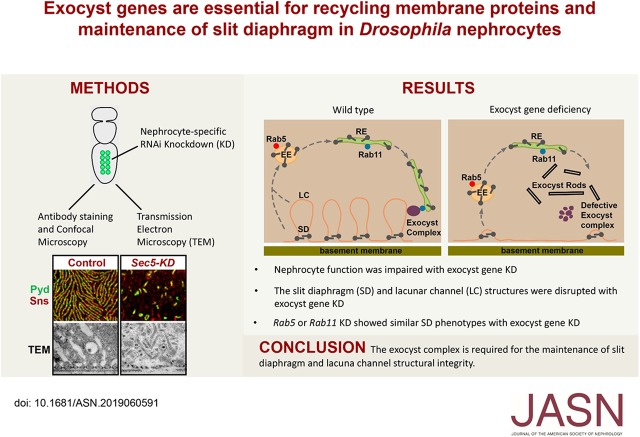
Significance Statement
Mutations in genes encoding the exocyst protein complex have been identified in patients with kidney disease, but the underlying mechanism of renal pathology is unclear. The authors demonstrated in Drosophila nephrocytes—counterparts to mammalian podocytes—that an intact exocyst complex is essential for normal function and structure. Silencing of various exocyst genes disrupted slit diaphragm structure and reduced infoldings of the nephrocyte plasma membrane (lacuna channels), the main sites of protein reabsorption. The authors also discovered abnormal electron-dense “exocyst rods,” structures formed by lacuna membrane proteins and slit diaphragm proteins that are not appropriately recycled because of deficiency of exocyst proteins. These findings are consistent with the exocyst complex playing a key role in maintenance of slit diaphragm and lacuna channel structural integrity via completion of the endosome recycling pathway.
Keywords: nephrocyte, exocyst complex, cell biology and structure, genetic renal disease, exocyst rod
Visual Abstract
Abstract
Background
Studies have linked mutations in genes encoding the eight-protein exocyst protein complex to kidney disease, but the underlying mechanism is unclear. Because Drosophila nephrocytes share molecular and structural features with mammalian podocytes, they provide an efficient model for studying this issue.
Methods
We silenced genes encoding exocyst complex proteins specifically in Drosophila nephrocytes and studied the effects on protein reabsorption by lacuna channels and filtration by the slit diaphragm. We performed nephrocyte functional assays, carried out super-resolution confocal microscopy of slit diaphragm proteins, and used transmission electron microscopy to analyze ultrastructural changes. We also examined the colocalization of slit diaphragm proteins with exocyst protein Sec15 and with endocytosis and recycling regulators Rab5, Rab7, and Rab11.
Results
Silencing exocyst genes in nephrocytes led to profound changes in structure and function. Abolition of cellular accumulation of hemolymph proteins with dramatically reduced lacuna channel membrane invaginations offered a strong indication of reabsorption defects. Moreover, the slit diaphragm’s highly organized surface structure—essential for filtration—was disrupted, and key proteins were mislocalized. Ultrastructural analysis revealed that exocyst gene silencing led to the striking appearance of novel electron-dense structures that we named “exocyst rods,” which likely represent accumulated membrane proteins following defective exocytosis or recycling. The slit diaphragm proteins partially colocalized with Sec15, Rab5, and Rab11.
Conclusions
Our findings suggest that the slit diaphragm of Drosophila nephrocytes requires balanced endocytosis and recycling to maintain its structural integrity and that impairment of the exocyst complex leads to disruption of the slit diaphragm and nephrocyte malfunction. This model may help identify therapeutic targets for treating kidney diseases featuring molecular defects in vesicle endocytosis, exocytosis, and recycling.
In the mammalian kidney, interdigitating podocyte foot processes spanned by extracellular slit diaphragm proteins mediate glomerular filtration of circulating proteins.1 Proximal tubule (PT) cells subsequently reabsorb proteins that pass through the glomerular filtration barrier.2,3 In Drosophila, pericardial nephrocytes (hereafter, nephrocytes) share remarkable structural, molecular, and functional similarities with both mammalian podocytes and PT cells, and they carry out both filtration and protein absorption functions.4–12
The mammalian podocyte slit diaphragm structure is a major component of the glomerular filtration barrier, and its disruption is associated with proteinuria and development of nephrotic syndrome.1,13 The slit diaphragm structure not only provides the physical barrier for glomerular filtration, but it also functions as a signaling platform and undergoes dynamic changes in its mechanotransduction and endocytosis functions through phosphorylation of Nephrin, an essential component of the slit diaphragm structure.14,15 In cultured podocytes, Nephrin, when dephosphorylated at the conserved Y1193 residue, undergoes β-arrestin2–mediated clathrin-dependent endocytosis.16 In mice, the podocyte-specific ablation of dynamin, synaptojanin, and endophilin, which are critical components of clathrin-mediated endocytosis, leads to endocytic defects, foot process effacement, severe proteinuria, and kidney failure.17 Recently, mutations in several genes involved in endocytosis have been identified in patients with nephrotic syndrome,18,19 suggesting that endocytosis plays an important role in glomerular function. However, because it is difficult to probe the slit diaphragm structure in high resolution in mammalian kidney tissue, the role of endocytosis in slit diaphragm structure maintenance in vivo still remains unclear.
The exocyst is a highly conserved octameric protein complex that mediates the tethering of secretory vesicles or recycling endosomes to the plasma membrane prior to membrane fusion.20–23 In kidney cells, it has been shown that exocyst proteins are required for the localization of polycystin-2 in the primary cilium.24–27 Acting through the primary cilium, the exocyst is essential for ciliogenesis, cystogenesis, and tubulogenesis.28 Mutations in exocyst complex genes have been identified in patients with kidney disease, such as Joubert syndrome.29 Recently, deletion of the Exoc4 gene was implicated in human nephrotic syndrome, and podocyte-specific deletion of Exoc5 in mice was shown to induce severe proteinuria and glomerular defects.30 However, the mechanism of how exocyst complex regulates podocyte function remains largely unknown.
The Drosophila nephrocyte, a relatively large and easily accessible cell type, is in multiple ways highly suited to the study of essential roles of renal genes that are conserved during evolution.10 Using a functional readout for Drosophila nephrocytes on the basis of in vivo uptake of fluorescently labeled hemolymph protein, we previously performed a large-scale RNA interference (RNAi) screen for fly genes that are essential for this process.8 In this study, we found that nephrocyte-specific silencing of each individual of the Drosophila exocyst complex genes, including Sec5, Sec6, Sec10, Sec15, or Exo84, was associated with dramatic impairment of protein uptake function and disruption of the nephrocyte slit diaphragm (NSD). Meanwhile, RNAi knockdown of the recycling endosome regulator Rab11 showed similar phenotypes, suggesting that the exocyst complex may be recruited to recycling endosomes via Rab11. Ultrastructure analysis using transmission electron microscopy (TEM) revealed complete loss of lacuna channels and formation of novel electron-dense structures, which we named “exocyst rods,” and this might reflect accumulated membrane structures with impaired exocytosis or recycling. Therefore, our results demonstrated the essential role of exocyst complex in regulating nephrocyte membrane protein recycling, which is essential for maintaining the integrity of the slit diaphragm structures.
Methods
Fly Strains
Flies were reared on standard food at 25°C. All UAS-Gal4 crosses were performed at 29°C. Dot-Gal4 was obtained from the Bloomington Drosophila Stock Center (BL; BL#67608). Transgenic RNAi lines were obtained from the BL and the Vienna Drosophila Resource Center. The following flies were used: UAS-Sec5RNAi (BL#27526), UAS-Sec6RNAi (BL#27314), UAS-Sec10RNAi (BL#27483), UAS-Sec15RNAi (BL#27499), UAS-Exo84RNAi (BL#28712), UAS-Sec3RNAi (Vienna Drosophila Resource Center #108085), UAS-Sec8RNAi (BL#57441), UAS-Exo70RNAi (BL#28041), UAS-Rab5RNAi (BL#34832), UAS-Rab7RNAi (BL#27051), UAS-Rab11RNAi (BL#42709), UASp-YFP-Rab5 (BL#24616), UASp-YFP-Rab7 (BL#23270), and UASp-YFP-Rab11 (BL#9790). Generation of MHC-ANF-RFP has been described in detail.8 Hand-GFP was used to label nephrocytes at all developmental stages.31
Atrium Natriuretic Factor-Red Fluorescent Protein Uptake Assay
The assay was performed as previously described.8 Briefly, ten female flies carrying MHC-ANF-RFP, Hand-GFP, and Dot-Gal4 transgenes were crossed to five male flies carrying a UAS-ExocystX-RNAi–targeting transgene at 25°C. Two days after crossing, flies were transferred to small egg collection cages with grape juice agar plates for 24 hours at 25°C. The collected embryos were maintained at 29°C, and red fluorescent protein (RFP) fluorescence in pericardial nephrocytes was recorded for second instar larvae and newly emerged adults (within 24 hours of eclosion). For quantification, 20 nephrocytes were analyzed from three larvae and three flies each per genotype. The results are presented as mean±SD. Results were analyzed by t test. P<0.05 was considered statistically significant.
Dextran Uptake Assay
Flies from Hand-GFP and Dot-Gal4 transgenic lines were crossed to flies from the UAS-RNAi transgenic lines at 25°C. One day after egg laying, embryos were shifted to 29°C. Dextran uptake by pericardial nephrocytes was assessed ex vivo in adult flies 1 day postemergence by dissecting heart tissues into Drosophila Schneider Medium (Thermo Fisher) and examining cells by fluorescence microscopy after a 20-minute incubation with Texas Red–labeled Dextran (10 kD, 0.05 mg/ml). For quantification, ≥20 nephrocytes were analyzed from three female flies each per genotype. The results are presented as mean±SD; t test was used to analyze the data. P<0.05 was considered statistically significant.
Antibodies and Fluorescent Immunochemistry
The mouse monoclonal anti-Pyd antibody (PYD2) was obtained from the Developmental Studies Hybridoma Bank. The chicken anti-Sns antibody was a gift from M. Krahn.32 The guinea pig anti-Sec15 antibody was provided by H. Bellen.33 The rabbit anti-Rab11 antibody was from A. Nakamura.34 The Alexa Fluor–conjugated secondary antibodies were ordered from Thermo Fisher. Pericardial nephrocytes were dissected in artificial hemolymph. For Sns and Pyd colabeling, nephrocytes were heat fixed in 100°C artificial hemolymph for 20 seconds as described previously.32 For Sec15 and Rab11 immunochemistry, nephrocytes were fixed in 8% paraformaldehyde (PFA) for 10 minutes. The nephrocytes were washed with 1× PBS with 0.1% Triton X-100 (PBST) three times, blocked in PBST+2% BSA for 1 hour, incubated with primary antibodies at 4°C overnight, washed with PBST three times, incubated with secondary antibodies at room temperature for 2 hours, washed three times, stained with 4′,6-diamidino-2-phenylindole, and mounted with Vectashield mounting medium (H-1000; Vector Laboratories).
Confocal Imaging, Image Quantification, and TEM
For atrium natriuretic factor (ANF)-RFP and Dextran uptake assays, the imaging was performed with a Zeiss Apotome.2 microscope using a 20× Plan-Apochromat 0.8 numerical aperture air objective. For quantitative comparisons of fluorescence intensity, standardized settings were used for all imaging. For immunofluorescence staining of nephrocytes, super-resolution confocal imaging was performed with a Zeiss LSM900 microscope using a 63× Plan-Apochromat 1.4 numerical aperture oil objective under Airyscan SR mode. ImageJ Software and Adobe Photoshop were used for image processing. Samples for TEM were prepared using standardized procedures. Briefly, third instar wandering larvae of the indicated genotypes were fixed using Sorensen phosphate buffer containing 4% paraformaldehyde and 2.5% glutaraldehyde. The processed samples were imaged using a Philips CM100 TEM.
Quantification of Slit Diaphragm Intervals, Lacunar Channels, and Exocyst Rods
The number of nephrocyte diaphragms in a 2500×1500-nm area of TEM images at ×46,000 magnification was counted from three different images. To quantify the total length of lacunae, we marked each lacuna with asterisks every 400 nm and then, counted the total number of asterisks in a 2500×1500-nm area of TEM images at ×46,000 magnification. The results are expressed as mean±SD. All statistical analyses were performed using GraphPad Prism software. The unpaired t test was performed with two-tailed P values and 95% confidence intervals. P<0.05 was considered statistically significant.
Results
Exocyst Genes Are Required for Protein and Particle Absorption by Nephrocytes
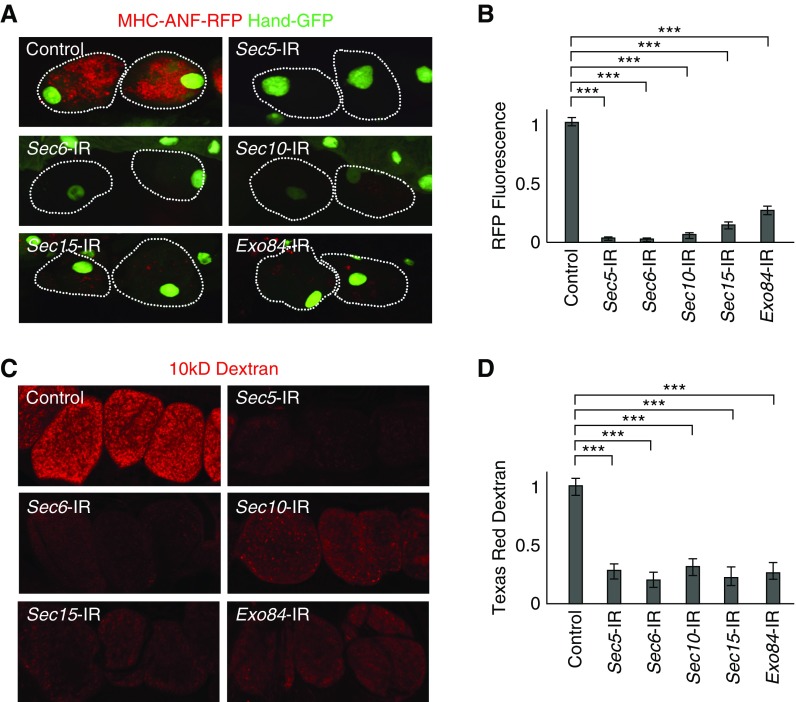
We previously performed a large-scale RNAi screen to identify fly genes essential for protein absorption by nephrocytes.8 Among the hundreds of genes identified in the course of this screen, Sec5, Sec6, Sec10, Sec15, and Exo84 encode subunits of the octameric exocyst complex, which also contains Sec3, Sec8, and Exo70. All eight of these subunits are highly conserved from flies to humans (Supplemental Table 1). To assess the functional consequences of exocyst gene silencing in nephrocytes, we quantitatively measured uptake of protein from the hemolymph in vivo.8 In flies carrying MHC-ANF-RFP, the myosin heavy chain promoter directs muscle cell expression of a rat ANF-RFP fusion protein that is secreted into the hemolymph. ANF-RFP is typically taken up by healthy nephrocytes, and the intracellular red fluorescence can be readily visualized and quantitated. The control image panel in Figure 1A shows normal levels of ANF-RFP fusion protein accumulated in wild-type nephrocytes of 1-day-old adult flies. To assess exocyst gene silencing effects on nephrocyte function, fly lines carrying UAS-RNAi transgenes targeting specific exocyst genes35 were crossed to flies carrying a Dot-Gal4 nephrocyte-specific “driver,”36 MHC-ANF-RFP. Newly emerged 1-day-old adult progeny flies of genotype Dot-Gal4; UAS-RNAi; MHC-ANF-RFP in which a given exocyst gene was silenced by RNAi in nephrocytes were tested for ANF-RFP absorption in vivo. As shown in Figure 1, A and B, silencing of any one of the exocyst genes Sec5, Sec6, Sec10, Sec15, or Exo84 led to severely reduced levels of RFP fluorescence within nephrocytes, suggesting that nephrocytes require the intact exocyst complex to function properly. These observations confirmed the initial identification of these exocyst genes as positive “hits” in our functional genetic screen.8 We also tested RNAi lines for the remaining genes: Sec3, Sec8, and Exo70 of the exocyst octameric complex. However, the phenotypes of Sec3 and Exo70 were much weaker, and Sec8 RNAi knockdown lead to severe nephrocyte defects at larvae stage and abolished nephrocytes in adult flies (data not shown), which could be due to varying knockdown efficiencies among those RNAi transgenic lines. Therefore, this study focuses on Sec5, Sec6, Sec10, Sec15, and Exo84 because RNAi inhibition of these genes in flies was found to be more consistent and efficient. Furthermore, we found that Sec15 protein level was reduced in not only Sec15- but also, Sec5-, Sec6-, Sec10-, and Exo84-silenced nephrocytes (Supplemental Figure 1). This suggests Sec15 is a key component to maintain the integrity and stability of the exocyst complex, which is consistent with previous findings in yeast.37
Figure 1.
The exocyst complex is required for hemolymph protein and Dextran uptake by Drosophila nephrocytes. (A) Nephrocytes of recently emerged (within the previous 24 hours) adult flies expressing high levels of ANF-RFP fusion protein in the hemolymph. Fluorescence microscopy showing myosin heavy chain-ANF-RFP–derived hemolymph ANF-RFP (red) accumulation in nephrocytes. Dotted lines (white) indicate nephrocyte cell boundaries as revealed by cytoplasmic green fluorescent protein (GFP) marker expression. Wild-type (control) flies normally expressing all exocyst complex component proteins accumulated abundant ANF-RFP. Subsequent panels show that adult nephrocytes expressing the indicated exocyst gene RNAi silencing construct (e.g., Sec5-IR) were severely compromised in terms of ANF-RFP accumulation. (B) Quantification of relative ANF-RFP fluorescence in nephrocytes expressing the indicated gene silencing RNAi construct (e.g., Sec5-IR). Total cellular red fluorescence expressed as a fraction of control nephrocyte fluorescence. For quantification, 20 nephrocytes were analyzed from each of three flies per indicated genotype. The results are presented as mean±SD. Statistics are by t test, and all gene silencing cases were found to be significantly reduced compared with control. ***P<0.001. (C) Effects of nephrocyte-specific exocyst gene silencing on accumulation of Texas Red–labeled 10-kD Dextran particles. Particles were readily taken up and accumulated by wild-type control nephrocytes in an ex vivo assay. Silencing of any of the exocyst genes clearly reduced levels of accumulated Dextran. (D) Quantification of relative Dextran-linked Texas Red fluorescence in nephrocytes expressing the indicated gene silencing RNAi construct (e.g., Sec5-IR). Total cellular red fluorescence expressed as a fraction of control nephrocyte fluorescence. For quantification, 20 nephrocytes were analyzed from each of three flies per indicated genotype. The results are presented as mean±SD. Statistics are by t test, and all gene silencing cases were found to be significantly reduced compared with control. ***P<0.001.
In an independent ex vivo functional assay, we tested the ability of dissected nephrocytes to absorb Texas Red–labeled 10-kD Dextran particles. Again, silencing of exocyst genes induced a marked deficiency in intracellular Texas Red fluorescence compared with control wild-type nephrocytes (Figure 1, C and D). These observations indicate that exocyst genes are required for normal nephrocyte absorption function.
The Exocyst Complex Is Essential for Normal NSD Component Localization and Cell Surface Cytoarchitecture
In mammalian kidney, the filtering function and reabsorption function are mainly performed by podocytes and PT cells, respectively. In Drosophila, nephrocytes combine both the filtering function and reabsorption function. The filtration apparatus of nephrocytes is characterized by the NSD, which is analogous to the slit diaphragm of the mammalian kidney at the molecular, structural, and functional levels. The NSD excludes larger hemolymph components from the lacunar channels. These channels are infoldings of the nephrocyte plasma membrane, and they are the main sites of protein reabsorption. The “mouth” of the channel is spanned by the extracellular domains of membrane protein components of the NSD to form the filter structure. The filtering function and reabsorption function of nephrocytes are closely connected: NSD is required for the formation of the lacunar channel, and disruption of NSD would also abolish the lacunae,4 whereas inhibition of protein reabsorption would partially reduce the number of NSD structures.7 Having demonstrated that exocyst genes are required for protein absorption, we further investigated whether the exocyst complex might also be essential for maintaining the integrity of the cytoarchitecture of the filtration apparatus.
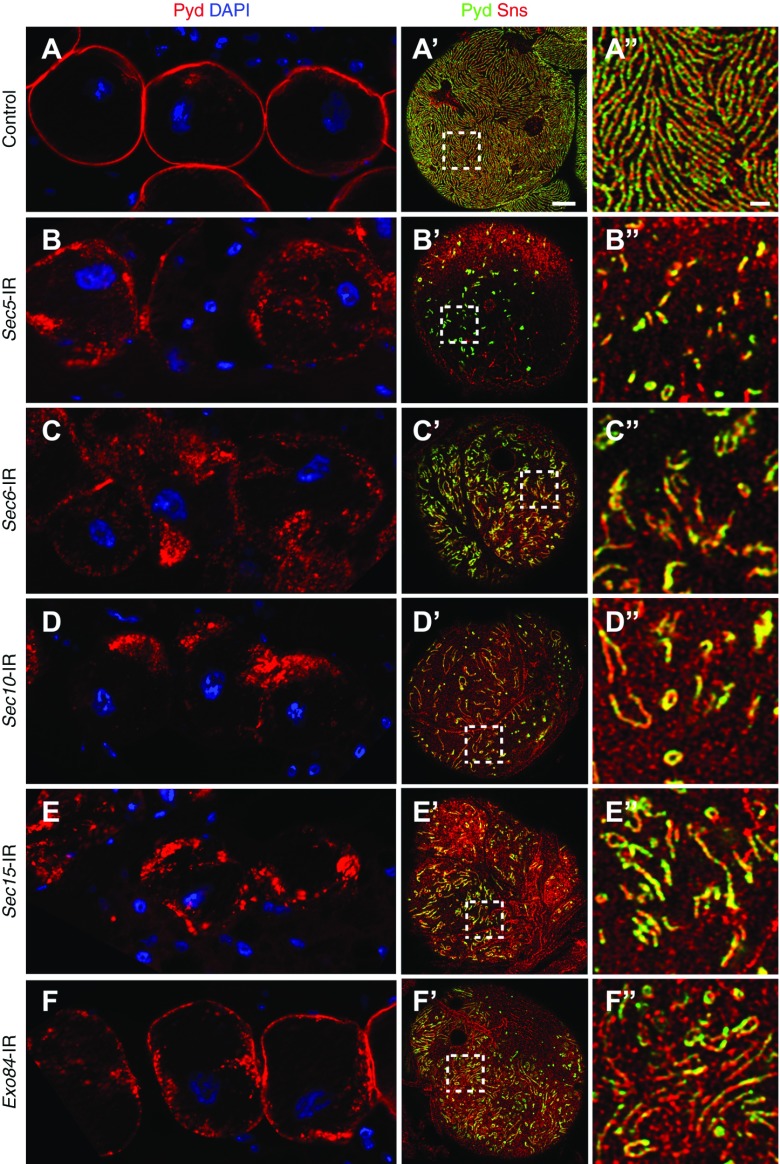
Pyd is the fly ortholog of the tight junction protein ZO-1 that interacts with Neph1 in the mammalian podocyte slit diaphragm.4 We reasoned that, if the exocyst complex is required for maintenance of NSD cytoarchitecture, knockdown of exocyst genes could result in dislocation of NSD components and associated proteins, including Pyd. As shown in Figure 2, knockdown of the exocyst genes resulted in dramatic mislocalization of Pyd at the nephrocyte cell surface. In wild-type nephrocytes imaged in medial plane sections, immunolabeling of Pyd illustrated a fine and continuously delineated circumferential ring. By contrast, in nephrocytes with a silenced exocyst gene, Pyd labeling is strikingly disorganized, ranging from patchy cell surface labeling to various degrees of intracellular aggregation. By high-resolution confocal imaging, we found that Pyd colocalized with Sns in the NSD structures on the nephrocyte cell surface. In wild-type nephrocytes, Pyd/Sns showed a very uniform and smoothly distributed fingerprint-like localization pattern (Figure 2, A′ and A″, Supplemental Figure 2). This pattern was largely disrupted with exocyst gene silencing, resulting in disorganized, shorter fingerprints and occasionally, ring-like structure (Figure 2, B′–F′ and B″–F″). There are some subtle differences in the pattern of Pyd/Sns distribution in cells with different exocyst gene silencing, which may reflect the differences in specific roles of the various members of the exocyst complex. In summary, the above results are consistent with a critical role for exocyst genes in maintaining the NSD cytoarchitecture on the nephrocyte cell surface.
Figure 2.
Exocyst gene silencing disrupts localization of the NSD-associated proteins Pyd and Sns. (A) In control (wild-type) adult fly nephrocytes, Pyd (red) was tightly localized to the cell margin in the medial optical section, defining a highly regular, continuous circumferential ring. (A′ and A″) Immunofluorescence detection of Pyd (green) and Sns (red) in the plane of the cell surface showed a highly regular and uniform fingerprint-like pattern of distribution in wild-type control adult nephrocytes. Scale bar, 5 µm in A′; 1 µm in A″. (B–F, B′–F′, and B″–F″) Silencing of any of the exocyst genes resulted in a clear disruption of normal Pyd localization at the both cell margin and cell surface. Cell nuclei are labeled by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue).
Defective Protein Uptake and Abnormal NSD Cytoarchitecture Resulting from Disrupted Vesicle Recycling
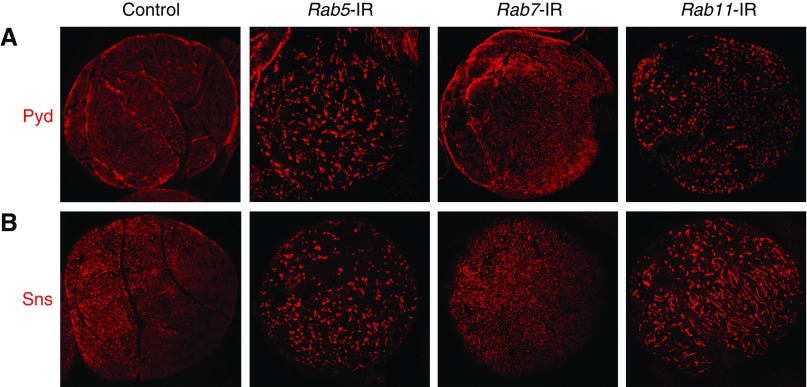
Many membrane-associated proteins undergo active endocytosis and were transported into early endosomes; then, they were either sorted into late endosomes or recycling endosomes.38,39 We previously systematically characterized nephrocyte vesicle trafficking functions of Rab proteins, which play essential roles in regulating endocytosis and recycling.40 Specifically, we reported that nephrocyte silencing of the Rab5 gene, which is required for early endosome biogenesis, prevented ANF-RFP uptake by nephrocytes by eliminating all intracellular vesicles and disrupting essential NSD structures. Rab11 gene silencing in turn blocked vesicle recycling, leading to a loss of protein uptake function similar to that shown in Figure 1. By contrast, Rab7 gene silencing blocked vesicle trafficking to lysosomes but resulted in no detectable (larvae) or minor effects (adult flies) on ANF-RFP uptake.40 We examined Pyd and Sns distribution on the surface of nephrocytes in which Rab5, Rab7, or Rab11 gene expression was silenced by RNAi (Figure 3) and found that Rab5 and Rab11 gene silencing was associated with severe Pyd and Sns mislocalization phenotypes highly similar to the phenotype of exocyst complex gene knockdown. Rab7 silencing, by contrast, did not seem to alter Pyd or Sns localization and showed even distribution of these proteins across the cell surface. As Rab5 and Rab11 are critical regulators of early endosomes and recycling endosomes, respectively, the above observations suggested that there is an endocytosis and recycling balance at baseline to maintain the functional localization of NSD proteins. We hypothesize that slit diaphragm proteins are endocytosed from NSD structures at the nephrocyte plasma membrane into early endosomes and then, sorted into Rab11-dependent recycling endosomes to maintain NSD cytoarchitecture.
Figure 3.
Pyd and Sns distribution is disrupted by Rab5 or Rab11 gene silencing. (A) Pyd and (B) Sns protein distribution on the cell surface of wild-type and Rab5-, Rab7-, and Rab11-silenced (Rab5-IR, Rab7-IR, and Rab11-IR, respectively) nephrocytes. Silencing of Rab5 and Rab11 but not Rab7 severely disrupted Pyd and Sns distribution.
To test this hypothesis, we investigated the association of Pyd with Rab5, Rab7, and Rab11 on the cell surface to determine whether NSD proteins move into the endocytic and recycling vesicles. As shown in Supplemental Figure 3, we detected partial colocalization of Pyd with Rab5 and to greater extent, with Rab11 at the cell surface, supporting that Pyd and presumably, other NSD component proteins undergo an endocytosis and recycling process to maintain the NSD. We noticed that the fraction of colocalization is small; probably only a small portion of Pyd (indicated by arrowheads in Supplemental Figure 3) is required to undergo this dynamic trafficking process to maintain the functional localization of Pyd proteins at the NSD as a whole. In contrast, Pyd did not colocalize with Rab7, the majority of which was observed well beneath the cell surface (Supplemental Figure 3B), suggesting very limited lysosome-mediated protein degradation of the NSD proteins.
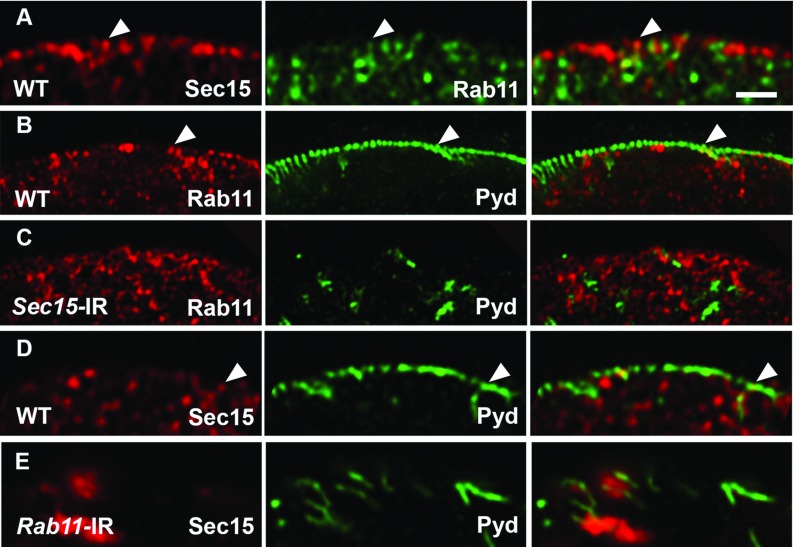
In both Drosophila and mammals, Sec15 interacts with Rab11 through its C-terminal domain.41,42 In wild-type nephrocytes, we found Sec15 localized in small vesicles close to the plasma membrane and partially colocalized with Rab11 (Figure 4, A and D). This suggests that Sec15-Rab11 interaction could mediate recruitment of the exocyst complex to the recycling endosomes. Furthermore, Sec15 and Rab11 partially colocalize with Pyd, consistent with a role for exocyst in mediating the recycling of NSD proteins to maintain the NSD (Figure 4, B and D). We also found that Rab11 silencing in nephrocytes resulted in aggregation of Sec15 protein (Figure 4E, Supplemental Figure 4), but Sec15 silencing did not influence the distribution of Rab11 (Figure 4, B and C), further supporting that the function of the exocyst complex is dependent on the recycling machinery. In summary, the exocyst complex interacts with Rab11 and thereby, mediates the recruitment of NSD proteins to recycling endosomes for functional recycling.
Figure 4.
Pyd colocalized with Rab11 and Sec15 on the nephrocyte cell surface, and Rab11 recruits Sec15 to recycling endosomes. (A) Wild-type (WT) adult nephrocyte immunodetection of Sec15 (red; left panel) and Rab11 (green; center panel). The merged images are shown in the right panel, with yellow indicating overlapping red and green fluorescence. Sec15 and Rab11 partially colocalize in vesicles close to the cell membrane. (B and C) Immunodetection of Rab11 (red) and Pyd (green) in (B) control (WT) and (C) Sec15-silenced nephrocytes. Note that Rab11 localization is similar between WT and Sec15-silenced nephrocytes. (D and E) Immunodetection of Sec15 (red) and Pyd (green) in (D) WT and (E) Rab11-silenced nephrocytes. (D) Sec15 partially colocalized with Pyd in WT nephrocytes. (E) Note that Sec15 formed aggregates in Rab11-silenced nephrocytes. Arrowheads indicate colocalization between two proteins. Scale bar, 1 μm.
Exocyst Gene Silencing Resulted in Reduction of Lacuna Channels, Disruption of NSD-Associated Cell Ultrastructure, and Accumulation of “Exocyst Rods,” a Novel Unique Electron-Dense Membranous Structure
Nephrocytes with exocyst gene knockdown fail to reabsorb proteins in the hemolymph (Figure 1). Although our findings demonstrate that NSD cytoarchitecture was disrupted, which leads to reduced reabsorption, whether the exocyst complex could directly affect the lacunar channel and thus, the reabsorption function is still unknown.
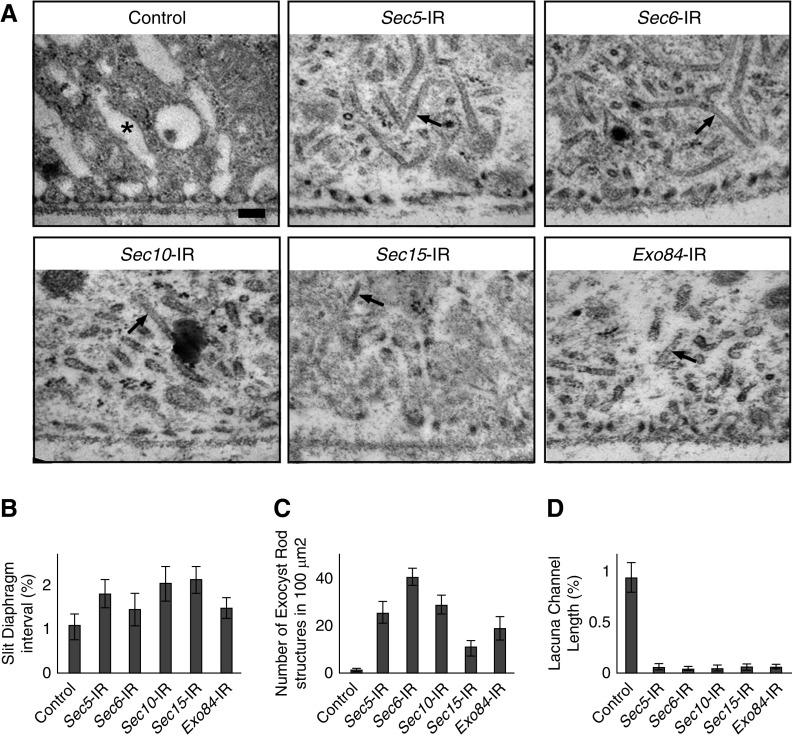
We used TEM to examine the ultrastructure of pericardial nephrocytes isolated from third instar larvae with exocyst gene knockdown. At this developmental stage, NSD cytoarchitecture at surface membrane remains largely intact in nephrocytes with exocyst gene knockdown, with slightly increased interval spacing between slit diaphragms (Figure 5). Silencing of any of the exocyst genes resulted in a striking reduction in the length of the lacuna channels (Figure 5, A and D). In fact, lacuna channels were almost completely absent. This phenotype occurred before the slit diaphragm defect and is consistent with a block in membrane recycling due to the absence of a functional exocyst complex to tether recycling vesicles to the lacuna channel (Figure 6).
Figure 5.
Exocyst gene silencing disrupts NSD structure and associated cytoarchitecture, eliminates lacuna channels, and induces formation of novel electron-dense structures. (A) TEM of wild-type third instar larva nephrocytes (control) shows the normal characteristic ultrastructural features of regularly spaced circumferential slit diaphragms spanning the openings of lacuna channel membrane invaginations. Exocyst gene silencing eliminated lacuna channels and altered the morphology of slit diaphragms and slit diaphragm spacing. The peripheral cytoplasm normally occupied by lacuna channels instead became filled with novel electron-dense tubular structures that we named “exocyst rods.” Arrows indicate a typical exocyst rod. *Typical lacuna channel. (B) Quantitative analysis of interval length between slit diaphragms showed an increase from the normal pattern. (C) The zipper-like electron-dense exocyst rod structures were extremely rare in wild-type nephrocytes, and abundance varied among nephrocytes expressing different exocyst gene-silencing constructs. (D) Quantitative analysis of lacuna channel numbers revealed that these ultrastructural features were almost completely eliminated by exocyst gene silencing.
Figure 6.
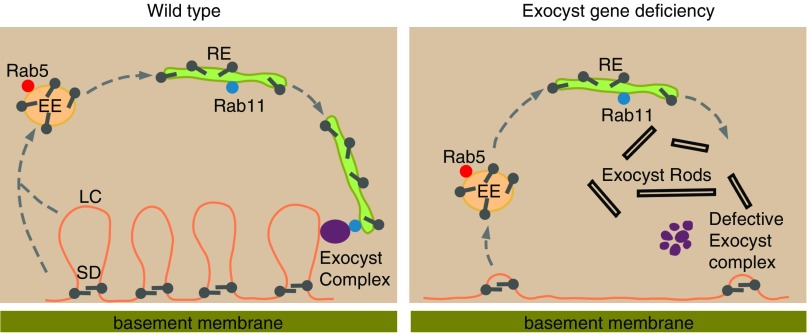
Schematic representation of exocyst gene silencing–induced disruption of membrane vesicle recycling that leads to loss of lacuna channels, altered slit diaphragm cytoarchitecture, and formation of electron-dense structures. The left panel shows that, in normal (wild-type) nephrocytes, plasma membrane and associated proteins from the lacunar channel (LC) and the slit diaphragm are endocytosed and went into Rab5-containing early endosomes (EEs). Some proteins will be sorted into late endosomes and then, lysosomes for degradation and amino acid recycling (not shown). LC membrane and slit diaphragm proteins are sorted into Rab11-containing recycling endosomes (REs). Rab11 recruits the exocyst complex, and the REs are tethered by the exocyst complex to be reincorporated into the LC and the slit diaphragm. The right panel depicts the effects of exocyst gene deficiency by nephrocyte-specific exocyst gene silencing. Here, the process of continuous endocytosis and recycling of LC membrane and slit diaphragm proteins is disrupted. The LC is depleted, and novel electron-dense exocyst rod structures are formed. Failure to recycle endocytosed slit diaphragm components leads to NSD structure and localization abnormalities. SD, slit diaphragm.
In the area that lacuna channels are normally formed, we observed accumulation of novel electron-dense structures that we named “exocyst rods” (Figure 5, A and C), which were not detectable in wild-type nephrocytes. The exocyst rod structures might reflect the accumulation of endosomal membranes harboring NSD proteins Pyd and Sns (Supplemental Figure 5) due to the impaired surface recycling. The composition, biogenesis, and significance of these intriguing exocyst rod structures warrant further investigation.
Discussion
Our previous in vivo functional genetic screen8 demonstrated that exocyst genes are critical for the protein absorption phenotype of nephrocytes. Here, our dedicated in vivo Drosophila model confirmed that nephrocyte-specific silencing of the exocyst complex genes results in impaired protein uptake due to a loss of lacuna channels and disrupted surface distribution of slit diaphragm components Pyd and Sns with resultant disrupted NSD ultrastructure.
Endocytosis is critical for normal podocyte physiology and function.17,43–45 It has been shown in a mouse model that deletion of genes required for clathrin-mediated endocytosis in podocytes resulted in proteinuria and kidney failure, with glomeruli exhibiting collapsed foot processes and features resembling FSGS.17 These observations suggest that the slit diaphragm is not static but requires dynamic removal and replacement or recycling of associated proteins to maintain its integrity.43–45 Recently, it was reported that podocyte-specific knockout of the gene encoding the central exocyst component Exoc5 led to severe proteinuria and pathologic glomerular features, including foot process effacement and loss of slit diaphragm.30 We characterized RNAi silencing effects for each of the eight genes of the exocyst complex and found that five of them showed strong and consistent phenotypes in nephrocytes, with reduced filtering/reabsorption function and disruption of slit diaphragm structure. Thus, the exocyst complex is critical for normal podocyte structure and function.
The exocyst complex tethers exocytic and recycling vesicles to the plasma membrane prior to membrane fusion.22,46 In Drosophila neurons, the exocyst has been shown to regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane.47 In oocytes, the exocyst protein Sec5 localizes to endocytic vesicles, and the exocyst was shown to be recruited to clathrin-coated pits to facilitate rapid recycling of proteins.48 We investigated the role of exocyst in the trafficking of NSD proteins and found that NSD protein Pyd partially colocalizes with the early endosome regulator Rab5 as well as with the exocyst complex subunit Sec15 and the recycling endosome regulator Rab11. Silencing of Rab5, Rab11, or exocyst complex genes in nephrocytes all disrupted the normal (wild-type) NSD cytoarchitecture. We propose that the NSD proteins undergo physiologic endocytosis from the plasma membrane, enter the early endosome with the help of Rab5, and then, are sorted into the Rab11-dependent recycling endosome. Through interaction with Sec15, Rab11 recruits the exocyst complex, thereby tethering the recycling endosome to the surface membrane, which then fuses and delivers the slit diaphragm proteins. Under pathologic conditions in which the exocyst complex is compromised, the recycling endosome cannot be tethered and fused to the surface membrane. Instead, the recycling endosomes harboring the cargo NSD proteins form the novel electron-dense exocyst rod structures inside the cell (Figure 6).
Finally, we investigated the role of exocyst in the tubular-like function of nephrocytes in absorbing hemolymph proteins, which were bound within the lacuna channel by a CUBN and AMN membrane coreceptor complex. CUBN and AMN are fly homologs to the mammalian CUBILIN and Megalin coreceptors found in PT cells. The cargo-coreceptor complex can be endocytosed together with the lacuna channel membrane components; then, they are either sorted into the late endosome and lysosome to be degraded or sorted into the recycling endosome to replenish the loss of lacuna channel membrane. We found that the exocyst gene deficiency blocks this membrane proteins recycle, thereby depleting the lacuna channel. The accumulated cargo-coreceptor complex and lacuna channel membrane components might also contribute to the accumulation of the exocyst rod structures (Figure 6).
In conclusion, we successfully used Drosophila nephrocyte-specific gene silencing as a model to elucidate the critical role of exocyst. We found the exocyst complex is essential in maintenance of the filtration and absorption functions of Drosophila nephrocytes, a cell type resembling mammalian podocytes and PT cells. This model will be beneficial to answer important further questions and to help identify therapeutic targets for the treatment of renal diseases with molecular defects in vesicle endocytosis, exocytosis, and recycling. Additional dedicated work is needed to help us understand the regulation of slit diaphragm proteins and the pathogenesis of exocyst deficiency in podocytes.
Disclosures
None.
Funding
Dr. Han was supported by National Institutes of Health grants R01DK098410 and R01DK120908.
Supplementary Material
Acknowledgments
We thank the Bloomington Drosophila Stock Center and the Vienna Drosophila Resource Center for Drosophila stocks. We thank Dotty Sorenson for assistance in electron microscopy (transmission electron microscopy) and Adam Richman and Joyce van de Leemput for editing of the manuscript.
Dr. Zhang performed the genetic screen, identified exocyst genes, and carried out the nephrocyte functional assay. Dr. Wen conceived the slit diaphragm structure analysis and carried out the immunofluorescence antibody staining and confocal microscopy. Dr. Fu and Dr. Zhu prepared samples for transmission electron microscopy analysis. Dr. Han supervised the project, analyzed the data, and wrote the manuscript. The manuscript was critically reviewed by all authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060591/-/DCSupplemental.
Supplemental Figure 1. Sec15 protein level decreased in exocyst gene-silenced nephrocytes.
Supplemental Figure 2. Pyd and Sns colocalize at nephrocyte slit diaphragm in nephrocytes.
Supplemental Figure 3. Pyd colocalized with Rab5 and Rab11 on the nephrocyte cell surface.
Supplemental Figure 4. Rab11 is required for Sec15 localization.
Supplemental Figure 5. Exocyst gene silencing disrupts Pyd and Sns membrane localization and leads to accumulation inside the nephrocyte.
Supplemental Table 1. Components of the human exocyst complex and the corresponding Drosophila homologs.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Christensen EI, Verroust PJ, Nielsen R: Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch 458: 1039–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, et al.: Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al.: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Zhao Y, Chao Y, Muir K, Han Z: Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol 24: 209–216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na J, Cagan R: The Drosophila nephrocyte: Back on stage. J Am Soc Nephrol 24: 161–163, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Chen X: The Drosophila nephrocyte has a glomerular filtration system. Nat Rev Nephrol 10: 491, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Helmstädter M, Simons M: Using Drosophila nephrocytes in genetic kidney disease. Cell Tissue Res 369: 119–126, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Helmstädter M, Huber TB, Hermle T: Using the Drosophila nephrocyte to model podocyte function and disease. Front Pediatr 5: 262, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Martin CE, Jones N: Nephrin signaling in the podocyte: An updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol (Lausanne) 9: 302, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, et al.: Beta-arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, et al.: Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermle T, Schneider R, Schapiro D, Braun DA, van der Ven AT, Warejko JK, et al.: GAPVD1 and ANKFY1 mutations implicate RAB5 regulation in nephrotic syndrome. J Am Soc Nephrol 29: 2123–2138, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorval G, Kuzmuk V, Gribouval O, Welsh GI, Bierzynska A, Schmitt A, et al.: TBC1D8B loss-of-function mutations lead to X-linked nephrotic syndrome via defective trafficking pathways. Am J Hum Genet 104: 348–355, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostov KE, Verges M, Altschuler Y: Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol 12: 483–490, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lipschutz JH, Mostov KE: Exocytosis: The many masters of the exocyst. Curr Biol 12: R212–R214, 2002 [DOI] [PubMed] [Google Scholar]
- 22.He B, Guo W: The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21: 537–542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei K, Li Y, Wang S, Shao G, Wang J, Ding Y, et al.: Cryo-EM structure of the exocyst complex. Nat Struct Mol Biol 25: 139–146, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, et al.: The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun 319: 138–143, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Zuo X, Guo W, Lipschutz JH: The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20: 2522–2529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, et al.: The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7: e1001361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachury MV, Seeley ES, Jin H: Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26: 59–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo X, Lobo G, Fulmer D, Guo L, Dang Y, Su Y, et al.: The exocyst acting through the primary cilium is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis. J Biol Chem 294: 6710–6718, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon-Salazar TJ, Silhavy JL, Udpa N, Schroth J, Bielas S, Schaffer AE, et al.: Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med 4: 138ra78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nihalani D, Solanki AK, Arif E, Srivastava P, Rahman B, Zuo X, et al.: Disruption of the exocyst induces podocyte loss and dysfunction. J Biol Chem 294: 10104–10119, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han Z, Olson EN: Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132: 3525–3536, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Hochapfel F, Denk L, Mendl G, Schulze U, Maaßen C, Zaytseva Y, et al.: Distinct functions of Crumbs regulating slit diaphragms and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci 74: 4573–4586, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta SQ, Hiesinger PR, Beronja S, Zhai RG, Schulze KL, Verstreken P, et al.: Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46: 219–232, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Nakamura A: The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development 135: 1107–1117, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al.: A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 37.TerBush DR, Maurice T, Roth D, Novick P: The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15: 6483–6494, 1996 [PMC free article] [PubMed] [Google Scholar]
- 38.Jovic M, Sharma M, Rahajeng J, Caplan S: The early endosome: A busy sorting station for proteins at the crossroads. Histol Histopathol 25: 99–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naslavsky N, Caplan S: The enigmatic endosome—sorting the ins and outs of endocytic trafficking. J Cell Sci 131: jcs216499, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Zhu JY, Zhang F, Richman A, Zhao Z, Han Z: Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes. Cell Tissue Res 368: 615–627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA: Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12: 879–885, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T: Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 279: 43027–43034, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Sampogna RV, Al-Awqati Q: Taking a bite: Endocytosis in the maintenance of the slit diaphragm. J Clin Invest 122: 4330–4333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soda K, Ishibe S: The function of endocytosis in podocytes. Curr Opin Nephrol Hypertens 22: 432–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swiatecka-Urban A: Endocytic trafficking at the mature podocyte slit diaphragm. Front Pediatr 5: 32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oztan A, Silvis M, Weisz OA, Bradbury NA, Hsu SC, Goldenring JR, et al.: Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell 18: 3978–3992, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, et al.: Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 9: 365–376, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Sommer B, Oprins A, Rabouille C, Munro S: The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster. J Cell Biol 169: 953–963, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.