Significance Statement
The CREDENCE randomized trial demonstrated that canagliflozin reduces risk of cardiovascular and renal events in people with type 2 diabetes and substantial albuminuria. The authors analyzed CREDENCE data to assess whether canagliflozin’s benefits are safely preserved in people with reduced eGFR, finding that the relative benefits for renal and cardiovascular outcomes appeared consistent among subgroups with initial eGFR ranging from 30 to <90 ml/min per 1.73 m2. Absolute benefit for renal outcomes was greater in subgroups with an initial eGFR of <60 ml/min per 1.73 m2. Safety outcomes were generally consistent among eGFR subgroups. Canagliflozin led to an acute eGFR drop, followed by relative stabilization of eGFR loss across subgroups. Canagliflozin’s benefits and safety are apparent across the eGFR range, including among those initiating treatment with eGFR as low as 30 ml/min per 1.73 m2.
Keywords: canagliflozin, SGLT2 inhibitor, chronic kidney disease, end-stage kidney disease, diabetes
Visual Abstract
Abstract
Background
Canagliflozin reduced renal and cardiovascular events in people with type 2 diabetes in the CREDENCE trial. We assessed efficacy and safety of canagliflozin by initial estimated glomerular filtration rate (eGFR).
Methods
CREDENCE randomly assigned 4401 participants with an eGFR of 30 to <90 ml/min per 1.73 m2 and substantial albuminuria to canagliflozin 100 mg or placebo. We used Cox proportional hazards regression to analyze effects on renal and cardiovascular efficacy and safety outcomes within screening eGFR subgroups (30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2) and linear mixed effects models to analyze the effects on eGFR slope.
Results
At screening, 1313 (30%), 1279 (29%), and 1809 (41%) participants had an eGFR of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2, respectively. The relative benefits of canagliflozin for renal and cardiovascular outcomes appeared consistent among eGFR subgroups (all P interaction >0.11). Subgroups with lower eGFRs, who were at greater risk, exhibited larger absolute benefits for renal outcomes. Canagliflozin’s lack of effect on serious adverse events, amputations, and fractures appeared consistent among eGFR subgroups. In all subgroups, canagliflozin use led to an acute eGFR drop followed by relative stabilization of eGFR loss. Among those with an eGFR of 30 to <45 ml/min per 1.73 m2, canagliflozin led to an initial drop of 2.03 ml/min per 1.73 m2. Thereafter, decline in eGFR was slower in the canagliflozin versus placebo group (–1.72 versus –4.33 ml/min per 1.73 m2; between-group difference 2.61 ml/min per 1.73 m2).
Conclusions
Canagliflozin safely reduced the risk of renal and cardiovascular events, with consistent results across eGFR subgroups, including the subgroup initiating treatment with an eGFR of 30 to <45 ml/min per 1.73 m2. Absolute benefits for renal outcomes were greatest in subgroups with lower eGFR.
Clinical Trial registry name and registration number
Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE), NCT02065791.
Sodium glucose cotransporter 2 (SGLT2) inhibitors were developed as glucose-lowering agents for people with type 2 diabetes mellitus. Their physiologic effect is exerted by inhibition of SGLT2 proteins on the luminal surface of proximal tubular cells, which they reach by filtration at the glomerulus.1 There they inhibit the reabsorption of sodium and glucose from the renal tubule, resulting in enhanced urinary sodium and glucose excretion. It is clear the effect of SGLT2 inhibitors on glucose lowering is attenuated at reduced eGFR levels2 and, as a consequence, it has been hypothesized that the effect of SGLT2 inhibitors on clinical benefit would likewise be attenuated at a lower eGFR. The original regulatory indications restricted the use of SGLT2 inhibitors to a lower eGFR limit of 45 or 60 ml/min per 1.73 m2 because of reduced efficacy in lowering blood glucose below these levels.3−6
Despite the attenuation of efficacy in lowering blood glucose in patients with impaired renal function, interest in studying canagliflozin for renal-protective effects in the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial was based on findings from early glycemic control studies in which favorable effects on lowering urinary albumin-creatinine ratio (UACR) and preserving eGFR were observed.7,8 The acute, modest decline in eGFR that was observed in previous studies attenuated over time and was consistent with a hemodynamically mediated effect reminiscent of those seen with angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy.9 The strong association of albuminuria with clinical renal outcomes and the concept that these agents might lower intraglomerular pressure led to the hypothesis that they may protect against the progression of diabetic kidney disease, including in people with lower eGFR, potentially independent of the glucose-lowering effect. The CREDENCE trial was designed to evaluate the benefits of canagliflozin on the risk of kidney failure and cardiovascular events, while also assessing safety, in people with type 2 diabetes at high risk of kidney disease progression.
Canagliflozin safely reduced renal and cardiovascular events in the CREDENCE population overall.10 In this secondary analysis of the CREDENCE trial, we investigated whether the effects of canagliflozin on clinically important kidney, cardiovascular, and safety outcomes were consistent across the broad range of included eGFR, including in the lower eGFR range of 30–45 ml/min per 1.73 m2 where glucose effects are minimal.
Methods
The CREDENCE study design11 has been published previously. In brief, CREDENCE was a randomized, double-blind, placebo-controlled, multicenter clinical trial assessing the effect of canagliflozin on clinically important renal, cardiovascular, and safety outcomes in people with type 2 diabetes and CKD.
Study Participants
Eligible participants were ≥30 years of age with type 2 diabetes mellitus, a glycated hemoglobin (HbA1c) level of 6.5%–12.0%, an eGFR of 30–<90 ml/min per 1.73 m2, UACR >300–5000 mg/g (>33.9–565.6 mg/mmol), and were receiving treatment with a stable maximum labeled or tolerated dose of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for ≥4 weeks before randomization. By design, approximately 60% of participants were to have a screening eGFR of 30 to <60 ml/min per 1.73 m2. Exclusion criteria included nondiabetic kidney disease, type 1 diabetes mellitus, and prior treatment of kidney disease with immunosuppression or a history of RRT.
Randomization, Study Treatment, and eGFR Categories
Participants were randomized to receive oral canagliflozin 100 mg daily or matching placebo. The protocol stipulated that study treatment be continued until the commencement of dialysis, receipt of a kidney transplant, occurrence of diabetic ketoacidosis, pregnancy, or receipt of disallowed therapy or study conclusion.
Eligibility criteria for the study included an eGFR of 30–90 ml/min per 1.73 m2. After screening, participants either proceeded to a 2-week single-blind placebo run-in or underwent an extended screening if required for various reasons including completing at least 4 weeks on a stable dose of renin-angiotensin blockade therapy. Participants who did not proceed directly to the 2-week run-in period had a repeat eGFR measurement at the beginning of the run-in period. The most proximate eGFR measurement (e.g., screening or week −2) to baseline was deemed the “screening” eGFR and was used to stratify randomization in the categories of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2. On the day of randomization, an additional baseline measurement of eGFR was performed. Background treatment intensification for glycemic management and cardiovascular protection according to practice guidelines was recommended.
Outcomes
The primary outcome for these analyses was the same as the primary trial10: the composite of ESKD (chronic dialysis for ≥30 days, kidney transplantation, or eGFR <15 ml/min per 1.73 m2 sustained for ≥30 days by central laboratory assessment), doubling of serum creatinine from baseline average of randomization and prerandomization values sustained for ≥30 days by central laboratory assessment, or death due to renal or cardiovascular disease. Secondary renal outcomes included the composite of ESKD, doubling of serum creatinine, or renal death; ESKD; doubling of serum creatinine; and the exploratory composite of initiation of RRT (initiation of chronic dialysis for ≥30 days or receipt of a kidney transplant) or renal death. Other efficacy outcomes included the composite of cardiovascular death or hospitalization for heart failure; the composite of cardiovascular death, myocardial infarction, or stroke; hospitalization for heart failure; cardiovascular death; and the composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure or unstable angina. Safety events were explored in this analysis where there were at least 10 events per eGFR subgroup and included all adverse events and serious adverse events, amputation, fracture, osmotic diuresis, and volume depletion. The renal and cardiovascular outcomes and selected safety outcomes were independently adjudicated.
Other outcomes for this study included eGFR slope, measured as the acute change in eGFR from baseline to week 3 (acute slope); the annualized chronic change in eGFR from week 3 until end of treatment (chronic slope); and the annualized change in eGFR from baseline to week 130 (total slope). The eGFR slope analyses used on-treatment measures to avoid the expected distortions due to modifications of the hemodynamic effect that occur when a study drug is discontinued. The CKD Epidemiology Collaboration formula was used to calculate the eGFR.
We also assessed the effect of canagliflozin on the intermediate outcomes of HbA1c, body weight, systolic BP, and UACR.
Observational Analysis of Participants Whose Last On-Treatment eGFR Was <30 ml/min per 1.73 m2
In an observational analysis, to illustrate the course of participants within the study, we assessed outcomes in participants whose last on-treatment eGFR was <30 ml/min per 1.73 m2 for the time period from their first eGFR <30 ml/min per 1.73 m2 until the end of the study. The outcomes reported in this way were those specified in the hierarchic testing sequence of the protocol, namely: the primary composite end point; the composite of cardiovascular death or hospitalization for heart failure; the composite of cardiovascular death, myocardial infarction, or stroke; hospitalization for heart failure; the renal composite of doubling of serum creatinine, ESKD, or renal death; and cardiovascular death.
Statistical Analyses
Analysis of the effects of canagliflozin on the primary outcome was prespecified in participants with screening eGFR categories of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2 using an intention-to-treat approach; analyses of other renal, cardiovascular, and safety outcomes by eGFR categories were post hoc. Hazard ratios (HRs) and 95% confidence intervals (CIs) for all outcomes were estimated using a Cox proportional hazards regression model within each eGFR stratum. We tested the heterogeneity of treatment effects across screening eGFR categories by adding eGFR categories as a covariable and an interaction term of treatment by eGFR categories to the relevant model. Annualized incidence rates were calculated per 1000 patient-years of follow-up. Absolute risk differences were calculated by subtracting the number of participants with an end point (per 1000 patients over follow-up) of placebo from those of canagliflozin. The heterogeneity of absolute risk reduction for cardiovascular or renal end points across screening eGFR subgroups was estimated using fixed effects meta-analysis. Linear mixed effects models for repeated measures were used to analyze changes in intermediate outcomes over time in the on-treatment analysis population (unless otherwise noted), assuming an unstructured covariance and adjusting for the baseline value, trial group, and trial visit. On-treatment eGFR slope was estimated using all central laboratory eGFR measurements from study day 1 up to the last dose of the study medication plus 2 days. The effects of canagliflozin on the mean on-treatment eGFR slope were analyzed by fitting a two-slope mixed effects linear spline model (with a knot at week 3) to eGFR values, with random intercept and random slopes for treatment. When the unstructured models failed to converge, a simplified model with a random intercept and a single random slope was used to account for the variation in eGFR trajectories across participants. The mean total slope was computed as a weighted combination of the acute and chronic slopes to reflect the mean rate of eGFR change to week 130. We also provide a visual representation of the pattern of change in mean eGFR using a restricted maximum likelihood repeated measures approach. This analysis included the fixed, categoric effects of treatment, visit, and treatment-by-visit interaction, as well as the continuous, fixed covariates of baseline eGFR and baseline eGFR-by-visit interaction. In the nonrandomized subgroup of participants defined by last on-treatment eGFR of <30 ml/min per 1.73 m2, the number of participants with the first event occurring on and after the first eGFR of <30 ml/min per 1.73 m2 were summarized by treatment group for the renal and cardiovascular outcomes. Given the post hoc nature of many of the analyses, P values have been presented for descriptive rather than inferential purposes, without adjustment for multiplicity. All analyses were performed using SAS version 9.4.
Results
The CREDENCE trial randomized 4401 participants with a median follow-up duration of 2.62 years (range 0.02–4.53 years) and was stopped for efficacy at the interim analysis on the advice of the Data Monitoring Committee. At baseline, participants had a mean age of 63 years, 34% were female, 67% were white, and 20% were Asian. The mean HbA1c was 8.3%, mean BP was 140/78 mm Hg, and 50% had a history of cardiovascular disease. The mean baseline eGFR was 56.2 ml/min per 1.73 m2 and median UACR was 927 mg/g (105 mg/mmol).
There were 1313 (30%), 1279 (29%), and 1809 (41%) participants with a screening eGFR of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2, respectively (Supplemental Table 1). Baseline characteristics for participants within each eGFR category were balanced between the groups randomized to interventional treatment or placebo (Supplemental Table 1). Participants with lower baseline eGFR were numerically more likely to be older, have a longer duration of diabetes, have greater insulin and diuretic use, and have higher levels of albuminuria (Supplemental Table 1).
Renal Time to Event Outcomes
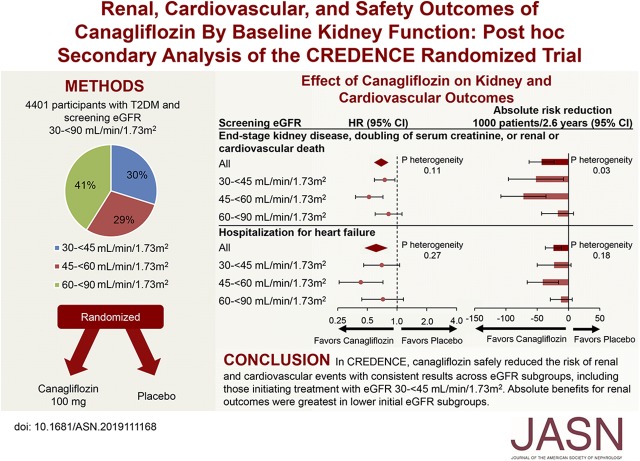
The effects of canagliflozin on the primary composite outcome of ESKD, doubling of serum creatinine, or renal or cardiovascular death (HR, 0.70; 95% CI, 0.59 to 0.82) was consistent in all eGFR categories (P interaction=0.11; Figure 1). Similarly, the effects of canagliflozin on the renal composite outcome of ESKD, doubling of serum creatinine, or renal death (HR, 0.66; 95% CI, 0.53 to 0.81), as well as ESKD, doubling of serum creatinine, and the composite of initiation of RRT or renal death were all consistent by baseline eGFR category, with no evidence that the results differed (all P interaction >0.11). Canagliflozin separately reduced the primary composite (HR, 0.75; 95% CI, 0.59 to 0.95) and the renal-specific composite (HR, 0.71; 95% CI, 0.53 to 0.94) in participants with a screening eGFR of 30 to <45 ml/min per 1.73 m2.
Figure 1.
Canagliflozin reduced renal events in all screening eGFR categories with greater absolute benefits in lower categories. *This outcome was exploratory.
Cardiovascular Outcomes
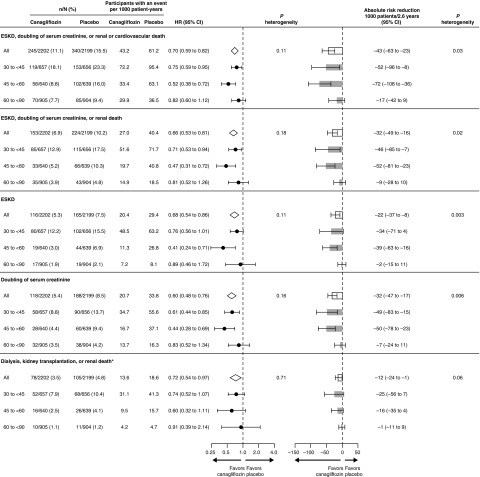
Across eGFR subgroups, canagliflozin consistently reduced cardiovascular death or hospitalization for heart failure; the composite of cardiovascular death, myocardial infarction, or stroke; and hospitalized heart failure; with all P values for interaction >0.25 (Figure 2). In particular, canagliflozin reduced the composite of cardiovascular death or hospitalization for heart failure (HR, 0.69; 95% CI, 0.50 to 0.94) in participants with screening eGFR of 30 to <45 ml/min per 1.73 m2.
Figure 2.
Canagliflozin reduced cardiovascular outcomes in all screening eGFR categories.
Safety
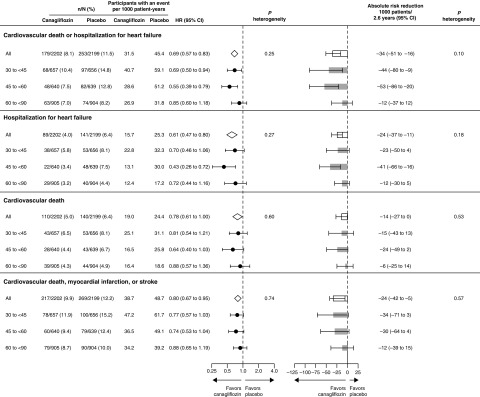
Canagliflozin led to fewer adverse events and serious adverse events overall, with consistent results across screening eGFR subgroups (P interaction=0.40 and 0.15, respectively; Figure 3). Rates of other adverse events including fractures and amputations were mostly not different among people randomized to canagliflozin or placebo overall, with consistent results across eGFR subgroups. The exceptions were volume depletion and osmotic diuresis events which were not more common with canagliflozin overall but there was some evidence that the effects differed among eGFR subgroups (P interaction=0.01 and 0.03, respectively). No unexpected safety signals were observed in patients with screening eGFR of 30 to <45 ml/min per 1.73 m2.
Figure 3.
The effect of canagliflozin on safety outcomes was generally consistent across screening eGFR categories. *Based on confirmed and adjudicated results.
Effects on eGFR Slope
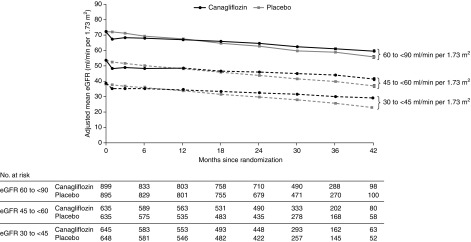
Canagliflozin led to an acute drop in eGFR at week 3 that was significant in every eGFR subgroup (all P<0.001), although the drop was least in those with screening eGFR of 30 to <45 ml/min per 1.73 m2 per year (P heterogeneity=0.02; Figure 4, Table 1). Thereafter, the eGFR of those randomized to placebo declined by 4.59 ml/min per 1.73 m2 per year with similar declines seen in all eGFR categories. Canagliflozin led to a slower eGFR decline in every eGFR category compared with placebo (all P<0.001), with no evidence the benefit differed among eGFR subgroups (P heterogeneity=0.65; Table 1). Canagliflozin improved total slope, the combined effect of the acute effect and chronic change in slope from baseline to week 130, overall and in every eGFR subgroup (all P<0.001) with no evidence the effect varied between eGFR subgroups (P heterogeneity=0.71; Table 1).
Figure 4.
Canagliflozin led to an acute drop in eGFR which was mildest in those with eGFR 30-<45 ml/min per 1.73 m2 at screening, and then to slower eGFR decline in every screening eGFR category. The slope lines cross at the point corresponding to 14.3, 11.2, and 8.7 months for those with initial eGFR of 60 to <90, 45 to <60, and 30 to <45 ml/min per 1.73 m2, respectively. The on-treatment eGFR includes all central laboratory eGFR measurements from study day 1 up to the last dose plus 2 days. The change from baseline in eGFR was analyzed using a restricted maximum likelihood repeated measures approach.
Table 1.
Effects of canagliflozin on eGFR slope by screening eGFR
| N Canagliflozin/Placebo | Canagliflozin | Placebo | Difference (95% CI) | P Value | P Interaction | |
|---|---|---|---|---|---|---|
| eGFR change from baseline to week 3 (ml/min per 1.73 m2) | ||||||
| All (unstructured) | 2179/2178 | −3.72 (0.25) | −0.55 (0.25) | −3.17 (–3.87 to –2.47) | <0.001 | |
| eGFR 30 to <45 | 645/648 | −2.45 (0.25) | −0.41 (0.25) | −2.03 (–2.73 to –1.34) | <0.001 | 0.02 |
| eGFR 45 to <60 | 635/635 | −4.08 (0.32) | −0.64 (0.31) | −3.44 (–4.32 to –2.57) | <0.001 | |
| eGFR 60 to <90 | 899/895 | −3.66 (0.32) | −0.39 (0.33) | −3.27 (–4.17 to –2.37) | <0.001 | |
| Annual eGFR change from week 3 to last available measurement (ml/min per 1.73 m2 per yr) | ||||||
| All (unstructured) | 2081/2095 | −1.85 (0.13) | −4.59 (0.14) | 2.74 (2.37 to 3.11) | <0.001 | |
| eGFR 30 to <45 | 611/622 | −1.72 (0.20) | −4.33 (0.20) | 2.61 (2.06 to 3.16) | <0.001 | 0.65 |
| eGFR 45 to <60 | 605/613 | −1.62 (0.23) | −4.58 (0.24) | 2.97 (2.32 to 3.61) | <0.001 | |
| eGFR 60 to <90 | 865/860 | −2.32 (0.23) | −4.92 (0.23) | 2.60 (1.97 to 3.23) | <0.001 | |
| Annual eGFR change from baseline to week 130 (ml/min per 1.73 m2 per yr) | ||||||
| All (unstructured) | 2179/2178 | −3.19 (0.15) | −4.71 (0.15) | 1.52 (1.11 to 1.93) | <0.001 | |
| eGFR 30 to <45 | 645/648 | −2.56 (0.21) | −4.35 (0.21) | 1.79 (1.20 to 2.38) | <0.001 | 0.71 |
| eGFR 45 to <60 | 635/635 | −3.11 (0.25) | −4.76 (0.25) | 1.65 (0.96 to 2.34) | <0.001 | |
| eGFR 60 to <90 | 899/895 | −3.61 (0.24) | −5.03 (0.24) | 1.42 (0.75 to 2.09) | <0.001 | |
Data in the columns of canagliflozin and placebo are mean (SEM). The effects of canagliflozin on the mean on-treatment eGFR slope were analyzed using a two-slope linear spline model for eGFR, with a knot at week 3 to account for separate acute (baseline to week 3) and chronic (week 3 to end of treatment) slopes. The full model also included random intercepts and acute and chronic slopes. When the full model failed to converge, a simplified model with a random intercept and a single random slope was used. The mean total slope was computed as a weighted combination of the acute and chronic slopes to reflect the mean rate of eGFR change to week 130.
In those with an eGFR of 30 to <45 ml/min per 1.73 m2, the group closest to a threshold for dialysis initiation, canagliflozin led to an acute drop in eGFR of 2.03 (95% CI, 1.34 to 2.73) ml/min per 1.73 m2 followed by an attenuation in eGFR decline of 2.61 (95% CI, 2.06 to 3.16) ml/min per 1.73m2 per year compared with those receiving placebo (mean decline [SD] 1.85 [0.13] in those assigned to canagliflozin compared with 4.59 [0.14] in those assigned to placebo).
Absolute Effects of Canagliflozin
Whereas the relative benefits of canagliflozin compared with placebo were generally consistent among the eGFR subgroups, the absolute benefits were greater in those with lower screening eGFR subgroups (all P heterogeneity <0.03) for all renal outcomes other than dialysis, transplantation, or renal death where the effects were consistent across subgroups (P heterogeneity=0.06; Figure 1). The absolute benefits for cardiovascular events did not clearly differ among eGFR subgroups except for the composite of cardiovascular death or hospitalized heart failure, where there was borderline evidence that the absolute benefits were greater in lower screening eGFR subgroups (P heterogeneity=0.096; Figure 2).
Effect on Intermediate Outcomes
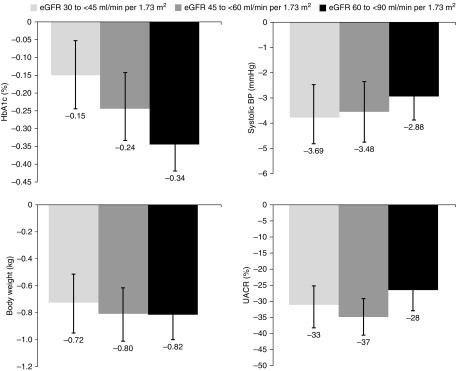
Canagliflozin reduced HbA1c, BP, body weight, and albuminuria compared with placebo in participants across screening eGFR subgroups (Figure 5, Supplemental Table 2). The glucose-lowering effect of canagliflozin was numerically less and reductions in BP were numerically greater in participants with lower initial eGFR compared with placebo, whereas reductions in body weight and albuminuria were similar across subgroups.
Figure 5.
Canagliflozin reduced HbA1c, BP, body weight, and albuminuria compared with placebo in participants across screening eGFR subgroups. Data are placebo-subtracted mean difference (95% CI), except for UACR where it is percentage change in the geometric mean of canagliflozin relative to placebo.
Experience of Those Experiencing Last On-Treatment eGFR <30 ml/min per 1.73 m2
In the CREDENCE trial, a substantial number of participants experienced an eGFR <30 ml/min per1.73 m2. For the subgroup of participants who ended with an on-treatment eGFR <30 ml/min per 1.73 m2 (n=929; canagliflozin, n=417; placebo, n=512), mean follow-up to the first eGFR <30 ml/min per 1.73 m2 was 12.9 months (canagliflozin, 11.7 months; placebo, 13.8 months), whereas mean follow-up thereafter was 19.3 months (canagliflozin, 20.5 months; placebo, 18.4 months). The relative number of events occurring after eGFR first fell to <30 ml/min per 1.73 m2 in the canagliflozin and placebo arms appeared similar to the trial overall (Supplemental Table 3). Because these analyses concern comparisons according to a postrandomization event (eGFR falling to <30 ml/min per 1.73 m2), they are not randomized and should be regarded as exploratory, but may be useful to illustrate the course of participants within the study.
Discussion
Canagliflozin consistently and safely prevented renal and cardiovascular events in participants with substantial albuminuria across eGFR categories of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2. These benefits were attained on the background of universal renin-angiotensin system blockade use. Although the relative benefits were consistent across eGFR categories, increasingly higher event rates were observed for both renal and cardiovascular events as eGFR levels declined, with greater absolute benefits in the lower eGFR subgroups. The beneficial effect of canagliflozin on the occurrence of clinical events was reinforced by the observed reduction in the chronic rate of renal functional decline, which was reduced by >50% in all three subgroups. In particular, canagliflozin attenuated the chronic decline in eGFR over time by 60% and 65% in those with initial eGFR 30 to <45 and 45 to <60 ml/min per 1.73 m2, respectively. Reassuringly, there was no excess of major safety concerns in participants with an eGFR of 30 to <45 ml/min per 1.73 m2 and observational analyses did not suggest differences in benefits as eGFR declined to <30 ml/min per 1.73 m2. Together, these findings make a compelling case for commencing canagliflozin in people with eGFR between 30 and 90 ml/min per 1.73 m2 and substantial albuminuria, and supporting the continuation of therapy below this threshold.
The effects of canagliflozin compared with placebo on intermediate outcomes were broadly consistent with those seen in previous studies. As expected, the glucose-lowering efficacy of canagliflozin was attenuated in patients with worsening renal function. However, the reductions in albuminuria, body weight, and BP were generally similar across eGFR subgroups. The initial acute drop in eGFR seen in CREDENCE is a well established response to canagliflozin treatment initiation8 and, together with the subsequent attenuation of eGFR decline, is consistent with reductions in intraglomerular pressure being a plausible contributing mechanism to renal protection.12−14 Other potential mechanisms for renoprotection are being actively studied.15−17 The data strongly suggest a glucose-independent mechanism of renal and cardiovascular benefit in CREDENCE.
Despite continuing uncertainty regarding the relative importance of several potential mechanisms, CREDENCE has established clear benefit for clinical renal outcomes.10 The important novel finding that kidney and cardiac protection is preserved in those in whom treatment is started with an eGFR between 30 and 45 ml/min per 1.73 m2 provides further insights into the potential mechanism of action. The finding of clinical benefits for important outcomes despite reduced effects on glycemic control raises important questions on whether these agents benefit kidney disease outcomes in nondiabetic settings. Ongoing trials recruiting people with nondiabetic kidney disease are likely to yield important further insights (NCT03036150, NCT03594110, NCT03190694).
Similarly, the benefits of SGLT2 inhibitors for preventing heart-failure hospitalizations in participants with predominantly preserved kidney function has been established in three large cardiovascular outcome trials,18−20 despite uncertainty on the precise mechanisms of heart-failure mitigation. These agents do have a natriuretic effect which is reflected in early reductions in BP and weight, and is a potential contributor to the early benefits for heart-failure hospitalization. However, the benefits continue to accumulate over time, despite stabilization of volume status. The CREDENCE trial has confirmed the absolute benefits for preventing heart-failure hospitalizations are greater in those with lower eGFR levels who are at greater risk of heart-failure events.
An important aspect of CREDENCE among the trials of SGLT2 inhibitors is that treatment was deliberately continued regardless of the eGFR falling to <30 ml/min per 1.73 m2. We provide observational reports of the events occurring once eGFR fell to <30 ml/min per 1.73 m2 in those whose eGFR remained <30 ml/min per 1.73 m2 at end of treatment, in analyses that are limited by their dependence on an outcome that occurs well after randomization. The ongoing DIAMOND (NCT03190694) and DAPA-CKD (NCT03036150) trials are recruiting participants with eGFR down to 25 ml/min per 1.73 m2, whereas the EMPA-Kidney trial (NCT03594110) includes those with an eGFR down to 20 ml/min per 1.73 m2. Together these trials will provide evidence of the effects of SGLT2 inhibitors in people with lower commencement eGFR levels. In the meantime, the consistency overall between our exploratory reports and the overall CREDENCE findings provide reassurance there is no reason to dismiss the CREDENCE protocolized approach of continuing treatment until the commencement of chronic dialysis or receipt of a renal transplant.
The CREDENCE study was designed to examine the effect of canagliflozin on outcomes of people at risk of progression of diabetic kidney disease. As such, its strengths include the inclusion of people with substantial albuminuria (who are at high risk of both renal and cardiovascular events) and stratified randomization by screening eGFR categories, so that a majority of participants had an eGFR of <60 ml/min per 1.73 m2, providing robust assessment of canagliflozin in people with reduced eGFR down to 30 ml/min per 1.73 m2. In addition, renal events were carefully evaluated with central assessment of eGFR, requirement for chronic outcomes to be documented as sustained, and adjudication of renal and other important events. The findings may not be generalizable to people commencing treatment with an eGFR <30 ml/min per 1.73 m2. Similarly, the findings apply to those with substantial albuminuria, although the concordance of the results with those of the CANVAS Program in which most participants had no or minor levels of albuminuria is reassuring. The trial was stopped early on grounds of clear efficacy for the primary end point which may have limited the power to assess the effect on secondary and safety end points. The analyses reported for participants who ended treatment with an eGFR <30 ml/min per 1.73 m2 are reported according to randomization arm but, because this cohort is defined by a postrandomization event, they are confounded and subject to biases including survival bias and collider bias, and should be regarded purely as hypothesis-generating data.
Canagliflozin safely prevents clinically important renal and cardiovascular events in people with diabetes, substantial albuminuria, and an eGFR at commencement of treatment of between 30 and 90 ml/min per 1.73 m2. These effects appear consistent across eGFR categories with greater absolute benefits for renal end points in lower eGFR categories. They support the expansion of canagliflozin treatment initiation to those with an eGFR of 30 to <45 ml/min per 1.73 m2, and the general continuation of treatment until the initiation of dialysis or receipt of kidney transplant.
Data Sharing Statement
Data from this study will be made available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and European Union and the study has been completed for 18 months.
Disclosures
Dr. Agarwal has received personal fees from Akebia, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, ER Squibb and Sons, Fresenius, Gilead, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson & Johnson, Opko, Otsuka, Reata, Relypsa, Sanofi, and Takeda; has served as associate editor of the American Journal of Nephrology and Nephrology, Dialysis, and Transplantation and as an author on UpToDate; and has received research funding from GlaxoSmithKline. Dr. Bajaj has received personal speaking honoraria for continuing medical education and research funding paid to LMC Healthcare for serving as principal investigator on clinical trials from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi; and has served as an editor for the Canadian Journal of Diabetes and as a columnist for Medscape. Dr. Bakris has received research funding paid to the University of Chicago for serving as principal investigator on national clinical trials for AbbVie, Bayer, CVRX, Janssen, Novo Nordisk, and Takeda; has served as a consultant for AbbVie, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Merck, Relypsa, Novo Nordisk, Nxstage Medical, Pfizer, Sanofi, and Takeda; and has served on a steering committee for Vascular Dynamics. Dr. Bakris has served as editor of the American Journal of Nephrology and Nephrology; editor in chief of UpToDate; nephrology and hypertension section editor of UpToDate; and has served as associate editor of Diabetes Care, Hypertension Research, and Nephrology, Dialysis, and Transplantation. Dr. Bull, Dr. Qiu, Dr. Rosenthal, and Dr. Sun are full-time employees of Janssen Research & Development, LLC. Dr. Cannon has received research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda; and has received consulting fees from Aegerion, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Corvidia, Eisai, Eli Lilly, GlaxoSmithKline, Innovent, Kowa, Merck, Pfizer, Regeneron, and Sanofi. Dr. Charytan has received fees paid by Janssen Pharmaceuticals to the Baim Institute for work on the CREDENCE trial steering committee and as scientific lead; and has received salary support from the Baim Institute for this work through October 2018. After that time, he received consulting fees from Baim. He has consulted for Amgen, Daiichi Sankyo, Douglas and London, Eli Lilly, Fresenius, Gilead, Medtronic/Covidien, Merck, Novo Nordisk, and Zoll; has served on data safety and monitoring boards for AstraZeneca and Allena Pharmaceuticals; and has served on a clinical effectiveness committee for Merck and PLC Medical. Dr. de Zeeuw has served on advisory boards and/or as speaker for Bayer, Boehringer Ingelheim, Fresenius, Mitsubishi Tanabe, and Mundipharma; has served on steering committees and/or as a speaker for AbbVie and Janssen; and has served on data safety and monitoring committees for Bayer. Dr. Di Tanna is a full-time employee of The George Institute. Dr. Greene has received consulting fees from Durect, Janssen, and Pfizer. Dr. Heerspink has served as a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, and Mitsubishi Tanabe; and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. Dr. Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Amgen, Baxter, Eli Lilly, and Merck Sharp & Dohme; serves on a steering committee sponsored by CSL; has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, and Vifor; and has spoken at scientific meetings sponsored by Janssen; with any consultancy, honoraria, or travel support paid to her institution. Dr. Levin serves as a scientific advisor to AstraZeneca, Boehringer Ingelheim, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); is on the data and safety monitoring board for NIDDK, Kidney Precision Medicine, University of Washington Kidney Research Institute Scientific Advisory Committee; and is funded by the Canadian Institute of Health Research and Kidney Foundation of Canada. She has received fees for time as CREDENCE National Coordinator from Janssen, directed to her academic team. Dr. Mahaffey has received research support from Afferent, Amgen, Apple, AstraZeneca, Cardiva Medical, Daiichi Sankyo, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, National Institutes of Health (NIH), Novartis, Sanofi, St. Jude, and Tenax; and has served as a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol-Myers Squibb, Elsevier, GlaxoSmithKline, Johnson & Johnson, MedErgy, Medscape, Mitsubishi, Myokardia, NIH, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing, and University of California, San Francisco. Dr. Neal is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; holds a research grant for this study from Janssen; has held research grants for other large-scale cardiovascular outcome trials from Merck Schering-Plough Roche, and Servier; and his institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards and/or the continuing medical education programs of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier. Dr. Oshima is supported by the Japan Society for the Promotion of Science Program for Fostering Globally Talented Researchers. Dr. Perkovic has received fees for advisory boards, steering committee roles, or scientific presentations from AbbVie, Astellas, AstraZeneca, Bayer, Baxter, BMS, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Sanofi, Servier, Tricida, and Vifor. Dr. Pollock has received honoraria for serving on advisory boards and as a speaker for AstraZeneca, Boehringer Ingelheim/Eli Lilly, Merck Sharp & Dohme. Dr. Wheeler has received consultancy fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Mitsubishi, Mundipharma, Napp, Ono Pharma, Tricidia, and Vifor Fresenius. Dr. Zhang has received consulting fees from Janssen. Dr. Zhou reports receiving a Scientia PhD Scholarship from the University of New South Wales, Sydney. Dr. Zinman has served as a consultant and received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and has received grant support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Funding
The CREDENCE study was sponsored by Janssen Research & Development, LLC. All of the authors received research support and consulting fees from Janssen in relation to their roles on the steering committee of the CREDENCE trial.
Supplementary Material
Acknowledgments
We thank all participants, investigators, and trial teams for their participation in the trial.
The CREDENCE study was sponsored by Janssen Research & Development, LLC, and was conducted collaboratively by the sponsor; an academic-led steering committee; and an academic research organization, George Clinical. Analyses were performed by George Clinical and independently confirmed by the sponsor.
Technical editorial assistance was provided by Alaina Mitsch and Kimberly Dittmar, of MedErgy, and was funded by Janssen Global Services, LLC. All authors reviewed and approved the manuscript.
Dr. Bajaj contributed to the conduct of the study and interpretation of the data; Dr. Agarwal, Dr. Bakris, Dr. Bull, Dr. Cannon, Dr. Charytan, Dr. de Zeeuw, Dr. Greene, Dr. Heerspink, Dr. Jardine, Dr. Levin, Dr. Mahaffey, Dr. Neal, Dr. Perkovic, Dr. Pollock, Dr. Qiu, Dr. Rosenthal, Dr. Wheeler, Dr. Zhang, and Dr. Zinman contributed to the design and conduct of the study and to the interpretation of the data; Dr. Di Tanna, Dr. Oshima, Dr. Sun, and Dr. Zhou contributed to the analysis and interpretation of data; Dr. Jardine wrote the first draft of the paper, had full access to the study design information, and had final responsibility for the decision to submit for publication; all authors provided input into subsequent drafts and approved the final version for submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Meg J. Jardine, Zien Zhou, Kenneth W. Mahaffey, Megumi Oshima, Rajiv Agarwal, George Bakris, Harpreet S. Bajaj, Scott Bull, Christopher P. Cannon, David M. Charytan, Dick de Zeeuw, Gian Luca di Tanna, Tom Greene, Hiddo J.L. Heerspink, Adeera Levin, Bruce Neal, Carol Pollock, Rose Qiu, Tao Sun, David C. Wheeler, Hong Zhang, Bernard Zinman, Norman Rosenthal, and Vlado Perkovic
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019111168/-/DCSupplemental.
Supplemental Material. CREDENCE Study Investigators.
Supplemental Table 1. Demographic and clinical characteristics by baseline eGFR.
Supplemental Table 2. Effects of canagliflozin on HbA1c, body weight, systolic BP, and urinary albumin-creatinine ratio according to screening eGFR.
Supplemental Table 3. Number of events in participants whose final eGFR was <30 ml/min per 1.73 m2 from the point their eGFR first fell below 30 ml/min per 1.73 m2.
References
- 1.Ghezzi C, Loo DDF, Wright EM: Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61: 2087–2097, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamout H, Perkovic V, Davies M, Woo V, de Zeeuw D, Mayer C, et al.: Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 40: 64–74, 2014 [DOI] [PubMed] [Google Scholar]
- 3. INVOKANA (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ, Janssen Pharmaceuticals, 2013.
- 4. FARXIGA (dapagliflozin) tablets, for oral use [package insert]. Princeton, NJ, Bristol-Myers Squibb Company, 2014.
- 5. JARDIANCE (empagliflozin) tablets, for oral use [package insert]. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, 2014.
- 6. STEGLATRO: Steglatro (ertugliflozin) [packet insert]. Heist-op-den-Berg, Belgium, Schering-Plough Labo NV.
- 7.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V: Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 28: 368–375, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al.: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, et al.; CREDENCE Trial Investigators: The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 46: 462–472, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al.: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML: Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70: 665–699, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Ortola FV, Ballermann BJ, Anderson S, Mendez RE, Brenner BM: Elevated plasma atrial natriuretic peptide levels in diabetic rats. Potential mediator of hyperfiltration. J Clin Invest 80: 670–674, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternlicht H, Bakris GL: Blood pressure lowering and sodium-glucose co-transporter 2 inhibitors (SGLT2is): More than osmotic diuresis. Curr Hypertens Rep 21: 12, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Esterline RL, Vaag A, Oscarsson J, Vora J: Mechanisms in endocrinology: SGLT2 inhibitors: Clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol 178: R113–R125, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Galla S, Cheng X, Yeo J-Y, Mell B, Singh V, et al.: Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 25: 677–689.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al.; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al.; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.