTP11) and macrophage content (CLIO-VT680) in arteriovenous fistulas (AVFs) and contralateral control jugular veins.
Supplemental Figure 7. In vivo AVF blood flow rate measurements and Kaplan-Meier survival analysis of fistula patency using a range of blood flow rates to define fistula occlusion.
References
- 1.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al.: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, et al.: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Thamer M, Lee TC, Wasse H, Glickman MH, Qian J, Gottlieb D, et al.: Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am J Kidney Dis 72: 10–18, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA, Allon M: Challenges in developing new therapies for vascular access dysfunction. Clin J Am Soc Nephrol 12: 2053–2055, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, et al.: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P: Biology of arteriovenous fistula failure. J Nephrol 20: 150–163, 2007 [PubMed] [Google Scholar]
- 7.Martinez L, Duque JC, Tabbara M, Paez A, Selman G, Hernandez DR, et al.: Fibrotic venous remodeling and nonmaturation of arteriovenous fistulas. J Am Soc Nephrol 29: 1030–1040, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, et al.: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, et al.: Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis 68: 455–464, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allon M, Greene T, Dember LM, Vita JA, Cheung AK, Hamburg NM, et al.; Hemodialysis Fistula Maturation Study Group: Association between preoperative vascular function and postoperative arteriovenous fistula development. J Am Soc Nephrol 27: 3788–3795, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al.; Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’shea SI, Lawson JH, Reddan D, Murphy M, Ortel TL: Hypercoagulable states and antithrombotic strategies in recurrent vascular access site thrombosis. J Vasc Surg 38: 541–548, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Salmela B, Hartman J, Peltonen S, Albäck A, Lassila R: Thrombophilia and arteriovenous fistula survival in ESRD. Clin J Am Soc Nephrol 8: 962–968, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gröntoft KC, Larsson R, Mulec H, Weiss LG, Dickinson JP: Effects of ticlopidine in AV-fistula surgery in uremia. Fistula Study Group. Scand J Urol Nephrol 32: 276–283, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Brahmbhatt A, Remuzzi A, Franzoni M, Misra S: The molecular mechanisms of hemodialysis vascular access failure. Kidney Int 89: 303–316, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang SY, Roan JN, Lin Y, Hsu CH, Chang SW, Huang CC, et al.: Rosuvastatin suppresses the oxidative response in the venous limb of an arteriovenous fistula and enhances the fistula blood flow in diabetic rats. J Vasc Res 51: 81–89, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Feng W, Chumley P, Allon M, George J, Scott DW, Patel RP, et al.: The transcription factor E26 transformation-specific sequence-1 mediates neointima formation in arteriovenous fistula. J Am Soc Nephrol 25: 475–487, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, et al.: MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol 22: 43–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez L, Tabbara M, Duque JC, Selman G, Falcon NS, Paez A, et al.: Transcriptomics of human arteriovenous fistula failure: Genes associated with nonmaturation. Am J Kidney Dis 74: 73–81, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong C, Bezhaeva T, Rothuizen TC, Metselaar JM, de Vries MR, Verbeek FP, et al.: Liposomal prednisolone inhibits vascular inflammation and enhances venous outward remodeling in a murine arteriovenous fistula model. Sci Rep 6: 30439, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B, Kilari S, Brahmbhatt A, McCall DL, Torres EN, Leof EB, et al.: CorMatrix wrapped around the adventitia of the arteriovenous fistula outflow vein attenuates venous neointimal hyperplasia. Sci Rep 7: 14298, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irish AB, Viecelli AK, Hawley CM, Hooi LS, Pascoe EM, Paul-Brent PA, et al.; Omega-3 Fatty Acids (Fish Oils) and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED) Study Collaborative Group: Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: A randomized clinical trial. JAMA Intern Med 177: 184–193, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Tanner NC, Da Silva A: Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst Rev (7): CD002786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönbeck U, Libby P: Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents? Circulation 109[Suppl 1]: II18–II26, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kessinger CW, Kim JW, Henke PK, Thompson B, McCarthy JR, Hara T, et al.: Statins improve the resolution of established murine venous thrombosis: Reductions in thrombus burden and vein wall scarring. PLoS One 10: e0116621, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez L, Duque JC, Escobar LA, Tabbara M, Asif A, Fayad F, et al.: Distinct impact of three different statins on arteriovenous fistula outcomes: A retrospective analysis. J Vasc Access 17: 471–476, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang HH, Chang YK, Lu CW, Huang CT, Chien CT, Hung KY, et al.: Statins improve long term patency of arteriovenous fistula for hemodialysis. Sci Rep 6: 22197, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter KE, Turner NA: Statins for the prevention of vein graft stenosis: A role for inhibition of matrix metalloproteinase-9. Biochem Soc Trans 30: 120–126, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Pisoni R, Barker-Finkel J, Allo M: Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol 5: 1447–1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Q, Li L, Yang S, Chu F: Impact of statins on arteriovenous fistulas outcomes: A meta-analysis. Ther Apher Dial 22: 67–72, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al.; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators: Intensive versus moderate lipid lowering with statins after acute coronary syndromes [published correction appears in N Engl J Med 354: 778, 2006]. N Engl J Med 350: 1495–1504, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Wong CY, de Vries MR, Wang Y, van der Vorst JR, Vahrmeijer AL, van Zonneveld AJ, et al. : Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J Vasc Surg 59: 192–201.e1, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Cui J, Kessinger CW, McCarthy JR, Sosnovik DE, Libby P, Thadhani RI, et al.: In vivo nanoparticle assessment of pathological endothelium predicts the development of inflow stenosis in murine arteriovenous fistula. Arterioscler Thromb Vasc Biol 35: 189–196, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, et al.: Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging 5: 607–615, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein-Merlob AF, Kessinger CW, Erdem SS, Zelada H, Hilderbrand SA, Lin CP, et al.: Blood accessibility to fibrin in venous thrombosis is thrombus age-dependent and predicts fibrinolytic efficacy: An in vivo fibrin molecular imaging study. Theranostics 5: 1317–1327, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang K, Francis SA, Aikawa E, Figueiredo JL, Kohler RH, McCarthy JR, et al.: Pioglitazone suppresses inflammation in vivo in murine carotid atherosclerosis: Novel detection by dual-target fluorescence molecular imaging. Arterioscler Thromb Vasc Biol 30: 1933–1939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, et al.: Miniaturized integration of a fluorescence microscope. Nat Methods 8: 871–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara T, Ughi GJ, McCarthy JR, Erdem SS, Mauskapf A, Lyon SC, et al.: Intravascular fibrin molecular imaging improves the detection of unhealed stents assessed by optical coherence tomography in vivo. Eur Heart J 38: 447–455, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JK, Choi SR, Lee WY, Park MJ, Lee HS, Song YR, et al. : Leptin, pre-existing vascular disease, and increased arteriovenous fistula maturation failure in dialysis patients. J Vasc Surg 64: 402–410.e1, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Simone S, Loverre A, Cariello M, Divella C, Castellano G, Gesualdo L, et al.: Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. J Nephrol 27: 555–562, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Chuang YC, Chen JB, Yang LC, Kuo CY: Significance of platelet activation in vascular access survival of haemodialysis patients. Nephrol Dial Transplant 18: 947–954, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Mailhac A, Badimon JJ, Fallon JT, Fernández-Ortiz A, Meyer B, Chesebro JH, et al.: Effect of an eccentric severe stenosis on fibrin(ogen) deposition on severely damaged vessel wall in arterial thrombosis. Relative contribution of fibrin(ogen) and platelets. Circulation 90: 988–996, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Browne LD, Bashar K, Griffin P, Kavanagh EG, Walsh SR, Walsh MT: The role of shear stress in arteriovenous fistula maturation and failure: A systematic review. PLoS One 10: e0145795, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra S, Fu AA, Misra KD, Glockner JF, Mukhopadhyay D: Wall shear stress measurement using phase contrast magnetic resonance imaging with phase contrast magnetic resonance angiography in arteriovenous polytetrafluoroethylene grafts. Angiology 60: 441–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misra S, Fu AA, Misra KD, Glockner JF, Mukhopadyay D: Evolution of shear stress, protein expression, and vessel area in an animal model of arterial dilatation in hemodialysis grafts. J Vasc Interv Radiol 21: 108–115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misra S, Fu AA, Puggioni A, Karimi KM, Mandrekar JN, Glockner JF, et al.: Increased shear stress with upregulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol 294: H2219–H2230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Undas A, Celinska-Löwenhoff M, Brummel-Ziedins KE, Brozek J, Szczeklik A, Mann KG: Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 25: 1524–1525, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Violi F, Calvieri C, Ferro D, Pignatelli P: Statins as antithrombotic drugs. Circulation 127: 251–257, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Chang CJ, Ko YS, Ko PJ, Hsu LA, Chen CF, Yang CW, et al.: Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int 68: 1312–1319, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Stein-Merlob AF, Hara T, McCarthy JR, Mauskapf A, Hamilton JA, Ntziachristos V, et al.: Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ Cardiovasc Imaging 10: e005813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourcier T, Libby P: HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol 20: 556–562, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Bush HL Jr., Jakubowski JA, Curl GR, Deykin D, Nabseth DC: The natural history of endothelial structure and function in arterialized vein grafts. J Vasc Surg 3: 204–215, 1986 [DOI] [PubMed] [Google Scholar]
- 53.Kwei S, Stavrakis G, Takahas M, Taylor G, Folkman MJ, Gimbrone MA Jr., et al.: Early adaptive responses of the vascular wall during venous arterialization in mice. Am J Pathol 164: 81–89, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duque JC, Martinez L, Tabbara M, Dvorquez D, Mehandru SK, Asif A, et al.: Arteriovenous fistula maturation in patients with permanent access created prior to or after hemodialysis initiation. J Vasc Access 18: 185–191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, et al.: Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int 84: 338–352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E: Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 100: 230–235, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Schwartz SM, deBlois D, O’Brien ER: The intima. Soil for atherosclerosis and restenosis. Circ Res 77: 445–465, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Jones KA, Wong GY, Jankowski CJ, Akao M, Warner DO: cGMP modulation of Ca2+ sensitivity in airway smooth muscle. Am J Physiol 276: L35–L40, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Motwani JG, Topol EJ: Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation 97: 916–931, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Roan JN, Fang SY, Chang SW, Hsu CH, Huang CC, Chiou MH, et al. : Rosuvastatin improves vascular function of arteriovenous fistula in a diabetic rat model. J Vasc Surg 56: 1381–1389.e1, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Miller GA, Hwang W, Preddie D, Khariton A, Savransky Y: Percutaneous salvage of thrombosed immature arteriovenous fistulas. Semin Dial 24: 107–114, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Manou-Stathopoulou S, Robinson EJ, Harvey JJ, Karunanithy N, Calder F, Robson MG: Factors associated with outcome after successful radiological intervention in arteriovenous fistulas: A retrospective cohort. J Vasc Access 20: 716–724, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu CC, Wen SC, Chen MK, Yang CW, Pu SY, Tsai KC, et al.: Radial artery approach for endovascular salvage of occluded autogenous radial-cephalic fistulae. Nephrol Dial Transplant 24: 2497–2502, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Briguori C, Colombo A, Airoldi F, Violante A, Focaccio A, Balestrieri P, et al.: Statin administration before percutaneous coronary intervention: Impact on periprocedural myocardial infarction. Eur Heart J 25: 1822–1828, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.