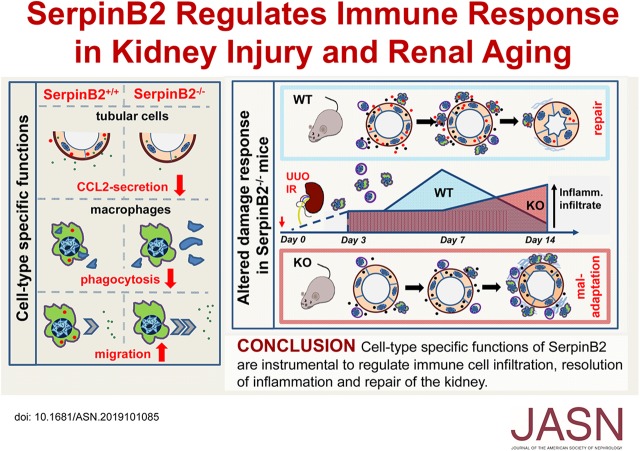
Significance Statement
Injured tubular cells activate a kidney survival program that includes complex crosstalk between tubular cells and macrophages. The authors show that SerpinB2, known to be expressed in activated macrophages, is also upregulated in stressed tubular cells. By subjecting knockout mice lacking SerpinB2 to renal stress, they show that SerpinB2 promotes proreparative adaptation of the kidney by two cell type–specific mechanisms: it enhances expression of the chemokine CCL2 in tubular cells, which supports transient intrarenal leukocyte accumulation, and it regulates function of macrophages by activating phagocytosis and inhibiting migration. These functions are crucial for timely resolution of inflammation, successful repair, and kidney homeostasis during aging. These findings suggest that SerpinB2 merits further exploration for its role in the human kidney in acute and chronic disease.
Keywords: acute renal failure, chronic kidney disease, Chronic inflammation, kidney tubule, interstitial fibrosis, macrophages
Visual Abstract
Abstract
Background
Expression of SerpinB2, a regulator of inflammatory processes, has been described in the context of macrophage activation and cellular senescence. Given that mechanisms for these processes interact and can shape kidney disease, it seems plausible that SerpinB2 might play a role in renal aging, injury, and repair.
Methods
We subjected SerpinB2 knockout mice to ischemia-reperfusion injury or unilateral ureteral obstruction. We performed phagocyte depletion to study SerpinB2’s role beyond the effects of macrophages and transplanted bone marrow from knockout mice to wild-type mice and vice versa to dissect cell type–dependent effects. Primary tubular cells and macrophages from SerpinB2 knockout and wild-type mice were used for functional studies and transcriptional profiling.
Results
Cultured senescent tubular cells, kidneys of aged mice, and renal stress models exhibited upregulation of SerpinB2 expression. Functionally, lack of SerpinB2 in aged knockout mice had no effect on the magnitude of senescence markers but associated with enhanced kidney damage and fibrosis. In stress models, inflammatory cell infiltration was initially lower in knockout mice but later increased, leading to an accumulation of significantly more macrophages. SerpinB2 knockout tubular cells showed significantly reduced expression of the chemokine CCL2. Macrophages from knockout mice exhibited reduced phagocytosis and enhanced migration. Macrophage depletion and bone marrow transplantation experiments validated the functional relevance of these cell type–specific functions of SerpinB2.
Conclusions
SerpinB2 influences tubule-macrophage crosstalk by supporting tubular CCL2 expression and regulating macrophage phagocytosis and migration. In mice, SerpinB2 expression seems to be needed for coordination and timely resolution of inflammation, successful repair, and kidney homeostasis during aging. Implications of SerpinB2 in human kidney disease deserve further exploration.
CKD is a major clinical problem and a growing public health burden worldwide. Despite intensive research, the number of therapeutic targets to prevent the development of CKD is limited. A common element in many forms of CKD is a prolonged or repetitive damage to tubular cells. When tubular cells are injured, they activate a survival program that can be adaptive and restore epithelial integrity or maladaptive, leading to the transition from AKI to CKD.1 The tubular survival program is incompletely understood but includes a cascade of cell-autonomous responses and a complex crosstalk with surrounding stromal and immune cells.2–4 The mononuclear phagocyte system, in particular macrophages, plays an important role in this crosstalk because they have emerged as key players in both the initial injury process and the subsequent recovery.5
An integral cell program that can promote both inflammation and failed recovery is cellular senescence, a persistent cell cycle arrest that occurs in the kidney with age and chronic and acute damage.6–10 We were interested in the role of SerpinB2 (or plasminogen activator inhibitor-2 [PAI-2]), a protein implicated in the interplay between cellular senescence, inflammation, and maladaptive repair.11–13 SerpinB2 was first described as a secreted protein inhibiting extracellular urokinase plasminogen activator (uPA), but it was later recognized that SerpinB2 also affects a variety of intracellular functions.14–16 SerpinB2 is known as a regulator of inflammatory processes with a strong expression in activated macrophages.17–21 SerpinB2 has been associated with a number of extrinsic inflammatory and autoimmune conditions, including asthma and infections,17,18,22 but there are no data so far on a potential involvement in kidney disease. Here, we investigated the role of SerpinB2 in kidney aging and injury.
Methods
Mice
SerpinB2+/− mice from Jackson Laboratory (B6.129S1-Serpinb2tm1Dgi/J; JAX stock #007234)23 were mated together to generate both homozygous Serpinb2 knockout and homozygous Serpinb2 wild-type littermates, thereby ensuring an identical genetic background. With these mice, a homozygous line was maintained for each genotype to obtain mice for experimental conditions. Mice were housed under standard conditions to breed wild-type and knockout mice. Genotyping PCR was done according to the Jackson Laboratory–recommended protocol. Male mice between 3 and 4 or 22 and 24 months (aged cohort) were used for experiments. All experimental protocols were in agreement with institutional and legislator regulations and approved by the local authorities.
Unilateral Ureteral Obstruction, Ischemia-Reperfusion Surgery, and Bone Marrow Transplantation
The mice used were 3- to 4-month-old male wild-type and SerpinB2−/− mice, and they were used for unilateral ureteral obstruction (UUO) and ischemia-reperfusion (IR) experiments. For UUO, mice were anesthetized with isoflurane, and the left ureter was ligated after a midabdominal incision. Mice were euthanized at 3, 7, and 14 days postsurgery to extract the kidneys. Representative kidney sections were snap frozen in liquid nitrogen for RNA and protein isolation, kept in 4% PFA overnight for paraffin embedding, or used to prepare single-cell suspensions for flow cytometry. For phagocytic cell depletion, 0.1 ml/10 g body wt of liposomal clodronate (Liposoma) was injected 24 hours before surgery. Control liposomes were used for vehicle-injected mice. For unilateral IR surgery, mice were anesthetized with isoflurane, and after a midabdominal incision, the left renal pedicle was clamped for 27 minutes while mice were kept on a warming plate at 37°C. Mice were euthanized after 3, 7, 14, and 21 days to extract kidneys. Bilateral IR followed the same protocol, but ischemia was restricted to 18 minutes to ensure survival. After bilateral IR, blood was collected at days 3, 7, and 14 for measurement of serum creatinine and urea with an automated system (Beckman Analyzer; Beckman Instruments GmbH). For bone marrow (BM) transplantation, lethal irradiations (10 Gy) were performed in 6-week-old wild-type and SerpinB2−/− mice. Mice were reconstituted with 1×106 unfractionized BM cells from both genotypes to obtain four recipient groups. Six weeks later, the BM-reconstituted recipients underwent UUO surgery.
Primary Tubular Epithelial Cells
Kidneys of male wild-type and SerpinB2−/− mice were extracted and dissected into small pieces for digestion in 0.125% collagenase type 1 solution (ThermoFisher Scientific) in M199 medium at 37°C for 45 minutes. The solution was then passed through a 40-µm cell strainer, and passed tubules were plated in REGM2 media (Promocell GmbH). When outgrowing primary tubular epithelial cells (PTECs) were 80% confluent, they were either subjected to γ-irradiation (10 Gy) for induction of senescence as previously described24,25 or treated with phorbol 12-myristate 13-acetate (PMA) at 100 ng/ml (Peprotech). For siRNA experiments, specific mouse Ccl2 or scrambled control siRNA (both Sigma-Aldrich) was used at 25 nM with TransIT-S2 as transfection reagent (Mirus Bio LLC). Efficient knockdown was verified by quantitative RT-PCR.
Senescence-Associated β-Galactosidase Staining
PTECs from wild-type and SerpinB2−/− kidneys were cultured and plated in six-well plates until 80% confluent. They were fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS buffer for 10 minutes, permeabilized with 1% Triton X-100, washed with PBS, and incubated at 37°C overnight with freshly prepared staining solution at pH 6.0 (40 mM citric acid, Na phosphate buffer, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM sodium chloride, 2 mM magnesium chloride, and 1 mg/ml X-gal prepared in dimethylformamide). For staining of whole kidneys, tissue was incubated in the staining solution for 4 hours and then, embedded with paraffin. Senescence-associated β-galactosidase (SA-β-gal)–positive area in 3-µm paraffin sections was determined by normalizing the blue-stained area against the total number of cells/tubules using ImageJ software. In case of costaining with SerpinB2 antibody, paraffin sections were cut and treated as described below.
BM-Derived Macrophage Isolation
Wild-type and SerpinB2−/− mice were euthanized. BM was flushed from femur and tibia and plated in RPMI media (Lonza) with 10% FCS and 100 ng/ml M-CSF (Peprotech). Media were changed after 24 hours. Differentiated bone marrow–derived macrophages (BMDMs) were passaged on day 7 and subsequently treated with LPS (100 ng/ml) and 20 ng/ml IFN-γ (both Peprotech) for M1-type macrophage stimulation.
Real-Time PCR
RNA was isolated from frozen kidney tissue or cultured cells using Nucleospin RNA Plus (Machery-Nagel) according to the manufacturer’s instructions. Reverse transcription was performed with M-MLV-Reverse transcription (Promega) and random primers (Promega). Amplified cDNA was used as a template for quantitative PCR. Levels of mRNA expression were determined by quantitative RT-PCR using a Roche Lightcycler 480 System with SYBR green master mix and specific primers as described in Table 1. Melting curves were examined to verify that a single product was amplified. The threshold cycle (Ct) for each individual PCR was determined by the instrument software, and Ct values obtained were normalized to β-Actin gene expression. Quantification of gene expression was done according to the 2−ΔΔCt method.
Table 1.
List of primers used for RT-PCR
| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| SerpinB2 | CTG CTA CCC GAA GGT TCT | GGA AGC AAC AGG AGC ATG C |
| Ngal | TGA AGG AAC GTT TCA CCC GCT TTG | ACA GGA AAG ATG GAG TGG CAG ACA |
| αSma | GTG CTA TGT CGC TCT GGA CTT TGA | ATG AAA GAT GGC TGG AAG AGG GTC |
| β-Actin | CCT CTA TGC CAA CAC AGT GC | CAT CGT ACT CCT GCT TGC TG |
| Ccl2 | TTT GAA TGT GAA GTT GAC CCG TAA | GAA GTG CTT GAG GTG GTT GT GG |
| Fn1 | GTG TGG TTT GGA CGA ATT CCA | CGT CAA ATA GCT GAC TCT TGG C |
| tPA | GATGAAGGTCTGGCTTTGGA | TAT GGA AGG TTG GCA TCT CC |
| uPA | GCC CAC AGACCTGATGC AT | TAGAGCCTT CTG GCC ACA CT |
| uPAR | AGG TGG TGA CAA GAG GCTGT | AGC TCT GGTCCAAAGAGGTG |
| Pai-1 | GCCAGATTTATCATCAATGACTGGG | GGAGAGGTGCACATCTTTCTCAAA G |
| Mmp2 | CTGATAACCTGGATGCCGTCGT | TGC TTC CAAACTTCACGCTCTT |
RNA Microarray
RNA was isolated from PMA-treated PTECs (100 ng/ml for 24 hours). Microarray expression analysis was done at the Hannover Medical School transcriptomics facility. Gene ontology analysis of microarray results was performed by using the web-based DAVID 6.7 tool.26 Prism 8 (GraphPad Software) was used to generate heat maps. The original microarray data are available at the Gene Expression Omnibus database with the accession number GSE139156.
Histology and Immunostainings
For evaluation of histologic damage, deparaffinized sections were stained with hematoxylin/eosin and picosirius red staining solution (Sigma-Aldrich). The picrosirius red staining was analyzed under polarized light, and the amount of birefringence was quantified in ten randomly chosen nonoverlapping fields (×200 magnification) using ImageJ software. For immunostaining, cells were fixed in 4% PFA and permeabilized with Triton X-100 (0.1%), or 3-µm paraffin sections of mouse kidneys were deparaffinized and boiled in citrate buffer, pH 6.0, for antigen retrieval. For double-labeling studies, primary antibodies (Table 2) raised in different species were used in a sequential fashion. Secondary fluorescence-labeled antibody or the ABC Vectastain kit (Vector Laboratories) was used for detection. Staining for SerpinB2, fibronectin (Fn), and α-smooth muscle actin (αSMA) and costaining of SA-β-gal/SerpinB2, p21/SerpinB2, and Ki67/SerpinB2 were quantified in ten randomly chosen nonoverlapping fields (×200 magnification) using ImageJ software or by counting tubular profiles under visual control.
Table 2.
List of primary antibodies
| Primary Antibodies | Catalog No. | Dilution | Company |
|---|---|---|---|
| SerpinB2 | NBP1–3318 | 1:50 | Novus Biologicals, CO |
| SerpinB2 | M20-sc8174 | 1:50 | Santa Cruz, CA |
| αSMA | 48938 | 1:200 | Cell Signaling, MA |
| Fn | NBP1–91258 | 1:200 | Novus Biologic, CO |
| CD45 | 550539 | 1:200 | BD Bioscience, CA |
| p21 | M19-sc471 | 1:200 | Santa Cruz, CA |
| Ki67 | Sp6 | 1:200 | ThermoFischer, MA |
CCL2 ELISA
Homogenized kidney lysates were centrifuged for 10 minutes at 11,000 rcf. Supernatants were collected, and CCL2-content was assessed by a CCL2-specific ELISA performed according to the manufacturer’s instructions (R&D Systems). Absorbance was measured at 495 nm on a Sunrise plate reader (Tecan, Männerdorf, Switzerland).
uPA Activity Assay
The uPA activity assay was performed as recommended by the manufacturer (Chemicon ECM600; Merck Millipore, Darmstadt, Germany). Briefly, homogenized kidney lysates were centrifuged for 10 minutes at 11,000 rcf, and the supernatant was collected. To evaluate the levels of tissue-associated uPA activity, tissue extracts were incubated in 96-well microtiter plates with the supplied chromogenic substrate. After incubation at 37°C for 24 hours, absorbance was assessed at 405 nm using a Sunrise plate reader (Tecan), including a standard curve with uPA-positive control (supplied with the kit) in each assay. uPA activity was normalized for total protein content.
Coculture Transwell Assay
After stimulating with LPS (100 ng/ml) and 20 ng/ml IFN-γ for 24 hours, BMDMs were starved overnight in RPMI media with 1% FCS. The next day, 2×105 BMDMs were plated in transwells (Corning) in a 12-well plate with 5-µm pore size with 0.5×106 PTECs in the lower chamber in RPMI, 1% FCS, and 1% PS medium. Cells were incubated at 37°C for 6 hours, and the transwells were subsequently immersed in 4% PFA to fix those cells that invaded to the bottom of the transwell. Bottom cells were stained using 0.1% solution of crystal violet in 70% ethanol. For invasion of BMDMs against a CCL2 gradient, 10 ng/ml recombinant CCL2 (Peprotech) was added to the bottom well for 1 hour. The number of invaded cells was then counted by imaging at ×200 magnification.
Phagocytosis Assay
In total, 2×105 BMDMs (wild type and SerpinB2−/−) were plated in 12-well plates and stimulated with LPS (100 ng/ml) and 20 ng/ml IFN-γ for 24 hours. Latex beads coated with FITC-rabbit IgG (Cayman Chemicals) were added in 1:500 dilution and incubated for 3 hours. Trypan blue (1:10) was added for 2 minutes to quench the unphagocytosed latex beads on the cell surface. The cells were then fixed with 4% PFA for 20 minutes. The nuclei were stained using Hoechst stain. The amount of phagocytosis was assessed by imaging at ×200 magnification.
Flow Cytometry
Representative tissue sections from contralateral and IR or UUO kidneys were used for flow cytometry. Tissues were weighed and digested in 1.5 ml digestion mix (450 U/ml collagenase type 1, 250 U/ml collagenase type 11, 120 U/ml hyaluronidase type 1, and 120 U/ml DNAse I; all ThermoScientific) at 37°C for 45 minutes to obtain a single-cell suspension. Cells were incubated with staining mix on ice (MHCII-FITC, 1:500; CD206-PE, 1:100; CD11b-PERCP Cy5.5, 1:100; F4/80-APC, 1:100; CD45.5–AF700, 1:100; and L/D-Qdot605, 1:500; all antibodies are from Biolegend) in Trucount tubes (BD Biosciences) and counted on an LSR II (Becton-Dickinson). Data were analyzed by FlowJo software (Tree Star Inc.). After gating for live CD45+ events, myeloid cell populations were assessed using the markers as depicted in Supplemental Figure 1. Absolute cell count was calculated using the weight of renal tissue in relation to Trucount tube bead counts.
Statistical Analyses
Results are expressed as means±SEMs. Statistical significance was determined by unpaired t test and two-way ANOVA with Bonferroni correction for multiple comparisons (GraphPad Software). Significances are represented by asterisks (*P<0.05; **P<0.01; *** P<0.001).
Results
Correlation between Tubular Cell Senescence and SerpinB2 Expression
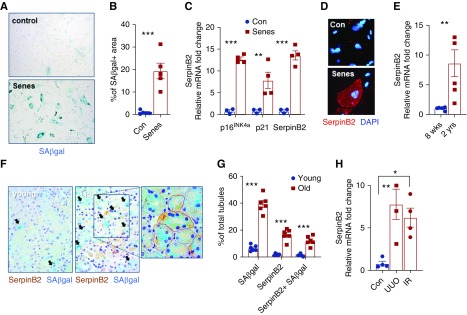
Cellular senescence as reflected by increased SA-β-gal activity and expression of 16INK4A/p21 and γH2AX+/Ki-67− nuclei (Figure 1, A–C, Supplemental Figure 2, A and B) was induced in PTECs via γ-irradiation.25 The senescence phenotype was associated with a significant induction of SerpinB2 (Figure 1, C and D). In keeping with this finding, we observed significantly more SerpinB2 expression in kidneys from aged (24-month-old) mice compared with young (2-month-old) mice (Figure 1E). By immunostaining, SerpinB2 was mainly found in tubular cells (Supplemental Figure 2C), of which the majority exhibited colocalization with senescence markers SA-β-gal and p21 (Figure 1, F and G, Supplemental Figure 2, D and E). A large proportion of tubules, which were, however, positive for SA-β-gal and p21, was negative for SerpinB2 (Figure 1G, Supplemental Figure 2E), indicating that SerpinB2 is not a prerequisite for senescence. SerpinB2 was also highly expressed in kidneys of young mice stressed by common disease models, renal IR, and UUO injury (Figure 1H). In UUO, tubular SerpinB2 expression correlated strongly with p21 expression, whereas coexpression with Ki67 was rare (Supplemental Figure 2, F–I).
Figure 1.
SerpinB2 is upregulated in senescent tubular epithelial cells. (A) Representative image for SA-β-gal staining and (B) quantification in nonsenescent (Con) versus γ-irradiation–induced senescent (Senes) primary tubular cells (n=5). (C) Quantitative RT-PCR of p16ink4a, p21, and SerpinB2 mRNA expression in control versus senescent PTECs (n=4). (D) Representative immunofluorescence staining image for SerpinB2 in control versus senescent PTECs. DAPI, 4′,6-diamidino-2-phenylindole. (E) Quantitative RT-PCR of SerpinB2 mRNA expression in kidneys from young (8-week-old) C57Bl/6J mice and old (2-year-old) C57Bl/6J mice (n=5). (F) Representative images from young and old kidneys showing costaining of SA-β-gal activity (arrows mark SA-β-gal staining) and SerpinB2 immunohistochemistry (brown); red dotted lines mark SerpinB2+/SA-β-gal+ tubules and blue dotted lines mark SerpinB2−/SA-β-gal+ tubules in the higher-magnification image detail of old kidney. (G) Quantification of tubular colocalization in young and old kidneys (n=6). (H) Quantitative RT-PCR of SerpinB2 mRNA in kidneys stressed by UUO and IR compared with control (n=4). Values are given as means±SEMs. Unpaired t test two tailed. Original magnification, ×200 in A, D, and E. *P<0.05; **P<0.01; ***P<0.001.
SerpinB2−/− Mice Show Increased Kidney Fibrosis and Inflammation in Aging
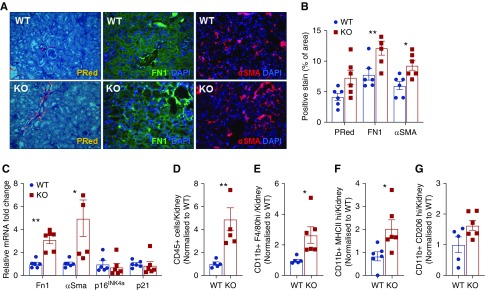
To explore the role of SerpinB2 during aging, we compared kidneys from old (22- to 24-month-old) wild-type and SerpinB2−/− mice, a strain without known renal pathology.23 Interestingly, we observed enhanced fibrosis as reflected by increased expression of αSma and Fn1 and a trend for increased collagen deposition by picrosirius red staining (Figure 2, A–C). In the same kidneys, we found no differences in senescence markers 16INK4A/p21 (Figure 2C). Given the strong association of kidney fibrosis and inflammation, we analyzed the presence of inflammatory cells by flow cytometry and found that aged SerpinB2−/− kidneys contained significantly more CD45+ cells than wild-type kidneys (Figure 2D). There were more total macrophages (CD11b+-F4/80high), were significantly more M1 type (F4/80+-MHCIIhigh), and was a trend for more M2-type macrophages (CD11b+-CD206high) in SerpinB2−/− kidneys (Figure 2, E–G). To explore the classic role of SerpinB2 as an inhibitor of the plasminogen activation system, we examined the renal expressions of uPA, uPA receptor, tissue plasminogen activator, PAI-1, and matrix metalloproteinase-2 as well as the activity of uPA in the same tissues, but we found no differences between wild-type and SerpinB2−/− kidneys (Supplemental Figure 3, A and B).
Figure 2.
SerpinB2 in aging kidney is protective and prevents immune cell accumulation. (A) Representative images of picrosirius red (PRed) and immunofluorescence staining for Fn1 and αSMA and (B) quantification in kidneys of old (22- to 24-month-old) wild-type (WT) and SerpinB2−/− (knockout [KO]) mice (n=6). DAPI, 4′,6-diamidino-2-phenylindole. (C) Quantitative RT-PCR of mRNA for Fn1, αSma, p16INK4a, and p21 in kidneys of old WT and KO mice (n=5). (D–G) Flow cytometry of kidney single-cell suspensions showing relative counts for (D) total CD45+ live leukocytes, (E) total macrophages (CD11b+-F4/80high), (F) M1-type macrophages (F4/80+-MHCIIhigh), and (G) M2-type macrophages (CD11b+-CD206high) in the kidneys from old WT and KO mice (n=6). Values are given as means±SEMs. Unpaired t test two tailed. Original magnification, ×200 in A. *P<0.05; **P<0.01.
SerpinB2−/− Mice Exhibit Increased Renal Damage and Altered Inflammation in UUO and IR Injury
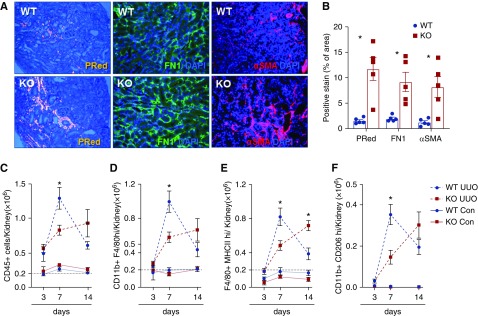
To investigate the effect of SerpinB2 deficiency under acute renal stress conditions, we subjected 3- to 4-month-old wild-type and SerpinB2−/− mice to UUO and IR. At 14 days of UUO, SerpinB2−/− kidneys showed comparatively higher expression of the tubular damage markers neutrophil gelatinase–associated lipocalin, αSma, and Fn1 and increased levels of collagen in picrosirius red staining (Figure 3, A and B, Supplemental Figure 3C). Similar results were obtained at days 3 and 7 of UUO (Supplemental Figure 3, D and E). Flow cytometry revealed similar leukocyte numbers in SerpinB2−/− and wild-type kidneys at 3 days of UUO (Figure 3, C–F), yet analysis of later time points revealed important differences. Wild-type kidneys showed significantly more total CD45+ leukocytes and macrophages on day 7. This pattern was reversed on day 14, with a significantly higher accumulation of inflammatory cells in SerpinB2−/− kidneys (Figure 3, C–F). No significant differences were found in senescence markers (16INK4A/p21) between the genotypes at any time point (data not shown), and we also found no differences in the expression of key players of the plasminogen activation system or in uPA activity (Supplemental Figure 3, F and G).
Figure 3.
SerpinB2 influences the outcome in UUO. (A) Representative images of picrosirius red (PRed) and immunofluorescence staining for Fn1 and αSMA and (B) quantification in kidney sections from wild-type (WT) and SerpinB2−/− (knockout [KO]) mice at 14 days after UUO (n=5). DAPI, 4′,6-diamidino-2-phenylindole. (C–F) Flow cytometry of (C) total CD45+ live leukocytes, (D) total macrophages (CD11b+-F4/80high), (E) M1-type macrophages (F4/80high-MHCIIhigh), and (F) M2-type macrophages (CD11b+-CD206high) in kidneys from WT and KO mice at indicated time points after UUO. Absolute counts of all cell populations in the contralateral (Con) kidneys are also shown for comparison. Values are given as means±SEMs (n=5 for each group). Unpaired t test two tailed. Original magnification, ×200 in A. *P<0.05.
Comparable results were obtained in the IR model, revealing enhanced expression of tubular damage and fibrosis markers in SerpinB2−/− kidneys (Supplemental Figure 4) together with significantly reduced functional recovery as judged by serum creatinine in mice with bilateral IR (Supplemental Figure 5, A and B). Very similar to UUO, leukocyte infiltration in IR kidneys was equal in both genotypes at day 3, whereas at day 7, SerpinB2−/− kidneys exhibited significantly fewer leukocytes, and on day 14, they had significantly more than the wild type (Supplemental Figure 5, C–F). No significant differences were found in senescence markers (16INK4A/p21; data not shown) or selected components of the plasminogen activation system (Supplemental Figure 5G).
SerpinB2 Regulates Leukocyte Accumulation by Stimulating Tubular Chemoattractant Expression
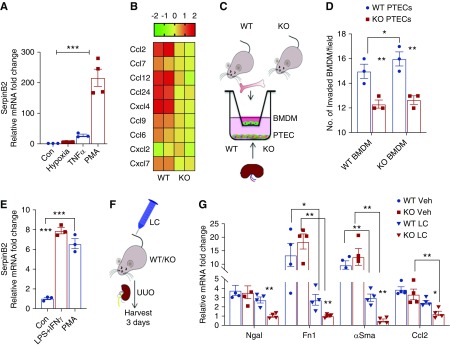
To explore the observed differences in immune cell dynamics, we addressed the effect of SerpinB2 on tubular chemokine expression in PTECs. SerpinB2 expression is very low in PTECs at baseline. Despite potential off-target effects, we decided to use the well known SerpinB2 inducer PMA, which promotes a strong SerpinB2 upregulation in PTECs (Figure 4A).27 We performed transcriptomic profiling of SerpinB2−/− versus wild-type PTECs. Importantly, the lack of SerpinB2 resulted in a suppressed expression of several cytokines/chemokines known to be crucial in the tubule-inflammatory axis (Figure 4B). Focusing on CCL2, which is a key chemoattractant for kidney monocyte/macrophage infiltration,28 we confirmed a significant reduction of CCL2 in SerpinB2−/− PTECs on the mRNA and protein levels (Supplemental Figure 6, A and B). To test how this could contribute to the reduced leukocyte infiltration seen at day 7 in UUO and IR, PTECs and BMDMs were cocultured in transwells (Figure 4C). SerpinB2−/− PTECs attracted significantly fewer BMDMs than wild-type PTECs (Figure 4D), supporting a role for reduced chemoattraction. A similar reduction in BMDM attraction was seen when CCL2 was antagonized in wild-type PTECs with Ccl2-specific siRNA (Supplemental Figure 6, C and D). In vivo, we also found significantly less CCL2 in SerpinB2−/− kidneys at day 7 of UUO and IR, with a congruent but nonsignificant decrease already at day 3 (Supplemental Figure 6, E and F). Apart from tubular cells, macrophages are major sources of CCL2 in inflamed kidneys.29 We, therefore, analyzed the effect of SerpinB2 on CCL2 expression in macrophages. Classic activation of BMDMs with LPS and IFN-γ, which resulted in strong SerpinB2 expression (Figure 4E), was not associated with differences in CCL2 expression between activated wild-type and SerpinB2−/− BMDMs (Supplemental Figure 7A).
Figure 4.
SerpinB2 regulates inflammatory crosstalk via upregulation of the chemoattractant pathway. (A) Quantitative RT-PCR to compare levels of SerpinB2 in PTECs after exposure to hypoxia, TNF-α, and PMA with control (Con; n=4). (B) Heat map showing differentially regulated chemokines and cytokines in PMA-treated PTECs from wild-type (WT) and SerpinB2−/− (knockout [KO]) mice (n=2). Values represent the mean log2 (ratio) of gene expression from microarray data. (C) Schematic representation of coculture experiment with BMDMs in the upper chamber and PTECs in the lower chamber. (D) Quantification of BMDMs invading the lower chamber as described in (C) (n=3). (E) Quantitative RT-PCR of SerpinB2 mRNA in BMDMs after stimulation with LPS/IFN-γ and PMA (n=3). (F) Schematic representation of liposomal clodronate (LC) injection for depletion of phagocytic monocytes/macrophages 24 hours before UUO surgery. (G) Quantitative RT-PCR for neutrophil gelatinase–associated lipocalin (Ngal), Fn1, αSma, and Ccl2 mRNA in UUO kidneys from WT and KO mice after vehicle (Veh) or LC treatment (n=4). Values are given as means±SEMs. (A and E) Unpaired t test two tailed and (D and G) two-way ANOVA with Bonferroni multiple comparison test. *P<0.05; **P<0.01; ***P<0.001.
Depletion of Macrophages before UUO Reverses the Kidney Phenotype in SerpinB2−/− Mice
To specifically address the role of tubular SerpinB2 beyond the effects of macrophages, we depleted mononuclear phagocytes by liposomal clodronate and subsequently performed UUO surgery in wild-type and SerpinB2−/− mice (Figure 4F). In addition to the expected reduction of kidney monocytes at 3 days of UUO (Supplemental Figure 7, B and C), we found an overall reduction in damage severity, which is a known feature of macrophage depletion (Figure 4G).30 More importantly, we observed that the phenotype of SerpinB2−/− kidneys was rescued and even reversed after liposomal clodronate treatment (Figure 4G). After macrophage depletion, SerpinB2−/− kidneys expressed significantly lower damage markers neutrophil gelatinase–associated lipocalin, Fn1, and αSma and had—in congruence with our tubular in vitro transcriptomic data—significantly lower CCL2 expression than wild-type kidneys (Figure 4G).
Cell-Autonomous Effects of SerpinB2 in Macrophages
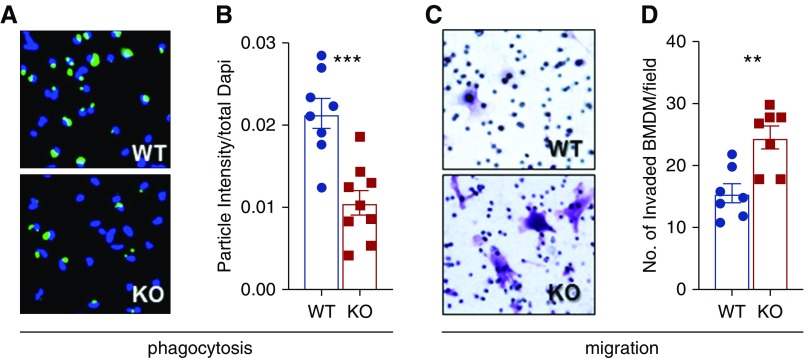
Although these data argue for specific effects of SerpinB2 in tubular cells, they also highlight the importance of macrophages that seem to dominate the tissue response under normal conditions. To explore the role of macrophages in more detail, we investigated cell-intrinsic effects of SerpinB2 in isolated macrophages. We observed a significant reduction in the phagocytic capacity of isolated SerpinB2−/− BMDMs (Figure 5, A and B). Additionally, we found significantly faster migration of SerpinB2−/− BMDMs when cells were challenged with recombinant CCL2 (Figure 5, C and D). Faster migration occurred despite unchanged intrinsic expression of the CCL2 receptor (Supplemental Figure 7A).
Figure 5.
SerpinB2 in macrophages stimulates phagocytosis and reduces macrophage migration. (A) Representative images of phagocytosed latex beads by LPS/IFN-γ–stimulated BMDMs from wild-type (WT) and SerpinB2−/− (knockout [KO]) mice, which are quantified in (B) (n=8). (C) Representative images and (D) quantification of BMDMs stained with crystal violet from WT and KO mice in a transwell experiment with BMDMs in the upper chamber and recombinant CCL2 in the lower chamber (n=8). Values are given as means±SEMs. (B and D) Unpaired t test two tailed. **P<0.01; ***P<0.001.
BM Transplantation Reveals Unique Cell Type–Specific Roles of SerpinB2
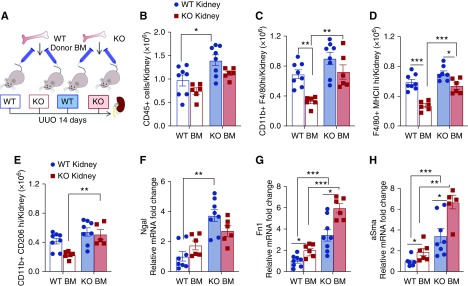
BM transplantation from wild-type and SerpinB2−/− mice into recipients of both genotypes followed by UUO 6 weeks later (Figure 6A) and analysis of kidneys from the resulting four groups of mice at 14 days allowed us to dissect cell type–dependent effects in kidney/tubular cells versus infiltrating BM-derived cells. In support of distinct cell type–specific mechanisms, which regulate cell attraction and invasion, we found the highest amount of infiltrating immune cells in wild-type kidneys (normal tubular CCL2 production) in combination with SerpinB2−/− BM (enhanced macrophage migration) (Figure 6, B–E, filled blue bars). Accordingly, we found the lowest immune infiltrate in the combination of SerpinB2−/− kidneys (reduced tubular CCL2 production) with wild-type BM (normal macrophage migration) (Figure 6, B–E, open red bars). The intermediate immune infiltrate in wild-type recipients of wild-type BM (Figure 6, B–E, open blue bars) was associated with the lowest degree of tubule-interstitial damage (Figure 6, F–H, Supplemental Figure 8), which highlights the balancing role of SerpinB2. Together, these findings indicate that SerpinB2 has distinct cell type–specific functions in tubular cells and macrophages, which regulate the delicate balance between inflammatory influx/activation, tissue repair, and immune resolution (Figure 7).
Figure 6.
SerpinB2 is important for balancing immune infiltration and adaptation. (A) Schematic representation of the BM transplantation model resulting in four separate groups: wild-type kidney (WT Kid)-WT BM, knockout (KO) Kid-WT BM, WT Kid-KO BM, and KO Kid-KO BM. (B–E) Flow cytometry showing relative counts for (B) CD45+ total live leukocytes, (C) total macrophages (CD11b+-F4/80high), (D) M1-type (F4/80+-MHCIIhigh) macrophages, and (E) M2-type macrophages (CD11b+-CD206high). (F–H) Quantitative RT-PCR for (F) neutrophil gelatinase–associated lipocalin (Ngal), (G) Fn1, and (H) αSma mRNA in kidneys of the four transplanted groups (n=8 for WT Kid-WT BM and WT Kid-KO BM; n=6 for KO Kid-WT BM and KO Kid-KO BM) normalized to the WT Kid-WT BM group. SerpinB2−/− is the KO. Values are given as means±SEMs. (B–H) Two-way ANOVA with Bonferroni multiple comparison test. *P<0.05; **P<0.01; ***P<0.001.
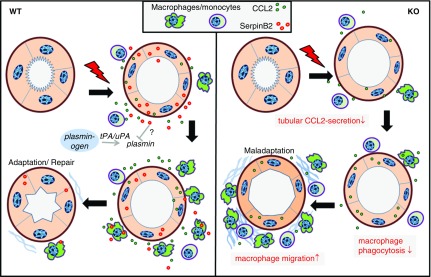
Figure 7.
SerpinB2 supports transient leukocyte accumulation and regulates macrophage function. Schematic representation of functional activities of SerpinB2 in injured kidney/tubules from wild-type (WT) and SerpinB2−/− (knockout [KO]) mice. tPA, tissue plasminogen activator, uPA, urokinase plasminogen activator. SerpinB2 from WT kidneys regulates CCL2 production which ensures timely immune cell migration and phagocytosis leading to adaptation or repair. Kidneys from KO mice have lower CCL2 production and exhibit macrophage dysfunctions like impaired phagocytosis and uninhibited migration leading to maladaptation post injury.
Discussion
Healing of the kidney requires a proreparative milieu to coordinate immune cell activation, tubular repair, and resolution of inflammation.3,5 We introduce SerpinB2 as a novel player in this triangle and demonstrate that SerpinB2 has beneficial effects for kidney outcome and healthy renal aging.
There are many mechanistic concepts about possible functions of SerpinB2, which are surprisingly heterogeneous and so far, have not resulted in a clear consensus.11,17,19,21,31,32 In vitro, we found that SerpinB2 was highly upregulated in cultured PTECs after γ-irradiation and after PMA treatment. In vivo, SerpinB2 was upregulated in tubular cells by a variety of stressors, such as aging, UUO, and IR. Tubular SerpinB2 was colocalized with markers of cellular senescence, but kidneys of SerpinB2−/− mice had no reduction in senescence. In contrast to previously described findings in other cell types,11,33 this does not support a direct prosenescent role of SerpinB2 in the kidney; however, the situation might be different if the tubular compartment is investigated in an isolated fashion. After macrophage depletion, we found evidence for reduced tubular UUO damage in SerpinB2−/− kidneys, suggesting an adverse effect of the protein on tubular cell integrity. Our findings suggest that these unfavorable effects are masked in the presence of macrophages, where beneficial immune regulatory functions of SerpinB2 dominate the tissue response.
Broad agreement exists on the effect of SerpinB2 on processes of the immune system.17–19,34 We extend these findings by providing several lines of evidence that SerpinB2 is involved in the regulation of kidney inflammation. After the early AKI phase, which is dominated by the release of damage-associated molecular patterns and neutrophil-driven inflammation, 35,36,37 tubular cells drive the immune response by actively secreting chemoattractants.2 We found that SerpinB2 is instrumental for efficient tubular chemokine synthesis. When SerpinB2 is lacking, synthesis of chemoattractants, in particular CCL2, is diminished, and accumulation of inflammatory cells is strongly delayed, similar to results observed in a nematode infection model.34
Another important finding is that SerpinB2 is crucial for the prevention of progression from acute to persistent inflammation. Our results suggest that the failure of this process is due to macrophage dysfunction in SerpinB2−/− mice. In agreement with a recent study, we observed uninhibited chemotactic migration in activated SerpinB2−/− BM macrophages.21 We propose that the bifunctional regulation of CCL2 activation in tubular cells, on the one hand, and inhibition of macrophage migration, on the other hand, permit regulatory fine tuning by which SerpinB2 can balance immune cell invasion and inflammatory resolution.38,39
In addition to changes in migration, we found significantly reduced phagocytosis in activated SerpinB2−/− BM macrophages. Disturbed phagocytosis can result in chronic inflammation and autoimmune disease.35,38 Of note, disturbed phagocytosis is also a major cause of chronic age-dependent inflammation,40 which makes phagocytic dysfunction a likely contributor to the exacerbated kidney inflammation and fibrosis seen in old SerpinB2−/− mice. Interestingly, another study that has investigated SerpinB2−/− peritoneal and not BMDMs found no difference in phagocytosis.21 Tissue- and site-dependent discrepancies in macrophages have been described in different conditions (e.g., aging), and they might also explain heterogeneous responses between kidney and liver.41,42
Although SerpinB2 has been originally described as an inhibitor of uPA/tissue plasminogen activator, published evidence for the effect of SerpinB2 on plasminogen activation is controversial and by far, not as clear as for the SerpinB2 paralog, PAI-1.31,43–46 In our study, we found no differences in uPA activity or expression of key members of the plasminogen activation system in SerpinB2−/− kidneys. Although this suggests that the kidney phenotype of SerpinB2−/− mice is independent of plasminogen activation, it has to be noted that we measured total kidney levels of gene expression and uPA activity and thus, cannot rule out localized differences (e.g., in interstitial space). Schroder et al.46 recently demonstrated a novel role for SerpinB2 in hemostasis, showing deregulated platelet activation and a hypercoagulation phenotype in SerpinB2−/− mice. Although we did not observe differences in platelet counts or renal fibrin deposition in SerpinB2−/− mice (data not shown), our experiments cannot exclude that subtle differences in coagulation might have contributed to the effects that we observed.
An additional confounder in the SerpinB2−/− phenotype could be SerpinB10, a protease inhibitor from the same superfamily as SerpinB2, which has recently been implicated in allergic asthma.47 Importantly, the coding gene for SerpinB10 contains a premature stop codon in C57BL/6 wild-type mice but not in SerpinB2−/− mice, which might cause enhanced SerpinB10 expression in SerpinB2−/− mice.21 Potential effects of SerpinB10 in the kidney are completely unknown and should be investigated by follow-up studies.
In summary, the results presented here emphasize crucial new roles for SerpinB2 in kidney disease and renal aging. Although expression of SerpinB2 in isolated tubular cells may be potentially maladaptive, we demonstrate that the immune-regulatory effects of SerpinB2 dominate the outcome by determining a timely activation and subsequent resolution of kidney inflammation. We show that SerpinB2 promotes proreparative adaptation of the kidney by two cell type–specific mechanisms. (1) SerpinB2 supports transient intrarenal leukocyte accumulation via enhanced expression of tubular CCL2. (2) SerpinB2 regulates macrophage function by activating phagocytosis and inhibiting migration. Our data suggest that these functions are crucial for a timely coordination and resolution of inflammation, successful repair, and kidney homeostasis during aging. As such, SerpinB2 may be regarded as a regulatory protein affecting various stages from acute kidney disease to CKD and tissue fibrosis.
Disclosures
None.
Funding
This work was supported by German Research Foundation grants SCHM 2146/6-1 and SFB738 (to Dr. Schmitt and Dr. Melk), the Dr. Werner Jackstädt Stiftung, and the Salisbury Cove Research Fund at MDI Biological Laboratory. Dr. Sen was supported by a Hannover Biomedical Research School stipend.
Supplementary Material
Acknowledgments
Technical assistance by Britta Gewecke is greatly appreciated.
Dr. Bräsen, Dr. Haller, Dr. Melk, Dr. Schmitt, and Dr. Sen designed the study; Dr. Helmke, Dr. Liao, Dr. Rong, Dr. Sen, and Dr. Sörensen-Zender carried out experiments; Dr. Schmitt, Dr. Sen, and Dr. von Vietinghoff analyzed the data; Dr. Schmitt and Dr. Sen made the figures. Dr. Schmitt, Dr. Sen, and Dr. von Vietinghoff wrote the manuscript; and Dr. Bräsen, Dr. Haller, Dr. Helmke, Dr. Liao, Dr. Melk, Dr. Rong, Dr. Schmitt, Dr. Sen, Dr. Sörensen-Zender, and Dr. von Vietinghoff approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101085/-/DCSupplemental.
Supplemental Figure 1. Gating strategy for flow cytometry of dissociated kidney cells.
Supplemental Figure 2. SerpinB2 and senescence markers in aged and stressed kidneys.
Supplemental Figure 3. Components of the plasminogen activation system and markers of kidney damage and fibrosis in aged and SerpinB2−/− kidneys after unilateral ureteral obstruction.
Supplemental Figure 4. Markers of kidney damage and fibrosis in SerpinB2−/− kidneys after IR.
Supplemental Figure 5. Components of the plasminogen activation system, markers of renal function and kidney leukocyte content in SerpinB2−/− kidneys after ischemia-reperfusion.
Supplemental Figure 6. CCL2 expression and bone marrow–derived macrophage attraction of primary tubular epithelial cells from SerpinB2−/− and wild-type kidneys and whole-kidney CCL2 expression in unilateral ureteral obstruction and ischemia-reperfusion.
Supplemental Figure 7. CCL and CCL2 receptor expression in bone marrow–derived macrophage and leukocyte counts after liposomal clodronate.
Supplemental Figure 8. Markers of kidney damage and fibrosis in kidneys from bone marrow transplantation experiments.
References
- 1.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, et al.: Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2: 94716, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi R, Yang C: Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis 9: 1126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castrop H: The role of renal interstitial cells in proximal tubular regeneration. Nephron 141: 265–272, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Huen SC, Cantley LG: Macrophages in renal injury and repair. Annu Rev Physiol 79: 449–469, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Jin H, Zhang Y, Ding Q, Wang SS, Rastogi P, Dai DF, et al.: Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 4: 125490, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt R, Melk A: Molecular mechanisms of renal aging. Kidney Int 92: 569–579, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Docherty MH, O’Sullivan ED, Bonventre JV, Ferenbach DA: Cellular senescence in the kidney. J Am Soc Nephrol 30: 726–736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, et al.: Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23: 1467–1473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DH, Wolstein JM, Pudasaini B, Plotkin M: INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol 302: F183–F191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh HH, Chen YC, Jhan JR, Lin JJ: The serine protease inhibitor serpinB2 binds and stabilizes p21 in senescent cells. J Cell Sci 130: 3272–3281, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al.: The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 14: 644–658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Herbert BS, Pan KH, Shay JW, Cohen SN: Disparate effects of telomere attrition on gene expression during replicative senescence of human mammary epithelial cells cultured under different conditions. Oncogene 23: 6193–6198, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Croucher DR, Saunders DN, Lobov S, Ranson M: Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer 8: 535–545, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Harris NLE, Vennin C, Conway JRW, Vine KL, Pinese M, Cowley MJ, et al.; Australian Pancreatic Cancer Genome Initiative: SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene 36: 4288–4298, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramnefjell M, Aamelfot C, Helgeland L, Akslen LA: Low expression of SerpinB2 is associated with reduced survival in lung adenocarcinomas. Oncotarget 8: 90706–90718, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JA, Cochran BJ, Lobov S, Ranson M: Forty years later and the role of plasminogen activator inhibitor type 2/SERPINB2 is still an enigma. Semin Thromb Hemost 37: 395–407, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Schroder WA, Le TT, Major L, Street S, Gardner J, Lambley E, et al.: A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J Immunol 184: 2663–2670, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Schroder WA, Major L, Suhrbier A: The role of SerpinB2 in immunity. Crit Rev Immunol 31: 15–30, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Shea-Donohue T, Zhao A, Antalis TM: SerpinB2 mediated regulation of macrophage function during enteric infection. Gut Microbes 5: 254–258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroder WA, Hirata TD, Le TT, Gardner J, Boyle GM, Ellis J, et al.: SerpinB2 inhibits migration and promotes a resolution phase signature in large peritoneal macrophages. Sci Rep 9: 12421, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhania A, Wallington JC, Smith CG, Horowitz D, Staples KJ, Howarth PH, et al.: Multitissue transcriptomics delineates the diversity of airway T cell functions in asthma. Am J Respir Cell Mol Biol 58: 261–270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty KM, Pearson JM, Yang AY, Westrick RJ, Baker MS, Ginsburg D: The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc Natl Acad Sci U S A 96: 686–691, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baisantry A, Bhayana S, Wrede C, Hegermann J, Haller H, Melk A, et al.: The impact of autophagy on the development of senescence in primary tubular epithelial cells. Cell Cycle 15: 2973–2979, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkenkamp B, Susnik N, Baisantry A, Kuznetsova I, Jacobi C, Sörensen-Zender I, et al.: In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS One 9: e88071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Petrova NV, Velichko AK, Razin SV, Kantidze OL: Small molecule compounds that induce cellular senescence. Aging Cell 15: 999–1017, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haller H, Bertram A, Nadrowitz F, Menne J: Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens 25: 42–49, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Sharkey D, Cantley LG: Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J Am Soc Nephrol 30: 1825–1840, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitamoto K, Machida Y, Uchida J, Izumi Y, Shiota M, Nakao T, et al.: Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 111: 285–292, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Gardiner EE, Medcalf RL: Is plasminogen activator inhibitor type 2 really a plasminogen activator inhibitor after all? J Thromb Haemost 12: 1703–1705, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Lee NH, Cho A, Park SR, Lee JW, Sung Taek P, Park CH, et al.: SERPINB2 is a novel indicator of stem cell toxicity. Cell Death Dis 9: 724, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sossey-Alaoui K, Pluskota E, Szpak D, Plow EF: The Kindlin2-p53-SerpinB2 signaling axis is required for cellular senescence in breast cancer. Cell Death Dis 10: 539, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao A, Yang Z, Sun R, Grinchuk V, Netzel-Arnett S, Anglin IE, et al.: SerpinB2 is critical to Th2 immunity against enteric nematode infection. J Immunol 190: 5779–5787, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundert F, Platen L, Iwakura T, Zhao Z, Marschner JA, Anders HJ: Immune mechanisms in the different phases of acute tubular necrosis. Kidney Res Clin Pract 37: 185–196, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosin DL, Okusa MD: Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders HJ: Immune system modulation of kidney regeneration--mechanisms and implications. Nat Rev Nephrol 10: 347–358, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Savill J: Resolution of inflammation: The beginning programs the end. Nat Immunol 6: 1191–1197, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe S, Alexander M, Misharin AV, Budinger GRS: The role of macrophages in the resolution of inflammation. J Clin Invest 129: 2619–2628, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oishi Y, Manabe I: Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis 2: 16018, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder WA, Gardner J, Le TT, Duke M, Burke ML, Jones MK, et al.: SerpinB2 deficiency modulates Th1∕Th2 responses after schistosome infection. Parasite Immunol 32: 764–768, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC: Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 13: 699–708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruithof EK, Baker MS, Bunn CL: Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood 86: 4007–4024, 1995 [PubMed] [Google Scholar]
- 44.Siefert SA, Chabasse C, Mukhopadhyay S, Hoofnagle MH, Strickland DK, Sarkar R, et al.: Enhanced venous thrombus resolution in plasminogen activator inhibitor type-2 deficient mice. J Thromb Haemost 12: 1706–1716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder WA, Anraku I, Le TT, Hirata TD, Nakaya HI, Major L, et al.: SerpinB2 deficiency results in a stratum corneum defect and increased sensitivity to topically applied inflammatory agents. Am J Pathol 186: 1511–1523, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Schroder WA, Le TT, Gardner J, Andrews RK, Gardiner EE, Callaway L, et al.: SerpinB2 deficiency in mice reduces bleeding times via dysregulated platelet activation. Platelets 30: 658–663, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Mo Y, Zhang K, Feng Y, Yi L, Liang Y, Wu W, et al.: Epithelial SERPINB10, a novel marker of airway eosinophilia in asthma, contributes to allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 316: L245–L254, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.