Significance Statement
Recent research into the pathophysiology of autosomal dominant polycystic kidney disease indicates that both signaling of primary cilia of tubular cells and immune cell infiltration play key roles. However, the reciprocal interactions between immune and tubular cells are not well characterized. The transcription factor STAT3, an important modulator of inflammatory response and a cilia component, is activated in polycystin 1 (PKD1)–deficient tubular cells and is suspected to promote cyst growth. In this work, the authors used murine models involving postdevelopmental ablation of Pkd1, Stat3, and cilia to assess STAT3’s role in the disease. They found that, contrary to previous assumptions, STAT3 does not appear to be a critical mediator of cyst growth, but instead acts in a feedback loop that restricts cilia-dependent renal inflammation by repressing proinflammatory cytokines.
Keywords: ADPKD, cilia, STAT3, inflammation
Abstract
Background
The inactivation of the ciliary proteins polycystin 1 or polycystin 2 leads to autosomal dominant polycystic kidney disease (ADPKD). Although signaling by primary cilia and interstitial inflammation both play a critical role in the disease, the reciprocal interactions between immune and tubular cells are not well characterized. The transcription factor STAT3, a component of the cilia proteome that is involved in crosstalk between immune and nonimmune cells in various tissues, has been suggested as a factor fueling ADPKD progression.
Method
To explore how STAT3 intersects with cilia signaling, renal inflammation, and cyst growth, we used conditional murine models involving postdevelopmental ablation of Pkd1, Stat3, and cilia, as well as cultures of cilia-deficient or STAT3-deficient tubular cell lines.
Results
Our findings indicate that, although primary cilia directly modulate STAT3 activation in vitro, the bulk of STAT3 activation in polycystic kidneys occurs through an indirect mechanism in which primary cilia trigger macrophage recruitment to the kidney, which in turn promotes Stat3 activation. Surprisingly, although inactivating Stat3 in Pkd1-deficient tubules slightly reduced cyst burden, it resulted in a massive infiltration of the cystic kidneys by macrophages and T cells, precluding any improvement of kidney function. We also found that Stat3 inactivation led to increased expression of the inflammatory chemokines CCL5 and CXCL10 in polycystic kidneys and cultured tubular cells.
Conclusions
STAT3 appears to repress the expression of proinflammatory cytokines and restrict immune cell infiltration in ADPKD. Our findings suggest that STAT3 is not a critical driver of cyst growth in ADPKD but rather plays a major role in the crosstalk between immune and tubular cells that shapes disease expression.
Autosomal dominant polycystic kidney disease (ADPKD), a leading Mendelian cause of kidney disease, accounts for 5% of patients with ESKD. Therapies allowing patients to avoid ESKD are currently lacking. Indeed, the only drug to have shown some efficacy to date only modestly reduces kidney function decline at the expense of considerable side effects.1 Thus, a better understanding of ADPKD pathophysiology is of major importance to delineate more effective therapies.
ADPKD is caused by inactivating mutations affecting either PKD1, which encodes polycystin 1, a putative orphan receptor, or PKD2, which encodes polycystin 2, a cation channel that interacts with polycystin 1.2 Although patients with ADPKD carry heterozygous germline PKD1 (or PKD2) inactivating mutations, cyst development is caused by somatic mutations affecting the remaining functional PKD1 (or PKD2) allele in a subset of tubular cells that will proliferate to form cysts.3 In adult mice, biallelic loss of Pkd1 in tubular cells results in a biphasic proliferative response leading first to noncystic kidney enlargement (precystic phase), followed by the rapid development of cysts (cystic phase).4,5 Although cyst formation is the consequence of a genetic defect in tubular cells, this process is not cell autonomous. Indeed, cystogenesis is associated with important modifications of the renal microenvironment, most importantly immune cell recruitment in the vicinity of Pkd1-deficient tubules, which, in turn, affects cyst growth and kidney function decline,6–10 as well as quantitative and qualitative alterations of the extracellular matrix,11,12 which are also thought to contribute to disease progression.
Primary cilia play an essential role in ADPKD.4,10 The primary cilium is a microtubule-based organelle that protrudes from the apical surface of tubular cells to integrate mechanical and chemical cues delivered by the urinary flow. The Polycystin 1/2 complex localizes to the primary cilium and this localization is essential to repress cyst formation.13,14 Remarkably, cilia ablation drastically suppressed both precystic cell proliferation and cyst formation in Pkd1 mutant animals.4,10 However, the nature of the deleterious signals delivered by cilia in this context are only starting to emerge. We and others have recently shown that cilia ablation prevents the induction of the macrophage chemoattractant CCL2 in response to Pkd1 disruption and that Ccl2 inactivation dampens renal macrophage infiltration and cyst formation in Pkd1 mutant mice.7,10 Although these results demonstrate that primary cilia control the communication between immune and tubular cells, the molecular network shaping the interaction between polycystin-deficient tubular cells and the neighboring immune cells remain poorly understood.
STAT3 is a pleiotropic transcription factor involved in a large spectrum of physiologic and pathophysiologic processes.15 STAT3 activation is principally mediated by its phosphorylation on tyrosine 705 by various cytosolic or membrane kinases, which promotes its nuclear accumulation and transcriptional activity. STAT3 represents an interesting candidate for immune-tubular cell communication because it stands at the crossroads of cilia signaling, immune regulation, and cyst growth. Numerous studies have pinpointed STAT3 as a general driver of both pro- and anti-inflammatory crosstalk between epithelial and neighboring immune cells.16,17 A recent proximity biotinylation-based proteomic screen identified STAT3 as a bona fide component of the ciliary machinery.18 In ADPKD and murine polycystic kidney disease models, phosphorylated STAT3 accumulates in the nucleus of cyst-lining epithelial cells.19,20 Furthermore, the cytosolic tail of polycystin 1 binds STAT3 in vitro and modulates its transcriptional activity.18 Lastly, the positive effect of STAT3 inhibitory drugs in orthologous models of the disease has led to the assumption that tubular STAT3 promoted cystogenesis.19,20 However, how STAT3 intersects with cilia signaling, renal inflammation, and cyst growth in the disease is not understood. The limited specificity of the inhibitors used in these studies20,21 and their effect on STAT3 activation in nontubular cells preclude definitive conclusions regarding the precise function of tubular STAT3 in polycystin-deficient tubular cells.
Considering these uncertainties, we aimed to clarify (1) the function of primary cilia in STAT3 activation in tubular cells, and (2) its subsequent function in cyst growth, renal inflammation, and kidney function decline.
Methods
Mice
All animal experiments were conducted according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as the German and French laws for animal welfare, and were approved by regional authorities. Mice were housed in a specific pathogen-free facility, fed ad libitum, and housed at constant ambient temperature in a 12-hour day/night cycle. Breeding and genotyping were done according to standard procedures.
Pkd1flox/flox mice (B6.129S4-Pkd1tm2Ggg/J, stock number 010671, C57BL/6 genetic background) and Ccl2-RFPflox/flox (B6.Cg-Ccl2tm1.1Pame/J, stock number 016849, C57BL/6 genetic background) were purchased from The Jackson Laboratory, Kif3aflox/flox mice (Kif3atm1Gsn, C57BL/6 genetic background) were kindly provided by Peter Igarashi and were crossed to Pax8rtTA (Tg[Pax8-rtTA2S*M2]1Koes)22 and TetOCre (Tg[tetO-cre]1Jaw)23 mice to generate an inducible tubule-specific Pkd1 knockout (further referred to as iPkd1ΔTub), Pkd1; Ccl2 knockout (further referred to as iPkd1ΔTub; iCcl2ΔTub), and Pkd1; Kif3a knockout (further referred to as iPkd1ΔTub; iKif3aΔTub) as previously described.10 Stat3flox/flox mice (Stat3tm1Vpo1, initially kindly provided by Valeria Poli, FVB/N genetic background)24 were backcrossed for three generations with iPkd1ΔTub mice on a pure C57BL/6 genetic background. The progeny was then intercrossed to generate mice with inducible tubule-specific Pkd1 knockout (further referred as iPkd1ΔTub) or Pkd1; Stat3 knockout (further referred to as iPkd1ΔTub; iStat3ΔTub) on a common mixed FVB/N; C57BL/6 genetic background.
To induce floxed alleles, recombination mice received doxycycline (ab141091; Abcam) in drinking water (2 mg/ml with 5% sucrose, protected from light) from postnatal day 28 (P28) to P42. Littermates (lacking either TetOCre or Pax8rtTA) were used as controls and received the same doxycycline regimen. Experiments were conducted on males.
Plasma Analyses
Retro-orbital blood was collected from anesthetized mice. Plasma BUN and plasma creatinine were measured using a Konelab 20i Analyzer (Thermo Scientific).
Morphologic Analysis
Mouse kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, and 4-µm sections were stained with Periodic acid–Schiff (PAS). Stained sections were imaged using an E800 microscope (Nikon) equipped with a Plan Neofluar 20×/0.30 NA objective and a digital camera Dx/m/1200 (Nikon) coupled to NIS software (Laboratory Imaging Ltd). PAS-stained, full-size kidney images were recorded using a whole-slide scanner Nanozoomer 2.0 (Hamamatsu) equipped with a 20×/0.75 NA objective coupled to NDPview software (Hamamatsu).
Cortical tubular lumen fractional area was measured with ImageJ software from PAS-stained, full-size kidney images. Interstitial infiltration score was evaluated by two independent observers in a blinded fashion to assess the overall infiltration of the whole-kidney section stained with PAS.
Cell Culture
Madin–Darby canine kidney (MDCK, kind gift of Prof. Kai Simons, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany) cells were cultured using DMEM supplemented with 10% FBS (F7524; Sigma-Aldrich) and 1% penicillin-streptomycin (15140122; Life Technologies). MDCK cells with a tetracycline-inducible Kif3a knockdown (further referred to as Kif3a-i), Ift88 knockdown (further referred to as Ift88-i), or control (further referred to as Luci-i) using a lentivirus-based transduction system have been previously described.10 A total of 150,000 cells/cm2 of each inducible MDCK cell line were seeded with or without 1 µg/ml doxycycline hyclate (ab141091) for 10 days on 12-mm transwell polycarbonate membranes (COSTAR, 3401). Proteins were extracted after 16 hours of serum deprivation.
Mouse inner medullary collecting duct (mIMCD-3) cells were grown in 50% DMEM high-glucose pyruvate (41966052; Gibco), 50% F-12 Nutrient Mixture (21765037; Gibco) supplemented with 10% FBS and 1% penicillin-streptomycin. mIMCD-3 cells with a stable Stat3 knockdown (further referred to as Stat3 sh1 and 2) have been already reported.24 A total of 30,000 mIMCD-3 cells per cm2 were seeded. Confluent mIMCD-3 cells were serum deprived for 16 hours and then stimulated with 1 µg/ml LPS (tlrl-3pelps; Invivogen). RNAs and proteins were extracted 24 hours after stimulation because this time point has been previously used to study the induction of NF-κB targets in tubular cells.25
All cells were regularly tested for mycoplasma contamination and were mycoplasma free.
Quantitative Real-Time PCR
Total RNAs were obtained from whole kidneys or cells using the NucleoSpin RNA Kit (Macherey Nagel) and reverse transcribed using the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). Quantitative PCR was performed with iTaq Universal SYBR Green Supermix (Bio-Rad) on the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) coupled to QuantStudio software version 1.3. Each biologic replicate was measured in technical duplicates. The primers used for quantitative real-time PCR are listed in Supplemental Table 1.
Immunohistochemistry
Sections (4 µm) of paraffin-embedded kidneys were incubated for 20 minutes at 95°C in 10 mM Tris base, 1 mM EDTA solution, and 0.05% Tween 20, pH 9.0, for pSTAT3Y705 staining or citrate buffer (ZUCD28; Zytomed) for the other antibodies, followed by avidin/biotin blocking (SP-2001; Vector Laboratories). Sections were incubated with primary antibody followed by biotinylated antibody, horseradish peroxidase–labeled streptavidin (1:500, 7100-05; Southern Biotech), and 3,3′-diaminobenzidine tetrahydrochloride (K3468; Dako) revelation. Stained sections were imaged using an E800 microscope equipped with a Plan Neofluar 20×/0.30 NA objective and a digital camera Dx/m/1200 coupled to NIS software.
Western Blot
Kidneys were lysed in 50 mM Tris, pH 8, 200 mM sodium chloride, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, and 1% SDS buffer. Cells were lysed in modified radioimmunoprecipitation assay lysis buffer (150 mM sodium chloride, 50 mM Tris hydrochloride, pH 7.5, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium laury sulfate). Nucleocytoplasmic fractionation was performed using the NePer kit (Thermo Fisher). Lysis buffers were supplemented with protease and protein phosphatase inhibitor tablets (A32959; Pierce). Protein content was determined with the Pierce BCA Protein Assay kit (A23225; Pierce). Equal amounts of protein were resolved on 4%–20% gradient gels (Bio-Rad) under reducing conditions, transferred and incubated with primary and secondary antibodies, and visualized using the ChemiDoc (Bio-Rad) imaging system coupled to Image Lab Software (Bio-Rad).
Antibodies
For Western Blotting
We used the following antibodies: pSTAT3Y705 (1:1000, 9145; Cell Signaling Technology), STAT3 (1:1000, 9132; Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (1:5000, MAB374; Millipore), α-Tubulin (1:20,000, T5168; Sigma), Lamin A/C (1:1000, ab108922; Abcam), and RELA (1:1000, 4764; Cell Signaling Technology).
For Immunostaining
We used the following antibodies: pSTAT3Y705 (1:100, 9145; Cell Signaling Technology), STAT3α (1:100, clone D1A5, 8768; Cell Signaling Technology), F4/80 (1:100, clone Cl:A3-1, MCA497R; Bio-Rad), CD3 (1:100, ab16669; Abcam), LY6B (1:100, ab53457; Abcam), CXCL10 (1:100, ab9807; Abcam), and KI67 (1:100, ab15580; Abcam).
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Staining
Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining was performed on 4-µm sections of paraffin-embedded kidneys using the In Situ Cell Death Detection Kit 5 (11684795910; Roche).
Statistical Analysis
Data were expressed as means or means±SEM. Differences between the experimental groups were evaluated using one- or two-way ANOVA as appropriate, followed by the Tukey–Kramer test when significant (P<0.05). When only two groups were compared, the two-tailed t test or Mann–Whitney test was used as appropriate. For Kaplan–Meier survival curves, the log-rank test was applied. The statistical analysis was performed using GraphPad Prism version 6 software. No samples were excluded from analyses. All image analyses (immunohistochemistry, immunofluorescence) and mouse phenotypic analyses were performed in a blinded fashion.
Results
Primary Cilia Regulate STAT3 Activation in Tubular Cells
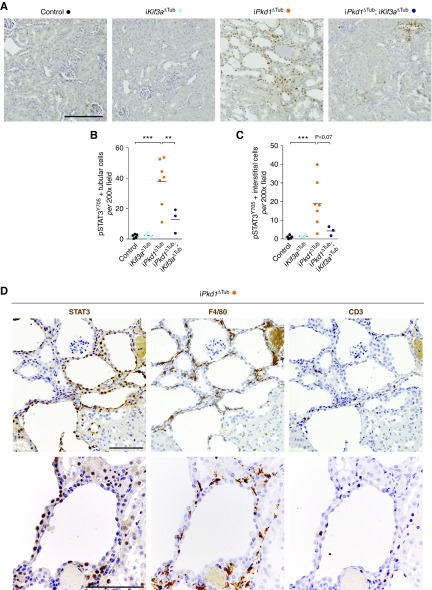
To investigate if STAT3 activation occurred at an early stage of the disease, we took advantage of transgenic mice that allowed the inducible inactivation of Pkd1 in tubular cells upon doxycycline treatment (iPkd1Δtub). At 12 weeks of age (6 weeks after the completion of doxycycline treatment), tubular cell proliferation is increased and kidneys are enlarged but minimally cystic.4,10 Immunolabeling of kidney sections from 12-week-old iPkd1Δtub and control mice with STAT3 phospho-specific antibody directed against tyrosine 705 revealed an increase in both tubular and interstitial phosphorylated STAT3-positive nuclei in polycystin-deficient kidneys compared with controls (Figure 1, A–C). Of note, most of the signal was observed in areas with mild tubular dilation and interstitial infiltration. Serial section staining with STAT3-, F4/80-, and CD3-specific antibodies revealed that STAT3 activation was mainly associated with macrophage recruitment (Figure 1D). Ablation of primary cilia in iPkd1Δtub mice through simultaneous deletion of Kif3a markedly blunted both tubular and interstitial STAT3 activation (Figure 1, A–C).
Figure 1.
Cilia ablation reduces STAT3 activation in response to Pkd1 inactivation. (A) Representative images and (B and C) quantification of pSTAT3Y705 immunostaining in kidneys from 12-week-old control, iKif3aΔTub, iPkd1ΔTub, and iPkd1ΔTub; iKif3aΔTub mice (6 weeks after the completion of doxycycline treatment). Scale bar, 50 µm. Each dot represents one individual mouse. One-way ANOVA followed by Tukey–Kramer test, **P<0.01, ***P<0.001. (D) Representative images of STAT3, F4/80, and CD3 staining on serial sections from 12-week-old control iPkd1ΔTub (n=3). Scale bar, 100 µm.
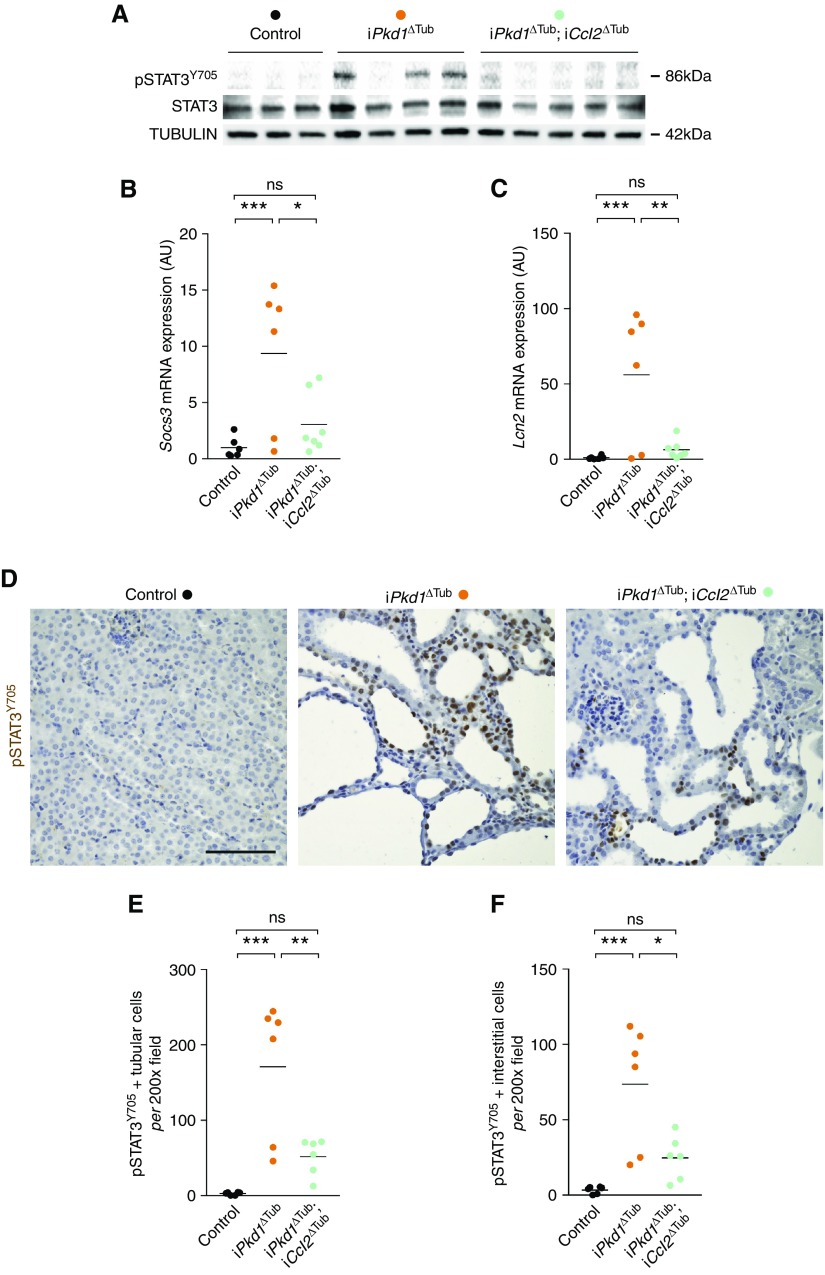
We then investigated the mechanisms allowing cilia-dependent STAT3 activation in vivo. Although the identification of STAT3 as a component of the primary cilia proteome renders a direct regulation of STAT3 by primary cilia plausible, primary cilia have been shown to orchestrate an inflammatory response through the induction of CCL2 in tubular cells. In the heart, CCL2 induces macrophage recruitment, which in turn activates STAT3 in cardiomyocytes through paracrine signaling.26,27 We evaluated the possibility of such a noncell-autonomous pathway to tubular STAT3 activation in vivo. We analyzed the effect of tubule-specific Ccl2 disruption on STAT3 in the polycystic kidney. We and others have previously shown that Ccl2 disruption reduced renal macrophage infiltration and cyst burden in polycystic kidneys.7,10 Indeed, Western blot of kidney homogenates revealed that Ccl2 inactivation strongly reduced renal STAT3Y705 phosphorylation (Figure 2A). In line with this observation, Ccl2 inactivation also reduced the mRNA abundance of the STAT3 transcriptional targets Socs3 and Lcn2, the expression of which correlated with the amount of phosphorylated STAT3 (Figure 2, B and C, Supplemental Figure 1). Immunolabeling confirmed that Ccl2 inactivation markedly reduced both interstitial and tubular STAT3 phosphorylation (Figure 2, D–F). Residual STAT3 activation in renal tubules was detected in the kidneys of iPkd1ΔTub; iCcl2ΔTub mice, but to a level that was not statistically different from control kidneys. To investigate if primary cilia can directly modulate STAT3 activation independently of immune cell recruitment, we analyzed STAT3 phosphorylation in tetracycline-inducible, Kif3a-silenced (Kif3a-i) or Ift88-silenced (Ift88-i) MDCK cells. We found that tetracycline treatment, which results in cilia ablation in both cell lines,28,29 modestly but significantly reduced STAT3 phosphorylation as compared with control cells expressing an inducible short hairpin RNA (shRNA)–targeting luciferase (Luci-i; Supplemental Figure 2, A and B).
Figure 2.
Ccl2 expression by tubular cells is required for STAT3 activation in response to Pkd1 disruption. (A) Western blot of STAT3 phosphorylation in kidney lysates from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iCcl2ΔTub mice (7 weeks after the completion of doxycycline treatment). Each lane indicates one individual mouse. (B and C) Quantification of (B) Socs3 and (C) Lcn2 mRNA abundance in kidneys from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iCcl2ΔTub mice. (D) Representative images and (E and F) quantification of pSTAT3Y705 immunostaining in kidneys from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iCcl2ΔTub mice. Scale bar, 50 µm. (B, C, E, and F) Each dot represents one individual mouse. One-way ANOVA followed by Tukey–Kramer test, *P<0.05, **P<0.01, ***P<0.001. AU, arbitrary units.
Collectively, these results indicate that, although primary cilia are direct positive regulators of STAT3 phosphorylation on Y705 in vitro, cilia-dependent STAT3 activation in polycystic kidneys is mainly mediated through paracrine mechanisms requiring CCL2 expression by tubular cells. Although CCL2’s main function in ADPKD is macrophages recruitment, we cannot totally exclude that distinct cell types expressing CCL2 receptors (CCR2 and CCR4) also contribute to STAT3 activation in tubular cells.
Tubule-Specific Stat3 Inactivation Does Not Prevent Precystic Kidney Growth in Response to Pkd1 Disruption
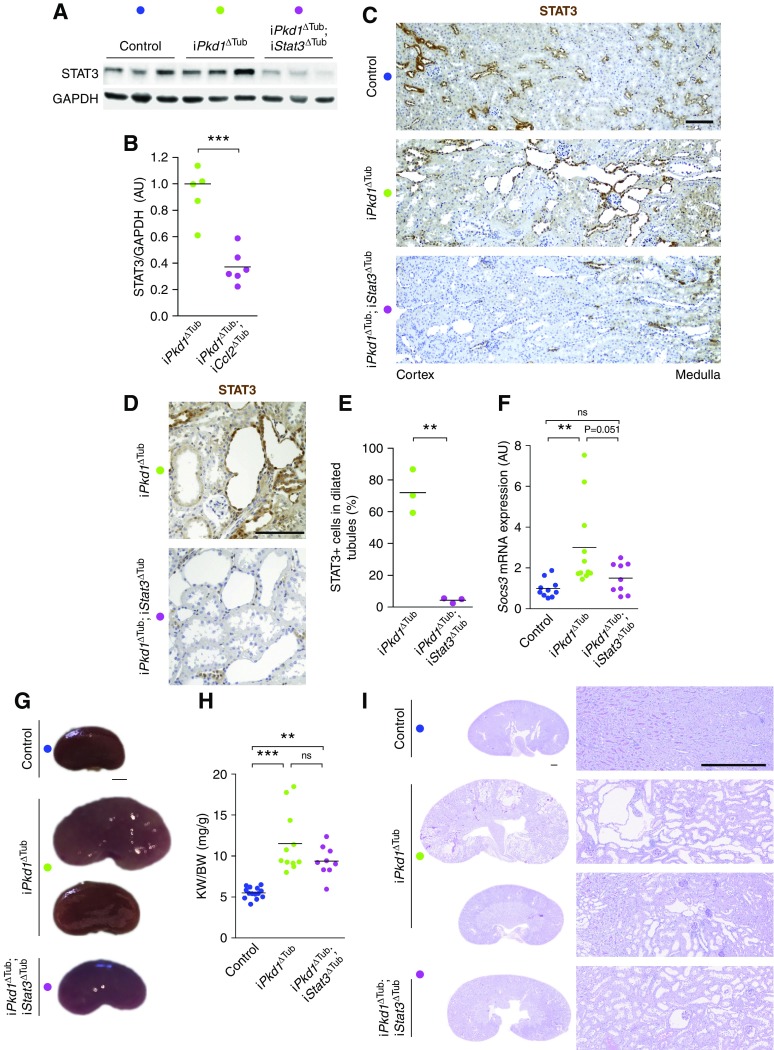
How CCL2-dependent immune cell recruitment promotes cyst growth remains unclear in ADPKD. Because STAT3 activation in tubular cells has been proposed to promote cyst growth, we investigated whether STAT3 activation in renal tubules is instrumental in ADPKD progression. To this end, we crossed iPkd1Δtub with Stat3flox/flox mice and derived mice to allow for the inactivation of Pkd1 alone (iPkd1Δtub) or in association with Stat3 (iPkd1Δtub; iStat3Δtub) specifically in tubular cells upon doxycycline treatment. At 13 weeks of age (7 weeks after the completion of doxycycline treatment), Western blot analysis of kidney homogenates revealed a significant reduction of STAT3 protein in kidneys from iPkd1Δtub; iStat3Δtub mice as compared with iPkd1Δtub mice (Figure 3, A and B). STAT3 immunolabeling confirmed the loss of STAT3 protein in renal tubular cells from iPkd1Δtub; iStat3Δtub mice (Figure 3C). In line with the reported activity of our inducible system, which incompletely targets distal convoluted tubule and the straight part of the proximal tubule,4 STAT3 expression was preserved in some tubular sections of the cortex and the outer medulla (Figure 3C). This notwithstanding, STAT3 expression was drastically reduced in dilated tubules from iPkd1Δtub; iStat3Δtub mice (Figure 3, D and E). In line with this observation, Stat3 tubular inactivation in polycystin-deficient mice markedly lessened Socs3 induction (Figure 3F). At this time point, both iPkd1Δtub and iPkd1Δtub; iStat3Δtub mice displayed kidney enlargement but overt polycystic kidneys was only observed in few iPkd1Δtub mice (Figure 3, G–I). Although iPkd1Δtub; iStat3Δtub kidneys tend to be smaller and less variable than iPkd1Δtub kidneys, this difference did not reach statistical significance. Collectively, these results demonstrate that Stat3 is dispensable for ADPKD early kidney growth.
Figure 3.
Tubule-specific Stat3 inactivation does not prevent early kidney growth in response to Pkd1 disruption. (A and B) Representative (A) Western blot and (B) quantification of STAT3 in kidney homogenates from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice (7 weeks after the completion of doxycycline treatment). Each lane indicates one individual mouse. Bars are mean±SEM of six mice per group. Two-tailed t test, ***P<0.0001. (C) STAT3 immunostaining of kidney sections from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Representative images of three mice per group. Scale bar, 100 µm. (D) Representative images and (E) quantification of STAT3 immunostaining in tubular cysts from 13-week-old iPkd1ΔTub and iPkd1ΔTub; iStat3ΔTub mice. (F) Quantification of Socs3 mRNA abundance in kidneys from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. (G) Representative kidneys from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Scale bar, 2 mm. (H) Kidney weight to body weight ratio (KW/BW) at 13 weeks. (I) Representative PAS-stained kidney sections from 13-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice at 13 weeks. Scale bar, 0.5 mm. Each dot represents one individual mouse. One-way ANOVA followed by Tukey–Kramer test, **P<0.01, ***P<0.001. AU, arbitrary units.
Tubule-Specific Stat3 Inactivation Does Not Prevent Cystic Kidney Growth nor Kidney Function Decline in Response to Pkd1 Disruption
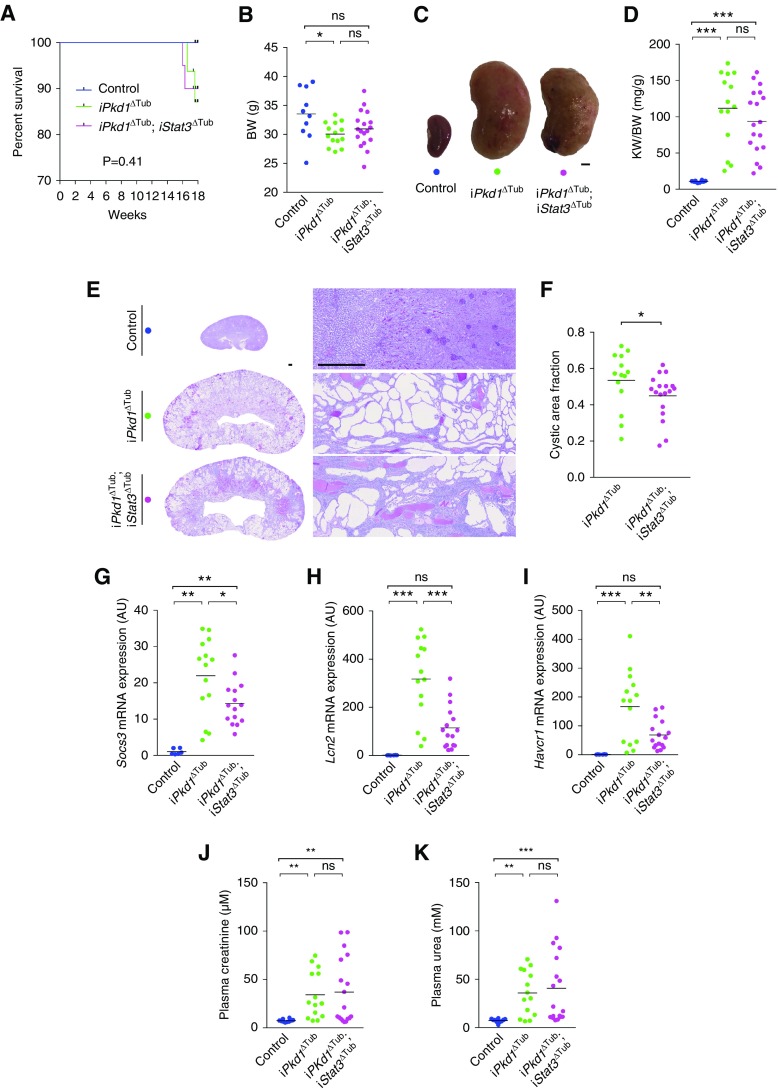
To assess the effect of loss of Stat3 function on overt cystogenesis and kidney function decline, we let iPkd1Δtub and iPkd1Δtub; iStat3Δtub mice age for 18 weeks (12 weeks after the completion of doxycycline treatment). Two of 16 iPkd1Δtub and two of 20 iPkd1Δtub; iStat3Δtub mice but none of the control mice died before the completion of the study (between 16 and 18 weeks; Figure 4A). iPkd1Δtub and iPkd1Δtub; iStat3Δtub mice displayed a parallel modest decline in body weight in comparison with control mice at euthanasia (Figure 4B). Macroscopic inspection revealed more irregular kidneys in iPkd1Δtub; iStat3Δtub than in iPkd1Δtub mice (Figure 4C), but overall both iPkd1Δtub and iPkd1Δtub; iStat3Δtub mice developed similar massive kidney enlargement as compared with control mice (Figure 4D). Microscopic inspection of the kidneys revealed that Stat3 deletion resulted in a slight but significant decrease of cystic area (Figure 4, E and F). In line with this modest effect on cyst burden, Stat3 disruption not only reduced the expression of the STAT3 transcriptional targets Socs3 and Lcn2, but also decreased the expression of Havcr1 transcript, which encodes KIM-1, a marker of tubular injury that positively correlates with cyst burden in patients with ADPKD (Figure 4, G–I).30 This slight reduction of cyst burden in Stat3 mutant animals was not associated with decreased tubular cell proliferation or increased apoptosis (Supplemental Figure 3). In spite of this reduction in cyst burden, both iPkd1Δtub and iPkd1Δtub; iStat3Δtub mice displayed a similar increase in plasma creatinine and urea compared with control mice (Figure 4, J and K), indicating that Stat3 tubular deletion did not prevent polycystic kidney function decline. Of note, the more severe reductions in kidney function were observed in iPkd1Δtub; iStat3Δtub animals (Figure 4, J and K). These results demonstrate that tubular STAT3 plays only a marginal role in cyst growth in ADPKD and that Stat3 tubular deletion does not prevent kidney function decline.
Figure 4.
Tubule-specific Stat3 inactivation does not prevent cystic kidney growth nor kidney function decline in response to Pkd1 disruption. (A) Kaplan–Meier survival curves of control, iPkd1ΔTub (n=16), and iPkd1ΔTub; iStat3ΔTub (n=20) mice. (B) Body weight (BW) of 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub animals (12 weeks after the completion of doxycycline treatment). (C) Representative kidneys from the same animals. Scale bar, 2 mm. (D) Kidney weight to body weight ratio (KW/BW) at 18 weeks. (E) Representative PAS staining of kidneys sections and (F) measurement of cysts area fraction from 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Scale bar, 0.5 mm. Mann–Whitney test, * P<0.05. Quantification of (G) Socs3, (H) Lcn2, and (I) Havcr1 mRNA abundance in kidneys from 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Measurement of (J) plasma creatinine and (K) urea from 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub animals. (B, D, F, G–K) Each dot represents one individual mouse. (B, D, G–K) One-way ANOVA followed by Tukey–Kramer test, *P<0.05, **P<0.01, ***P<0.001. AU, arbitrary units.
Tubule-Specific Stat3 Inactivation Increases Polycystic Kidney Inflammation
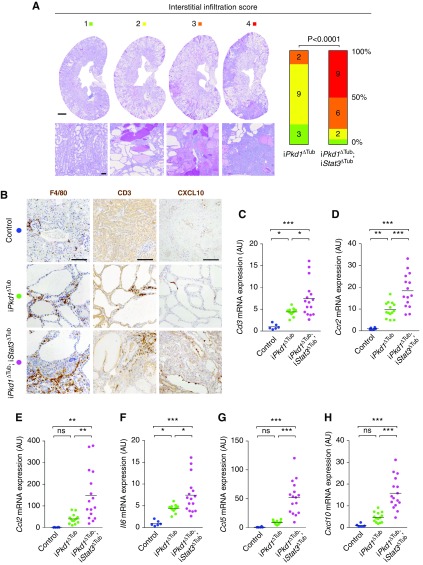
The fact that iPkd1Δtub; iStat3Δtub mice presented with severe renal function impairment in spite of a reduction in cyst burden suggested that Stat3 deletion could somehow worsen kidney damage without accelerating cyst growth. Indeed, we were surprised to observe a notable increase in interstitial inflammation in iPkd1Δtub; iStat3Δtub mice (Figure 4E). Repeated semiquantitative, blinded assessment of the severity of the inflammatory infiltrates confirmed this difference (Figure 5A). Immunolabeling identified both T cells (CD3+) and macrophages (F4/80+) in the large inflammatory areas of iPkd1Δtub; iStat3Δtub mice, whereas neutrophils (LY6B+) were rarely observed (Figure 5B, Supplemental Figure 4A). Consistently, we observed that Stat3 inactivation was associated with a significant increase in the renal abundance of Cd3 (expressed by all T cells), Cd4 and Cd8 (mainly expressed by CD4 and CD8 T cells, respectively), and Ccr2 (expressed by infiltrating macrophages) transcripts (Figure 5, C and D, Supplemental Figure 4, B and C).
Figure 5.
Tubule-specific Stat3 inactivation increases polycystic kidney inflammation. (A) Representative examples of interstitial inflammation scoring and distribution of renal interstitial inflammation scores in PAS-stained kidneys from 18-week-old iPkd1ΔTub and iPkd1ΔTub; iStat3ΔTub mice. Numbers in the bars indicate number of mice per score; P is the P value from Mann–Whitney test. (B) Representative images of F4/80, CD3, and CXCL10 immunostaining of kidney sections from 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Scale bar, 100 µm. (C–H) Quantification of (C) Cd3, (D) Ccr2, (E) Ccl2, (F) Il6, (G) Ccl5, and (H) Cxcl10 mRNA abundance in kidneys from 18-week-old control, iPkd1ΔTub, and iPkd1ΔTub; iStat3ΔTub mice. Each dot represents one individual mouse. One-way ANOVA followed by Tukey–Kramer test, *P<0.05, **P<0.01, ***P<0.001.
A heightened inflammatory response has been observed after STAT3 ablation in hematopoietic cells, osteoblasts, and keratinocytes.31,32 In macrophages, the proinflammatory consequences of Stat3 deletion are linked to an excessive activation of NF-κB signaling resulting in increased expression of NF-κB target genes.33 We therefore investigated the effect of Stat3 deletion on the expression of selected NF-κB targets. Whereas Pkd1 loss of function led to an increase in the abundance of Ccl2, Il6, Ccl5, and Cxcl10 transcripts, Stat3 inactivation amplified this phenomenon (Figure 5, E–H). This was particularly evident for Ccl5 and Cxcl10, whose expression was six- and threefold higher in iPkd1Δtub; iStat3Δtub than in iPkd1Δtub kidneys, respectively. CXCL10 immunostaining localized maximal CXCL10 expression to tubular cells of iPkd1Δtub; iStat3Δtub kidneys (Figure 5B). In contrast, we did not detect any significant difference in Tnfa, Il1b, or Cxcl3 mRNA abundance between iPkd1Δtub and iPkd1Δtub; iStat3Δtub kidneys (Supplemental Figure 4, D–F), indicating that Stat3 deletion led to the upregulation of specific inflammatory mediators. In macrophages, STAT3 anti-inflammatory effects have been linked to the transcriptional repression of the E2 ubiquitin–conjugating enzyme UB2N, which stabilizes NF-κB signaling, resulting in the nuclear accumulation of the transcription factor RELA.32 However, contrary to its published effect in macrophages, tubule-specific Stat3 inactivation was not associated with an increase in Ub2n mRNA abundance in Pkd1-deficient kidneys, suggesting alternative mechanisms (Supplemental Figure 4G).
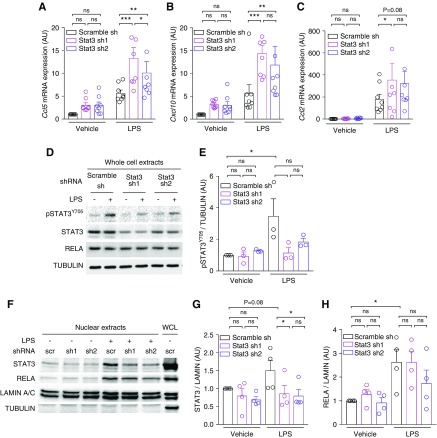
To investigate if STAT3 directly functions as a repressor of NF-κB–induced proinflammatory cytokine in tubular cells, we analyzed the effect of STAT3 depletion on the induction of Il6, Ccl2, Ccl5, and Cxcl10 expression by LPS, a major activator of NF-κB, in tubular mIMCD-3 cells. Although we were not able to detect Il6 transcript in those cells, LPS treatment induced a robust induction of Ccl2, Ccl5, and Cxcl10 transcripts (Figure 6, A–C). Strikingly, STAT3 depletion led to a proportional increase in the abundance of Ccl5 and Cxcl10 mRNA in response to LPS (Figure 6, A and B). Ccl2 mRNA levels showed the same trend as Ccl5 and Cxl10, but a statistically significant increase was restricted to the cell line with a profound STAT3 depletion (Figure 6C). Interestingly, LPS treatment led to STAT3 phosphorylation and nuclear accumulation, which was reduced by STAT3 depletion (Figure 6, D–G). Contrary to macrophages, STAT3 depletion in mIMCD-3 did not increase the nuclear accumulation of RELA (Figure 6, F and G). These results indicate that STAT3 dampens Ccl5 and Cxcl10 induction by LPS without affecting RELA nuclear accumulation. Collectively, these results indicate that STAT3 represses the expression of proinflammatory cytokines and restricts immune cell infiltration in ADPKD.
Figure 6.
Stat3 inactivation in tubular cells increases inflammatory cytokine transcriptional response. (A–C) Quantification of (A) Ccl5, (B) Cxcl10, and (C) Ccl2 mRNA abundance in mIMCD3 cells stably expressing shRNAs targeting STAT3 (sh1 and sh2) or a scramble control shRNA (scr) treated with LPS or vehicle for 24 hours. Bars are mean±SEM of eight independent experiments. (D) Representative Western blot and (E) quantification of three independent experiments of STAT3 phosphorylation and RELA expression in whole-cell lysates from mIMCD3 cells expressing the indicated shRNAs, 24 hours after LPS or vehicle treatment. (F) Representative Western blot and quantification of four independent experiments of (G) STAT3 and (H) RELA expression in nuclear extracts from mIMCD3 cells expressing the indicated shRNAs, 24 hours after LPS or vehicle treatment. Whole-cell lysates (WCL) served as a positive control for tubulin labeling. (A–C, E, G, and H). Dots represent independent experiments. Two-way paired ANOVA followed by Tukey–Kramer test, *P<0.05, **P<0.01, ***P<0.001.
Discussion
Although multiple intrinsic defects in tubular cell homeostasis are observed in ADPKD,34–36 accumulating evidence demonstrates that interstitial inflammation also plays an important role in the disease.6–8,10,37,38 Yet the nature of the factors mediating communication between tubular and immune cells and their effect on disease expression are not well characterized. Two distinct pathways have been shown to promote macrophage accumulation in polycystic kidneys. First, polycystin deficiency triggers monocyte migration to the kidney through the induction of CCL2.7,10 Second, kidney injury has been shown to promote cyst progression by triggering CSF1-dependent resident macrophage proliferation.37 In turn, infiltrating macrophages drive tubular cell proliferation, possibly by releasing arginine metabolites.8 In this context, our results unravel an unanticipated anti-inflammatory feedback loop orchestrated by STAT3 in ADPKD.
Previous reports have documented a robust activation of STAT3 in cyst-lining epithelial cells from patients with ADPKD and related murine models.19,20,39 Although in vitro results suggested that cleaved polycystin 1 may directly activate STAT3,39,40 the mechanisms governing STAT3 activation in cystic tubular cells remained poorly understood. Our results indicate that the bulk of STAT3 activation in response to Pkd1 disruption in vivo occurs through a noncell-autonomous process requiring a cilia-dependent induction of CCL2. Acting on its receptor CCR2, CCL2 is a major driver of macrophage recruitment, which occurs at an early step of the disease.7,8,38 Thus, our results imply that infiltrating macrophages directly or indirectly promote STAT3 activation in tubular cells. Such noncell-autonomous activation of STAT3 by neighboring macrophages has been documented in distinct settings including cancers,41–45 heart failure,27 or pancreatitis.46 Different molecular pathways have been reported, including the direct expression of paracrine activator of STAT3 such as oncostatin M,27 IL-6,47 IL-8,42 and TGF-β,46 or the indirect upregulation of STAT3-activating receptors,48 mirroring the multiplicity of STAT3 activation mechanisms.
In most extrarenal settings, paracrine activation of STAT3 by macrophages plays a detrimental effects in the disease, promoting tissue injury27 or tumor growth/metastasis.41–45 In contrast to these situations, our data indicate that tubular Stat3 is mostly dispensable for cyst growth in ADPKD, but dampens immune cell infiltration of polycystic kidneys. The absence of a notable effect of Stat3 tubular deletion on kidney enlargement and kidney function decline in our model sharply contrasts with the positive effect of STAT3 inhibitory compounds previously reported in Pkd1 mutant mice.19,20 This discrepancy may be caused by off-target effects or the consequence of inadequate target specificity of these drugs.20,21,49 Alternatively, the beneficial effect of these drugs may be linked to Stat3 inhibition in nontubular cells. Indeed, pericystic interstitial cells also display STAT3 activation in ADPKD. Importantly, different interstitial cell types have been shown to affect cyst growth, including macrophages6,8 and CD8+ T cells.38 Interestingly, STAT3 activation has been reported in pericystic macrophages in ADPKD and STAT3 inhibitory compounds reduce the ability of cultured macrophages to induce tubular cell proliferation through paracrine signaling in vitro.50 It is therefore plausible that the therapeutic effects of STAT3 inhibitory drugs are linked to the inhibition of STAT3 in macrophages. However, the proper evaluation of STAT3 functions in macrophages in ADPKD represents a complicated task: conditional Stat3 inactivation in macrophages leads to the spontaneous development of inflammatory bowel disease precluding the use of such an approach in a murine model of ADPKD.51 Thus, our results do not rule out that STAT3 inhibition may have a benefit in patients with ADPKD through its effect on nontubular cells.
Unexpectedly, we observed that Stat3 deletion in renal tubules markedly increased immune cell infiltration of polycystic kidneys. Mechanistically, STAT3 depletion resulted in the upregulation of Ccl5 and Cxcl10 transcripts in polycystic kidneys and in cultured tubular cells. Although surprising, this observation is reminiscent of the well characterized anti-inflammatory functions of STAT3 in macrophages. In those cells, STAT3 activation in response to IL-10 restricts NF-κB–dependent proinflammatory signaling.31,32 The molecular bases of this anti-inflammatory effect involves the transcriptional regulation of factor(s) that modulate(s) NF-κB signaling.31 More specifically, the transcriptional repression of UB2N ubiquitin ligase by STAT3 appears to play a major role in restricting NF-κB signaling in macrophages.32 Indeed, in macrophages, Stat3 inactivation increases UB2N abundance, which in turn stabilizes NF-κB signaling and promotes RELA nuclear accumulation. Of note, STAT3 anti-inflammatory functions have also been documented in nonimmune cells. Indeed, the deletion of STAT3 in intestinal epithelial cells leads to an exacerbated inflammatory response after intestinal injury.17 We did not fully investigate the underlying mechanisms of STAT3 anti-inflammatory function in tubular cells, but our results indicate some specificities: contrary to STAT3 anti-inflammatory functions in macrophages, the anti-inflammatory effect of STAT3 in tubular cells is not mediated by the repression of Ub2n transcript, nor is it associated with RELA nuclear accumulation. Although the molecular intermediates allowing STAT3 to restrict polycystic kidney inflammation remain unclear, our results clearly expand STAT3 anti-inflammatory function to tubular cells. They further suggest that this apparently conserved function of STAT3 is related to distinct tissue-specific mechanisms.
A striking aspect of our study is that tubular Stat3 disruption decoupled renal inflammation from cyst growth. Indeed, previous studies have consistently shown that reducing renal inflammation through macrophage depletion or CCL2/CCR2 inhibition led to a proportional decrease in cyst burden. Why increased macrophage infiltration does not sustain cyst growth in Stat3-deficient animals remain unclear. It may indicate a direct effect of STAT3 on tubular cell proliferation, as suggested by some in vitro studies.19,52 Considering the role played by CD8+ T cells in restricting cyst growth,38 the massive increase in T cell infiltration induced by Stat3 inactivation could also be involved. Of note, the marked renal infiltration by immune cells observed in iPkd1Δtub; iStat3Δtub mice may contribute to nephron loss and explain why the reduction in cyst burden and tubular injury markers observed in response to Stat3 disruption does not translate into improved renal function.
Stemming from our results, we propose a novel model for STAT3 function in ADPKD: cilia-dependent CCL2 expression promotes immune cell recruitment, which in turn induces directly or indirectly STAT3 phosphorylation in tubular cells. Although STAT3 activation is dependent on both cilia and CCL2, which are drivers of cyst growth, STAT3 is, however, not essential for disease progression but functions in a feedback loop that restrains kidney inflammation by downregulating inflammatory cytokines (see visual abstract). It should be mentioned that, because our experiments were conducted on males, we cannot formally exclude sex-specific functions for STAT3 in the disease.
The identification of V2R inhibition as a valid target to reduce cyst growth in human has opened the area of specific therapy in ADPKD.53,54 Although this represents an important cornerstone on the road to cure the disease, V2R inhibition has only a modest efficacy and important side effects. Thus, the search for more potent and better tolerated drugs remains central to the field.55 Over the last 20 years, joint research efforts allowed the identification of a large panel of pathways that are activated in growing cysts, thereby providing multiple potential target for molecular targeted therapies in ADPKD.56 Although concordant evidence identified STAT3 as an interesting candidate, robust evaluation of STAT3 activation mechanisms and functions in ADPKD was lacking. In this context, our study, which relied on a state-of-the-art model of tubular-specific inactivation of Stat3 and Pkd1 in adult mice, clearly indicates that tubular STAT3 is not a critical driver of cyst formation. More generally, our results exemplify the critical contribution of complex genetic conditional models to the rigorous evaluations of potential therapeutic targets in ADPKD.
Disclosures
None.
Funding
Dr. Bienaimé and Dr. Terzi were supported by Institut National de la Santé et de la Recherche Médicale, Université de Paris, Assistance Publique - Hôpitaux de Paris, and Agence Nationale de la Recherche grants 16-CE14-0023-01 and 19-CE14-0016-01. Dr. Kuehn was supported by Deutsche Forschungsgemeinschaft grant 1504/5-1. Dr. Viau was supported by Fondation pour la Recherche Médicale grant ARF20150934110.
Supplementary Material
Acknowledgments
We thank the animal facility (Laboratoire d'Expérimentation Animale et de Transgénèse, Société Fédérative de Recherche Necker, INSERM US24, Paris, France), the histology facility (Société Fédérative de Recherche Necker, INSERM US24, Paris, France), and the mice physiology facility (Cordelier Research Center, Paris, France) for technical assistance.
Dr. Bienaimé and Dr. Viau designed the study; Dr. Baaziz, Dr. Bienaimé, Dr. Mazloum, Mr. Nguyen, and Dr. Viau performed experiments and analyzed data; Dr. Bienaimé, Dr. Kuehn, Dr. Terzi, and Dr. Viau drafted and revised the manuscript; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019090959/-/DCSupplemental.
Supplemental Figure 1. Correlation between STAT3 phosphorylation and Socs3 and Lcn2 mRNA.
Supplemental Figure 2. Primary cilia promote STAT3 phosphorylation in tubular cells in vitro.
Supplemental Figure 3. STAT3 ablation does not reduce cell proliferation or increase apoptosis.
Supplemental Figure 4. STAT3 disruption has a selective impact on renal inflammation in ADPKD.
Supplemental Table 1. Primers used for RT-qPCR.
References
- 1.Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al.: A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol 29: 2458–2470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong ACM, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases: Autosomal dominant polycystic kidney disease: the changing face of clinical management [published correction appears in Lancet 385: 2576, 2015]. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Tan AY, Zhang T, Michaeel A, Blumenfeld J, Liu G, Zhang W, et al. : Somatic mutations in renal cyst epithelium in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 2139–2156, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonhard WN, Zandbergen M, Veraar K, van den Berg S, van der Weerd L, Breuning M, et al.: Scattered deletion of PKD1 in kidneys causes a cystic snowball effect and recapitulates polycystic kidney disease. J Am Soc Nephrol 26: 1322–1333, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, et al.: Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassini MF, Kakade VR, Kurtz E, Sulkowski P, Glazer P, Torres R, et al.: Mcp1 promotes macrophage-dependent cyst expansion in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 2471–2481, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Chen M, Zhou J, Lv J, Song S, Fu L, et al. : Interactions between macrophages and cyst-lining epithelial cells promote kidney cyst growth in Pkd1-deficient mice. J Am Soc Nephrol 29: 2310–2325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, et al.: Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viau A, Bienaimé F, Lukas K, Todkar AP, Knoll M, Yakulov TA, et al.: Cilia-localized LKB1 regulates chemokine signaling, macrophage recruitment, and tissue homeostasis in the kidney. EMBO J 37: e98615, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joly D, Morel V, Hummel A, Ruello A, Nusbaum P, Patey N, et al.: Beta4 integrin and laminin 5 are aberrantly expressed in polycystic kidney disease: Role in increased cell adhesion and migration. Am J Pathol 163: 1791–1800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayakumar S, Dang S, Marinkovich MP, Lazarova Z, Yoder B, Torres VE, et al.: Aberrant expression of laminin-332 promotes cell proliferation and cyst growth in ARPKD. Am J Physiol Renal Physiol 306: F640–F654, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, et al.: Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 5: 5482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS: ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol 168: 1288–1298, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillmer EJ, Zhang H, Li HS, Watowich SS: STAT3 signaling in immunity. Cytokine Growth Factor Rev 31: 1–15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarnicki A, Putoczki T, Ernst M: Stat3: linking inflammation to epithelial cancer - more than a “gut” feeling? Cell Div 5: 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willson TA, Jurickova I, Collins M, Denson LA: Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm Bowel Dis 19: 512–525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, et al.: Proteomics of primary cilia by proximity labeling. Dev Cell 35: 497–512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, et al.: Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, et al. : Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Ball DP, Lewis AM, Williams D, Resetca D, Wilson DJ, Gunning PT: Signal transducer and activator of transcription 3 (STAT3) inhibitor, S3I-201, acts as a potent and non-selective alkylating agent. Oncotarget 7: 20669–20679, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, et al.: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al.: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bienaimé F, Muorah M, Yammine L, Burtin M, Nguyen C, Baron W, et al.: Stat3 controls tubulointerstitial communication during CKD. J Am Soc Nephrol 27: 3690–3705, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, et al.: Protein overload stimulates RANTES production by proximal tubular cells depending on NF-κ B activation. Kidney Int 53: 1608–1615, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, et al.: Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol 152: 101–111, 1998 [PMC free article] [PubMed] [Google Scholar]
- 27.Richter M, Lautze H-JJ, Walther T, Braun T, Kostin S, Kubin T: The failing heart is a major source of circulating FGF23 via oncostatin M receptor activation. J Heart Lung Transplant 34: 1211–1214, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, et al.: Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12: 1115–1122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehlke C, Janusch H, Hamann C, Powelske C, Mergen M, Herbst H, et al.: A cilia independent role of Ift88/polaris during cell migration. PLoS One 10: e0140378, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petzold K, Poster D, Krauer F, Spanaus K, Andreisek G, Nguyen-Kim TDL, et al.: Urinary biomarkers at early ADPKD disease stage. PLoS One 10: e0123555, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, et al.: General nature of the STAT3-activated anti-inflammatory response. J Immunol 177: 7880–7888, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Hu H, Greeley N, Jin J, Matthews AJ, Ohashi E, et al.: STAT3 restrains RANK- and TLR4-mediated signalling by suppressing expression of the E2 ubiquitin-conjugating enzyme Ubc13. Nat Commun 5: 5798, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchins AP, Diez D, Miranda-Saavedra D: The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief Funct Genomics 12: 489–498, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, et al.: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Boctor S, Barisoni LMC, Gusella GL: Inactivation of integrin-β1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J Am Soc Nephrol 26: 888–895, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al.: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman KA, Song CJ, Li Z, Lever JM, Crossman DK, Rains A, et al. : Tissue-resident macrophages promote renal cystic disease. J Am Soc Nephrol 30: 1841–1856, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleczko EK, Marsh KH, Tyler LC, Furgeson SB, Bullock BL, Altmann CJ, et al.: CD8+ T cells modulate autosomal dominant polycystic kidney disease progression. Kidney Int 94: 1127–1140, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talbot JJ, Song X, Wang X, Rinschen MM, Doerr N, LaRiviere WB, et al.: The cleaved cytoplasmic tail of polycystin-1 regulates Src-dependent STAT3 activation. J Am Soc Nephrol 25: 1737–1748, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, et al.: Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A 108: 7985–7990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang S, Liu Z, Yang L, Liu J, Xiu M: Increased Six1 expression in macrophages promotes hepatocellular carcinoma growth and invasion by regulating MMP-9. J Cell Mol Med 23: 4523–4533, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Valeta-Magara A, Gadi A, Volta V, Walters B, Arju R, Giashuddin S, et al. : Inflammatory breast cancer promotes development of M2 tumor-associated macrophages and cancer mesenchymal cells through a complex chemokine network. Cancer Res 79: 3360–3371, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo L, Cheng X, Chen H, Chen C, Xie S, Zhao M, et al.: Induction of breast cancer stem cells by M1 macrophages through Lin-28B-let-7-HMGA2 axis. Cancer Lett 452: 213–225, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Li E, Xu Z, Zhao H, Sun Z, Wang L, Guo Z, et al.: Macrophages promote benzopyrene-induced tumor transformation of human bronchial epithelial cells by activation of NF-κB and STAT3 signaling in a bionic airway chip culture and in animal models. Oncotarget 6: 8900–8913, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu X-T, Dai Z, Song K, Zhang Z-J, Zhou Z-J, Zhou S-L, et al.: Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol 46: 587–596, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Xiao X, Fischbach S, Zhang T, Chen C, Sheng Q, Zimmerman R, et al.: SMAD3/Stat3 signaling mediates β-Cell epithelial-mesenchymal transition in chronic pancreatitis-related diabetes. Diabetes 66: 2646–2658, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao S, Hu J, Wu X, Liang Z: PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/β-catenin signaling pathway. Biomed Pharmacother 108: 618–624, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Yao R-R, Li J-H, Zhang R, Chen R-X, Wang Y-H: M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol 16: 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepist EI, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, et al.: Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int 86: 350–357, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peda JD, Salah SM, Wallace DP, Fields PE, Grantham CJ, Fields TA, et al. : Autocrine IL-10 activation of the STAT3 pathway is required for pathological macrophage differentiation in polycystic kidney disease. Dis Model Mech 9: 1051–1061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugimoto K: Role of STAT3 in inflammatory bowel disease. World J Gastroenterol 14: 5110–5114, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M: Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci Rep 9: 4491, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torres VE, Higashihara E, Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, et al.; TEMPO 3:4 Trial Investigators: Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: Results from the TEMPO 3:4 trial. Clin J Am Soc Nephrol 11: 803–811, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. ; REPRISE Trial Investigators: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, et al.: Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: A position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31: 337–348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.