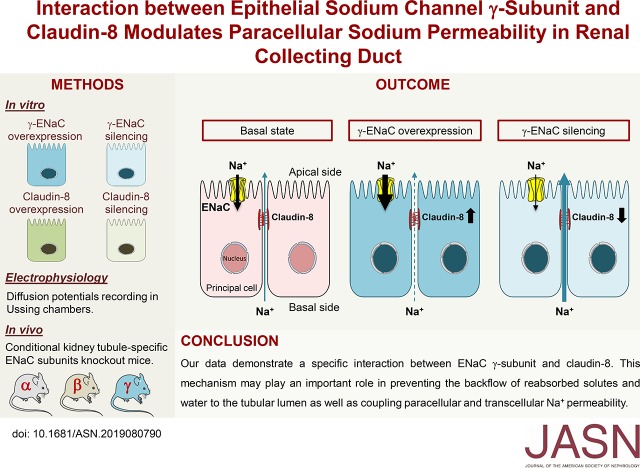
Significance Statement
In the renal collecting duct, fluid and solute reabsorption are the result of both transcellular and paracellular transport. Although the mechanisms of transcellular transport have been extensively studied, less is known regarding the regulation of the paracellular pathway. The authors investigated the physiologic role and regulation of the transmembrane protein claudin-8 in cultured mouse cortical collecting duct cell models and in knockout mice lacking kidney tubule–specific expression of the epithelial sodium channel γ-subunit, discovering an interaction between the γ-subunit and claudin-8. This interaction modulates paracellular permeability to sodium and may play an important role in preventing the backflow of reabsorbed solutes and water to the tubular lumen, as well as in coupling paracellular and transcellular sodium transport.
Keywords: principal cell, epithelial sodium channel, tight junction, sodium reabsorption, paracellular ion permeability
Visual Abstract
Abstract
Background
Water and solute transport across epithelia can occur via the transcellular or paracellular pathways. Tight junctions play a key role in mediating paracellular ion reabsorption in the kidney. In the renal collecting duct, which is a typical absorptive tight epithelium, coordination between transcellular sodium reabsorption and paracellular permeability may prevent the backflow of reabsorbed sodium to the tubular lumen along a steep electrochemical gradient.
Methods
To investigate whether transcellular sodium transport controls tight-junction composition and paracellular permeability via modulating expression of the transmembrane protein claudin-8, we used cultured mouse cortical collecting duct cells to see how overexpression or silencing of epithelial sodium channel (ENaC) subunits and claudin-8 affect paracellular permeability. We also used conditional kidney tubule–specific knockout mice lacking ENaC subunits to assess the ENaC’s effect on claudin-8 expression.
Results
Overexpression or silencing of the ENaC γ-subunit was associated with parallel and specific changes in claudin-8 abundance. Increased claudin-8 abundance was associated with a reduction in paracellular permeability to sodium, whereas decreased claudin-8 abundance was associated with the opposite effect. Claudin-8 overexpression and silencing reproduced these functional effects on paracellular ion permeability. Conditional kidney tubule–specific ENaC γ-subunit knockout mice displayed decreased claudin-8 expression, confirming the cell culture experiments' findings. Importantly, ENaC β-subunit or α-subunit silencing or kidney tubule–specific β-ENaC or α-ENaC knockout mice did not alter claudin-8 abundance.
Conclusions
Our data reveal the specific coupling between ENaC γ-subunit and claudin-8 expression. This coupling may play an important role in preventing the backflow of reabsorbed solutes and water to the tubular lumen, as well as in coupling paracellular and transcellular sodium permeability.
Body fluid homeostasis and BP control are dependent on renal sodium chloride (NaCl) handling. This is mainly achieved by glomerular filtration followed by NaCl reabsorption along the successive segments of the kidney tubule. In contrast with the proximal and distal tubule where sodium ion (Na+) and chloride ion (Cl−) reabsorptions are coupled, the Na+ reabsorption is separated from that of Cl− in the collecting duct (CD). In the CD, luminal Na+ is reabsorbed by principal cells via the apical epithelial sodium channel (ENaC) and basolateral Na+/potassium ion (K+)-ATPase, whereas Cl− reabsorption is mostly mediated by the β-type intercalated cell via the pendrin anion exchanger1–3 but also through the paracellular pathway.1 The driving force for paracellular Cl− reabsorption is the lumen-negative transepithelial potential generated by the unidirectional transepithelial Na+ current from the luminal to the interstitial side. However, the negative transepithelial potential generated by Na+ entry via ENaC combined with the interstitial-to-lumen Na+ concentration gradient that progressively increases along the CD favors the paracellular backflow of reabsorbed Na+. Indeed, a net Na+ secretion can be observed in isolated mice cortical CDs (CCDs) perfused in the presence of a physiologic basal-to-apical Na+ concentration gradient.4 This Na+ backflow may compromise Na+ reabsorption efficiency by the CD.
The CD is a typical tight epithelium that displays several layers of interconnected tight junctions (TJs). The TJ barrier comprises a tissue-specific arrangement of claudins associated with accessory components such as occludin, junctional adhesion molecules, cingulin, and paracingulin.5 The claudin tetraspan family comprises at least 28 different transmembrane proteins (20–28 KDa) with tissue-specific expression. Paracellular permeability is determined by the tissue-specific combination and ratio of various claudin isoforms that generate paracellular channels or barriers.5,6 In the kidney, claudins display a nephron segment–specific expression pattern. Claudin-8 is one of the major isoforms expressed in the CD system,7,8 where it likely participates to the generation of a high electrical resistance and barrier to Na+ diffusion.9 Interestingly, previous work showed that aldosterone, which stimulates Na+ reabsorption, was associated with claudin-8 upregulation and reduced paracellular Na+ backflow in the colon.10 Several studies also suggest that TJ formation and permeability are modulated by Na+/K+-ATPase activity and intracellular Na+.11–13 However, the Na+-dependent control of TJ composition and permeability in renal epithelial cells remains an open question.
Using gene silencing and overexpression of ENaC subunits and claudin-8 or physiologic regulation of ENaC by vasopressin and hyperosmotic stress in cultured CD principal cells, as well as conditional kidney tubule–specific ENaC subunit knockout (KO) mice, we showed that variations of the ENaC γ-subunit abundance in the CD are associated with parallel changes in claudin-8 abundance, leading to alterations in paracellular permeability. This coupling between the ENaC γ-subunit and claudin-8 abundance may play an important role in preventing the backflow of reabsorbed solutes and water.
Methods
Cell Culture and Electrical Measurements
mCCDcl1 cells were grown on permeable filters (Transwell; Corning Costar, Cambridge, MA) and were grown to confluence in a 1:1 mixture of DMEM and F12 medium as previously described.14 Potential difference was measured in the absence of any drug (except otherwise noted) using a Millicell-ERS Voltohmmeter (Millipore, Billerica, MA).
γ-ENaC-TetOn-mCCD cells overexpressing the ENaC γ-subunit in a doxycycline (Dox)-inducible manner were grown in the presence of G418 (400 μg/ml), hygromycin (300 μg/ml), and Dox (1.25 μg/ml) to maintain the selection. Before experiments Dox was removed from the culture medium for 4 days to allow the expression of γ-ENaC to return to basal levels, followed by Dox treatment or not for 1.5 days to induce γ-ENaC overexpression. For ion-substitution experiments, apical culture medium was substituted with: (1) NaCl medium (135 mM NaCl, 4 mM potassium chloride [KCl], 14 mM sodium bicarbonate [NaHCO3], 0.3 mM magnesium chloride [MgCl2], 0.45 mM magnesium sulfate, 0.5 mM monosodium phosphate, 0.4 mM disodium phosphate), (2) medium in which NaCl was replaced by choline chloride, or (3) medium in which NaCl was replaced by sodium gluconate.
mpkCCDcl4 cells were grown on permeable filters (Transwell) to confluence in a 1:1 mixture of DMEM and F12 medium as previously described.15 Cells were exposed to 0.1 nM [deamino-Cys1, D-Arg8]-Vasopressin (dDAVP) or vehicle for 24 hours (basal side) before cell lysis. For hyperosmotic challenge experiments, hypertonic medium (500 mOsmol/kg) was made by replacing a fraction of the iso-osmotic medium (300 mOsmol/kg) with NaCl-enriched medium (apical and basal sides).
Constructs, Viral Particle Production, and Cell Transduction
For gene silencing, α-ENaC, β-ENaC, γ-ENaC, claudin-8, or scramble short hairpin RNA (shRNA) (Table 1) was inserted into the plasmid pLKO.1 (8453; Addgene). For gene overexpression, mouse claudin-2, claudin-8, and claudin-10 cDNAs were transferred from pGEM vector (Promega) to modified pSF-lenti vector (Sigma) in which puromycin resistance has been replaced by geneticin resistance. pLKO.1 or pSF-lenti together with the two helper plasmids psPAX2 (12260; Addgene) and pMD2.G (12259; Addgene) were transiently cotransfected in packaging HEK293T cells using the calcium phosphate precipitation method. Lentiviral particles were collected after 48 hours and γ-ENaC-TetOn-mCCD or mCCDcl1 cells were transduced. Stable polyclonal cell lines were selected using puromycin (2 μg/ml) which was applied 72 hours after transduction.
Table 1.
Sequences of scramble shRNA, shRNAs targeting claudin-8, and shRNAs targeting ENaC subunits
| Name | Forward | Reverse |
|---|---|---|
| shScr | CCGGGCGCGATAGCGCTAATAATTTCTCGAGAAATTATTAGCGCTATCGCGCTTTTTG | AATTCAAAAAGCGCGATAGCGCTAATAATTTCTCGAGAAATTATTAGCGCTATCGCGC |
| sh-Cl-8–248 | CCGGCTGAAAGGAGCAACAGTTACTCGAGTAACTGTTGCTCCTTTCAGTTTTTG | AATTCAAAAACTGAAAGGAGCAACAGTTACTCGAGTAACTGTTGCTCCTTTCAG |
| sh-Cl-8–512 | CCGGCTGCCTTCATCGAAAGTAACTCGAGTTACTTTCGATGAAGGCAGTTTTTG | AATTCAAAAACTGCCTTCATCGAAAGTAACTCGAGTTACTTTCGATGAAGGCAG |
| sh-γ-ENaC-1 | CCGGCCCAGCCAACAGTATTGAGATCTCGAGATCTCAATACTGTTGGCTGGGTTTTTG | AATTCAAAAACCCAGCCAACAGTATTGAGATCTCGAGATCTCAATACTGTTGGCTGGG |
| sh-γ-ENaC-7 | CCGGCCCGTCACAAACATCTACAATCTCGAGATTGTAGATGTTTGTGACGGGTTTTTG | AATTCAAAAACCCGTCACAAACATCTACAATCTCGAGATTGTAGATGTTTGTGACGGG |
| sh-α-ENaC-1 | CCGGCGAGATGCTATCCTTGCAGAACTCGAGTTCTGCAAGGATAGCATCTCGTTTTTG | AATTCAAAAACGAGATGCTATCCTTGCAGAACTCGAGTTCTGCAAGGATAGCATCTCG |
| sh-α-ENaC-2 | CCGGGCACCCTTAATCCTTACAGATCTCGAGATCTGTAAGGATTAAGGGTGCTTTTTG | AATTCAAAAAGCACCCTTAATCCTTACAGATCTCGAGATCTGTAAGGATTAAGGGTGC |
| sh-β-ENaC-1 | CCGGGCCTATCTTCTACCCTGATTACTCGAGTAATCAGGGTAGAAGATAGGCTTTTTG | AATTCAAAAAGCCTATCTTCTACCCTGATTACTCGAGTAATCAGGGTAGAAGATAGGC |
| sh-β-ENaC-2 | CCGGGCCTCTGAGGATTGGATCTTACTCGAGTAAGATCCAATCCTCAGAGGCTTTTTG | AATTCAAAAAGCCTCTGAGGATTGGATCTTACTCGAGTAAGATCCAATCCTCAGAGGC |
shScr, scramble shRNA.
RNA Extraction and Real-Time PCR
Total RNA from cultured cells was extracted using the EZNA Total RNA Kit I (Promega) according to the manufacturer’s instructions. RNA concentration and purity was measured using Nanodrop. One microgram of RNA was used to synthesize cDNA using qScript reverse transcriptase (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer’s instructions. Primers are listed in Table 2. PCR was performed on 3 μl of cDNA diluted 1:10 (v/v) using 0.5 μM of each primer and 7 μl of SYBR Green Master Mix (Applied Biosystems, Foster City, CA) to obtain a final reaction volume of 14 μl. Duplicate amplification reactions were performed with an ABI StepOne sequence detection system (Applied Biosystems). Analysis of the data has been done using the ABI Prism software (Applied Biosystems) and P0 was used as an internal standard. Fold difference in cDNA abundance (F) was calculated using the formula F=2(Ct1−Ct2) where Ct1 and Ct2 are the number of cycles required to reach the threshold of amplicon abundance for experimental and control conditions, respectively.
Table 2.
Sequences of primers used for real-time PCR
| Name | Forward | Reverse |
|---|---|---|
| P0 | 5′-AATCTCCAGAGGCACCATTG-3′ | 5′-GTTCAGCATGTTCAGCAGTG-3′ |
| α-ENaC | 5′-CAGACTTGGAGCTTTGACAAGGA-3′ | 5′-ACTTCTCTGTGCCTTGTTTATATGTGTT-3′ |
| β-ENaC | 5′-CAGACTGGGCCTATGCTATCTAAA-3′ | 5′-ACATGCTGAGGCAGGTCTCTCT-3′ |
| γ-ENaC | 5′-CCGAGATCGAGACAGCAATGT-3′ | 5′-CGCTCA GCTTGA AGGATTCTG-3′ |
| Claudin-8 | 5′-GTGCTGCGTCCGTCTTGGCT-3′ | 5′-TCGTCCCCCGTGCATCTGGT-3′ |
| Claudin-4 | 5′-AAGTGCACCAACTGC ATGGA-3′ | 5′-GGCTCCGGCGGTGATC-3′ |
| Claudin-7 | 5′-AAG CGA AGA AGG CCC GAA TA-3′ | 5′-GCA AGA CCT GCC ACA ATG AA-3′ |
| ZO-1 | 5′-TTATGCGCAGTGGTATCCAATT-3′ | 5′-TCCGGACACAACCTCATCCT-3′ |
Western Blotting
Equal amounts of protein from cultured cells or kidney cortex were separated by 4%–12% SDS-PAGE (Invitrogen, Basel, Switzerland) and transferred to polyvinylidene difluoride membranes (Immobilion-P; Millipore), as previously described.14 After incubation with primary antibodies (Table 3), membranes were incubated with anti-rabbit or anti-mouse IgG antibody coupled to horseradish peroxidase (Transduction Laboratories, Lexington, KY), and the antigen-antibody complexes were detected by enhanced chemiluminescence (Advansta, Menlo Park, CA). Protein abundance was quantified with ImageJ software (National Institutes of Health). Results are expressed as the ratio of the densitometry of the band of interest to the loading control.
Table 3.
Antibodies used for Western blots
| Name | Species | dilution | Supplier |
|---|---|---|---|
| Claudin-8 | Rabbit | 1:500 | Thermo Fisher |
| Claudin-4 | Mouse | 1:500 | Thermo Fisher |
| Claudin-7 | Rabbit | 1:500 | Thermo Fisher |
| Claudin-2 | Mouse | 1:500 | Thermo Fisher |
| Claudin-10 | Rabbit | 1:500 | Thermo Fisher |
| ZO-1 | Rabbit | 1:1000 | Thermo Fisher |
| α-ENaC | Rabbit | 1:1000 | Prof. J. Loffing36 |
| β-ENaC | Rabbit | 1:500 | StressMarq |
| γ-ENaC | Rabbit | 1:500 | StressMarq |
| β-Actin | Mouse | 1:10,000 | Sigma |
| GAPDH | Mouse | 1:20,000 | Millipore |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell-Surface Protein Biotinylation
Cell-surface proteins were biotinylated by adding serum-free medium containing 1.0 mg/ml Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL) to the apical and basolateral side of the filter for 1 hour at 4°C. The free unreacted Sulfo-NHS-SS-Biotin was quenched by incubation with PBS-complete medium (2.7 mM KCl, 1.5 mM monopotassium phosphate, 137 mM NaCl, 6.4 mM disodium phosphate, 0.1 mM calcium chloride [CaCl2], and 1 mM MgCl2) containing 0.1% BSA (wt/vol) for 30 minutes at 4°C. Cells were then lysed in 20 mM Tris-hydrochloride, pH 8.0, 150 mM NaCl, and 5 mM EDTA and the protein content was determined using the bicinchoninic acid protein assay (Pierce). Equal amounts of proteins were precipitated by streptavidin-agarose beads (Pierce) and analyzed by Western blotting.
Measurement of Diffusion Potentials
Cultured mCCDcl1 or γ-ENaC-TetOn-mCCD cells were seeded onto 1.12 cm2 Snapwell polyester filters (Snapwell; Corning Costar, Cambridge, MA) (250,000 cells/cm2) and incubated in medium for 7 days. The Snapwell rings were then detached and mounted in Ussing chambers (model P2300; Physiologic Instruments, San Diego, CA). The Ussing chambers were connected to a VCC MC6 multichannel voltage/current clamp via silver/silver chloride electrodes and 3 M KCl agar bridges. The apical and basolateral half-chambers were separately filled with buffer A (120 mM NaCl, 10 mM NaHCO3, 5 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, pH 7.4). The fluid volume on each side was 5 ml. Transepithelial potentials between the apical and basal hemichambers were recorded using a Quick Data Acquisition DI100 USB board (Physiologic Instruments) with the transepithelial current clamped at 0 µA during the whole experiment. Cells were equilibrated for 1 hour in buffer A. Diffusion potentials were measured by replacing half the buffer in the basal side with buffer B (240 mM mannitol, 10 mM NaHCO3, 5 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, pH 7.4) to measure the diffusion potential of NaCl, with buffer C (120 mM sodium gluconate, 10 mM NaHCO3, 5 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, pH 7.4) to measure the chloride/gluconate diffusion potential, or with buffer D (120 mM choline chloride, 10 mM NaHCO3, 5 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, pH 7.4) for the sodium/choline diffusion potential measurements. These buffers were used to obtain half the concentration (65 mM) of the salt indicated by their names, mannitol was used at a concentration of 130 mM (to maintain osmolarity) and pH was adjusted to 7.4 with hydrochloric acid. To ensure ion flux was occurring via a paracellular pathway, we added 100 μM amiloride (Sigma) to the apical compartment for 30 minutes before performing diffusion potential measurements. During all of the experiment, buffers were maintained at 37°C and bubbled constantly with a mixture of 95% oxygen and 5% carbon dioxide. Peak values of diffusion potentials were measured and used to prepare the figures.
Animals
Kidney cortex samples of adult conditional kidney tubule–specific ENaC subunit KO mice were kindly provided by Dr. Edith Hummler (Department of Pharmacology and Toxicology, University of Lausanne, Lausanne, Switzerland). Animal maintenance and protocols were done as described.16–18 Animal experiments were performed according to the guidelines of the local ethical committee and after authorization of the cantonal authorities.
Immunofluorescence
Cells grown to confluence on polycarbonate filters were fixed (together with filters) with ice-cold methanol for 2 minutes at −20°C and then washed with PBS. After washing for 30 minutes, blocking of nonspecific binding sites was done with PBS containing 2% BSA (PBS-BSA) at room temperature. Finally, cells were incubated overnight at 4°C with antibodies against claudin-4 and claudin-8 diluted 1:500 in 0.2% PBS-BSA followed by 1 hour incubation with Alexa Fluor 488–conjugated goat anti-mouse (catalog number A-11017; Invitrogen) diluted 1:500 in PBS-BSA. Samples were mounted on microscope slides using Vectashield mounting medium (Maravai Life Science, San Diego, CA) with 4′,6-diamidino-2-phenylindole for nuclear counterstaining. Fluorescence images were acquired using a LSM 710 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany) using 488-nm and 561-nm ray lasers. Distance between the Z-slices was 0.25 µm. From 5 to 10 Z-stack images were processed using ImageJ software.
Kidney cryosection sections (5 µm thick) were used for immunofluorescence analysis. Nonspecific binding was blocked with PBS-BSA for 20 minutes followed by washing three times for 10 minutes with 0.1% Tris-buffered saline/Tween 20 wash buffer. The sections were incubated with primary antibody against claudin-8 or γ-ENaC (diluted in PBS-BSA) overnight at 4°C followed by 1 hour incubation with fluorescent-labeled secondary antibody (goat anti-rabbit IgG Alexa Fluor 488; 11008) at room temperature. Coverslips were mounted with a hydrophilic mounting media (n-propyl-gallate, P-3101; Sigma Chemical, St. Louis, MO). Confocal imaging and z-stacks were performed on a Nikon A1R confocal microscope using 488-nm and 561-nm ray lasers. Z-stack images were further processed using ImageJ software.
Statistical Analyses
Results are shown as the mean±SEM from n independent experiments. The normal distribution of the population from which sample data were extracted was determined by a Shapiro–Wilk test. For parametric data, statistical differences were assessed using a two-tailed unpaired t test for comparison between two groups or one-way ANOVA with Tukey multiple pairwise comparison test for comparisons between more than two groups. For nonparametric data, statistical differences were assessed using the Mann–Whitney test for comparison between two groups or by the Kruskal–Wallis test with a Dunn multiple pairwise comparison test for comparisons between more than two groups. A P value <0.05 was considered significant.
Results
ENaC γ-Subunit Overexpression Modulates Claudin-8 Expression and Paracellular Transport in Cultured CD Principal Cells
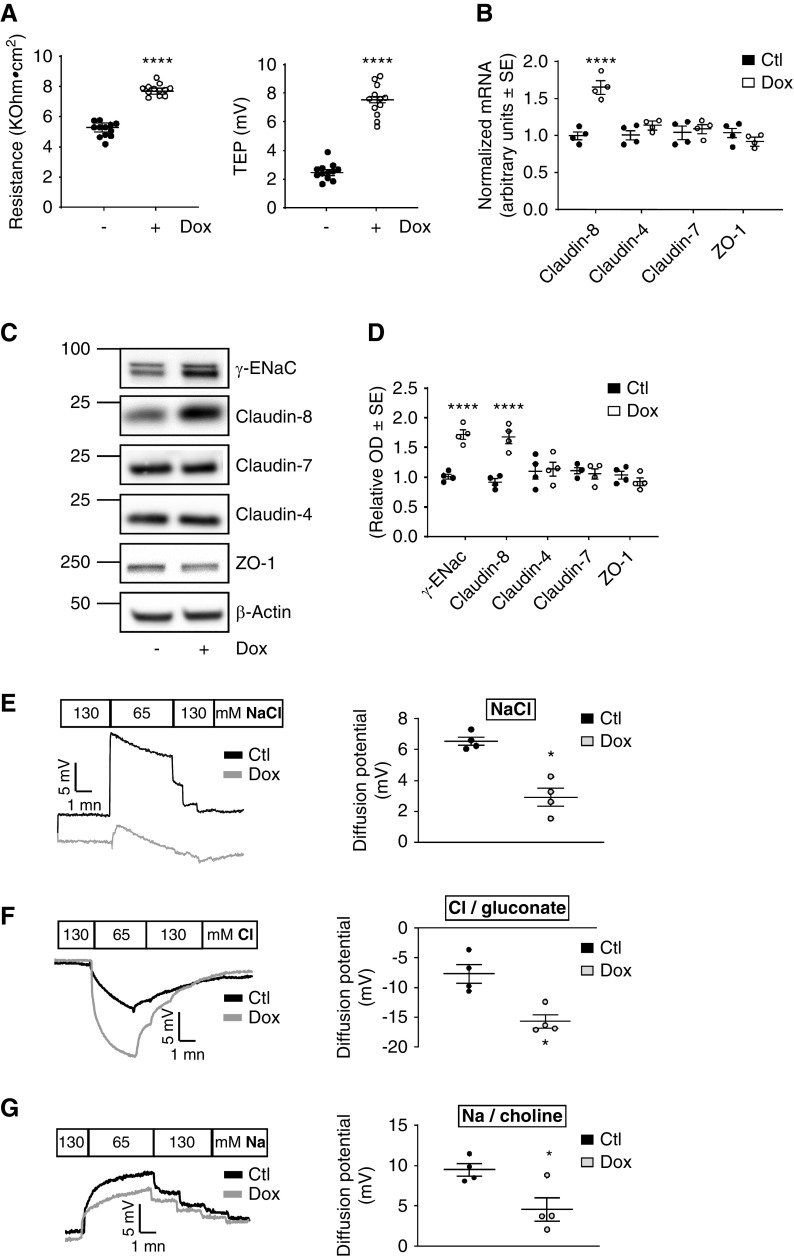
We recently established an inducible γ-ENaC-TetOn-mCCD cell line derived from mCCDcl1 cells19 that conditionally overexpresses wild-type mouse γ-ENaC in response to Dox in a CD principal cell line.14 Surprisingly, we found that γ-ENaC resulted in a significant increase in transepithelial resistance associated with the expected increase in transepithelial potential difference. This finding is unexpected because transepithelial resistance should rather decrease when apical membrane conductance is increased by ENaC overexpression. We hypothesized this increase in transepithelial resistance is due to a change in TJ composition and permeability. We then asked whether the overexpression of γ-ENaC influences the expression of claudin-8, claudin-4, and claudin-7—the major TJ core components found along the CD. As expected, enhanced apical Na+ entry by γ-ENaC overexpression was associated with increased apical negative transepithelial potential difference (Figure 1A, right panel). We consistently showed that this increase in transcellular Na+ transport was also associated with enhanced transepithelial resistance (Figure 1A, left panel) and increased claudin-8 mRNA and protein levels, but claudin-4, claudin-7, and the TJ-associated protein ZO-1 mRNA and protein levels remained unchanged (Figure 1, B–D). Control experiments demonstrated that Dox modified neither transepithelial potential difference and resistance nor claudin-8 expression level in parental mCCDcl1 cells expressing the reverse tetracycline transactivator protein alone (Supplemental Figure 1).
Figure 1.
ENaC γ-subunit overexpression increases transepithelial resistance and claudin-8 expression in cultured CD principal cells. (A–C) γ-ENaC-TetOn-mCCD cells were grown to confluence on filters and treated or not with 1.25 μg/ml Dox for 2.5 days. (A) Measured transepithelial potential difference (TEP; right panel, lumen negative) and resistance (R; left panel). (B) Claudin-4, claudin-7, claudin-8, and ZO-1 mRNA level assessed by real-time PCR. (C) Representative immunoblots showing the effect of Dox treatment on γ-ENaC, claudin-4, claudin-7, claudin-8, and ZO-1 protein abundance. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. (D) Bar graphs depicting relative densitometric quantification of immunoblots from four independent experiments. (E–G) Representative diffusion potential recording in Ussing chambers under a zero-current clamp (left panels). The transepithelial voltage is shown as basolateral potential referenced to the apical side. The diffusion potentials were determined from the change in transepithelial voltage upon switching the basolateral side from symmetrical bathing solutions (130 mM) to a 65 mM concentration gradient of (E) NaCl, (F) Cl−/gluconate, and (G) Na+/choline measured in the presence of amiloride to inhibit most of the transcellular ion transport. A positive diffusion potential indicates that Na+ permeability is greater than Cl− permeability, whereas a negative diffusion potential indicates that Cl− permeability is greater than Na+ permeability. Results are means±SEM from four independent experiments. Statistical analysis was performed by (A) t test, (B and D) one-way ANOVA, and (E–G) Mann–Whitney U test. *P<0.05, ***P<0.01. Ctl, control.
To determine the electrophysiologic properties of the paracellular pathway in these cells, monolayers of control and γ-ENaC–overexpressing mCCD cells were mounted in Ussing chambers and diffusion potentials were monitored under current-clamp conditions. As shown in Figure 1E, the magnitude of diffusion potential (mV) after a 130 mM (apical) to 65 mM (basolateral) NaCl dilution was decreased in γ-ENaC–overexpressing cells compared with control cells, indicating a decrease in the paracellular conductance to Na+ and/or an increase in the paracellular conductance to Cl−. The dilution of the basolateral side with sodium gluconate (65 mM Cl−) induced a large negative diffusion potential, indicating that relative Cl−/gluconate over Na+ permeability was increased in γ-ENaC–overexpressing cells compared with control cells (Figure 1F). The substitution of NaCl with choline chloride confirmed the decreased permeability to Na+ in γ-ENaC–overexpressing cells because the diffusion potential of Na+/choline was decreased in these cells as shown in Figure 1G.
These results indicate that ENaC γ-subunit overexpression primarily modulates claudin-8 expression levels in cultured mouse CD cells. The increase in claudin-8 abundance resulted in a significant increase in paracellular selectivity to Cl− ions over Na+ ions, suggesting that claudin-8 may form a paracellular barrier to Na+.
ENaC γ-Subunit Silencing Alters Claudin-8 Expression and Paracellular Permeability in Cultured CD Principal Cells
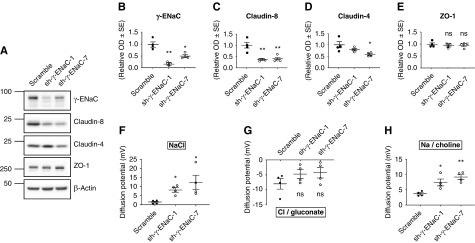
Further examining the effect of ENaC γ-subunit expression levels on claudin-8 abundance in vitro, we investigated the silencing of this subunit. We transduced cultured mCCDcl1 cells19 with lentiviruses encoding shRNAs that specifically target the ENaC γ-subunit. Two different shRNA constructs (sh-γ-ENaC-1 and sh-γ-ENaC-7) that efficiently silenced the ENaC γ-subunit were obtained (Figure 2, A and B, Supplemental Figure 2A). Claudin-8 mRNA and protein abundance were strongly decreased in cells transduced with sh-γ-ENaC-1 and sh-γ-ENaC-7 compared with scramble shRNA–transduced control cells (Figure 2, A and C, Supplemental Figure 2B). Figure 2D shows a significant decrease in claudin-4 with sh-γ-ENaC-7 and a nonsignificant trend to a decrease with sh-γ-ENaC-1. The TJ-associated protein ZO-1 abundance was not significantly altered (Figure 2, A and E, Supplemental Figure 2D).
Figure 2.
ENaC γ-subunit silencing decreases claudin-8 expression in cultured CD principal cells. mCCDcl1 cells transduced with lentiviruses encoding either scramble shRNA (scramble) or shRNAs targeting mouse γ-ENaC (sh-γ-ENaC 1 and sh-γ-ENaC 7) were grown to confluence on filters. (A) Representative immunoblots showing the effect of γ-ENaC silencing on γ-ENaC, claudin-8, claudin-4, and ZO-1 protein levels. β-Actin was used as the loading control. (B–E) Relative densitometric quantification of immunoblots (shown in [A]) from four independent experiments. (F–H) diffusion potentials measurements in Ussing chambers under a zero-current clamp. The diffusion potentials are normalized to results obtained in scramble shRNA–transduced cells and were determined from the change in transepithelial voltage upon switching the basolateral side from symmetrical bathing solutions (130 mM) to a 65 mM concentration gradient of (F) NaCl, (G) Cl−/gluconate, and (H) Na+/choline measured in the presence of amiloride to inhibit most of the transcellular ion transport. A positive diffusion potential indicates that Na+ permeability is greater than Cl− permeability whereas a negative diffusion potential indicates that Cl− permeability is greater than Na+ permeability. Results are means±SEM from four independent experiments. Statistical analysis was performed by (B–E) one-way ANOVA and (F–H) Kruskal–Wallis test. *P<0.05, **P<0.01.
We then assessed the electrophysiologic characteristics of mCCDcl1 cells with γ-ENaC silencing. The magnitude of NaCl diffusion potential was increased in both sh-γ-ENaC-1 and sh-γ-ENaC-7 cells compared with control cells, indicating an increase in the paracellular conductance to Na+ and/or a decrease in the paracellular conductance to Cl− (Figure 2F). Figure 2G shows a nonsignificant trend toward decreased Cl−/gluconate diffusion potentials in γ-ENaC–silencing cells. In contrast, the Na+/choline diffusion potentials were increased in sh-γ-ENaC-1 and sh-γ-ENaC-7 cells, reflecting higher paracellular permeability to Na+ in these cells (Figure 2H).
These results indicate that γ-ENaC silencing is associated with a significant decrease in claudin-8 abundance which is associated with an increase in the paracellular permeability to Na+. The effect seems to be specific to the ENaC γ-subunit because claudin-8 abundance remained unchanged after ENaC α-subunit silencing (Supplemental Figure 3) and ENaC β-subunit silencing (Supplemental Figure 4) except for cells transduced with sh-β-ENaC-1, where the ENaC γ-subunit and claudin-8 abundance are both decreased. This decrease in claudin-8 abundance in cells transduced with sh-β-ENaC-1 most likely relied on decreased ENaC γ-subunit expression but not on ENaC β-subunit silencing, because claudin-8 abundance remained unchanged in cells transduced with sh-β-ENaC-2 (Supplemental Figure 4).
Effect of Claudin-8 Overexpression on Transepithelial Resistance and Paracellular Transport in Cultured CD Principal Cells
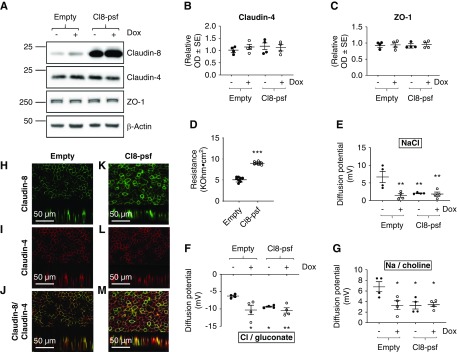
To assess whether claudin-8 abundance is a major determinant of transepithelial resistance in CD epithelium, we constitutively overexpressed claudin-8 in γ-ENaC-TetOn-mCCD cells. Real-time PCR (data not shown) and Western blot analysis (Figure 3A) confirmed claudin-8 overexpression. Targeting of overexpressed claudin-8 to the plasma membrane was further confirmed by biotinylation of cell-surface proteins (Supplemental Figure 5A) and immunofluorescence imaging (Figure 2, H and K). Overexpression of claudin-8 resulted in a significant increase in transepithelial resistance (Figure 3D) and did not alter claudin-4 and ZO-1 abundance (Figure 3, B, C, I and L). We also examined the electrophysiologic characteristics of cells overexpressing claudin-8. The diffusion potential measurements across confluent monolayers show a reduction in NaCl diffusion potentials (Figure 3E), reflecting a decreased paracellular conductance to Na+ or an increased paracellular conductance to Cl−. The basolateral dilution of Cl− induced an increased Cl−/gluconate diffusion potential in claudin-8–overexpressing cells (Figure 3F), indicating that the sodium-to-chloride permeability is decreased in these cells compared with control cells. The diffusion potential of Na+/choline confirms the decreased paracellular permeability to Na+ in claudin-8–overexpressing cells (Figure 3G).
Figure 3.
Claudin-8 overexpression increases transepithelial resistance and decreases paracellular sodium-to-chloride permeability in cultured CD principal cells. γ-ENaC-TetOn-mCCD cells were first transduced with empty lentiviruses (empty) or those encoding wild-type mouse claudin-8 (Cl8-psf). Transduced cells were grown to confluence on filters and treated or not with 1.25 μg/ml Dox for 2.5 days. (A) Representative immunoblots confirming claudin-8 overexpression and unchanged claudin-4 and ZO1 abundance. β-Actin was used as the loading control. (B and C) Bar graphs depicting relative densitometric quantification of immunoblots (shown in [A]) from four independent experiments. (D) Measured transepithelial resistance (TER). (E–G) diffusion potentials recording in Ussing chambers under a zero-current clamp. The diffusion potentials were determined from the change in transepithelial voltage upon switching the basolateral side from symmetrical bathing solutions (130 mM) to a 65 mM concentration gradient of (E) NaCl, (F) Cl−/gluconate, and (G) Na+/choline measured in the presence of amiloride to inhibit most of the transcellular ion transport. A positive diffusion potential indicates that Na+ permeability is greater than Cl− permeability whereas a negative diffusion potential indicates that Cl− permeability is greater than Na+ permeability. (H–M) Immunofluorescence staining of claudin-8 (green channel) and claudin-4 (red channel) in confluent monolayers. (J and M) Representative images of a monolayer double stained with rabbit claudin-8 antibody and mouse claudin-4 antibody. Lower part of each panel is an optical section obtained from Z-stack. Results are means±SEM from four independent experiments. Statistical analysis was performed by (B–D) one-way ANOVA and (E–G) Kruskal–Wallis test. *P<0.05, **P<0.01, ***P<0.001.
These results confirm the barrier function to Na+ played by claudin-8 in mCCD cells.
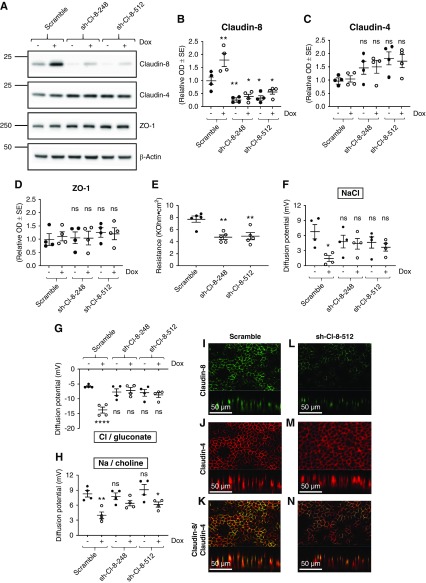
Effect of Claudin-8 Silencing on Transepithelial Resistance and Paracellular Transport in Cultured CD Principal Cells
Further analyzing the function of claudin-8, we transduced cultured γ-ENaC-TetOn-mCCD cells with lentiviruses encoding shRNAs that specifically target claudin-8. Two different shRNAs (sh-Cl-8-248 and sh-Cl-8-512) that efficiently silenced claudin-8 were obtained as confirmed by Western blot (Figure 4, A and B), immunofluorescence (Figure 4, I and L), and by cell-surface protein biotinylation (Supplemental Figure 5D). Basal transepithelial resistance of cell monolayers were significantly decreased in sh-Cl-8-248 and sh-Cl-8-512 cell models compared with control cells transduced with empty vector (Figure 4E). Claudin-8 silencing slightly but not significantly increased claudin-4 abundance (Figure 4, A, C, J and M) and did not alter ZO-1 abundance (Figure 4, A and D). Figure 4F shows that basal NaCl diffusion potential measured across confluent monolayers was unchanged under baseline conditions, whereas the large decrease in NaCl diffusion potential observed in response to ENaC γ-subunit overexpression observed in control cells was blunted in claudin-8–silenced cells. The basal Cl−/gluconate diffusion potential was slightly but not significantly increased in claudin-8–silencing cells compared with control cells. However, the decrease in sodium-to-chloride paracellular permeability induced by γ-ENaC overexpression was abolished by claudin-8 silencing because there was no significant difference in Cl−/gluconate diffusion potentials between induced and noninduced cells (with and without Dox, respectively) (Figure 4G). In contrast, the silencing of claudin-8 did not alter the basal diffusion potential and incompletely prevented the decrease in diffusion potentials of Na+ observed in response to γ-ENaC overexpression (Figure 4H).
Figure 4.
Claudin-8 silencing decreases transepithelial resistance and increases paracellular sodium-to-chloride permeability in cultured CD principal cells. γ-ENaC-TetOn-mCCD cells transduced with lentiviruses encoding either scramble shRNA (scramble) or shRNAs targeting mouse claudin-8 (shCl8-248 and shCl8-512) were grown to confluence on filters and treated or not with 1.25 μg/ml Dox for 2.5 days to induce ENaC γ-subunit overexpression. (A) Representative immunoblots showing the effect of claudin-8 silencing and Dox treatment on claudin-8, claudin-4, and ZO-1 protein levels. β-Actin was used as the loading control. (B–D) Bar graphs depicting relative densitometric quantification of immunoblots (shown in [A]) from four independent experiments. (E) Measured transepithelial resistance (TER). (F–H) Epithelial monolayers acquired simultaneously in Ussing chambers under a zero-current clamp. The diffusion potentials are normalized to scramble (− Dox) and were determined from the change in transepithelial voltage upon switching the basolateral side from symmetrical bathing solutions (130 mM) to a 65 mM concentration gradient of (F) NaCl, (G) Cl−/gluconate, and (H) Na+/choline measured in the presence of amiloride to inhibit most of the transcellular ion transport. A positive diffusion potential indicates that Na+ permeability is greater than Cl− permeability whereas a negative diffusion potential indicates that Cl− permeability is greater than Na+ permeability. (I–N) Immunofluorescence staining of claudin-8 (green channel) and claudin-4 (red channel) in confluent monolayers. (K and N) Representative images of a monolayer double stained with rabbit claudin-8 antibody and mouse claudin-4 antibody. Lower part of each panel is an optical section obtained from Z-stack. Results are means±SEM from four independent experiments. Statistical analysis was performed by (B–E) one-way ANOVA and (F–H) Kruskal–Wallis test. *P<0.05, **P<0.01.
These results show that claudin-8 is an important determinant of the high basal transepithelial resistance observed in mCCD cells and confirm the major role played by this claudin in the observed coupling between γ-ENaC subunit expression and paracellular NaCl permeability.
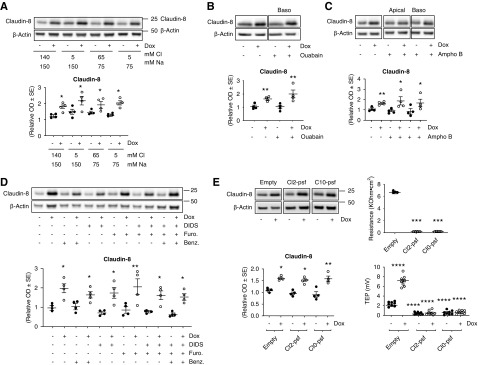
Effect of Sodium and Chloride Transport on Claudin-8 Abundance
Overexpression and silencing of the ENaC γ-subunit may lead to changes in intracellular sodium and chloride concentrations, as well as apical membrane potential changes. These changes might alter signaling pathways and modulate the expression of cell membrane proteins as demonstrated for the WNK pathway and NCC.20 We first investigated whether the change observed in claudin-8 abundance is linked to Na+ or Cl− transport. We compared the effect of the replacement of the apical medium by iso-osmotic buffers containing various concentrations of Na+ and Cl−. Supplemental Figure 6A shows the effect of decreasing the apical Na+ and Cl− concentration on transepithelial potential difference and Figure 5A shows that modifying apical Na+ or/and Cl− concentration did not alter claudin-8 expression in γ-ENaC-TetOn-mCCD cells despite changes in transepithelial ion transport. We then examined the effect of pharmacologic blockade of transcellular Na+ transport by blocking the sodium channel ENaC with 10 μM benzamil (added to the apical side) and inhibiting the Na+/K+-ATPase pump with 1 μM ouabain (added to the basolateral side). Neither benzamil nor ouabain altered claudin-8 abundance (Figure 5, B and D), whereas ion transport was actually decreased as indicated by the inhibited transepithelial potential difference shown in Supplemental Figure 6, B and C. Increasing membrane permeability and increasing intracellular Na+ concentration with amphotericin B (50 μg/ml) affected the transepithelial potential difference (Supplemental Figure 6D) but had also no effect on claudin-8 abundance (Figure 5D). We also tested the blockade of chloride transport with 10 μM furosemide and/or 100 μM 4,4ʹ-diisothiocyanatostilbene-2,2ʹ-disulfonic acid, associated or not with sodium transport blockade (with benzamil) that did not produce any effect on claudin-8 abundance (Figure 5D), despite clear effects on the transepithelial potential difference (Supplemental Figure 6E).
Figure 5.
Neither altered sodium and chloride transport nor transepithelial potential influence claudin-8 abundance. γ-ENaC-TetOn-mCCD cells were grown to confluence on filters and treated or not with 1.25 μg/ml Dox for 2.5 days to induce ENaC γ-subunit overexpression. (A–D) Cells were grown to confluence before apical medium was removed and replaced for 24 hours by iso-osmotic medium containing different concentrations of NaCl, Na-gluconate, or choline-Cl (A). Cells were grown to confluence and were also treated for 24 hours with 1 μM ouabain (B), 50 μg/ml amphotericin B (Ampho B) (C), 10 μM benzamil or chloride transport blockers (10 μM furosemide [Furo] and 100 μM 4,4ʹ-diisothiocyanatostilbene-2,2ʹ-disulfonic acid [DIDS]) (D) . γ-ENaC-TetOn-mCCD cells were transduced with empty lentiviruses (empty) or those encoding wild-type mouse claudin-2 (Cl2-psf) or wild-type mouse claudin-10 (Cl10-psf). Transduced cells were grown to confluence on filters and treated or not with 1.25 μg/ml Dox for 2.5 days. Representative immunoblots showing the effect of different treatments on claudin-8 abundance are shown in upper panels, Western blot quantifications are shown in lower panels, measured transepithelial resistance (TER) is shown in right-upper panels, and measured transepithelial potential difference (TEP; lumen negative) is shown in right-lower panels. Results are means±SEM from four independent experiments. Statistical analysis was performed by one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
We then decided to investigate whether the transepithelial potential regulated claudin-8 abundance. We generated new cell lines, Cl2-psf and Cl10-psf, that respectively overexpress claudin-2 and claudin-10—two pore-forming claudins. These two claudins were constitutively transduced in γ-ENaC-TetOn-mCCD cells. We validated the overexpression by Western blot (data not shown) and by biotinylation (Supplemental Figure 5). As expected, the overexpression of claudin-2 and claudin-10 dramatically decreased the transepithelial potential difference and the transepithelial resistance (Figure 5E) as a result of the alteration of both tightness and paracellular charge selectivity. However, no effect on claudin-8 abundance was observed in these two overexpression models (Figure 5E).
These results indicate that claudin-8 expression is regulated neither by sodium and chloride transport nor by the variation of electrical potential and the expression levels of pore-forming claudin-2 and -10.
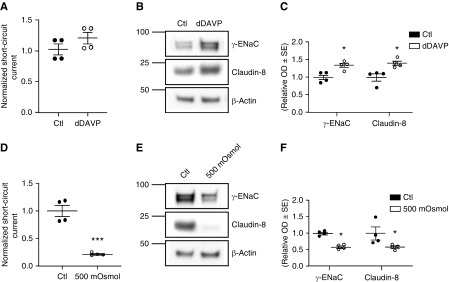
Physiologic Changes in ENaC γ-Subunit Abundance Modulates Claudin-8 Expression in Cultured CD Principal Cells
To investigate potential physiologic linkage between transcellular and paracellular pathways in vitro, without genetic manipulations, we assessed the upregulation of the ENaC γ-subunit in vasopressin-responsive mpkCCDcl4 cells, a second model of mouse CD cells.21,22 As expected, 1 day of 0.1 nM dDAVP incubation upregulated γ-ENaC and enhanced apical Na+ entry. This effect was associated with an increase in short-circuit current (Figure 6A) and increased ENaC γ-subunit abundance (Figure 6, B and C). Moreover, application of dDAVP increased claudin-8 abundance (Figure 6, B and C), confirming our results obtained in the γ-ENaC-TetOn-mCCD cell line.
Figure 6.
Vasopressin and hyperosmotic stress modulate claudin-8 expression in mpkCCD cells. mpkCCDcl4 cells were grown to confluence on filters and were exposed or not to (A–C) 0.1 nM [deamino-Cys1, D-Arg8]-Vasopressin (dDAVP) or (D–E) hypertonic medium (500 mOsmol/kg) for 24 hours. (A) Short-circuit current changes in response to dDAVP exposure. (B) Representative immunoblots showing the effect of dDAVP treatment on ENaC γ-subunit and claudin-8 protein abundance. β-Actin was used as a loading control. (C) Bar graphs depicting relative densitometric quantification of immunoblots from four independent experiments. (D) Effect of hyperosmotic stress on short-circuit current. (E) Representative immunoblots showing the effect of hyperosmotic challenge on the ENaC γ-subunit and claudin-8 protein abundance. β-Actin was used as a loading control. (F) Bar graphs depicting relative densitometric quantification of immunoblots from four independent experiments. Results are means±SEM from four independent experiments. Statistical analysis was performed by Mann–Whitney U test. *P<0.05, ***P<0.001. Ctl, control.
We next investigated the downregulation of the ENaC γ-subunit by hyperosmotic stress, one of the main regulators of ENaC abundance and surface expression.23 The use of hypertonic medium (500 osmol/kg NaCl) for 24 hours dramatically decreased the short-circuit current (Figure 6D) and ENaC γ-subunit abundance (Figure 6, E and F). This decrease was again accompanied by a decrease in claudin-8 abundance (Figure 6, E and F).
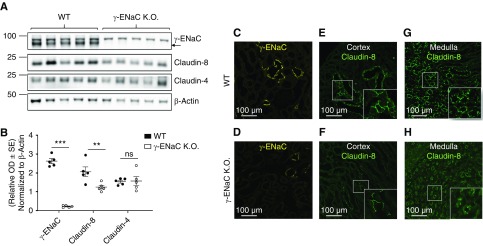
The Nephron-Specific KO of γ-ENaC Is Associated with a Decrease in Claudin-8 Abundance in Mouse Kidney
To investigate potential physiologic interactions between transcellular and paracellular pathways in vivo, we assessed claudin-4 and claudin-8 protein abundance in Dox-induced, kidney tubule–specific, γ-ENaC KO mice.18 As expected, in kidney cortex, Western blot analyses revealed the absence of γ-ENaC protein in 4-week-old γ-ENaC KO mice as compared with control wild-type littermates (Figure 7, A and B); this was accompanied by a significant decrease in claudin-8 abundance that is specifically expressed along the collecting system (connecting tubule and CD).9 This decrease in claudin-8 protein expression was specific because abundance of claudin-4, which is mostly expressed along the collecting system, remained unchanged.
Figure 7.
Nephron-specific γ-ENaC KO decreases claudin-8 abundance. (A) Representative immunoblots showing the effect of γ-ENaC KO on claudin-8 and claudin-4 abundance in kidney cortex. β-Actin was used as a loading control. (B) Quantification of protein expression levels normalized to β-actin protein expression levels. (C–H) Representative images of the confocal immunofluorescence detection of γ-ENaC (yellow) and claudin-8 (green) in cortical and medullary CD in (C, E and G) wild-type (WT) or (D, F, and H) γ-ENaC KO mice. Results are presented as mean±SEM from five animals and data were analyzed by t test. **P<0.01, ***P<0.001.
Immunofluorescence staining of kidney sections from wild-type and γ-ENaC KO mice confirmed the Western blot results with a large decrease in staining of γ-ENaC (Figure 7, C and D) associated with decreased claudin-8 staining in both cortex and medulla (Figure 7, E–H).
These results confirm our in vitro results and indicate that γ-ENaC KO is associated with a significant decrease in claudin-8 abundance. It should be mentioned that this effect is specific to γ-ENaC because claudin-8 abundance remained unchanged in Dox-induced and kidney tubule–specific α-ENaC16 and β-ENaC17 KO mice (Supplemental Figure 7).
Discussion
The renal CD is characterized by an electrically tight epithelium. The paracellular pathway functions primarily as an ion diffusion barrier, protecting transepithelial gradients for solutes and preventing them from passive interstitium-to-lumen backflow. In this study, we have shown for the first time that stimulation of transcellular Na+ transport by overexpression of the ENaC γ-subunit resulted in increased transepithelial resistance and decreased paracellular Na+ permeability in renal cultured CD principal cells. We also demonstrated that transepithelial resistance was decreased when we reduced transcellular Na+ transport by silencing of γ-ENaC in renal cultured CD principal cells. This observed modulation of transepithelial resistance accompanying the changes in transcellular Na+ transport is certainly linked to variations of paracellular resistance. Indeed, according to Ohm law, the transcellular component of the transepithelial resistance should decrease when ENaC activity increases, and vice versa. Increased or decreased paracellular resistance leads to a bidirectional decrease or increase of paracellular permeability to water and solutes, respectively.24–26 Therefore, interaction between transepithelial Na+ reabsorption and paracellular resistance may prevent the luminal backflow of Na+ and water reabsorbed by the CD. This mechanism might be especially relevant in the medullary CD, which is characterized by a steep interstitial-to-luminal Na+ and osmolality gradient.
A large body of experimental evidence has shown that claudins, a core component of TJ complexes, can act as ion-selective paracellular channels or paracellular ion-selective permeability barriers.27–30 Claudins are commonly classified as barrier or pore based on whether their overexpression or silencing increases or decreases transepithelial resistance. Claudin-8 is highly and specifically expressed along the electrically tight aldosterone-sensitive distal nephron, including the CD, but is not detected in the proximal tubule and thick ascending limb which are electrically leaky epithelia,9 indicating that it may function as a diffusion barrier. The functional role of claudin-8 was confirmed by overexpression and silencing experiments showing that transepithelial resistance varies in parallel with claudin-8 protein levels. Overexpression of claudin-8 increased transepithelial resistance whereas claudin-8 knockdown decreased transepithelial resistance. Furthermore, we have shown for the first time that overexpression of claudin-8 resulted in a decrease in the paracellular conductance to Na+, emphasizing the functional importance of claudin-8 as a Na+ barrier. In contrast, loss of claudin-8 did not detectably alter the paracellular permeability to Na+ in transduced mCCD cells. This effect could be explained by the residual expression of claudin-8 detected by immunofluorescence.
The modulation of paracellular permeability relies, at least in part, on transcriptional regulation of the expression levels of claudins. We showed that claudin-8 is specifically coregulated with γ-ENaC subunit abundance whereas two other claudins strongly expressed in CD, i.e., claudin-4 and claudin-7,9 are not. Claudin-8 silencing prevented the increased transepithelial resistance induced by increased γ-ENaC subunit abundance. Therefore, regulation of claudin-8 most likely accounts, at least in part, for the changes in transepithelial resistance accompanying the variations of γ-ENaC subunit expression levels. The simultaneous changes in claudin-8 mRNA and protein abundance suggest that claudin-8 expression is controlled at the level of transcription or/and mRNA stability.
Experiments performed in MDCK cells suggested that claudin-8 is required to stabilize claudin-4, which generates a barrier to Na+ but facilitates Cl− diffusion.31 The NaCl wastage observed in claudin-4 or claudin-8 KO mice, which may result from impaired paracellular Cl− reabsorption that leads to increased luminal Na+ retention and eventually hypotension, was taken as additional evidence supporting the interdependence of these two claudins.32,33 However, we did not found any dependence of the expression levels of claudin-4 on claudin-8 abundance. In mCCD cells, claudin-4 abundance and cell-surface expression remained unchanged under conditions with increased or decreased claudin-8 expression levels. In mouse kidney cortex, claudin-4 abundance was unchanged despite claudin-8 downregulation in kidney tubule–specific ENaC γ-subunit KO mice. Our observations rather suggest that the reported stabilization of claudin-4 by association with claudin-8 can be observed in a specific cellular context or under some experimental conditions but does not represent a universal mechanism.
We showed that claudin-8 expression was independent of Na+ and Cl− transport via active, secondary active, or passive transporters in γ-ENaC-TetOn-mCCD cells. Overexpression of claudin-2 and claudin-10, two pore-forming claudins, induced a dramatic decrease in the transepithelial potential but did not alter claudin-8 abundance. We conclude that claudin-8 expression is being regulated neither by Na+ and Cl− transport nor by the variation of electrical potentials or abundance of pore-forming claudins-2 or -10. In contrast, our results indicate that the modulation of claudin-8 expression relies on γ-ENaC protein abundance. Indeed, in mCCDcl1 cells, overexpression or silencing of the ENaC γ-subunit are associated with parallel changes in claudin-8 expression levels whereas, in mpkCCDcl4 cells, the increased or decreased expression of γ-ENaC induced by vasopressin treatment or hyperosmotic challenge, respectively, was associated with parallel changes in claudin-8 abundance. It should be mentioned that claudin-8 has been identified as a vasopressin-induced mRNA in an RNA-sequencing study performed in mpkCCDcl4 cells.34 Moreover, in conditional kidney tubule–specific γ-ENaC KO mice,18 claudin-8 abundance is decreased. This effect seems to be specific to the ENaC γ-subunit because claudin-8 abundance remained unchanged in conditional kidney tubule–specific α-ENaC and β-ENaC KO mice.16,17 The mechanisms of the interaction between the ENaC γ-subunit and claudin-8 expression levels in the CD are the subject of future investigations. One may speculate that γ-ENaC may directly or indirectly modulate the activity of protein kinase(s) via direct association, as described for β-ENaC and IKKβ35 or with regulatory proteins (see speculated mechanism in Supplemental Figure 8).
Our results show modulation of claudin-8 expression levels leading to a change in TJ permeability in response to sustained variations of transcellular Na+ transport. This study further demonstrates that TJs are highly dynamic structures that undergo functional and structural changes in response to physiologic stimuli.
Disclosures
None.
Funding
This work was supported by the National Center of Competence in Research Kidney Control of Homeostasis grant 31003A_156736/1 (to Dr. Feraille), and Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation) grants 31003A_175471/1 (to Dr. Feraille) and 31003A_144198/1 (to Dr. Hummler).
Supplementary Material
Acknowledgments
We thank Prof. Johannes Loffing (Institute of Anatomy, University of Zurich) for kindly providing the α-ENaC subunit antibody and Dr. Dominique Loffing (Institute of Anatomy, University of Zurich) for kindly providing kidney cryosections of wild-type and γ-ENaC KO mice. We also thank Dr. Suresh Ramakrishnan for his reading and helpful comments of the draft versions.
Dr. Feraille, Dr. Sassi, and Dr. Wang designed the study; Dr. Boscardin, Ms. Chassot, Dr. Crambert, Dr. Dizin, Dr. Hummler, Dr. Komarynets, Dr. Olivier, Ms. Roth, Dr. Sassi, and Dr. Wang carried out experiments; Dr. Sassi and Dr. Wang analyzed the data; Ms. Chassot, Dr. Sassi, and Dr. Wang made the figures; Dr. Feraille, Dr. Sassi, Dr. Wang drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080790/-/DCSupplemental.
Supplemental Figure 1. Doxycycline alone does not alter transepithelial resistance and claudin-8 expression.
Supplemental Figure 2. ENaC γ-subunit silencing decreases claudin-8 expression in cultured collecting duct principal cells.
Supplemental Figure 3. Effect of ENaC α-subunit silencing on claudin-8 abundance in cultured collecting duct principal cells.
Supplemental Figure 4. Effect of ENaC β-subunit silencing on claudin-8 abundance in cultured collecting duct principal cells.
Supplemental Figure 5. Cell-surface protein biotinylation from cells overexpressing and silencing claudin-8 and cells overexpressing claudin-2 and claudin-10.
Supplemental Figure 6. Effect of sodium and chloride transport on measured transepithelial potential difference.
Supplemental Figure 7. Effect of nephron-specific α-ENaC and β-ENaC knockout on claudin-8 abundance.
Supplemental Figure 8. Speculated mechanism of interaction between ENaC γ-subunit and claudin-8.
References
- 1.Sansom SC, Weinman EJ, O’Neil RG: Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol 247: F291–F302, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, et al.: Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17: 2153–2163, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, et al.: Dietary Cl(-) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Morla L, Doucet A, Lamouroux C, Crambert G, Edwards A: The renal cortical collecting duct: A secreting epithelium? J Physiol 594: 5991–6008, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balda MS, Matter K: Tight junctions at a glance. J Cell Sci 121: 3677–3682, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE: Structure and function of claudins. Biochim Biophys Acta 1778: 631–645, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Li WY, Huey CL, Yu ASL: Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 286: F1063–F1071, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hou J: The kidney tight junction (Review). Int J Mol Med 34: 1451–1457, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu ASL: Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke J-D, et al.: Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun 378: 45–50, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, et al.: Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 12: 3717–3732, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajasekaran AK, Rajasekaran SA: Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol 285: F388–F396, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK: Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G124–G133, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y-B, Leroy V, Maunsbach AB, Doucet A, Hasler U, Dizin E, et al.: Sodium transport is modulated by p38 kinase-dependent cross-talk between ENaC and Na,K-ATPase in collecting duct principal cells. J Am Soc Nephrol 25: 250–259, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasler U, Vinciguerra M, Vandewalle A, Martin P-Y, Féraille E: Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16: 1571–1582, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, et al.: Severe salt-losing syndrome and hyperkalemia induced by adult nephron-specific knockout of the epithelial sodium channel α-subunit. J Am Soc Nephrol 27: 2309–2318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boscardin E, Perrier R, Sergi C, Maillard M, Loffing J, Loffing-Cueni D, et al.: Severe hyperkalemia is rescued by low-potassium diet in renal βENaC-deficient mice. Pflugers Arch 469: 1387–1399, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Boscardin E, Perrier R, Sergi C, Maillard MP, Loffing J, Loffing-Cueni D, et al.: Plasma potassium determines NCC abundance in adult kidney-specific γENaC knockout. J Am Soc Nephrol 29: 977–990, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaeggeler H-P, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, et al.: Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marunaka Y, Eaton DC: Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am J Physiol 260: C1071–C1084, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, et al.: Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Crambert G, Ernandez T, Lamouroux C, Roth I, Dizin E, Martin PY, et al.: Epithelial sodium channel abundance is decreased by an unfolded protein response induced by hyperosmolality. Physiol Rep 2: e12169, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanning AS, Mitic LL, Anderson JM: Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol 10: 1337–1345, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, et al.: Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem 274: 20982–20988, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Anderson JM, Van Itallie CM: Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuse M, Sasaki H, Fujimoto K, Tsukita S: A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143: 391–401, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Itallie C, Rahner C, Anderson JM: Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, et al.: Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Yu ASL, Enck AH, Lencer WI, Schneeberger EE: Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278: 17350–17359, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Renigunta A, Yang J, Waldegger S: Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A 107: 18010–18015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, et al.: The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A 111: E3766–E3774, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J: KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A 112: 4340–4345, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epithelial Systems Biology Laboratory, National Heart, Lung, and Blood Institute: RNA polymerase II ChIP-seq and RNA-seq in mpkCCD cells: response to vasopressin. Available at: https://helixweb.nih.gov/ESBL/Database/Vasopressin/. Accessed January 10, 2020
- 35.Lebowitz J, Edinger RS, An B, Perry CJ, Onate S, Kleyman TR, et al.: Ikappab kinase-β (ikkbeta) modulation of epithelial sodium channel activity. J Biol Chem 279: 41985–41990, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.