Significance Statement
Identifying novel biomarkers of kidney function decline in children may have clinical value and help elucidate the biologic mechanisms of CKD progression. In the CKiD prospective cohort study, the authors evaluated 651 children with CKD and measured biomarkers in plasma collected 5 months after enrollment. After multivariable adjustment, risk of CKD progression was significantly higher among children with concentrations of a biomarker of tubular injury (KIM-1) or either of two biomarkers of inflammation (TNF receptor–1 [TNFR-1] and TNFR-2) in the highest quartile compared with those with concentrations in the lowest quartile for the respective biomarker. Use of plasma KIM-1, TNFR-1, and TNFR-2 as biomarkers of ongoing tubular damage and inflammation may identify children at increased risk of CKD progression.
Keywords: chronic kidney disease, pediatric nephrology, Chronic inflammation, renal injury
Visual Abstract
Abstract
Background
After accounting for known risk factors for CKD progression in children, clinical outcomes among children with CKD still vary substantially. Biomarkers of tubular injury (such as KIM-1), repair (such as YKL-40), or inflammation (such as MCP-1, suPAR, TNF receptor-1 [TNFR-1], and TNFR-2) may identify children with CKD at risk for GFR decline.
Methods
We investigated whether plasma KIM-1, YKL-40, MCP-1, suPAR, TNFR-1, and TNFR-2 are associated with GFR decline in children with CKD and in subgroups defined by glomerular versus nonglomerular cause of CKD. We studied participants of the prospective CKiD Cohort Study which enrolled children with an eGFR of 30–90 ml/min per 1.73 m2 and then assessed eGFR annually. Biomarkers were measured in plasma collected 5 months after study enrollment. The primary endpoint was CKD progression, defined as a composite of a 50% decline in eGFR or incident ESKD.
Results
Of the 651 children evaluated (median age 11 years; median baseline eGFR of 53 ml/min per 1.73 m2), 195 (30%) had a glomerular cause of CKD. Over a median follow-up of 5.7 years, 223 children (34%) experienced CKD progression to the composite endpoint. After multivariable adjustment, children with a plasma KIM-1, TNFR-1, or TNFR-2 concentration in the highest quartile were at significantly higher risk of CKD progression compared with children with a concentration for the respective biomarker in the lowest quartile (a 4-fold higher risk for KIM-1 and TNFR-1 and a 2-fold higher risk for TNFR-2). Plasma MCP-1, suPAR, and YKL-40 were not independently associated with progression. When stratified by glomerular versus nonglomerular etiology of CKD, effect estimates did not differ significantly.
Conclusions
Higher plasma KIM-1, TNFR-1, and TNFR-2 are independently associated with CKD progression in children.
Progression of CKD in children can lead to ESKD which is associated with adverse cardiovascular outcomes and mortality rates significantly higher than those observed in the general pediatrics population.1 After accounting for the known risk factors for CKD progression, including proteinuria and serum creatinine concentration, there remains a substantial variation in the risk of clinical outcomes among children with CKD.
Focused research is required on CKD progression in children, as there are unique concerns of growth and development, and the cause of CKD in children is markedly different than in adults.2 The majority of CKD in children is due to congenital anomalies of the kidneys and urinary tract such as obstructive uropathy, kidney dysplasia, or reflux nephropathy.3,4 Children also can develop CKD from glomerular disease such as FSGS or IgA nephropathy.
Previous studies of adults with CKD have identified plasma biomarkers of tubular injury and inflammation that are associated with disease progression.5–10 Most biomarker studies in children with CKD have been cross-sectional.11 Few studies have evaluated biomarkers in longitudinal cohorts to predict CKD progression over time. Schaefer et al.12 studied serum soluble urokinase receptor (suPAR) in 898 children with CKD, and reported that a higher suPAR level was associated with an increased risk of CKD progression after multivariable adjustment, but only in participants with a baseline eGFR>40 ml/min per 1.73 m2.
In addition to providing new information that could help guide clinical care, resource allocation, and clinical trial enrollment, novel biomarkers may also improve our understanding of CKD pathophysiology in children.13 In this present investigation we examined biomarkers of tubular injury (kidney injury molecule–1 [KIM-1]), inflammation (monocyte chemoattractant protein–1 [MCP-1], TNF receptor–1 [TNFR-1], TNFR-2, suPAR), and repair (YKL-40) to understand their associations with CKD progression in children. This biomarker panel represents diverse biologic pathways, which may provide insights into the heterogeneous long-term outcomes in children with CKD. Using the CKD in Children (CKiD) multicenter cohort study, we hypothesized that several of these plasma biomarkers would be associated with CKD progression and that kidney disease subtypes would be an important modifying factor.
Methods
Study Cohort
The CKiD study is a prospective cohort study of children with CKD enrolled from January of 2005 to December of 2014 in two recruitment waves at 54 participating medical centers in the United States and Canada.14,15 Briefly, children were enrolled if they were between the ages of 6 months and 16 years old and had an eGFR of 30–90 ml/min per 1.73 m2. Children were excluded if they had a history of renal, solid-organ, or bone marrow transplantation; dialysis within 3 years; or history of cancer. Participants underwent annual study visits to assess height, weight, medication use, BP, eGFR, and proteinuria. Written, informed consent was obtained from all parents or legal guardians, along with assent, when appropriate, from children. The CKiD study was approved by the institutional review board of each participating institution. The CKiD study is registered at ClinicalTrials.gov with the identifier NCT00327860.
Study Participants and Study Design
Children in the CKiD study were eligible for inclusion in the analysis if they had sufficient volume of stored plasma and had data on BP, eGFR, proteinuria, or body mass index (BMI) at study entry (Supplemental Figure 1). Participants underwent annual study visits to assess height, weight, medical history, medication use, BP, eGFR, and proteinuria. For this study, we defined the baseline for analyses as the first CKiD study visit. The primary outcome was the time to the composite event of a 50% decline in eGFR or incident ESKD (dialysis or transplantation). We extrapolated or interpolated eGFR to get the time of 50% decline for the composite. If eGFR was missing, we imputed it to determine the outcome status. Participants not experiencing the event were censored at the last visit when they had active data collection or March 1, 2018, whichever came first. The primary exposures were measured biomarker levels in stored plasma, collected a median of 5 months (interquartile range [IQR], 4–6) after the baseline visit.
Variables
We determined the eGFR using the CKiD estimating equation which is based on serum creatinine concentration, cystatin C, and BUN concentrations.16 Hypertension was defined as a systolic or diastolic BP ≥95th percentile for age, sex, and height.17 Proteinuria was defined as the log2-transformed urine protein-to-creatinine ratio. BMI was age and sex standardized. Kidney disease subtypes were classified according to cause: glomerular (FSGS, hemolytic uremic syndrome, systemic immunologic disease, chronic GN, familial nephritis, IgA nephropathy, membranoproliferative GN, Henoch–Schonlein nephritis, other) or nonglomerular (obstructive uropathy, aplastic kidney, dysplastic kidney, reflux nephropathy, autosomal recessive polycystic kidney disease, renal infarct, other).14
CKD Biomarker Measurements
Biospecimens were centrifuged and plasma was aliquoted. Barcoded aliquots of plasma were stored at −80°C until biomarker measurement. The six biomarkers were selected on the basis of a literature review of prior CKD biomarker research and pilot studies within the CKD Biomarker Consortium with consideration for pathophysiologic mechanisms of CKD progression.5,6,8,18–20 All assays were performed blinded to clinical outcomes. The plasma biomarkers were measured in duplicate using the Meso Scale Discovery (MSD) platform (Meso Scale Discovery, Gaithersburg, MD) and the mean value was used in the analysis. Biomarker measurements were repeated on a participant’s plasma sample if two or more analytes had intra-assay coefficients of variation >15%. After repeating measurements, the intra- and interassay coefficients of variation were all <10% (Supplemental Table 1). Because we recognized that differences between assay methods used may lead to differences in results and inferences, we performed extensive assay validation testing (Supplemental Methods). This included comparing the measurement of biomarker levels using MSD with a different multiplex platform Luminex, and we found the two different assays to have very high correlation, with an R2≥0.92 for each biomarker.
Statistical Analyses
Continuous variables were compared with the Wilcoxon rank sum test. The Spearman correlations between biomarkers, age, eGFR, and proteinuria were estimated in the total sample. For the primary analysis, we estimated the unadjusted and adjusted incidence rates of CKD progression events by quartile of plasma biomarker concentration. Adjusted incidence rates were estimated using Poisson regression, controlling for age, glomerular dianosis, proteinuria and eGFR at study entry. Each of these covariates was stratified as adjusted incidence rates were estimated using Poisson regression models. We then used Cox proportional hazards models to assess the association between quartiles of each plasma biomarker and CKD progression in separate models, after adjustment for known predictors of GFR decline. We controlled for baseline values of age, sex, BMI, glomerular diagnosis, proteinuria, hypertension status, and eGFR at study entry. Secondary analyses examine the associations stratified by both type of kidney disease (glomerular/nonglomerular) and baseline eGFR (above/below 60). The presence of interaction between continuous biomarker level and covariates was assessed by adding two-way interaction terms to the best fitting Cox regression model including: 1) log linear biomarkers and glomerular diagnosis and 2) log linear biomarkers and eGFR. The Akaike information criterion guided the selection of the best fit model, evaluating linear, polynomial, and spline relationships between the biomarker level and progression.
To evaluate the predictive value of the biomarkers, we used several discrimination metrics to determine the ability of values in the highest quartile of each biomarker to discriminate between participants who would and would not develop the composite outcome: Uno’s concordance statistic for the survival context, net reclassification improvement (NRI), and integrated discrimination improvement (IDI).21,22 We estimated the performances of the clinical model (incorporating age, sex, glomerular diagnosis, BMI z-score, urine protein-to-creatinine ratio, hypertension, eGFR) and the clinical model plus each individual biomarker for CKD progression. We also combined biomarkers that were independently associated with CKD progression and tested discrimination by comparing the performances of these biomarkers in combinations.
To examine the robustness of the primary analysis results, we used Bootstrap to resample the data with replacement 500 times and calculated the median of the estimates of coefficients adjusted for all covariates with a 95% confidence interval (95% CI). Although all six tested biomarkers were selected on the basis of literature reviews, we also evaluated significance compared with a conservative Bonferroni P=0.008 (0.05 divided by 6) to account for multiple testing. Analyses were performed using SAS 9.4 for Windows (SAS Institute Inc., NC) and R (R Core Team Version 3.5.1).
Results
Study Participants
The study sample consisted of 651 children, 195 with a glomerular cause of CKD and 456 with a nonglomerular cause of CKD (Table 1).14 In the overall cohort, the median age was 11 years (IQR, 8–15), 38% were female, 20% were black, 13% were Hispanic, and the median eGFR at study entry was 53 ml/min per 1.73 m2 (IQR, 40–67). At study entry, participants had a diagnosis of CKD for a median of 8.2 years (IQR, 3.9–12.4). The median follow-up time was 5.7 years (IQR, 3.6–7.9). During this time, 223 (34%) children developed the composite outcome; 138 (21%) with incident ESKD and 85 (13%) with a 50% decline in eGFR (Table 2).
Table 1.
Baseline characteristics by type of CKD
| Characteristic | Overall (n=651) | Glomerular Disease (n=195) | Nonglomerular Disease (n=456) |
|---|---|---|---|
| Age, yr | 11 [8–15] | 14 [11–16] | 10 [7–14] |
| Sex (male) | 404 (62) | 106 (54) | 298 (65) |
| Black race | 131 (20) | 59 (30) | 72 (16) |
| Hispanic ethnicity | 86 (13) | 27 (14) | 59 (13) |
| Premature birth (<36 wk) | 74 (12) | 21 (11) | 53 (12) |
| eGFR | 53 [40–67] | 62 [45–78] | 50 [38–62] |
| Time with CKD, yr | 8.2 [3.9–12.4] | 3.4 [1.3–7.6] | 9.5 [6.3–13.2] |
| Hypertension | 117 (18) | 30 (15) | 87 (19) |
| BUN | 24 [18–32] | 23 [17–30] | 25 [18–33] |
| Serum albumin level | 4.4 [4.1–4.6] | 4.2 [3.8–4.5] | 4.4 [4.2–4.6] |
| Hemoglobin level | 12.7 [11.7–13.7] | 12.3 [11.5–13.5] | 12.8 [11.8–13.7] |
| Urine protein/creatinine, mg/g | 0.32 [0.12–0.94] | 0.61 [0.21–1.76] | 0.24 [0.11–0.69] |
| Treatment with RAAS inhibitor | 370 (57) | 159 (82) | 211 (46) |
Hypertension is defined as a systolic or diastolic BP ≥95 percentile for age, height, and sex. Sample size differs due to missing data: time with CKD (n=640), hemoglobin level (n=639), Hispanic ethnicity (n=643), premature (n=627), and serum albumin level (n=649). Median [IQR] or frequency (percentage, %). RAAS, renin-angiotensin-aldosterone system.
Table 2.
CKD progression events during follow-up by type of CKD
| Characteristic | Overall (n=651) | Glomerular Disease (n=195) | Nonglomerular Disease (n=456) |
|---|---|---|---|
| Follow-up time, yr | 5.7 [3.6–7.9] | 4.6 [2.7–6.0] | 6.2 [4.0–9.1] |
| Event type | |||
| CKD progression | 223 (34) | 74 (38) | 149 (33) |
| Dialysis or transplantation | 138 (21) | 40 (21) | 98 (22) |
| 50% decline in eGFR | 85 (13) | 34 (17) | 51 (11) |
Median [IQR] or frequency (percentage, %).
Baseline Biomarker Levels and Participant Characteristics
The baseline characteristics are displayed by biomarker quartile in Supplemental Table 2. Spearman correlations among baseline biomarker levels and key predictors of CKD progression are shown in Supplemental Table 3. TNFR-1 and TNFR-2 were highly correlated (Rho=0.85). All biomarkers were inversely correlated with eGFR, with the strongest relationship between eGFR and TNFR-1 (Rho=−0.74). Plasma KIM-1 levels were higher in participants with glomerular causes of CKD compared with those with nonglomerular causes of CKD, whereas plasma MCP-1, TNFR-1, TNFR-2, suPAR, and YKL-40 levels were higher in nonglomerular causes of CKD (P<0.05) (Supplemental Table 4).
Association of Biomarkers with CKD Progression
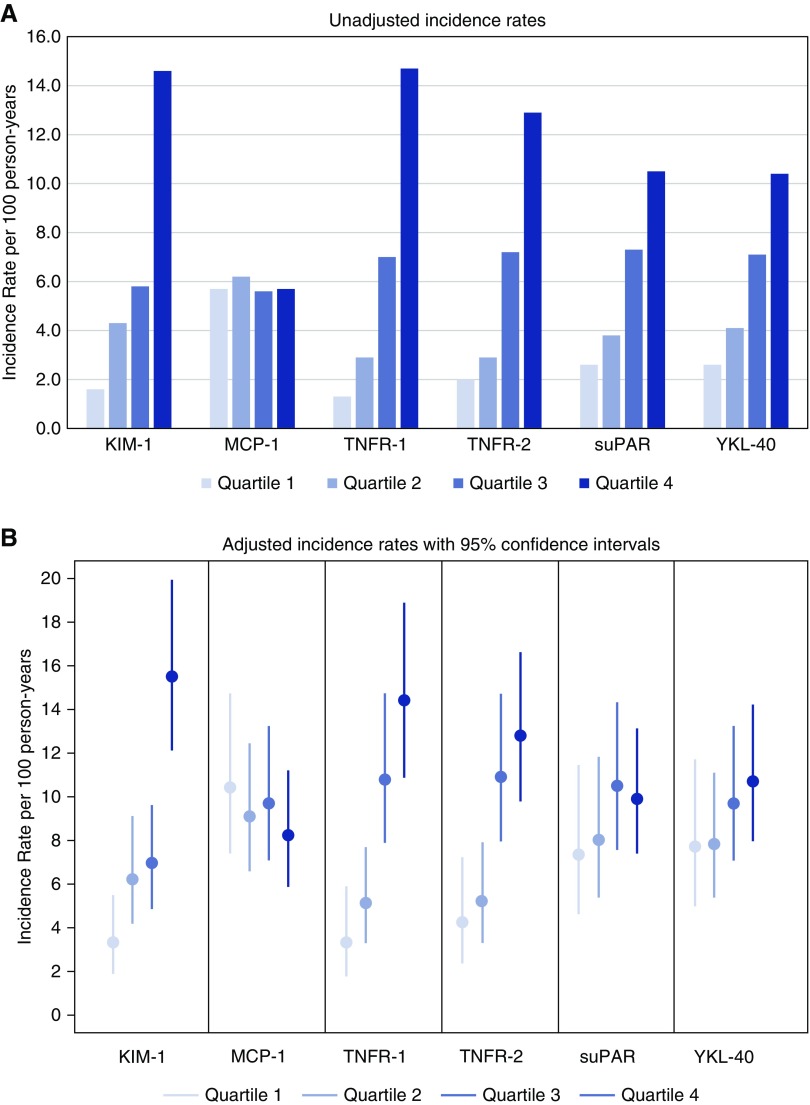
All baseline biomarkers except for MCP-1 were significantly higher in patients with CKD progression compared with those without CKD progression (P<0.001 for all except MCP-1) (Supplemental Figure 2). To study the risk of CKD progression by baseline biomarker levels, we examined the incidence rate as well as the cumulative risk of CKD progression according to biomarker quartiles (Figure 1, Supplemental Figure 3). The greatest number of CKD progression events occurred in the fourth quartile for most biomarkers except MCP-1. The unadjusted incidence rate was nine times higher for KIM-1, with 14.6 events per 100 person-years in quartile 4 versus 1.6 events per 100 person-years in quartile 1. This pattern was similar for TNFR-1, with 14.5 events per 100 person-years in quartile 4 versus 1.5 events per 100 person-years in quartile 1.
Figure 1.
All of the biomarkers except for MCP-1 had the greatest number of CKD progression events in the fourth quartile. The unadjusted (A) and adjusted (B) incidence rates of CKD progression events according to quartile of plasma biomarker concentration. Estimates of adjusted incidence rates were controlled for age, glomerular diagnosis, proteinuria, and eGFR at baseline.
After adjustment for age, sex, BMI z-score, and glomerular diagnosis, as well as hypertension status, proteinuria, and eGFR at study entry, children with plasma KIM-1, TNFR-1, or TNFR-2 concentrations in the highest quartile were at significantly higher risk of CKD progression compared with those with biomarker concentrations in the lowest quartile (KIM-1 adjusted hazard ratio [aHR], 4.29 [95% CI, 2.49 to 7.38]; TNFR-1 aHR, 4.14 [95% CI, 2.11 to 8.11]; TNFR-2 aHR, 2.55 [95% CI, 1.45 to 4.51]), which also crossed the Bonferroni threshold (Table 3). The medians and 95% CIs of aHRs among 500 Bootstrap samples were similar to the observed ones (Table 3).
Table 3.
Unadjusted and adjusted hazard ratios for the risk of CKD progressiona according to baseline plasma biomarker levels
| Biomarker | Biomarker Alone | Adjusted Model (plus age, sex, glomerular diagnosis, BMI z-score, hypertension status) | Adjusted Model (plus log2 uPr/Cr) | Fully Adjusted Model (plus eGFR) | Bootstrap Results of Fully Adjusted Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Overall (n=651), 223 events | ||||||||||
| KIM-1 | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 2.63 | (1.52 to 4.56) | 2.50 | (1.44 to 4.35) | 2.18 | (1.25 to 3.79) | 1.93 | (1.11 to 3.37) | 2.00 | (1.22 to 3.55) |
| Quartile 3 | 3.62 | (2.14 to 6.14) | 3.37 | (1.98 to 5.73) | 2.31 | (1.35 to 3.94) | 1.67 | (0.97 to 2.86) | 1.74 | (1.03 to 3.09) |
| Quartile 4 | 9.69 | (5.86 to 16.02) | 9.16 | (5.43 to 15.46) | 5.99 | (3.49 to 10.26) | 4.29 | (2.49 to 7.38) | 4.54 | (2.79 to 8.41) |
| MCP-1 | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 1.11 | (0.76 to 1.62) | 1.27 | (0.86 to 1.89) | 1.12 | (0.75 to 1.66) | 1.15 | (0.77 to 1.7) | 1.15 | (0.76 to 1.74) |
| Quartile 3 | 1.03 | (0.71 to 1.5) | 1.32 | (0.88 to 1.98) | 1.06 | (0.71 to 1.58) | 1.00 | (0.66 to 1.49) | 1.02 | (0.65 to 1.54) |
| Quartile 4 | 1.06 | (0.73 to 1.54) | 1.32 | (0.88 to 1.99) | 1.20 | (0.8 to 1.8) | 0.84 | (0.55 to 1.26) | 0.85 | (0.53 to 1.37) |
| TNFR-1 | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 1.91 | (1.03 to 3.54) | 2.34 | (1.26 to 4.37) | 2.11 | (1.13 to 3.93) | 1.43 | (0.75 to 2.72) | 1.49 | (0.78 to 2.89) |
| Quartile 3 | 4.75 | (2.71 to 8.31) | 7.09 | (3.98 to 12.63) | 5.05 | (2.83 to 9.01) | 3.01 | (1.63 to 5.56) | 3.07 | (1.76 to 6.38) |
| Quartile 4 | 10.40 | (6.06 to 17.85) | 15.81 | (9 to 27.75) | 9.65 | (5.45 to 17.08) | 4.14 | (2.11 to 8.11) | 4.19 | (2.03 to 9.31) |
| TNFR-2 | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 1.37 | (0.77 to 2.42) | 1.64 | (0.92 to 2.91) | 1.58 | (0.89 to 2.82) | 1.14 | (0.64 to 2.05) | 1.19 | (0.64 to 2.24) |
| Quartile 3 | 3.81 | (2.32 to 6.24) | 4.64 | (2.81 to 7.66) | 3.82 | (2.3 to 6.35) | 2.14 | (1.25 to 3.67) | 2.16 | (1.27 to 4.34) |
| Quartile 4 | 6.74 | (4.17 to 10.9) | 8.76 | (5.37 to 14.29) | 5.98 | (3.63 to 9.84) | 2.55 | (1.45 to 4.51) | 2.65 | (1.46 to 5.08) |
| suPAR | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 1.33 | (0.81 to 2.18) | 1.55 | (0.94 to 2.55) | 1.35 | (0.82 to 2.22) | 0.89 | (0.54 to 1.48) | 0.91 | (0.56 to 1.47) |
| Quartile 3 | 2.69 | (1.71 to 4.24) | 3.24 | (2.04 to 5.13) | 2.59 | (1.62 to 4.12) | 1.15 | (0.7 to 1.88) | 1.18 | (0.7 to 2.11) |
| Quartile 4 | 3.92 | (2.53 to 6.09) | 4.69 | (3 to 7.34) | 3.35 | (2.12 to 5.3) | 1.15 | (0.68 to 1.95) | 1.18 | (0.71 to 2.21) |
| YKL-40 | ||||||||||
| Quartile 1 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| Quartile 2 | 1.49 | (0.92 to 2.42) | 1.55 | (0.96 to 2.53) | 1.13 | (0.69 to 1.84) | 0.83 | (0.51 to 1.37) | 0.86 | (0.53 to 1.4) |
| Quartile 3 | 2.54 | (1.63 to 3.98) | 2.89 | (1.83 to 4.57) | 2.15 | (1.35 to 3.43) | 1.13 | (0.69 to 1.84) | 1.16 | (0.71 to 1.96) |
| Quartile 4 | 3.97 | (2.57 to 6.11) | 4.37 | (2.78 to 6.87) | 3.05 | (1.93 to 4.83) | 1.33 | (0.81 to 2.18) | 1.33 | (0.83 to 2.41) |
Data are presented as HR (95% CI). Adjusted for age, sex, glomerular diagnosis, BMI z-score, proteinuria/creatinine ratio, hypertension status, and baseline eGFR. uPr/Cr, urine protein/creatinine ratio; Ref. referent; suPAR, soluble urokinase receptor.
The primary outcome of CKD progression is defined as a composite of 50% decline in eGFR or ESKD.
Association of Biomarkers with CKD Progression Stratified by Glomerular versus Nonglomerular Causes of CKD
We assessed the relationship between baseline biomarker levels and CKD progression stratified by glomerular versus nonglomerular cause of kidney disease. There was no significant difference in the effect estimates by glomerular (KIM-1 fourth versus first quartile aHR, 2.49 [95% CI, 0.73 to 8.49]; TNFR-1 aHR, 6.48 [95% CI, 2.11 to 19.86]; TNFR-2 aHR, 4.60 [95% CI, 1.79 to 11.82]) versus nonglomerular (KIM-1 aHR, 4.49 [95% CI, 2.43 to 8.31]; TNFR-1 aHR, 2.30 [95% CI, 0.99 to 5.36]; TNFR-2 aHR, 1.46 [95% CI, 0.71 to 3.01]) causes of kidney disease (Table 4). In continuous biomarker models, the interactions between the biomarkers and diagnosis were NS for any of the biomarkers (interaction P values for KIM-1, MCP-1, TNFR-1, TNFR-2 suPAR, and YKL-40 of 0.544, 0.882, 0.388, 0.230, 0.416, and 0.060, respectively) (Supplemental Table 5).
Table 4.
aHRs for the risk of CKD progression according to plasma biomarker levels by glomerular or nonglomerular disease diagnosis
| Biomarkera | KIM-1 | MCP-1 | TNFR-1 | TNFR-2 | suPAR | YKL-40 |
|---|---|---|---|---|---|---|
| Glomerular (n=195), 74 events | ||||||
| Q1 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Q2 | 1.68 (0.41 to 6.87) | 1.45 (0.74 to 2.84) | 1.12 (0.38 to 3.31) | 1.08 (0.39 to 3.01) | 1.27 (0.57 to 2.85) | 0.55 (0.26 to 1.17) |
| Q3 | 0.68 (0.17 to 2.73) | 1.01 (0.46 to 2.23) | 4.79 (1.75 to 13.1) | 3.43 (1.37 to 8.59) | 1.29 (0.51 to 3.23) | 0.90 (0.38 to 2.12) |
| Q4 | 2.49 (0.73 to 8.49) | 0.87 (0.42 to 1.81) | 6.48 (2.11 to 19.86) | 4.60 (1.79 to 11.82) | 1.42 (0.56 to 3.6) | 1.20 (0.55 to 2.63) |
| Nonglomerular (n=456), 149 events | ||||||
| Q1 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Q2 | 1.95 (1.06 to 3.59) | 0.86 (0.52 to 1.42) | 1.44 (0.65 to 3.22) | 0.94 (0.45 to 1.96) | 0.79 (0.41 to 1.52) | 1.17 (0.58 to 2.38) |
| Q3 | 1.96 (1.09 to 3.53) | 0.88 (0.54 to 1.43) | 1.92 (0.89 to 4.15) | 1.37 (0.69 to 2.69) | 1.22 (0.65 to 2.26) | 1.51 (0.75 to 3.04) |
| Q4 | 4.49 (2.43 to 8.31) | 0.72 (0.43 to 1.19) | 2.30 (0.99 to 5.36) | 1.46 (0.71 to 3.01) | 1.08 (0.56 to 2.08) | 1.73 (0.85 to 3.53) |
Data are presented as aHR (95% CI). Adjusted for age, sex, glomerular diagnosis, BMI, hypertension, proteinuria, and baseline eGFR. suPAR, soluble urokinase receptor; Q, quartile; Ref. referent.
The primary outcome of CKD progression is defined as a composite of 50% decline in eGFR or ESKD.
Association of Biomarkers with CKD Progression Stratified by Baseline eGFR
We examined how the baseline eGFR modified the association of each biomarker with CKD progression. Children with plasma KIM-1, TNFR-1, or TNFR-2 concentrations in the highest quartile were at a significantly higher risk of CKD progression compared with the lowest quartile, in both the baseline eGFR≥60 (KIM-1 aHR, 10.37 [95% CI, 2.03 to 52.97]; TNFR-1 aHR, 13.94 [95% CI, 3.88 to 50.09]; TNFR-2 aHR, 6.1 [95% CI, 1.98 to 18.86]) and the GFR<60 ml/min per 1.73 m2 (KIM-1 aHR, 3.74 [95% CI, 2.1 to 6.65]; TNFR-1 aHR, 2.72 [95% CI, 1.18 to 6.28], TNFR-2 aHR, 1.99 [95% CI, 1.01 to 3.9]) subgroups (Supplemental Table 6). There was no significant difference in the effect estimates in those with a baseline eGFR≥60 versus <60 ml/min per 1.73 m2. However, there were only 30 events in the higher GFR group, limiting the power to evaluate these associations. In models with continuous biomarker levels and interactions with baseline eGFR, there were no significant interactions for any of the biomarkers (interaction P values for KIM-1, MCP-1, TNFR-1, TNFR-2 suPAR, and YKL-40 of 0.796, 0.976, 0.735, 0.948, 0.827, and 0.794, respectively).
Discrimination of Biomarkers for CKD Progression
Because neither glomerular diagnosis nor baseline eGFR modified the association between biomarkers and CKD progression, we evaluated the predictive value of the biomarkers in the overall model. Table 5 displays the C-statistic, IDI, and NRI for the clinical model alone and the model including each biomarker to predict CKD progression in the subsequent 12 years of follow-up. The clinical model, which consisted of age, sex, BMI, glomerular diagnosis, hypertension status, proteinuria, and eGFR at study entry, yielded a C-statistic of 0.76 (95% CI, 0.73 to 0.80). When plasma KIM-1 was added to the clinical model, the C-statistic increased by 0.02 (95% CI, 0.01 to 0.03), whereas adding other markers made no or a negligible difference in the C-statistic. The best discrimination by the C-statistic was obtained by the addition of both KIM-1 and TNFR-1 to the clinical models, with an increase of 0.03 (95% CI, 0.01 to 0.04) in the C-statistic. The IDI did not significantly improve with the addition of KIM-1 or TNFR-1. The NRI was 0.27 with the addition of KIM-1, indicating modest improvement over the clinical model. However, addition of TNFR-1 did not result in improvement according to the NRI. All NRI and IDI estimates had 95% CIs that included 0, indicating uncertainty as to whether there was any improvement in the predicted probabilities with the addition of any of the biomarkers.
Table 5.
Discrimination and calibration of CKD progression from biomarker values in the highest quartile
| Model | Uno C-Statistic | Difference in C-Statistic (95% CI) | IDI (95% CI) | Continuous NRI (95% CI) |
|---|---|---|---|---|
| Clinical model (as reference) | 0.76 (0.73 to 0.80) | |||
| Clinical model plus biomarker | ||||
| KIM-1 | 0.78 (0.75 to 0.81) | 0.02 (0.01 to 0.03) | 0.05 (−0.01 to 0.10) | 0.27 (−0.25 to 0.42) |
| MCP-1 | 0.76 (0.73 to 0.80) | 0 (−0.01 to 0.01) | 0.01 (−0.02 to 0.05) | 0.07 (−0.28 to 0.41) |
| TNFR-1 | 0.77 (0.74 to 0.80) | 0.01 (−0.01 to 0.02) | 0 (−0.04 to 0.04) | −0.05 (−0.37 to 0.40) |
| TNFR-2 | 0.76 (0.73 to 0.80) | 0 (−0.01 to 0.01) | 0 (−0.03 to 0.04) | 0.10 (−0.24 to 0.42) |
| suPAR | 0.76 (0.73 to 0.80) | 0 (−0.01 to 0.01) | 0 (−0.03 to 0.02) | −0.03 (−0.35 to 0.37) |
| YKL-40 | 0.76 (0.73 to 0.80) | 0 (−0.01 to 0.01) | 0 (−0.03 to 0.01) | −0.17 (−0.47 to 0.34) |
| Clinical model plus biomarker combinations | ||||
| Plus KIM-1 and TNFR-1 | 0.79 (0.75 to 0.82) | 0.03 (0.01 to 0.04) | 0.04 (−0.02 to 0.10) | 0.13 (−0.24 to 0.48) |
| Plus TNFR-2 | 0.78 (0.75 to 0.82) | 0.02 (0.01 to 0.04) | 0.05 (−0.02 to 0.14) | 0.23 (−0.16 to 0.53) |
| Plus MCP-1 | 0.79 (0.75 to 0.82) | 0.03 (0.01 to 0.04) | 0.05 (0 to 0.14) | 0.28 (−0.15 to 0.50) |
| Plus suPAR | 0.79 (0.75 to 0.82) | 0.03 (0.01 to 0.04) | 0.05 (−0.02 to 0.13) | 0.26 (−0.21 to 0.50) |
| Plus YKL-40 | 0.79 (0.75 to 0.82) | 0.03 (0.01 to 0.04) | 0.04 (−0.03 to 0.10) | 0.14 (−0.27 to 0.48) |
| Clinical with all biomarkers | 0.79 (0.75 to 0.82) | 0.03 (0.01 to 0.04) | 0.07 (0 to 0.19) | 0.37 (−0.19 to 0.54) |
Discussion
We observed that a one-time baseline measurement of circulating KIM-1, TNFR-1, or TNFR-2 was associated with the risk of CKD progression even after adjusting for established risk factors including hypertension, proteinuria, and eGFR. Plasma MCP-1, suPAR, and YKL-40 were not independently associated with CKD progression in our study. After multivariable adjustment, children with KIM-1, TNFR-1, or TNFR-2 concentrations in the highest quartile were at a four-, four-, and two-fold higher risk, respectively, of progressing to the composite end point compared with those in the lowest quartile. In stratified quartile analyses, we observed that TNFR-1 and TNFR-2 were independently associated with CKD progression in children with glomerular causes of CKD, whereas KIM-1 was independently associated with CKD progression in children with nonglomerular causes of CKD. The addition of both plasma KIM-1 and TNFR-1 to the baseline clinical model led to a modest increase in discrimination for the composite outcome.
To our knowledge, this is the first study to describe independent associations of plasma KIM-1, TNFR-1, and TNFR-2 with CKD progression in children. Previous studies demonstrating an association between these biomarkers and eGFR decline had been restricted to adults and focused predominantly on patients with diabetic nephropathy and multiple comorbidities.5,8,9 However, these findings in adult studies may not be generalizable to children whose primary causes of CKD are different, notably due to congenital anomalies of the kidney and urinary tract. Furthermore, we studied participants from the CKiD cohort who represent a diverse group of kidney diseases with >30 primary diagnoses including both glomerular and nonglomerular causes of CKD. Remarkably, our findings for plasma KIM-1, TNFR-1, and TNFR-2 concentrations in a heterogeneous pediatric CKD cohort were consistent with previous studies in adults with diabetic nephropathy, despite the markedly different baseline characteristics, comorbidities, and disease pathophysiology.5,8,9
We observed that plasma KIM-1 was independently associated with CKD progression in the entire sample. KIM-1 is a glycoprotein receptor expressed in the apical membrane of proximal tubular cells that mediates the uptake of apoptotic cells and oxidized lipids.23,24 KIM-1 is expressed with any form of proximal tubular injury.25 Proximal tubular injury is an important contributor to CKD progression. After tubular injury, KIM-1 is thought to enter the interstitium and then the blood circulation when epithelial permeability increases and tubular cell polarity is lost.6,26 Prior studies have documented that in both nondiabetic and diabetic adult cohorts plasma KIM-1 has been an independent predictor of CKD progression.5–7
We also observed that plasma TNFR-1 and TNFR-2 concentrations are independently associated with CKD progression in the overall sample and in the subsample of children with glomerular causes of CKD. The plasma TNF receptors (TNFRs) have been shown to predict the rate of GFR decline in an adult cohort with minimal CKD at baseline as well as multiple prospective studies of diabetic kidney disease.5,8,9,27,28 It is notable that the associations and effect sizes of TNFR-1 and TNFR-2 in prior diabetic kidney disease research were very similar to our results.5 The robust association between plasma TNFRs and CKD progression across multiple glomerular causes of CKD suggests that TNFR marks a common biologic pathway that directly contributes to the rate of GFR decline. We also noted that plasma TNFR-1 and TNFR-2 concentrations were highly correlated, with a correlation coefficient of 0.85, which underscores the close relationship and similar information indicated by plasma concentrations of these two receptors.
TNFR-1 and TNFR-2 are cell surface receptors that mediate the action of TNFα, a potent proinflammatory cytokine and a key driver of the acute phase reaction. TNFR-1 is expressed in a wide variety of cells including glomerular and peritubular endothelial cells and acts as an integral mediator of local inflammation.29 TNFR-2 is expressed primarily in lymphocytes.30 Animal studies have shown that systemic administration of TNFα causes direct glomerular injury.31 TNFα knockout mice with GN had a delay in onset of proteinuria and do not develop glomerular crescents.32 Interestingly, as compared with the TNFRs, TNFα has been shown to have a weaker association with the risk of CKD progression.8 The exact mechanism by which TNFRs contribute to CKD progression remains unclear, but experimental models demonstrate that the TNFRs are essential in the pathway of endothelial injury and glomerular damage.29,33 This mechanism of glomerular damage involving the TNF pathway is consistent with the independent association with CKD progression that we observed with the TNFRs in children with glomerular causes of CKD as compared with nonglomerular disease.
We observed that plasma suPAR was associated with CKD progression, but the association was attenuated upon adjusting for eGFR and proteinuria. Our findings are in contrast to those reported by Schaefer et al., who noted that suPAR was associated with eGFR decline in children with a baseline eGFR>40 ml/min per 1.73 m2. Our results for plasma suPAR did not vary according to baseline eGFR<60 and eGFR≥60 ml/min per 1.73 m2. Several differences between our study and that of Schaefer et al. may explain the divergent findings. We studied a racially and ethnically diverse CKD cohort (20% black, 13% Hispanic), whereas their study cohort was predominantly white. Another important difference was the measurement of plasma suPAR with a multiplex electrochemiluminescence assay in this study, whereas Schaefer et al. measured serum suPAR with an ELISA. The suPAR results presented herein from CKiD differ from results from the Emory Cardiovascular Biobank cohort34 as well as an analysis from CKiD where plasma suPAR was measured with the Quantikine ELISA immunoassay.35 As compared with the Quantikine assay, our data demonstrated a very similar pattern of significant effect estimates in unadjusted and partially adjusted Cox models, although results differed in fully adjusted models. Only after adjusting for eGFR in the fully adjusted model did we observe no significant effect for children in the fourth quartile in our study (suPAR aHR, 1.15 [95% CI, 0.68 to 1.95]), whereas the Quantikine assay CKiD data showed that children in the fourth quartile of plasma suPAR levels experienced an increased risk of CKD progression when compared with the first quartile in the fully adjusted model (suPAR aHR, 1.74 [95% CI, 1.03 to 2.95]). Our study is the first that we are aware of that examined suPAR and the TNFRs in the same cohort, so that direct head-to-head comparisons could be made.
We observed that the highest quartiles of plasma KIM-1, TNFR-1, and TNFR-2 concentrations in Cox regression models have high point estimates in children with a baseline eGFR≥60 ml/min per 1.73 m2. Additionally, we observed that YKL-40 was only associated with CKD progression in children with a baseline eGFR≥60 ml/min per 1.73 m2. These findings highlight the opportunity to develop biomarkers for children with a higher baseline GFR, because eGFR and proteinuria are less-reliable predictors of CKD progression in this group and there is substantially more kidney function to preserve. As such, in children with a baseline eGFR≥60 ml/min per 1.73 m2, novel biomarkers may have better performance because the variability and heterogeneity of progressive CKD is greater.36
Our investigation has several limitations. We stratified the cohort by both disease cause and baseline eGFR to understand the bounds of associations between biomarkers and progression. We performed these stratified analyses because the type of kidney disease and the baseline eGFR are important determinants in the rate and pattern of CKD progression. However, the interaction P value was NS in these subgroup analyses. Additionally, by stratifying, we reduced our sample size and the number of composite outcome events in each subgroup, which can result in less-stable estimates and a higher risk of chance findings. These less-stable estimates in subgroup analyses were reflected in the wider 95% CIs observed. We also acknowledge the multiple statistical testing performed in our investigation and the risk of chance (false-positive) findings. Notably, there are currently no standard assays to measure the studied analytes and we used a validated MSD multiplex assay to complete the biomarker measurements. Additionally, with our current sample size we are underpowered to study specific diagnoses or treatments. For instance, for the glomerular disease group the most common diagnoses include FSGS (n=57 with 29 of 57 events), hemolytic uremic syndrome (n=36 with ten of 36 events), and systemic immunologic disease such as SLE nephritis (n=25 with five of 25 events). These sample sizes limit the extent of diagnosis-specific analyses. Our research is also limited by the use of eGFR to evaluate kidney function, which can differ from a directly measured GFR but is consistent with what is used in clinical practice to assess the stage of CKD.
In conclusion, we demonstrated that plasma KIM-1, TNFR-1, and TNFR-2 concentrations were independently associated with CKD progression in children. However, in subgroup analyses TNFR-1 was only independently associated with CKD progression in children with glomerular disease. Although KIM-1 may be a byproduct of tubular injury, TNFRs are part of a proinflammatory pathway that may mediate CKD progression. As such, KIM-1, TNFR-1, and TNFR-2, as biomarkers of tubular damage and inflammation, respectively, may be identifying ongoing causative mechanisms of CKD progression.
Disclosures
Dr. Abraham, Dr. Furth, Dr. Gonzales, Dr. Greenberg, Dr. Kimmel, Dr. Sabbisetti, Dr. Schelling, and Dr. Xu declare no financial interest. Dr. Abraham reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) during the conduct of the study. Dr. Bonventre is cofounder and holds equity in Goldfinch Bio. He is coinventor on KIM-1 patents assigned to Partners Healthcare, received grant funding from Boehringer Ingelheim, and holds equity in Dicerna, Goldilocks, Innoviva, Medibeacon, Medssenger, Rubius, Sensor-Kinesis, Sentien, Theravance, Thrasos, and VeriNano and has received consulting income from Aldeyra, Angion, Biomarin, Praxis, PTC, and Sarepta. Dr. Bonventre’s interests were reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict of interest policies. Dr. Coca reports personal fees from Bayer, CHF Solutions, Goldfinch, Janssen, Quark, Relypsa, and Takeda; and personal fees and other from pulseData and RenalytixAI, outside the submitted work. Dr. Feldman reports grants from the National Institutes of Health–NIDDK, during the conduct of the study; and other from the American Journal of Kidney Disease and Kyowa Hakko Kirin Co., Ltd., outside the submitted work. Dr. Parikh reports other from Akebia Therapeutics, Inc., Genfit Biopharmaceutical Company, and Renaltix AI; and grants from the NIDDK and the National Heart, Lung and Blood Institute, outside the submitted work. Dr. Shlipak is a Scientific Advisor for TAI Diagnostics. Dr. Waikar reports personal fees from Barron and Budd (versus Fresenius), Bunch and James, Cerus, CVS, GE Health Care, GSK, Harvard Clinical Research Institute (aka Baim), JNJ, Kantum Pharma, Mallinckrodt, Mass Medical International, Pfizer, the Public Health Advocacy Institute, Roth Capital Partners, Strataca, Takeda, Verbio, Wolters Kluewer; and grants and personal fees from Allena Pharmaceuticals, outside the submitted work.
Funding
This research was supported by National Institutes of Health (NIH) career development grant K08DK110536 (to Dr. Greenberg). Dr. Parikh is supported by NIH K24DK090203, R01HL085757, U01DK082185, and P30DK079310-07 O’Brien Center Grant. Dr. Furth is supported by NIH grants K24DK078737 and U01DK66174. Dr. Schrauben is supported by NIH grant K23DK118198-01A1. Dr. Xu, Dr. Schelling, Dr. Feldman, Dr. Sabbisetti, Dr. Gonzalez, Dr. Coca, Dr. Sabbisetti, Dr. Bonventre, Dr. Parikh, and Dr. Furth receive support from the CKD Biomarkers Consortium (National Institute of Diabetes and Digestive and Kidney Diseases grants U01 DK085689, U01 DK102730, U01 DK103225, and U01 DK085660).
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the CKD in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Dr. Warady), the Children’s Hospital of Philadelphia (Dr. Furth), and the Central Biochemistry Laboratory (George Schwartz) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz) at the Johns Hopkins Bloomberg School of Public Health. The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid. The CKD Biomarker consortium members include: Dr. Vasan S. Ramachandran, Dr. Jeffrey Schelling, Dr. Michelle Denburg, Dr. Susan Furth, Dr. Bradley Warady, Dr. Joseph Bonventre, Dr. Sushrut Waikar, Dr. Venkata Sabbisetti, Dr. Josef Coresh, Dr. Morgan Grams, Dr. Casey Rebholz, Dr. Alison Abraham, Dr. Chirag Parikh, Dr. Steven Coca, Dr. Eugene Rhee, Dr. Paul L. Kimmel, Dr. John W. Kusek, Dr. Brad Rovin, Dr. Michael G. Shlipak, Dr. Mark Sarnak, Dr. Orlando M. Gutiérrez, Dr. Joachim Ix, Dr. Ruth Dubin, Dr. Tom Hostetter, Dr. Rajat Deo, Dr. Harold I. Feldman, Dr. Dawei Xie, Dr. Haochang Shou, Mr. Shawn Ballard, Ms. Krista Whitehead, Dr. Heather Collins, Dr. Jason H. Greenberg, and Dr. Peter Ganz.
Dr. Greenberg, Dr. Xu, Dr. Schelling, Dr. Feldman, Dr. Coca, Dr. Waikar, Dr. Vasan, Dr. Shlipak, Dr. Kimmel, Dr. Bonventre, Dr. Parikh, and Dr. Furth designed the study. Dr. Sabbisetti, Dr. Gonzalez, and Dr. Bonventre carried out experiments and acquired data. Dr. Greenberg, Dr. Xu, and Dr. Furth analyzed the data. Dr. Greenberg and Dr. Xu made the figures. Dr. Greenberg, Dr. Xu, Dr. Schelling, Dr. Feldman, Dr. Sabbisetti, Dr. Gonzalez, Dr. Coca, Dr. Schrauben, Dr. Waikar, Dr. Vasan, Dr. Shlipak, Dr. Warady, Dr. Kimmel, Dr. Bonventre, Dr. Parikh, and Dr. Furth drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Biomarkers of CKD in Children,” on pages 894–896.
Contributor Information
Collaborators: Jason H. Greenberg, Alison G. Abraham, Yunwen Xu, Jeffrey R. Schelling, Harold I. Feldman, Venkata S. Sabbisetti, Mariana Cardenas Gonzalez, Steven Coca, Sarah J. Schrauben, Sushrut S. Waikar, Ramachandran S. Vasan, Michael G. Shlipak, Bradley Warady, Paul L. Kimmel, Joseph V. Bonventre, Michelle Denburg, Chirag R. Parikh, Susan Furth, and on behalf of the CKD Biomarkers Consortium
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019070723/-/DCSupplemental.
Supplemental Table 1. Biomarker intra- and interassay coefficients of variation.
Supplemental Table 2. Baseline characteristics by biomarker quartiles.
Supplemental Table 3. Spearman correlations of biomarker concentrations and baseline participant characteristics.
Supplemental Table 4. Median biomarker concentration by glomerular versus nonglomerular diagnosis.
Supplemental Table 5. Adjusted hazard ratios for the risk of CKD progression according to doubling of plasma biomarker levels.
Supplemental Table 6. Adjusted hazard ratios for the risk of CKD progression according to plasma biomarker levels by baseline GFR>60 versus GFR<60.
Supplemental Figure 1. Study population flow chart.
Supplemental Figure 2. Baseline biomarker concentrations by CKD progression.
Supplemental Figure 3. Kaplan–Meier curve of risk of CKD progression by plasma biomarker quartile.
References
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, et al.: US renal data system 2012 annual data report. Am J Kidney Dis 61[Suppl 1]: A7, e1–e476, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JH, Parikh CR: Biomarkers for diagnosis and prognosis of AKI in children: One size does not fit all. Clin J Am Soc Nephrol 12: 1551–1557, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in Children) prospective cohort study: A review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avner ED, Harmon WE, Niaudet P, Yoshikawa N: Pediatric nephrology, Baltimore, Williams & Wilkins, 2009 [Google Scholar]
- 5.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al.: Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al.: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alderson HV, Ritchie JP, Pagano S, Middleton RJ, Pruijm M, Vuilleumier N, et al.: The associations of blood kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin with progression from CKD to ESRD. Clin J Am Soc Nephrol 11: 2141–2149, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al.: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al.: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, et al.: Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89: 459–467, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg JH, Kakajiwala A, Parikh CR, Furth S: Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol 33: 925–933, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer F, Trachtman H, Wühl E, Kirchner M, Hayek SS, Anarat A, et al.; ESCAPE Trial Consortium and the 4C Study Group: Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr 171: e172914, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu RK, Chawla LS, Wheeler DS, Goldstein SL: Renal angina: An emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol 27: 1067–1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al.: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al.: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al.: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al.; SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN: Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140: e20171904, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al.: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, et al.: Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol 24: 309–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesch GH: MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 294: F697–F701, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ: On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 30: 1105–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno H, Tian L, Cai T, Kohane IS, Wei LJ: A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med 32: 2430–2442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonventre JV: Kidney injury molecule-1: A translational journey. Trans Am Clin Climatol Assoc 125: 293–299; discussion 299, 2014 [PMC free article] [PubMed] [Google Scholar]
- 24.Bonventre JV: Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Schnaper HW: The tubulointerstitial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis 24: 107–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers BD, Chui F, Hilberman M, Michaels AS: Transtubular leakage of glomerular filtrate in human acute renal failure. Am J Physiol 237: F319–F325, 1979 [DOI] [PubMed] [Google Scholar]
- 27.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA: Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 87: 812–819, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatraju PK, Zelnick LR, Shlipak M, Katz R, Kestenbaum B: Association of soluble TNFR-1 concentrations with long-term decline in kidney function: The multi-ethnic study of atherosclerosis. J Am Soc Nephrol 29: 2713–2721, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Lamki RS, Mayadas TN: TNF receptors: Signaling pathways and contribution to renal dysfunction. Kidney Int 87: 281–296, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Murphy K, Weaver C: Janeway’s Immunobiology, New York, NY, Garland Science/Taylor & Francis Group, LLC, 2016 [Google Scholar]
- 31.Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, et al.: Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol 134: 419–430, 1989 [PMC free article] [PubMed] [Google Scholar]
- 32.Le Hir M, Haas C, Marino M, Ryffel B: Prevention of crescentic glomerulonephritis induced by anti-glomerular membrane antibody in tumor necrosis factor-deficient mice. Lab Invest 78: 1625–1631, 1998 [PubMed] [Google Scholar]
- 33.Vielhauer V, Stavrakis G, Mayadas TN: Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest 115: 1199–1209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weidemann DK, Abraham AG, Roem JL, Furth SL, Warady BA: Plasma soluble urokinase plasminogen activator receptor (suPAR) and CKD progression in children. Am J Kidney Dis 2020. 10.1053/j.ajkd.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayek SS, Quyyumi AA, Reiser J: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 374: 891, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Schnaper HW: Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol 29: 193–202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.