Abstract
Growing evidence indicates that oxidative and endoplasmic reticular stress, which trigger changes in ion channels and inflammatory pathways that may undermine cellular homeostasis and survival, are critical determinants of injury in the diabetic kidney. Cells are normally able to mitigate these cellular stresses by maintaining high levels of autophagy, an intracellular lysosome-dependent degradative pathway that clears the cytoplasm of dysfunctional organelles. However, the capacity for autophagy in both podocytes and renal tubular cells is markedly impaired in type 2 diabetes, and this deficiency contributes importantly to the intensity of renal injury. The primary drivers of autophagy in states of nutrient and oxygen deprivation—sirtuin-1 (SIRT1), AMP-activated protein kinase (AMPK), and hypoxia-inducible factors (HIF-1α and HIF-2α)—can exert renoprotective effects by promoting autophagic flux and by exerting direct effects on sodium transport and inflammasome activation. Type 2 diabetes is characterized by marked suppression of SIRT1 and AMPK, leading to a diminution in autophagic flux in glomerular podocytes and renal tubules and markedly increasing their susceptibility to renal injury. Importantly, because insulin acts to depress autophagic flux, these derangements in nutrient deprivation signaling are not ameliorated by antihyperglycemic drugs that enhance insulin secretion or signaling. Metformin is an established AMPK agonist that can promote autophagy, but its effects on the course of CKD have been demonstrated only in the experimental setting. In contrast, the effects of sodium-glucose cotransporter–2 (SGLT2) inhibitors may be related primarily to enhanced SIRT1 and HIF-2α signaling; this can explain the effects of SGLT2 inhibitors to promote ketonemia and erythrocytosis and potentially underlies their actions to increase autophagy and mute inflammation in the diabetic kidney. These distinctions may contribute importantly to the consistent benefit of SGLT2 inhibitors to slow the deterioration in glomerular function and reduce the risk of ESKD in large-scale randomized clinical trials of patients with type 2 diabetes.
Keywords: diabetic nephropathy, antihyperglycemic drugs, autophagy
Type 2 diabetes is accompanied by an inexorable decline in renal function, but the pathogenesis of diabetic CKD is not fully understood. Classically, physicians have long believed that hyperglycemia triggers changes in intrarenal hemodynamics that lead to glomerular hyperfiltration and injury.1 This conceptual framework underpins the use of drugs that inhibit the renin-angiotensin system, which reduce intraglomerular pressures, presumably by acting to reduce efferent arteriolar tone.2 Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors have favorable effects on the development and progression of CKD,3 but renal function still deteriorates in patients with diabetes despite the use of these drugs.
Sodium-glucose cotransporter–2 (SGLT2) inhibitors exert striking effects to slow the deterioration of glomerular function and reduce the likelihood of progression to ESKD in patients with diabetes already receiving inhibitors of the renin-angiotensin system.4 SGLT2 inhibitors also can lower intraglomerular filtration pressures, which has been attributed to their influence on the proximal reabsorption of sodium.5 It has been proposed that this action of SGLT2 inhibitors (via tubuloglomerular feedback) negates the effects of hyperglycemia, producing excessive afferent arteriolar vasodilatation.6 However, this hypothesis has been recently disputed,7 and, experimentally, knockout of SGLT2 attenuates glomerular hyperfiltration without preventing renal injury.8 Importantly, SGLT2 inhibitors exert favorable effects on the course of diabetic CKD at GFR levels that abolish the actions of these drugs on glycosuria and their effects to reduce glomerular filtration pressures.9,10 These observations have raised important questions concerning the central role of intrarenal hemodynamic events in mediating the effects of SGLT2 inhibitors on the development of diabetic CKD.
In light of this uncertainty, growing evidence suggests that a critical determinant of injury in the diabetic kidney is oxidative and endoplasmic reticulum stress.11 Oxidative stress follows the overproduction of reactive oxygen species that can result from the deleterious effects of hyperglycemia and glucose intermediates on the structural and functional integrity of mitochondria and peroxisomes.12,13 Endoplasmic reticulum stress may be triggered by the diabetes-related accumulation of intracellular glucose and lipid pools, as well as unfolded proteins that result from glycation.14,15 Both forms of cellular stress are highly prominent in the diabetic kidney and can lead to inflammasome activation, the production of proinflammatory cytokines, or the stimulation of other injurious pathways that lead to cellular dysfunction and loss, as well as to inflammation and fibrosis.16,17 These derangements can affect all kidney cell types, and thus may play a role in the development of podocyte effacement, mesangial expansion, tubulointerstitial inflammation and fibrosis, and renal tubular cell injury and atrophy that is characteristic of diabetic CKD.18
Role of Autophagy in Diabetic CKD
When oxidative and endoplasmic reticulum stress occurs in the kidney (regardless of cause), its intensity and consequences are normally ameliorated by activation of the housekeeping pathway known as autophagy.19 Autophagy is an intracellular lysosome-dependent degradative pathway, which maintains cellular homeostasis. Typically triggered by states of nutrient deprivation, autophagy involves the identification and disposal of dysfunctional cellular constituents, particularly mitochondria and peroxisomes, which are the major sources of reactive oxygen species.20,21 The damaged organelles that are targeted for disposal are encircled by double-layered membranes to form autophagosomes. When the autophagosomes fuse with lysosomes, breakdown of the cargo leads to neutralization of injurious effects, and its molecules are recycled for reuse. Importantly, autophagy can be activated selectively to clear cells of accumulated debris, excessive stores of glucose and lipids, unfolded proteins, and dysfunctional subcellular constituents, which contribute to the pathogenesis of disease.20 Tissue-specific overexpression of autophagy genes is sufficient to extend lifespan, indicating that the activation of autophagy underlies the effects of fasting to prolong life in a broad range of animal species.22
Autophagy is typically activated by energy deprivation, and it is depressed in states of nutrient overabundance. Type 2 diabetes is characterized by excessive glucose and lipid stores,23 and the intracellular accumulation of glucose and fatty acid intermediates undermines organellar stability and promotes the formation of unfolded proteins.24 The organelles that are the primary targets for injury, the mitochondria and peroxisomes, are the two most important oxygen-consuming cellular constituents and underlie the enormous oxygen demands of the kidney that are driven by the reabsorptive processes of the renal tubules.25 Accordingly, mitochondrial and peroxisomal dysfunction plays an important role in the genesis of CKD, especially in diabetes.25,26
Cells might be able to mitigate the oxidative and endoplasmic reticulum stresses produced in the diabetic kidney by stimulating autophagic flux, and podocytes and renal tubules normally maintain high levels of autophagy to sustain their structural and functional integrity.27,28 However, the capacity for autophagy in both podocytes and renal tubular cells is markedly impaired in type 2 diabetes, and this deficiency contributes importantly to determining the intensity of renal injury.29–31 The diminution of autophagic flux is related to hyperglycemia and to advanced glycation end products and lipids that accumulate because of deficiencies in glucose and fatty acid oxidation31–33; these metabolic intermediates can, in turn, be cleared if autophagic flux is enhanced.34 The inadequacy of the autophagic response to cellular stress is a hallmark of diabetic CKD.35,36 Experimentally, the course of nephropathy can be ameliorated if autophagy is augmented31,37,38; conversely, it is accelerated if the capacity for autophagy is weakened further.39,40 Clinically, blood and urine biomarkers of autophagic proteins are depressed in the patients with diabetic kidney disease,41,42 and renal biopsy specimens of patients with insulin resistance exhibit molecular evidence of autophagy suppression.43
Molecular Determinants of Autophagic Flux in the Kidney and Their Derangements in Type 2 Diabetes
Numerous molecular pathways are capable of modulating autophagy in the kidney, but it is noteworthy that the low-energy sensors sirtuin-1 (SIRT1) and AMP-activated protein kinase (AMPK) act as the primary drivers of autophagy in states of nutrient deprivation. SIRT1 is responsive to levels of NAD, serves as a redox rheostat and a primary response to caloric impoverishment, and is a master regulator of glucose homeostasis.44,45 AMPK discerns the balance between ATP and ADP/AMP in the cytosol; its activation leads to the breakdown of energy stores, thereby promoting the generation of ATP.46 Additionally, oxygen deprivation leads to increased expression and activity of the hypoxia-inducible factors HIF-1α and HIF-2α; their downstream effects act to promote the delivery of oxygen and reduce its utilization.47 The effects of SIRT1, AMPK, and hypoxia-inducible factors on autophagy are opposed by activation of the PI3K/Akt/mTOR pathway, which is stimulated by energy overabundance and prioritizes cell growth over survival.48–50
These enzymes and transcription factors control the activity of hundreds of genes that play a critical role in maintaining cellular homeostasis, and they interact in various ways to promote autophagic flux. The interaction of HIF-1α with Beclin 1 promotes autophagosome formation,51 and AMPK enhances the maturation of autophagosomes and their fusion with lysosomes,52 a process that is opposed by signaling through the PI3K/AKt/mTOR pathway.53 Interestingly, SIRT1 and HIF-2α may act primarily to enhance the autophagy of selected cellular constituents. For example, SIRT1 promotes the clearance of damaged mitochondria,54 whereas HIF-2α stimulates the degradation of dysfunctional peroxisomes.55 Furthermore, whereas AMPK depresses the activity of HIF-1α,56 HIF-1α promotes autophagy in a manner that is AMPK-independent.57,58 In contrast, the actions of SIRT1 and HIF-2α serve to stimulate and reinforce each other.59,60
Nutrient and Oxygen Deprivation Signaling and Renoprotection
Importantly, SIRT1 and AMPK exert renoprotective effects that are both dependent on and independent of their effects to promote autophagy. SIRT1 and AMPK are important inducers of autophagic flux in the kidney, and this augmentation is accompanied by a striking amelioration of glomerular and tubular injury in a variety of models of renal stress, including those related to diabetes, aging, ischemia, and drug-related nephrotoxicity.61–64 Furthermore, these nutrient deprivation transcription factors can inhibit the generation of reactive oxygen species and activation of the inflammasome in ways that are independent of their autophagy-promoting actions.65 Both SIRT1 and AMPK act directly to maintain mitochondrial homeostasis, preserve peroxisome integrity and functionality, and enhance the activity of antioxidant mechanisms.66,67 Additionally, both SIRT1 and AMPK interact directly with a key subunit of NFκB to inhibit its actions, thereby attenuating activation of the NLRP3 inflammasome and muting inflammation-mediated cellular injury.68,69 Conversely, signaling through the P13K/AKT/mTOR pathway can impair autophagic flux in the kidney.70 The pathway also reduces antioxidant activity and promotes oxidative stress and can interact directly with NFκB to stimulate proinflammatory pathways and cell death.71–73
Derangements in Nutrient and Oxygen Deprivation Signaling in Diabetic CKD
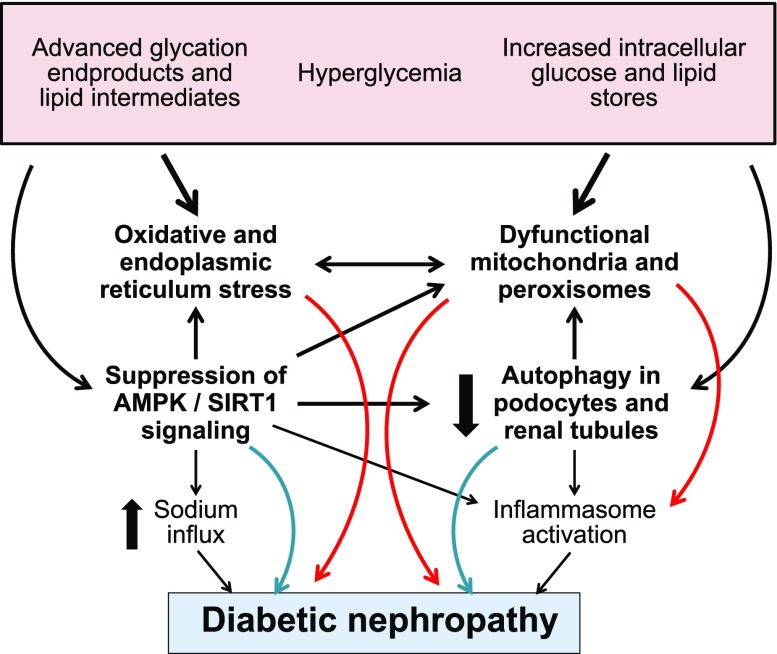
Type 2 diabetes is characterized by marked suppression of the expression and activity of both SIRT1 and AMPK simultaneously with hyperactivation of mTOR, thereby leading to a striking diminution in autophagic flux in both glomerular podocytes and renal tubules and markedly increasing their susceptibility to renal injury (Figure 1).28–33,74 Hyperglycemia inhibits phosphorylation of the catalytic isoform AMPK-α,75 and inhibition and activation of the noncatalytic subunit AMPK-β1 lead to worsening and improvement in the development of diabetic CKD, respectively.76,77 Similarly, high levels of environmental glucose act to suppress SIRT1 signaling in podocytes and renal tubules,78,79 but SIRT1 activation mitigates glycemia-related renal dysfunction and injury.80–82 Conversely, Akt/mTOR signaling is hyperactivated in the diabetic kidney and can trigger proinflammatory pathways and promote renal injury43,71,83,84; inhibiting mTOR with rapamycin can ameliorate the development of diabetic CKD.85,86 All of these derangements—acting in concert—reduce the kidney’s ability to maintain cellular homeostasis and health in states of energy overabundance; however, the loss of SIRT1 function seems to be particularly important.87 Several epidemiologic studies have shown that polymorphisms in the gene encoding SIRT1 increase the risk of CKD in patients with type 2 diabetes.88–90 Additionally, serum levels of SIRT1 are decreased in these patients, and this decline parallels the development of albuminuria.91
Figure 1.
Proposed mechanisms by which type 2 diabetes can lead to cellular stress and CKD. AMPK, AMP-activated protein kinase.
The role of hypoxia-inducible factors HIF-1α and HIF-2α in autophagy and the pathogenesis of diabetic CKD is complex. The kidney’s enormous oxygen requirements are heightened by type 2 diabetes, because renal oxygen consumption is driven by proximal tubular sodium reabsorption,92 which is markedly increased in the diabetic kidney.93 This excessive consumption may explain the renal hypoxia that has been seen in diabetes, both experimentally and clinically,94,95 and this, in turn, may activate HIF-1α signaling. Diabetes can also lead to activation of HIF-1α by a direct effect of hyperglycemia and advanced glycation end products on HIF-1α transcription.96,97 Regardless of its genesis, the increased activity of HIF-1α has been proposed as a cause of cellular dysfunction and activation of proinflammatory pathways in podocytes, mesangial cells, and renal tubular epithelial cells,98,99 thus potentially implicating HIF-1α in the pathogenesis of glomerulosclerosis and CKD.100 However, both HIF-1α and HIF-2α act to promote autophagic flux and thus attenuate oxidative stress and ischemia- and diabetes-related renal injury57,101–103; HIF-1α promotes the clearance of damaged mitochondria,47 whereas HIF-2α enhances the lysosome-mediated removal of dysfunctional peroxisomes.55 These actions may explain why pharmacologic enhancement of hypoxia-inducible factor signaling can prevent renal injury and the development of diabetic and nondiabetic CKD, especially when the disease process is well established.104–108 Therefore, although the available evidence is conflicting, it is possible that the intensity of the HIF response in the diabetic kidney modulates the development of renal injury in an isoform- and time-dependent manner.
Effects of Nutrient Deprivation Signaling on Renal Sodium Transport
Nutrient deprivation signaling not only influences podocyte and renal tubular health, but it also modulates transmembrane sodium transport in the kidney. In general, sodium movement into cells is increased by transporters than enhance sodium influx (e.g., the sodium-hydrogen exchanger [NHE] and the epithelial sodium channel [ENaC]), and it is decreased by transporters that promote sodium egress out of cells (e.g., sodium-potassium ATPase). An imbalance in these transporters can lead to a nonphysiologic increase in intracellular sodium; this impairs the ability of mitochondria to generate ATP and deploy antioxidant defense mechanisms,109–111 thus contributing to oxidative stress and cell death. Consequently, overactivity of HE or ENaC or suppression of sodium-potassium ATPase can cause increases in intracellular sodium that can trigger cellular demise.112–116 Oxidative stress in the kidney stimulates transporters driving sodium influx (e.g., NHE or ENaC), while inhibiting those involved in sodium efflux (e.g., sodium-potassium ATPase).117–119
Importantly, type 2 diabetes is characterized by activation of both NHE and ENaC and decreased activity of sodium-potassium ATPase120–122; the resulting increase in intracellular sodium leads to mitochondrial stress and cell loss, thus contributing to the development of CKD. Furthermore, if the sodium influx mechanisms (e.g., NHE3) are activated in the proximal renal tubules, the resulting enhancement of sodium reabsorption can lead to a reduction in sodium delivery to the macula densa. This, through tubuloglomerular feedback facilitated by the ENaC, could cause afferent arteriolar vasodilatation and glomerular hyperfiltration, with its attendant risks of glomerular injury.93,123 In experimental models, inhibition of sodium influx (i.e., NHE and ENaC) and/or enhancement of sodium efflux (i.e., sodium-potassium ATPase) attenuates the adverse changes in the kidneys that are produced by nutrient overabundance.124–126
It is therefore noteworthy that nutrient deprivation signaling and the P13K/Akt/mTOR pathway modulate the function of ion channels in the kidney.127,128 Both SIRT1 and AMPK downregulate ENaC and other mechanisms of sodium reabsorption in the renal tubules, but they increase the activity of sodium-potassium ATPase to promote sodium efflux.129–133 Conversely, mTOR promotes sodium influx through activation of ENaC.134 Overall, there is an inverse relationship between autophagic activity and the activation of mechanisms that promote the entry of sodium into cells. Increased autophagic activity leads to enhanced degradation of ENaC and decreased expression of NHE3,135 whereas increased NHE3 activity impairs autophagy.136 Conversely, there is a direct relationship between the activation of nutrient and oxygen deprivation signaling and the upregulation of mechanisms that enhance sodium efflux (e.g., sodium-potassium ATPase)—that is, activation of AMPK/SIRT1 stimulates sodium-potassium ATPase,133,137 and activation of sodium-potassium ATPase promotes autophagic flux.138 Additionally, inhibition of sodium-potassium ATPase is accompanied by downregulation of HIF-1α,139–141 and conversely, activation of HIF-1α/HIF-2α leads to inhibition of sodium-hydrogen exchange.142
Therefore, the totality of evidence suggests that there is a close linkage between enhanced nutrient and oxygen deprivation signaling (AMPK/SIRT/HIF-1α/HIF-2α) and the attenuation of sodium transport mechanisms that leads to increases in intracellular sodium that can undermine mitochondrial stability. Furthermore, by inhibiting sodium influx in the proximal tubule, activation of nutrient deprivation signaling may also ameliorate the sodium hyper-reabsorption and the abnormalities in tubuloglomerular feedback that are seen in diabetes and that have been implicated in the development of CKD.93,143,144
Effects of Antihyperglycemic Drugs on Nutrient and Oxygen Deprivation Signaling and on Autophagic Flux in the Diabetic Kidney
Because hyperglycemia and advanced glycation end products can injure the kidney, long-term treatment with antihyperglycemic agents might be expected to slow CKD progression. In randomized, controlled clinical trials, glycemic control in patients with type 1 diabetes has prevented the development of proteinuria,145 and prolonged treatment with glucose-lowering drugs, combined with intensive treatment of other risk factors, has decreased the likelihood of progression to ESKD in type 2 diabetes.146
Effects of Insulin-Signaling Antihyperglycemic Drugs on Autophagic Flux and the Clinical Course of Diabetic CKD
Despite these favorable effects, the benefits of antihyperglycemic drugs per se on the course of CKD in type 2 diabetes remain unclear. In the U.K. Prospective Diabetes Study 33 trial, patients receiving insulin or sulfonylureas did not show amelioration of CKD (as reflected by proteinuria or doubling of serum creatinine), despite the drugs’ blood glucose–lowering effects.147 Furthermore, in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), and the Veterans Affairs Diabetes Trial (VADT), patients who received intensive glycemic control with insulin, sulfonylureas, and thiazolidinediones showed little or no reduction in proteinuria and no preservation of renal function (as assessed by serum creatinine) compared with patients who received less aggressive glucose lowering.148–150 Although the ADVANCE trial found a reduction in ESKD,149 this finding was on the basis of few events and the other two trials observed no such reduction. Long-term therapy with glucagon-like peptide–1 receptor agonists and dipeptidyl peptidase–4 inhibitors has yielded little or no slowing of the rate of decline in glomerular function and no reduction in the risk of major adverse renal outcomes, despite achieving a decrease in albuminuria.151–154 Therefore, on the basis of the totality of evidence, drugs that promote insulin secretion or signaling have not demonstrated consistently favorable effects on the development of diabetic CKD, even when glucose lowering has been maintained for many years.
Are these disappointing results related to a lack of a benefit of insulin secreatogues or sensitizers on autophagic flux? Any lowering of blood glucose might be expected to increase SIRT1/AMPK signaling, and both liraglutide and pioglitazone appear to promote autophagy in isolated renal tubular cells or in experimental nondiabetic CKD.155,156 However, in patients with type 2 diabetes, these drugs increase circulating levels of insulin and insulin stimulates the PI3K/Akt/mTOR pathway in the kidney, an action that is preserved in states of insulin resistance.157,158 Such an effect is accompanied by the suppression of autophagy genes as well as an enhancement of sodium reabsorption.159 Glucagon-like peptide–1 signaling can impair autophagic flux under conditions of nutrient overabundance.160 Additionally, dipeptidyl peptidase–4 inhibitors potentiate the actions of the stem cell chemokine stromal cell–derived factor 1, as well as signaling through the latter’s receptor, CXCR4,161,162 and CXCR4 agonism depresses autophagic flux.163 For all of these reasons, antihyperglycemic drugs that promote insulin signaling may be unable to ameliorate the deficient autophagic flux in the diabetic kidney, and they thus may be limited in their effects to favorably influence the course of diabetic CKD.
Effects of Metformin on Nutrient Deprivation Signaling and Autophagic Flux in the Diabetic Kidney
Although metformin is routinely used as first-line therapy to achieve glycemic control in type 2 diabetes, its benefit on the clinical course of diabetic CKD has not been established.164,165 The drug does not ameliorate (and it may exacerbate) albuminuria,166,167 and it has not been shown to reduce the risk of serious adverse renal events. In patients at risk of developing diabetes, metformin has not reduced the risk of developing CKD, even though it decreases the likelihood of the development of diabetes.168
In contrast to this lack of evidence for a beneficial effect of metformin on diabetic CKD from trials or observational studies in the clinical setting, the drug has been shown to ameliorate the development of CKD in animal models of diabetes. This benefit has been attributed to the drug’s actions to mitigate oxidative stress, inflammation, podocyte and tubular injury, and fibrosis.169–173 The mechanisms that underlie these potential benefits of metformin are unknown, but the drug is an established agonist of AMPK. Although metformin has also been reported to stimulate SIRT1 and inhibit PI3K/Akt/mTOR signaling,174,175 it is doubtful that these secondary effects mediate the drug’s actions. Metformin inhibits gluconeogenesis and ketogenesis, as well as mitochondrial respiratory-chain complex 1,176,177 and such actions are inconsistent with enhanced signaling through SIRT1.178,179 Therefore, it is not surprising that numerous studies have shown that it is metformin’s effect on AMPK signaling that underlies its ability to promote autophagic flux and alleviate cellular stress in different renal cell types.180,181 The effect on AMPK also appears to explain the drug’s ability to ameliorate the development of the inflammation and fibrosis that characterizes experimental kidney disease in states of nutrient overabundance.182 However, it is not clear whether AMPK signaling alone can ameliorate the course of diabetic CKD in the clinical setting.164,165
Effects of SGLT2 Inhibitors on Nutrient Deprivation Signaling and Autophagic Flux in the Diabetic Kidney
The most persuasive evidence for a benefit of any glucose-lowering drug on the development and clinical course of diabetic CKD exists for the SGLT2 inhibitors. Large-scale randomized, placebo-controlled trials have demonstrated the ability of SGLT2 inhibitors to reduce diabetes-related deterioration in glomerular function, as well as to decrease the risk of serious adverse renal events and ESKD.4 The magnitude of this benefit is clinically impressive and evident after only 1–3 years of treatment; this benefit is not related to these drugs’ effects on glycemic control.
Although SGLT2 inhibitors can lower intraglomerular filtration pressures,5 their actions on arteriolar and efferent arteriolar tone have been debated and disputed.6,7 Importantly, interference with the actions of SGLT2 can attenuate glomerular hyperfiltration without attenuating renal injury,8 and SGLT2 inhibitors’ renoprotective effects are apparent even when there is sufficient glomerular dysfunction to abolish the actions of these drugs on glycosuria and on intrarenal hemodynamics.9,10
SGLT2 is a biologic indicator of nutrient excess, and thus there is an inverse relationship between SGLT2 activity and the expression of SIRT1 and AMPK in the kidney. High levels of renal tubular glucose promote the expression of SGLT2 but reduce the expression of SIRT179,183; increases in SGLT2 promote oxidative stress in the kidney.184 At the same time, hypoxia causes simultaneous downregulation of SGLT2 but upregulation of AMPK.57 Most interestingly, experimental knockout of SGLT2 in the renal proximal tubule appears to promote autophagic flux.185 These observations suggest that proximal tubular SGLT2 may be an entry point for therapeutic interventions that aim to stimulate nutrient deprivation signaling, i.e., SIRT1 and AMPK. In light of this conceptual framework, it is noteworthy that inhibition of SGLT2 induces a fasting-like transcriptional paradigm186 that is characterized by loss of calories in the urine, shrinkage of adipose tissue depots, and promotion of gluconeogenesis and ketogenesis (the classic biomarkers of starvation). Additionally, SGLT2 inhibitors appear to induce a state of hypoxia mimicry (akin to that produced by cobalt187) that is characterized by increased erythropoietin and erythrocytosis.188
Importantly, the ketonemia and erythrocytosis produced by SGLT2 inhibitors may provide important molecular clues concerning the mechanisms that may underlie the renoprotective effects of SGLT2 inhibitors. A critical determinant of increased ketone body production appears to be the activation of SIRT1 signaling, because SIRT1 promotes both gluconeogenesis and fatty acid oxidation, which are the two most important pathways for ketogenesis.45,189,190 Additionally, SIRT1 signaling activates the rate-limiting step in ketone body synthesis.191 At the same time, the principal driver of erythropoietin synthesis and erythrocytosis is the activation of the hypoxia-inducible factors HIF-1α and HIF-2α, and HIF-2α (the isoform most closely linked to red blood cell production192) is activated by SIRT1.59,60
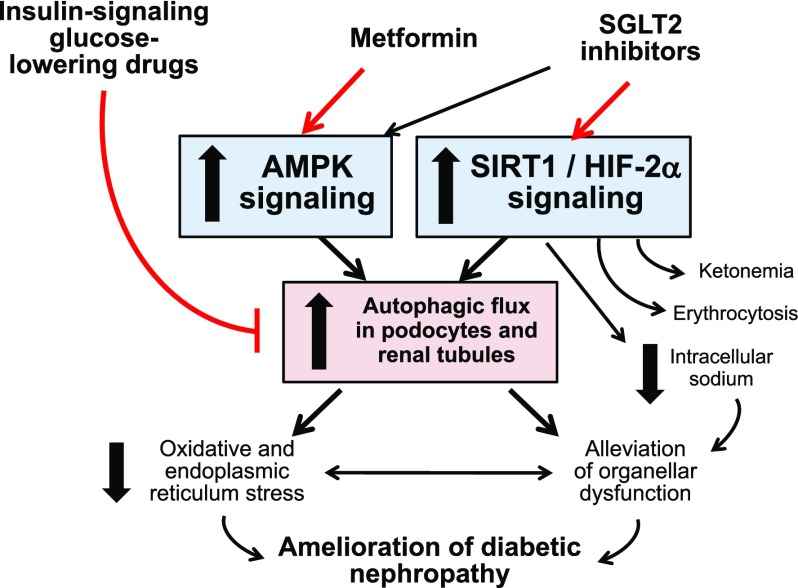
In light of their effects to promote ketogenesis and erythropoiesis (the two hallmarks of SIRT1 signaling), it is not surprising that studies show that SGLT2 inhibitors enhance the activation of SIRT1 in diverse tissues, including the kidney,79,183,193–195 and there is evidence that SGLT2 inhibitors may directly interact with SIRT1.193 The combined activation of SIRT1/HIF-2α may underlie the ability of SGLT2 inhibitors to promote autophagic flux (Figure 2).55,185,196–198 Additionally, SGLT2 inhibitors may influence HIF-1α in a manner that favorably affects the course of CKD.199,200 The hypothesis that SGLT2 inhibitors exert their effects to slow diabetic CKD progression primarily by modulating signaling through low energy–triggered enzymes and transcription factors (SIRT1 and hypoxia-inducible factors) and by stimulating autophagy is worthy of further study.
Figure 2.
Mechanisms by which antihyperglycemic drugs may influence nutrient and oxygen deprivation signaling, autophagic flux and the course of diabetic nephropathy. AMPK, AMP-activated protein kinase; SGLT2, sodium-glucose cotransporter 2.
Similarities and Differences in the Effects of Metformin and SGLT2 Inhibitors on Nutrient Deprivation Signaling in the Diabetic Kidney
Importantly, the effects of SGLT2 inhibitors on nutrient deprivation signaling and autophagy can be distinguished from those of metformin, which acts primarily as an AMPK agonist and does not induce a state of fasting or hypoxia mimicry. Although canagliflozin can activate AMPK directly, empagliflozin and dapagliflozin may not exert such an effect201,202; nevertheless, an action of these SGLT2 inhibitors to promote SIRT1 signaling may lead indirectly to stimulation of AMPK, at least to a modest degree.196,203 These observations raise the possibility that AMPK activation may contribute to the renoprotective benefits of SGLT2 inhibitors.204 The findings of the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA-REG OUTCOME) trial support this possibility, showing that metformin partially attenuated empagliflozin’s beneficial effects on the clinical course of nephropathy (53% risk reduction in metformin nonusers versus 32% risk reduction in metformin users, interaction P=0.01).205 It should be noted, however, that patients who were receiving metformin still exhibited a substantial benefit from SGLT2 inhibitors, suggesting that the major renoprotective effect of empagliflozin is not mediated through AMPK.
The effects of SGLT2 inhibitors and metformin on nutrient and oxygen deprivation signaling can be distinguished in other ways. Ketogenesis (a consequence of SIRT1 activation45,189,190) is seen with SGLT2 inhibitors and not with metformin, presumably because AMPK activation inhibits gluconeogenesis.176,206 Furthermore, SGLT2 inhibitors can interfere with sodium transport mechanisms (e.g., NHE3),207 and they cause a decrease in intracellular sodium, at least in cardiomyocytes.208 These effects are not seen with metformin, which does not reduce cytosolic sodium209 and has minimal effects on sodium reabsorption, even in salt-sensitive states.210 Finally, SGLT2 inhibitors promote erythropoietin synthesis and erythrocytosis,211,212 whereas metformin use is accompanied by a decrease in hematocrit in clinical trials.213 The latter effect is likely explained by an action of metformin (and other AMPK agonists) to inhibit HIF-1α.56,214–216 In contrast, the effects of SGLT2 inhibitors on HIF-1α remain uncertain, with studies reporting that these drugs decrease HIF-1α activity in the diabetic kidney and increase its activity in nondiabetic renal injury.119,200 In light of this uncertainty about the effect of SGLT2 inhibitors on HIF-1α, the erythrocytosis that routinely accompanies use of SGLT2 inhibitors in the clinical setting may be more readily explained by activation of HIF-2α as a result of enhanced SIRT1 signaling.59,60 In statistical mediation analyses, the intensity of erythrocytosis has predicted the reduction in the risk of heart failure events with SGLT2 inhibitors,211,212 but the strength of any association between erythrocytosis and the lowering of the risk of adverse renal events in clinical trials of these drugs has yet to be explored.
Conclusions
The principal mechanisms driving renal injury in type 2 diabetes appear to be an increase in oxidative and endoplasmic reticulum stress, exacerbated by an impairment of the kidney’s autophagic capacity to neutralize these injurious processes and their adverse effects on renal health. This deficiency is related to an effect of diabetes to depress nutrient deprivation signaling (SIRT1 and AMPK) in both podocytes and renal tubular cells—and, conceivably, diabetes may also impair the adaptive and enhance the maladaptive actions of hypoxia-inducible factors. Interestingly, these derangements are not effectively ameliorated by antihyperglycemic drugs that enhance insulin secretion or signaling. In striking contrast, however, both metformin and SGLT2 inhibitors are poised to augment autophagic flux and thereby mitigate oxidative stress and inflammation in the renal parenchyma.
Yet, these two classes of drugs differ meaningfully in their effects on nutrient deprivation signaling. Metformin acts primarily as an AMPK agonist, whereas the effects of SGLT2 inhibitors may be best explained by postulating that they induce a fasting- and hypoxia-like transcriptional paradigm that manifests primarily by enhanced SIRT1/HIF-2α signaling. Activation of these transcription factors may explain the actions of SGLT2 inhibitors to promote ketogenesis and erythrocytosis in the clinical setting, effects not seen when patients are treated with metformin. Interestingly, other drugs that promote SIRT1 (e.g., resveratrol) reduce oxidative stress, promote autophagy, and ameliorate renal abnormalities in experimental diabetes.217,218 Drugs that induce autophagy by activation of HIFs (such as cobalt) can also induce autophagy219 and prevent the development of diabetic CKD.105 Therefore, although the mechanisms of action of metformin and SGLT2 inhibitors may overlap, SGLT2 inhibitors exert molecular effects and clinical benefits on the course of CKD in type 2 diabetes that are distinct from other antihyperglycemic agents.
Disclosures
Dr. Packer has recently consulted for Abbvie, Actavis, Akcea, Amgen, AstraZeneca, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, NovoNordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M: Consequences of glomerular hyperfiltration: The role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143: 38–42, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Kastner PR, Hall JE, Guyton AC: Control of glomerular filtration rate: Role of intrarenally formed angiotensin II. Am J Physiol 246: F897–F906, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al.; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al.: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Layton AT, Vallon V, Edwards A: Modeling oxygen consumption in the proximal tubule: Effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al.: Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140: 303–315, 2019 [DOI] [PubMed] [Google Scholar]
- 7.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, et al. : The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 97: 202–212, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al.: SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Giacco F, Brownlee M: Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Díaz AG: Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol 2018: 1875870, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Han Y, Liu J, Song P, Xu X, Zhao L, et al.: Mitochondria: A novel therapeutic target in diabetic nephropathy. Curr Med Chem 24: 3185–3202, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Lee K, Wang N, He JC: The role of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab Rep 17: 17, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Maekawa H, Inagi R: Organelle crosstalk in the kidney. Kidney Int 95: 1318–1325, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR: The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton) 21: 736–744, 2016 [DOI] [PubMed] [Google Scholar]
- 17.El-Horany HE, Abd-Ellatif RN, Watany M, Hafez YM, Okda HI: NLRP3 expression and urinary HSP72 in relation to biomarkers of inflammation and oxidative stress in diabetic nephropathy patients. IUBMB Life 69: 623–630, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Ma KL, Zhang Y, Liu J, Wu Y, Hu ZB, Ruan XZ, et al.: Establishment of an inflamed animal model of diabetic nephropathy. Int J Biol Sci 10: 149–159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaushal GP, Chandrashekar K, Juncos LA: Molecular interactions between reactive oxygen species and autophagy in kidney disease. Int J Mol Sci 20: E3791, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine B, Kroemer G: Biological functions of autophagy genes: A disease perspective. Cell 176: 11–42, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Packer M, Codogno P: Development of autophagy inducers in clinical medicine. J Clin Invest 125: 14–24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen M, Rubinsztein DC, Walker DW: Autophagy as a promoter of longevity: Insights from model organisms. Nat Rev Mol Cell Biol 19: 579–593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Y, Sun H, Sun Z: Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis 16: 126, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosokawa K, Takata T, Sugihara T, Matono T, Koda M, Kanda T, et al.: Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int J Mol Sci 21: E190, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamekhi Amiri F: Intracellular organelles in health and kidney disease. Nephrol Ther 15: 9–21, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Bhargava P, Schnellmann RG: Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bork T, Liang W, Yamahara K, Lee P, Tian Z, Liu S, et al.: Podocytes maintain high basal levels of autophagy independent of mtor signaling [published ahead of print December 23, 2019]. Autophagy doi:10.1080/15548627.2019.1705007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki C, Tanida I, Oliva Trejo JA, Kakuta S, Uchiyama Y: Autophagy deficiency in renal proximal tubular cells leads to an increase in cellular injury and apoptosis under normal fed conditions. Int J Mol Sci 21: E155, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai S, Yamamoto T, Takabatake Y, Takahashi A, Namba-Hamano T, Minami S, et al.: Proximal tubule autophagy differs in type 1 and 2 diabetes. J Am Soc Nephrol 30: 929–945, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei W, An XR, Jin SJ, Li XX, Xu M: Inhibition of insulin resistance by PGE1 via autophagy-dependent FGF21 pathway in diabetic nephropathy. Sci Rep 8: 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Chen Y, Tan X, Zhang L, Zhang H, Li Z, et al.: Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J Pathol 245: 235–248, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu WJ, Shen TT, Chen RH, Wu HL, Wang YJ, Deng JK, et al.: Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J Biol Chem 290: 20499–20510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al.: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi A, Takabatake Y, Kimura T, Maejima I, Namba T, Yamamoto T, et al.: Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66: 1359–1372, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Choi ME: Autophagy in diabetic nephropathy. J Endocrinol 224: R15–R30, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka Y, Kume S, Kitada M, Kanasaki K, Uzu T, Maegawa H, et al.: Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res 2012: 628978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Feng Z, Xie J, Wen F, Jv M, Liang T, et al.: Podocyte-specific knockin of PTEN protects kidney from hyperglycemia. Am J Physiol Renal Physiol 314: F1096–F1107, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Lenoir O, Jasiek M, Hénique C, Guyonnet L, Hartleben B, Bork T, et al.: Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11: 1130–1145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Li QX, Wang XJ, Zhang C, Duan YQ, Wang ZY, et al.: β-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis 7: e2183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, et al.: Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65: 755–767, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Naguib M, Rashed LA: Serum level of the autophagy biomarker Beclin-1 in patients with diabetic kidney disease. Diabetes Res Clin Pract 143: 56–61, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Matboli M, Azazy AEM, Adel S, Bekhet MM, Eissa S: Evaluation of urinary autophagy transcripts expression in diabetic kidney disease. J Diabetes Complications 31: 1491–1498, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, et al.: Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol 24: 1769–1781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, et al.: A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456: 269–273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardie DG, Ross FA, Hawley SA: AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schönenberger MJ, Kovacs WJ: Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front Cell Dev Biol 3: 42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laplante M, Sabatini DM: mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SY, Cai GY, Chen XM: Energy restriction in renal protection. Br J Nutr 120: 1149–1158, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, et al.: Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell Mol Life Sci 75: 669–688, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu N, Li X, Tan R, An J, Cai Z, Hu X, et al.: HIF-1α/Beclin1-mediated autophagy is involved in neuroprotection induced by hypoxic preconditioning. J Mol Neurosci 66: 238–250, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang M, Park R, Kim H, Namkoong S, Jo D, Huh YH, et al.: AMPK contributes to autophagosome maturation and lysosomal fusion. Sci Rep 8: 12637, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munson MJ, Ganley IG: MTOR, PIK3C3, and autophagy: Signaling the beginning from the end. Autophagy 11: 2375–2376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, et al.: Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157: 882–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter KM, Schönenberger MJ, Trötzmüller M, Horn M, Elsässer HP, Moser AB, et al.: Hif-2α promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab 20: 882–897, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Treins C, Murdaca J, Van Obberghen E, Giorgetti-Peraldi S: AMPK activation inhibits the expression of HIF-1α induced by insulin and IGF-1. Biochem Biophys Res Commun 342: 1197–1202, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Li H, Satriano J, Thomas JL, Miyamoto S, Sharma K, Pastor-Soler NM, et al.: Interactions between HIF-1α and AMPK in the regulation of cellular hypoxia adaptation in chronic kidney disease. Am J Physiol Renal Physiol 309: F414–F428, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Long M, Zhang S, Cheng Z, Zhao X, He F, et al.: Hypoxia inducible factor-1α regulates autophagy via the p27-E2F1 signaling pathway. Mol Med Rep 16: 2107–2112, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen R, Xu M, Hogg RT, Li J, Little B, Gerard RD, et al.: The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem 287: 30800–30811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA: Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem 286: 13869–13878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, et al.: Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci Rep 6: 23975, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang LT, Chen BL, Wu CT, Huang KH, Chiang CK, Hwa Liu S: Protective role of AMP-activated protein kinase-evoked autophagy on an in vitro model of ischemia/reperfusion-induced renal tubular cell injury. PLoS One 8: e79814, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM: Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 208: 410–421, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, et al.: Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al.: AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, et al.: Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab 26: 884–896.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, et al.: Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285: 13045–13056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A: Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25: 1939–1948, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Li X, Zhang W, He J, Xu B, Lei B, et al.: Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 83: 256–270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du C, Zhang T, Xiao X, Shi Y, Duan H, Ren Y: Protease-activated receptor-2 promotes kidney tubular epithelial inflammation by inhibiting autophagy via the PI3K/Akt/mTOR signalling pathway. Biochem J 474: 2733–2747, 2017 [DOI] [PubMed] [Google Scholar]
- 71.Lu Q, Zhou Y, Hao M, Li C, Wang J, Shu F, et al.: The mTOR promotes oxidative stress-induced apoptosis of mesangial cells in diabetic nephropathy. Mol Cell Endocrinol 473: 31–43, 2018 [DOI] [PubMed] [Google Scholar]
- 72.Zhang HT, Wang WW, Ren LH, Zhao XX, Wang ZH, Zhuang DL, et al.: The mTORC2/Akt/NFκB pathway-mediated activation of TRPC6 participates in adriamycin-induced podocyte apoptosis. Cell Physiol Biochem 40: 1079–1093, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, et al.: Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood 117: 4273–4283, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Muratsubaki S, Kuno A, Tanno M, Miki T, Yano T, Sugawara H, et al.: Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes. Sci Rep 7: 5311, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al.: A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Choy SW, Fraser SA, Katerelos M, Galic S, Kemp BE, Mount PF, et al.: Absence of the β1 subunit of AMP-activated protein kinase reduces myofibroblast infiltration of the kidneys in early diabetes. Int J Exp Pathol 100: 114–122, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salatto CT, Miller RA, Cameron KO, Cokorinos E, Reyes A, Ward J, et al.: Selective activation of AMPK β1-containing isoforms improves kidney function in a rat model of diabetic nephropathy. J Pharmacol Exp Ther 361: 303–311, 2017 [DOI] [PubMed] [Google Scholar]
- 78.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM, et al.: Role of transcription factor acetylation in diabetic kidney disease. Diabetes 63: 2440–2453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Umino H, Hasegawa K, Minakuchi H, Muraoka H, Kawaguchi T, Kanda T, et al.: High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep 8: 6791, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong Q, Zhang L, Das B, Li Z, Liu B, Cai G, et al.: Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int 93: 1330–1343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Zheng ZJ, Jia YJ, Yang YL, Xue YM: Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J Transl Med 16: 146, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, et al.: Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19: 1496–1504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng D, Tao M, Liang X, Li Y, Jin J, He Q: p66Shc regulates podocyte autophagy in high glucose environment through the Notch-PTEN-PI3K/Akt/mTOR pathway [published ahead of print October 25, 2019]. Histol Histopathol doi:10.14670/HH-18-178 [DOI] [PubMed] [Google Scholar]
- 84.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, et al.: mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, et al.: The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun 384: 471–475, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, et al.: Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27: 495–502, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Sun W, Cheng Y, Xu Z, Cai L: Role of sirtuin-1 in diabetic nephropathy. J Mol Med (Berl) 97: 291–309, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maeda S, Koya D, Araki SI, Babazono T, Umezono T, Toyoda M, et al.: Association between single nucleotide polymorphisms within genes encoding sirtuin families and diabetic nephropathy in Japanese subjects with type 2 diabetes. Clin Exp Nephrol 15: 381–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang K, Sun M, Shen J, Zhou B: Transcriptional coactivator p300 and silent information regulator 1 (SIRT1) gene polymorphism associated with diabetic kidney disease in a Chinese cohort. Exp Clin Endocrinol Diabetes 125: 530–537, 2017 [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Wei J, Hou X, Liu H, Guo F, Zhou Y, et al.: SIRT1 rs10823108 and FOXO1 rs17446614 responsible for genetic susceptibility to diabetic nephropathy. Sci Rep 7: 10285, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shao Y, Ren H, Lv C, Ma X, Wu C, Wang Q: Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine 55: 130–138, 2017 [DOI] [PubMed] [Google Scholar]
- 92.Torelli G, Milla E, Faelli A, Costantini S: Energy requirement for sodium reabsorption in the in vivo rabbit kidney. Am J Physiol 211: 576–580, 1966 [DOI] [PubMed] [Google Scholar]
- 93.Hallow KM, Gebremichael Y, Helmlinger G, Vallon V: Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: A modeling analysis. Am J Physiol Renal Physiol 312: F819–F835, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al.: Tubular injury in a rat model of type 2 diabetes is prevented by metformin: A possible role of HIF-1α expression and oxygen metabolism. Diabetes 60: 981–992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang ZJ, Kumar R, Banerjee S, Hsu CY: Blood oxygen level-dependent (BOLD) MRI of diabetic nephropathy: Preliminary experience. J Magn Reson Imaging 33: 655–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, et al.: High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int 78: 48–59, 2010 [DOI] [PubMed] [Google Scholar]
- 97. Bondeva T, Heinzig J, Ruhe C, Wolf G: Advanced glycated end-products affect HIF-transcriptional activity in renal cells. Mol Endocrinol 27: 1918–1933, 2013. [DOI] [PMC free article] [PubMed]
- 98.Nayak BK, Shanmugasundaram K, Friedrichs WE, Cavaglierii RC, Patel M, Barnes J, et al.: HIF-1 mediates renal fibrosis in OVE26 type 1 diabetic mice. Diabetes 65: 1387–1397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu J, Wang W, Zhang F, Li PL, Boini KM, Yi F, et al.: Hypoxia inducible factor-1α mediates the profibrotic effect of albumin in renal tubular cells. Sci Rep 7: 15878, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumann B, Hayashida T, Liang X, Schnaper HW: Hypoxia-inducible factor-1α promotes glomerulosclerosis and regulates COL1A2 expression through interactions with Smad3. Kidney Int 90: 797–808, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lv B, Hua T, Li F, Han J, Fang J, Xu L, et al.: Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res 9: 2492–2499, 2017 [PMC free article] [PubMed] [Google Scholar]
- 102.Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye Z, et al.: Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis 9: 338, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP: Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci 86: 115–123, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, et al.: Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant 25: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, et al.: Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol 26: 328–338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou C, Miyata T: Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant 23: 1166–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Kong KH, Oh HJ, Lim BJ, Kim M, Han KH, Choi YH, et al.: Selective tubular activation of hypoxia-inducible factor-2α has dual effects on renal fibrosis. Sci Rep 7: 11351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu X, Fang Y, Liu H, Zhu J, Zou J, Xu X, et al.: The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol Dial Transplant 27: 3110–3119, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Murphy E, Eisner DA: Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res 104: 292–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sedova M, Blatter LA: Intracellular sodium modulates mitochondrial calcium signaling in vascular endothelial cells. J Biol Chem 275: 35402–35407, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Wiczer BM, Marcu R, Hawkins BJ: KB-R7943, a plasma membrane Na(+)/Ca(2+) exchanger inhibitor, blocks opening of the mitochondrial permeability transition pore. Biochem Biophys Res Commun 444: 44–49, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pedersen SF: The Na+/H+ exchanger NHE1 in stress-induced signal transduction: Implications for cell proliferation and cell death. Pflugers Arch 452: 249–259, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Wärntges S, Gröne HJ, Capasso G, Lang F: Cell volume regulatory mechanisms in progression of renal disease. J Nephrol 14: 319–326, 2001 [PubMed] [Google Scholar]
- 114.Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T, et al.: Aberrant ENaC activation in Dahl salt-sensitive rats. J Hypertens 27: 1679–1689, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Burlaka I, Nilsson LM, Scott L, Holtbäck U, Eklöf AC, Fogo AB, et al.: Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric kidney disease. Kidney Int 90: 135–148, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Orlov SN, Platonova AA, Hamet P, Grygorczyk R: Cell volume and monovalent ion transporters: Their role in cell death machinery triggering and progression. Am J Physiol Cell Physiol 305: C361–C372, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Jia Z, Zhuang Y, Hu C, Zhang X, Ding G, Zhang Y, et al.: Albuminuria enhances NHE3 and NCC via stimulation of mitochondrial oxidative stress/angiotensin II axis. Oncotarget 7: 47134–47144, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pinto V, Pinho MJ, Hopfer U, Jose PA, Soares-da-Silva P: Oxidative stress and the genomic regulation of aldosterone-stimulated NHE1 activity in SHR renal proximal tubular cells. Mol Cell Biochem 310: 191–201, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Luo Q, Cui H, Deng H, Kuang P, Liu H, Lu Y, et al.: Histopathological findings of renal tissue induced by oxidative stress due to different concentrations of fluoride. Oncotarget 8: 50430–50446, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang CT, Wu MS, Tian YC, Chen KH, Yu CC, Liao CH, et al.: Enhancement of epithelial sodium channel expression in renal cortical collecting ducts cells by advanced glycation end products. Nephrol Dial Transplant 22: 722–731, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Nordquist L, Shimada K, Ishii T, Furuya DT, Kamikawa A, Kimura K: Proinsulin C-peptide prevents type-1 diabetes-induced decrease of renal Na+-K+-ATPase alpha1-subunit in rats. Diabetes Metab Res Rev 26: 193–199, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Klisic J, Nief V, Reyes L, Ambuhl PM: Acute and chronic regulation of the renal Na/H+ exchanger NHE3 in rats with STZ-induced diabetes mellitus. Nephron, Physiol 102: 27–35, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Monu SR, Ren Y, Masjoan-Juncos JX, Kutskill K, Wang H, Kumar N, et al.: Connecting tubule glomerular feedback mediates tubuloglomerular feedback resetting after unilateral nephrectomy. Am J Physiol Renal Physiol 315: F806–F811, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong H, Sun J, Xue W, Tian P, Ding X, Yan H, et al.: Protective effect of truncated Na+/K+-ATPase β on ischemia/reperfusion-induced renal injury in rats. Exp Biol Med (Maywood) 239: 677–685, 2014 [DOI] [PubMed] [Google Scholar]
- 125.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al.: Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: Possible contribution of fat-derived factors. J Am Soc Nephrol 17: 3438–3446, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Prasad V, Lorenz JN, Miller ML, Vairamani K, Nieman ML, Wang Y, et al.: Loss of NHE1 activity leads to reduced oxidative stress in heart and mitigates high-fat diet-induced myocardial stress. J Mol Cell Cardiol 65: 33–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lang F, Föller M: Regulation of ion channels and transporters by AMP-activated kinase (AMPK). Channels (Austin) 8: 20–28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sørensen MV, Saha B, Jensen IS, Wu P, Ayasse N, Gleason CE, et al.: Potassium acts through mTOR to regulate its own secretion. JCI Insight 5: 126910, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ho PY, Li H, Cheng L, Bhalla V, Fenton RA, Hallows KR: AMPK phosphorylation of the β1Pix exchange factor regulates the assembly and function of an ENaC inhibitory complex in kidney epithelial cells. Am J Physiol Renal Physiol 317: F1513–F1525, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, et al.: Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, et al.: Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang D, Li S, Cruz P, Kone BC: Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate α-ENaC transcription in collecting duct. J Biol Chem 284: 20917–20926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Funk JA, Schnellmann RG: Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 273: 345–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lang F, Pearce D: Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol Dial Transplant 31: 200–205, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eaton DC, Malik B, Bao HF, Yu L, Jain L: Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi H, Zhao X, Ding Z, Han C, Jiang Y, Qian W, et al.: Na+/H+ exchanger regulates amino acid-mediated autophagy in intestinal epithelial cells. Cell Physiol Biochem 42: 2418–2429, 2017 [DOI] [PubMed] [Google Scholar]
- 137.Xiao J, Zhu S, Guan H, Zheng Y, Li F, Zhang X, et al.: AMPK alleviates high uric acid-induced Na+-K+-ATPase signaling impairment and cell injury in renal tubules. Exp Mol Med 51: 1–14, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Felippe Gonçalves-de-Albuquerque C, Ribeiro Silva A, Ignácio da Silva C, Caire Castro-Faria-Neto H, Burth P: Na/K Pump and beyond: Na/K-ATPase as a modulator of apoptosis and autophagy. Molecules 22: E578, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei D, Peng JJ, Gao H, Li H, Li D, Tan Y, et al.: Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells. Int J Mol Sci 14: 7273–7285, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang W, Li G, Li W, Wang P, Xiu P, Jiang X, et al.: Sodium orthovanadate overcomes sorafenib resistance of hepatocellular carcinoma cells by inhibiting Na+/K+-ATPase activity and hypoxia-inducible pathways. Sci Rep 8: 9706, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang XS, Xu ZW, Yi TL, Xu RC, Li J, Zhang WB, et al.: Ouabain suppresses the growth and migration abilities of glioma U-87MG cells through inhibiting the Akt/mTOR signaling pathway and downregulating the expression of HIF-1α. Mol Med Rep 17: 5595–5600, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang P, Li L, Zhang Z, Kan Q, Gao F, Chen S: Time-dependent activity of Na+/H+ exchanger isoform 1 and homeostasis of intracellular pH in astrocytes exposed to CoCl2 treatment. Mol Med Rep 13: 4443–4450, 2016 [DOI] [PubMed] [Google Scholar]
- 143.Huang DY, Gao H, Boini KM, Osswald H, Nürnberg B, Lang F: In vivo stimulation of AMP-activated protein kinase enhanced tubuloglomerular feedback but reduced tubular sodium transport during high dietary NaCl intake. Pflugers Arch 460: 187–196, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Hasegawa K: Novel tubular-glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide Oshima Award Address 2017. Clin Exp Nephrol 23: 987–994, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al.; Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 146.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 147.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 148.Ismail-Beigi F, Craven TE, O’Connor PJ, Karl D, Calles-Escandon J, Hramiak I, et al.; ACCORD Study Group: Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 81: 586–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, et al.; ADVANCE Collaborative Group: Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 150.Agrawal L, Azad N, Emanuele NV, Bahn GD, Kaufman DG, Moritz TE, et al.; Veterans Affairs Diabetes Trial (VADT) Study Group: Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 34: 2090–2094, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al.; LEADER Steering Committee and Investigators: Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017 [DOI] [PubMed] [Google Scholar]
- 152.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al.; SUSTAIN-6 Investigators: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844, 2016 [DOI] [PubMed] [Google Scholar]
- 153.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, et al.: Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 40: 69–76, 2017 [DOI] [PubMed] [Google Scholar]
- 154.Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, et al.; TECOS Study Group: Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: Outcomes from TECOS. Diabetes Care 39: 2304–2310, 2016 [DOI] [PubMed] [Google Scholar]
- 155.Xue L, Pan Z, Yin Q, Zhang P, Zhang J, Qi W: Liraglutide promotes autophagy by regulating the AMPK/mTOR pathway in a rat remnant kidney model of chronic renal failure. Int Urol Nephrol 51: 2305–2313, 2019 [DOI] [PubMed] [Google Scholar]
- 156.Xi X, Zou C, Ye Z, Huang Y, Chen T, Hu H: Pioglitazone protects tubular cells against hypoxia/reoxygenation injury through enhancing autophagy via AMPK-mTOR signaling pathway. Eur J Pharmacol 863: 172695, 2019 [DOI] [PubMed] [Google Scholar]
- 157.Nakamura M, Tsukada H, Seki G, Satoh N, Mizuno T, Fujii W, et al. : Insulin promotes sodium transport but suppresses gluconeogenesis via distinct cellular pathways in human and rat renal proximal tubules. Kidney Int 97: 316–326, 2020 [DOI] [PubMed] [Google Scholar]
- 158.Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G: Selective insulin resistance in the kidney. BioMed Res Int 2016: 5825170, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paula-Gomes S, Gonçalves DA, Baviera AM, Zanon NM, Navegantes LC, Kettelhut IC: Insulin suppresses atrophy- and autophagy-related genes in heart tissue and cardiomyocytes through AKT/FOXO signaling. Horm Metab Res 45: 849–855, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Guo H, Wang B, Li H, Ling L, Niu J, Gu Y: Glucagon-like peptide-1 analog prevents obesity-related glomerulopathy by inhibiting excessive autophagy in podocytes. Am J Physiol Renal Physiol 314: F181–F189, 2018 [DOI] [PubMed] [Google Scholar]
- 161.Jackson EK, Zhang Y, Gillespie DD, Zhu X, Cheng D, Jackson TC: SDF-1α (stromal cell-derived factor 1α) induces cardiac fibroblasts, renal microvascular smooth muscle cells, and glomerular mesangial cells to proliferate, cause hypertrophy, and produce collagen. J Am Heart Assoc 6: e007253, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Packer M: Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc Diabetol 17: 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Coly PM, Gandolfo P, Castel H, Morin F: The autophagy machinery: A new player in chemotactic cell migration. Front Neurosci 11: 78, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 [DOI] [PubMed] [Google Scholar]
- 165.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 854–865, 1998 [PubMed] [Google Scholar]
- 166.De Jager J, Kooy A, Lehert P, Bets D, Wulffelé MG, Teerlink T, et al.: Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: A randomized, placebo-controlled trial. J Intern Med 257: 100–109, 2005 [DOI] [PubMed] [Google Scholar]
- 167.Lachin JM, Viberti G, Zinman B, Haffner SM, Aftring RP, Paul G, et al.; ADOPT Study Group: Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol 6: 1032–1040, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diabetes Prevention Program Research Group: Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 3: 866–875, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang S, Xu H, Yu X, Wu Y, Sui D: Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med 14: 383–390, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kang Z, Zeng J, Zhang T, Lin S, Gao J, Jiang C, et al.: Hyperglycemia induces NF-κB activation and MCP-1 expression via downregulating GLP-1R expression in rat mesangial cells: Inhibition by metformin. Cell Biol Int 43: 940–953, 2019 [DOI] [PubMed] [Google Scholar]
- 171.Yao XM, Ye SD, Xiao CC, Gu JF, Yang D, Wang S: Metformin alleviates high glucose-mediated oxidative stress in rat glomerular mesangial cells by modulation of p38 mitogen-activated protein kinase expression in vitro. Mol Med Rep 12: 520–526, 2015 [DOI] [PubMed] [Google Scholar]
- 172.Kim J, Shon E, Kim CS, Kim JS: Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res 2012: 210821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA: Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 192: 233–242, 2011 [DOI] [PubMed] [Google Scholar]
- 174.Cuyàs E, Verdura S, Llorach-Parés L, Fernández-Arroyo S, Joven J, Martin-Castillo B, et al.: Metformin is a direct SIRT1-activating compound: Computational modeling and experimental validation. Front Endocrinol (Lausanne) 9: 657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li Y, Chen C, Yao F, Su Q, Liu D, Xue R, et al.: AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys 558: 79–86, 2014 [DOI] [PubMed] [Google Scholar]
- 176.Fulgencio JP, Kohl C, Girard J, Pégorier JP: Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem Pharmacol 62: 439–446, 2001 [DOI] [PubMed] [Google Scholar]
- 177.Fontaine E: Metformin-induced mitochondrial complex I inhibition: Facts, uncertainties, and consequences. Front Endocrinol (Lausanne) 9: 753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gillum MP, Erion DM, Shulman GI: Sirtuin-1 regulation of mammalian metabolism. Trends Mol Med 17: 8–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang HH, Qin GJ, Li XL, Zhang YH, Du PJ, Zhang PY, et al.: SIRT1 overexpression in skeletal muscle in vivo induces increased insulin sensitivity and enhanced complex I but not complex II-V functions in individual subsarcolemmal and intermyofibrillar mitochondria. J Physiol Biochem 71: 177–190, 2015 [DOI] [PubMed] [Google Scholar]
- 180.Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q: Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol 500: 110628, 2020 [DOI] [PubMed] [Google Scholar]
- 181.Xu J, Liu LQ, Xu LL, Xing Y, Ye S: Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis. Clin Exp Pharmacol Physiol 47: 599–608, 2020 [DOI] [PubMed] [Google Scholar]
- 182.Kim D, Lee JE, Jung YJ, Lee AS, Lee S, Park SK, et al.: Metformin decreases high-fat diet-induced renal injury by regulating the expression of adipokines and the renal AMP-activated protein kinase/acetyl-CoA carboxylase pathway in mice. Int J Mol Med 32: 1293–1302, 2013 [DOI] [PubMed] [Google Scholar]
- 183.Sasaki M, Sasako T, Kubota N, Sakurai Y, Takamoto I, Kubota T, et al.: Dual regulation of gluconeogenesis by insulin and glucose in the proximal tubules of the kidney. Diabetes 66: 2339–2350, 2017 [DOI] [PubMed] [Google Scholar]
- 184.Maeda S, Matsui T, Takeuchi M, Yamagishi S: Sodium-glucose cotransporter 2-mediated oxidative stress augments advanced glycation end products-induced tubular cell apoptosis. Diabetes Metab Res Rev 29: 406–412, 2013 [DOI] [PubMed] [Google Scholar]
- 185.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, et al.: Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, et al.: SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4: 123130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goldberg MA, Dunning SP, Bunn HF: Regulation of the erythropoietin gene: Evidence that the oxygen sensor is a heme protein. Science 242: 1412–1415, 1988 [DOI] [PubMed] [Google Scholar]
- 188.Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, et al.: Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 141: 704–707, 2020 [DOI] [PubMed] [Google Scholar]
- 189.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al.: Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu TF, Vachharajani VT, Yoza BK, McCall CE: NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 287: 25758–25769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E: SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1,2 and HMGCS1,2. Aging (Albany NY) 3: 635–642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eckardt KU, Kurtz A: Regulation of erythropoietin production. Eur J Clin Invest 35: 13–19, 2005 [DOI] [PubMed] [Google Scholar]
- 193.Ying Y, Jiang C, Zhang M, Jin J, Ge S, Wang X: Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging (Albany NY) 11: 2822–2835, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A: Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond) 133: 2415–2430, 2019 [DOI] [PubMed] [Google Scholar]
- 195.Kim JW, Lee YJ, You YH, Moon MK, Yoon KH, Ahn YB, et al.: Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and α-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes [published online ahead of print Nov 26, 2018]. J Cell Biochem doi:10.1002/jcb.28141 [DOI] [PubMed] [Google Scholar]
- 196.Aragón-Herrera A, Feijóo-Bandín S, Otero Santiago M, Barral L, Campos-Toimil M, Gil-Longo J, et al.: Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol 170: 113677, 2019 [DOI] [PubMed] [Google Scholar]
- 197.Xu C, Wang W, Zhong J, Lei F, Xu N, Zhang Y, et al.: Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol 152: 45–59, 2018 [DOI] [PubMed] [Google Scholar]
- 198.Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, et al.: Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep 6: e13741, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim S, Jo CH, Kim GH: Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens Res 42: 1905–1915, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, et al.: Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep 9: 14754, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, et al.: Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep 8: 5276, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al.: The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65: 2784–2794, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J: Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15: 335–346, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Inoue MK, Matsunaga Y, Nakatsu Y, Yamamotoya T, Ueda K, Kushiyama A, et al.: Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol Metab Syndr 11: 57, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Inzucchi SE, Fitchett D, Jurišić-Eržen D, Woo V, Hantel S, Janista C, et al.; EMPA-REG OUTCOME® Investigators: Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes Metab 22: 631–639, 2020 [DOI] [PubMed] [Google Scholar]
- 206.Lee JM, Seo WY, Song KH, Chanda D, Kim YD, Kim DK, et al.: AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J Biol Chem 285: 32182–32191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G: Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, et al.: Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61: 722–726, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ségalen C, Longnus SL, Baetz D, Counillon L, Van Obberghen E: 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside reduces glucose uptake via the inhibition of Na+/H+ exchanger 1 in isolated rat ventricular cardiomyocytes. Endocrinology 149: 1490–1498, 2008 [DOI] [PubMed] [Google Scholar]
- 210.Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, et al.: Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 8: 227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al.: How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41: 356–363, 2018 [DOI] [PubMed] [Google Scholar]
- 212.Li JW, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, et al.: Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 8: 57–66, 2020 [DOI] [PubMed] [Google Scholar]
- 213.Diabetes Prevention Program Research Group: Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35: 731–737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li X, Li J, Wang L, Li A, Qiu Z, Qi LW, et al.: The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol 173: 2001–2015, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Al-Hashem F, Al-Humayed S, Amin SN, Kamar SS, Mansy SS, Hassan S, et al.: Metformin inhibits mTOR-HIF-1α axis and profibrogenic and inflammatory biomarkers in thioacetamide-induced hepatic tissue alterations. J Cell Physiol 234: 9328–9337, 2019 [DOI] [PubMed] [Google Scholar]
- 216.Guimarães TA, Farias LC, Santos ES, de Carvalho Fraga CA, Orsini LA, de Freitas Teles L, et al.: Metformin increases PDH and suppresses HIF-1α under hypoxic conditions and induces cell death in oral squamous cell carcinoma. Oncotarget 7: 55057–55068, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang T, Chi Y, Kang Y, Lu H, Niu H, Liu W, et al.: Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J Cell Physiol 234: 5033–5043, 2019 [DOI] [PubMed] [Google Scholar]
- 218.Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, et al.: Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract 212: 310–318, 2016 [DOI] [PubMed] [Google Scholar]
- 219.Chen R, Jiang T, She Y, Xu J, Li C, Zhou S, et al.: Effects of cobalt chloride, a hypoxia-mimetic agent, on autophagy and atrophy in skeletal C2C12 myotubes. BioMed Res Int 2017: 7097580, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]