Abstract
Data on causes of community-onset bloodstream infection in Myanmar are scarce. We aimed to identify etiological agents of bloodstream infections and patterns of antimicrobial resistance among febrile adolescents and adults attending Yangon General Hospital (YGH), Yangon, Myanmar. We recruited patients ≥12 years old with fever ≥38°C who attended YGH from 5 October 2015 through 4 October 2016. A standardized clinical history and physical examination was performed. Provisional diagnoses and vital status at discharge was recorded. Blood was collected for culture, bloodstream isolates were identified, and antimicrobial susceptibility testing was performed. Using whole-genome sequencing, we identified antimicrobial resistance mechanisms of Enterobacteriaceae and sequence types of Enterobacteriaceae and Streptococcus agalactiae. Among 947 participants, 90 (9.5%) had bloodstream infections (BSI) of which 82 (91.1%) were of community-onset. Of 91 pathogens isolated from 90 positive blood cultures, we identified 43 (47.3%) Salmonella enterica including 33 (76.7%) serovar Typhi and 10 (23.3%) serovar Paratyphi A; 20 (22.0%) Escherichia coli; 7 (7.7%) Klebsiella pneumoniae; 6 (6.6%), Staphylococcus aureus; 4 (4.4%) yeasts; and 1 (1.1%) each of Burkholderia pseudomallei and Streptococcus agalactiae. Of 70 Enterobacteriaceae, 62 (88.6%) were fluoroquinolone-resistant. Among 27 E. coli and K. pneumoniae, 18 (66.6%) were extended-spectrum beta-lactamase (ESBL)-producers, and 1 (3.7%) each were AmpC beta-lactamase- and carbapenemase-producers. Fluoroquinolone resistance was associated predominantly with mutations in the quinolone resistance-determining region. blaCTX-M-15 expression was common among ESBL-producers. Methicillin-resistant S. aureus was not detected. Fluoroquinolone-resistant, but not multiple drug-resistant, typhoidal S. enterica was the leading cause of community-onset BSI at a tertiary hospital in Yangon, Myanmar. Fluoroquinolone and extended-spectrum cephalosporin resistance was common among other Enterobactericeae. Our findings inform empiric management of severe febrile illness in Yangon and indicate that measures to prevent and control enteric fever are warranted. We suggest ongoing monitoring and efforts to mitigate antimicrobial resistance among community-onset pathogens.
Author summary
Bloodstream infection (BSI) is common among persons seeking healthcare for severe febrile illness in low-and middle-income countries. Data on community-onset BSI are few for some countries in Asia, including Myanmar. Such data are needed to inform empiric antimicrobial treatment of patients and to monitor and control antimicrobial resistance. We performed a one year, prospective study collecting information and blood cultures from patients presenting with fever at a tertiary referral hospital in Yangon, Myanmar. We found that almost 10% of participants had a bloodstream infection, and that Salmonella enterica serovars Typhi and Paratyphi A were the most common pathogens. Typhoidal Salmonella were universally resistant to ciprofloxacin. More than half of Escherichia coli and Klebsiella pneumoniae were resistant to extended-spectrum cephalosporins and resistance to carbapenems was also identified in some isolates. We show that typhoid and paratyphoid fever are common, and fluoroquinolone resistance is widespread. Extended-spectrum cephalosporin resistance is common in E. coli and K. pneumoniae and carbapenem resistance is present. Our findings inform empiric antimicrobial management of severe febrile illness, underscore the value of routine use of blood cultures, indicate that measures to prevent and control enteric fever are warranted, and suggest a need to monitor and mitigate antimicrobial resistance among community-acquired pathogens.
Introduction
Fever is a common reason for seeking healthcare in South-East Asia [1, 2]. Bloodstream infection (BSI) is an important cause of severe febrile illness [3] and requires urgent and appropriate antimicrobial therapy to avert death [4]. The causes of bacteremia and patterns of antimicrobial resistance among bloodstream isolates may vary considerably by location and change over time. Data are needed to inform empiric antimicrobial regimens and to identify emerging pathogens and antimicrobial resistance problems. A 2012 systematic review of community-acquired bloodstream infections in South and South-East Asia identified just 17 studies [5], and only one from Myanmar [6], indicating major data gaps in the region.
Lack of clinical microbiology services is an important barrier to understanding BSIs in low-resource areas [7]. Where blood cultures can be performed, they may be subject to quality concerns or may not be obtained systematically from patients who could potentially benefit. Furthermore, laboratory records frequently lack sufficient detail to make the epidemiologically important distinction between hospital-acquired and community-onset infections. Antimicrobial resistance is a growing problem globally and especially in South and South-East Asia among hospital-acquired infections [8]. Moreover, community-acquired pathogens with concerning patterns of antimicrobial resistance, including extended-spectrum cephalosporin-resistant S. enterica Typhi [9] and other Enterobacteriaceae [10–12], are increasingly identified.
In order to improve knowledge on the epidemiology of community-onset BSI in Myanmar, we sought to identify the bacterial and fungal etiology, and antimicrobial susceptibility of bloodstream isolates from adolescent and adult patients with febrile illness attending the Yangon General Hospital (YGH), Yangon, Myanmar. We also determined genetic mechanisms of antimicrobial resistance among Enterobacteriaceae bloodstream isolates.
Methods
Ethics statement
The study protocol was reviewed and approved by Ethics Review Committees of University of Medicine 1, and the Department of Medical Research, Yangon, Myanmar, and the Human Ethics Committee of the University of Otago (reference number: H15/045). We sought and obtained written informed consent from guardians or caregivers for patients aged between 12 to 18 years of age, those who were illiterate, or were unconscious at presentation. For all others, written consent was sought and obtained from the patient.
Setting
Yangon, the largest city and former capital of Myanmar, is situated in the Yangon Region (Fig 1) with a population of 5.16 million [13]. YGH is a 2,000-bed hospital and is the largest civilian tertiary referral hospital in Myanmar receiving patients directly from the community as well as by referral from other hospitals nationwide. YGH provides free medical and surgical care services to outpatients and inpatients aged ≥12 years. Following triage in the Department of Emergency Medicine, febrile patients without surgical conditions are referred to the Medical Observation (MO) Unit for pre-admission care or referral for outpatient management.
Fig 1.
Map of South and South-East Asia showing Myanmar (panel A) and Yangon Region (panel B). Reprinted from Oo WT et al [14].
Participants
We prospectively identified participants among adolescent and adult patients seeking healthcare at the Medical Observation (MO) unit at YGH from 5 October 2015 through 4 October 2016. Adolescent and adult patients aged ≥12 years with an oral temperature of ≥38° C seen at the MO unit were eligible for enrolment. Febrile patients returning for the same illness episode were excluded from the study.
Clinical data and sample collection
Using a standardized case report form, trained medical graduates collected demographic, clinical history, and physical examination data from consenting participants. We assessed the participant’s severity status at the time of enrollment by calculating quick sequential organ failure assessment (qSOFA) score as defined by Sepsis-3 Task Force [15]. Accordingly, we assigned one point each for three clinical criteria: respiratory rate ≥22 per minute, systolic blood pressure ≤100 mmHg, and Glasgow coma scale <15. A high qSOFA was defined as a score ≥2 [15]. Provisional diagnoses of the hospital clinical team were recorded and coded using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes [16].
Prior to commencing hospital antimicrobial therapy and after skin cleansing and disinfection, team members collected blood from each consenting participant and 8–10 mL were inoculated aseptically into BacT/ALERT standard aerobic (SA) blood culture bottles (bioMérieux, Marcy l’Etoile, France).
Blood culture, isolate identification, and antimicrobial susceptibility testing
We performed blood culture processing, isolate identification, and antimicrobial susceptibility testing (AST) at the Microbiology Laboratory Section, YGH. Blood culture bottles were assessed for volume adequacy by comparing the weight before and after inoculation. Inoculated BacT/ALERT SA bottles were loaded into the BacT/ALERT 3D 60 Microbial Detection system (bioMérieux, Marcy l’Etoile, France) and incubated at 37°C for 5 days. Bottles flagging positive were sub-cultured following standard methods. Positive bottles with no growth on subculture were tested for the presence of pneumococcal antigen using BinaxNOW Streptococcus pneumoniae antigen card rapid immunochromatographic assay (Alere Ltd., Auckland, New Zealand) [17]. Isolate identification and AST were done by VITEK2 Compact 60 system (bioMérieux, Marcy l’Etoile, France). Clinical and Laboratory Standards Institute (CLSI) criteria [18, 19] were used for AST interpretation at YGH and results were reported to YGH clinicians responsible for patient care. We tested all isolates that were identified by the VITEK2 as Burkholderia spp. by latex agglutination assay for Burkholderia pseudomallei, as previously described [20]. We classified blood culture isolates of commensal organisms and gram-negative bacteria that are unlikely to cause BSI as likely contaminants. Likely contaminants included Achromobacter spp., Bacillus spp. other than B. anthracis, Brevibacterium spp., coagulase-negative staphylococci, Corynebacterium spp. other than C. diphtheriae, Dermacoccus spp., Kocuria spp., and Micrococcus spp. [21]. AST for fungal isolates was not performed.
For external quality control, isolate identification and AST were confirmed at Southern Community Laboratories (SCL), Dunedin, New Zealand. Matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (Microflex LT, Bruker Daltonics, Billerica, MA, USA) and the BD Phoenix system (Becton and Dickenson, Franklin Lakes, New Jersey, USA) were used. B. pseudomallei isolates was not confirmed at SCL since the organism could not be imported into New Zealand. The European Committee for Antimicrobial Susceptibility Testing (EUCAST) disc diffusion method was used for AST determination when the Phoenix system was unable to provide a result. E-tests (Thermo Fisher Scientific, Auckland, New Zealand) were used to determine the minimum inhibitory concentrations (MICs) of ciprofloxacin, nalidixic acid, and azithromycin for isolates identified as S. enterica. At SCL, EUCAST clinical breakpoints [22] were used for AST interpretation. Final AST results were interpreted according to EUCAST criteria where available [22]. Susceptibility results for antimicrobial agents against Burkholderia spp., and those for nalidixic acid and azithromycin for S. enterica, for which EUCAST breakpoints do not exist, were interpreted by CLSI criteria [19].
At SCL, screening for ESBL, AmpC, and carbapenemase production among gram-negative isolates and phenotypic confirmation were performed following EUCAST methods [23]. ESBL-, carbapenemase-, and AmpC-producing organisms were defined on the results of the phenotypic confirmatory tests [23]. For all bacteria except S. enterica, we defined multidrug resistance (MDR) as acquired non-susceptibility (resistant or intermediate susceptibility) to at least one agent in ≥3 antimicrobial classes and extensive drug resistance (XDR) as non-susceptibility to at least one agent in all but 2 or fewer antimicrobial classes [24]. The definition of MDR for S. enterica followed the conventional definition of resistance to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole [25].
Whole-genome sequencing and detection of antimicrobial resistance mechanisms, sequence types, and phylogenetic analysis
We performed whole-genome sequencing (WGS) for Enterobacteriaceae and Streptococcus agalactiae. DNA was extracted using the Nucleospin Microbial DNA kit (Machery Nagel, Düren, Germany) according to the manufacturer’s instructions [26]. Quality of DNA was assessed with the Nanodrop One spectrophotometer (Thermo Fisher Scientific, Auckland, New Zealand) and Qubit fluorometry (Thermo Fisher Scientific, Auckland, New Zealand). WGS of extracted DNA was performed by Illumina HiSeq (Illumina Inc., Melbourne, Australia) with 2x125bp PE v4 sequencing chemistry at New Zealand Genomics Ltd, Palmerston North, New Zealand. Bioinformatic analysis of WGS data was done with the Nullarbor pipeline version 1.2 [27] to detect antimicrobial resistance genes and to determine sequence types (STs) of isolates. Reference genomes used are listed in the S1 Table. To detect mutations in the quinolone resistance determining regions (QRDR) among ciprofloxacin-resistant E. coli, sequences of gyrA, gyrB, parC, and parE of isolates were compared to those of E. coli strain K-12 substrain MG1655 (GenBank accession number: NC_000913.3) using CLC sequence viewer version 8.0 (Qiagen, Hilden, Germany).
Phylogenetic analysis was performed as previously described [28]. Recombinant regions were filtered from the core genome SNP alignment and a maximum likelihood phylogenetic tree generated using Gubbins [29]. Trees were then rooted using the minimal ancestor deviation method [30].
We have submitted sequence data for S. enterica Typhi and Paratyphi A isolates to GenBank under BioProject PRJNA493305 [28] and E. coli, K. pneumoniae, and S. agalactiae to BioProject PRJNA624724. Accession numbers and metadata for E. coli, K. pneumoniae, and S. agalactiae are provided in supporting information (S1–S3 Appendices).
Statistical methods
Clinical and laboratory data were entered into Microsoft Access 2013 and Excel 2013 (Microsoft Corporation, Redmond, Washington, USA). Data were analyzed using STATA 13.1 version (Stata Corporation, College Station, TX, USA). Descriptive data were calculated for continuous variables. Proportions of BSIs and antimicrobial resistance patterns of identified pathogens were calculated. The X2 test or Fisher’s exact test, as appropriate, were used to compare proportions.
We defined a BSI for any positive blood culture yielding pathogenic bacteria or fungi obtained from a febrile participant. For the purpose of statistical analyses, we classified BSIs as community onset BSI (CO BSI) and hospital-acquired BSI (HA BSI). CO BSI was defined as any BSI from outpatients or inpatients who came directly from the community to YGH, or had received <48 hours of healthcare at another healthcare facility, whereas HA BSI were defined as any BSI acquired at the transferring hospital where the participant was hospitalized for >48 hours [31]. To distinguish BSI originated in the community but with healthcare exposure other than hospital admission, we further subclassified CO BSI into healthcare-associated BSI (HCA BSI) and community-acquired BSI (CA BSI), defining each based on modifications of published definitions [32]. To calculate blood culture volume adequacy, we defined the manufacturer’s recommended volume of 10mL±20% or 2mL as ‘adequate.’ Blood volumes less than ‘adequate’ were regarded as ‘underfilled’ and those more than adequate as ‘over-filled.’ To assess seasonal variations of BSIs, we defined ‘wet season’ as May through October and ‘dry season’ as November through April [33].
We examined associations between participant qSOFA score on presentation at the MO unit and vital status at discharge. Odds ratios were calculated by univariate logistic regression to investigate associations between exposure and outcome variables, and to examine associations between season and proportions of pathogens isolated. The two-sided P value of <0.05 was considered significant.
Results
Study population, participant demographics, and clinical data
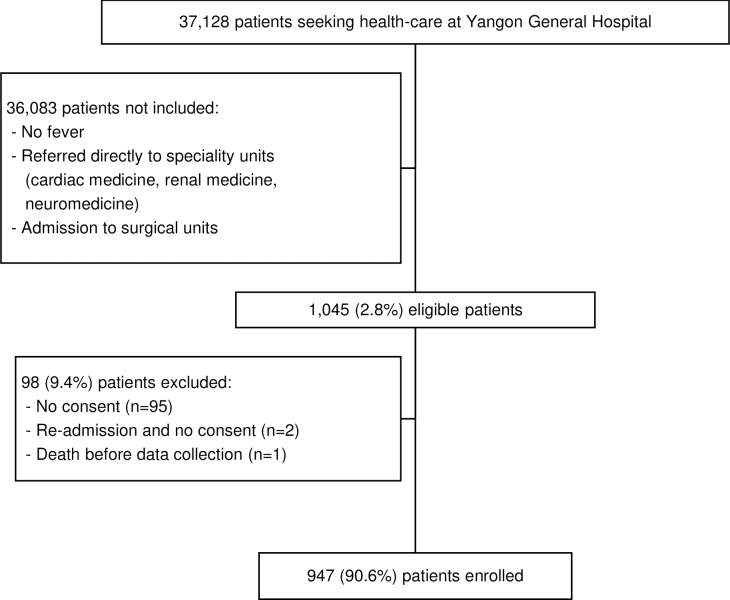
Of 37,128 patients seen at the MO unit, YGH from 5 October 2015 through 4 October 2016, 1,045 (2.8%) were eligible for inclusion, among which 947 (90.6%) consented and were enrolled in the study (Fig 2). Of 947 participants, 671 (70.9%) resided in the Yangon Region, and 428 (45.2%) were transferred from other healthcare facilities. Among 428 participants transferred, 219 (51.2%) came from healthcare centers with inpatient facilities and 209 (50.8%) from rural health centers or private clinics. Participants’ demographics and clinical characteristics are shown in Table 1. The median (range) age of participants was 37 (12, 94) years and 446 (47.1%) were female. Of participants, comorbidities included 122 (12.9%) hypertension, 92 (9.7%) tuberculosis, and 80 (8.4%) diabetes mellitus (Table 1). ICD-10 admission diagnoses of study participants included 329 (34.7%) unspecified fever, 100 (10.6%) enteric fever, 62 (6.5%) lower respiratory tract infection, 49 (5.2%) meningitis or encephalitis, and 35 (3.7%) septicemia (Table 1).
Fig 2. Flow diagram for enrolling febrile patients at Yangon General Hospital, 2015–2016.
Table 1. Demographics and clinical characteristics of febrile patients attending Yangon General Hospital, 2015–16.
| Characteristic | Participants without BSI (n = 857) | Participants with BSI (n = 90) | Total participants (n = 947) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, (range) | n (%) | Median (range) | n (%) | Median (range) | n (%) | ||||
| Age, years | 38 (12, 87) | 33 (13, 94) | 37 (12, 94) | ||||||
| Gender | |||||||||
| - Male | 448 (52.3) | 53 (58.9) | 501 (52.9) | ||||||
| - Female | 409 (47.7) | 37 (41.1) | 446 (47.1) | ||||||
| Duration of fever, days | 5 (1, 30) | 5 (1, 28) | 5 (1, 30) | ||||||
| Presenting symptoms | |||||||||
| - Fever (>38°C) | 857 (100.0) | 90 (100.0) | 947 (100.0) | ||||||
| - Headache | 357 (41.7) | 47 (52.2) | 404 (42.7) | ||||||
| - Cough | 257 (30.0) | 23 (25.6) | 280 (29.6) | ||||||
| - Vomiting | 240 (28.0) | 35 (38.9) | 275 (29.0) | ||||||
| - Diarrhoea | 110 (12.8) | 10 (11.1) | 120 (12.7) | ||||||
| - Dysuria | 79 (9.2) | 9 (10.0) | 88 (9.3) | ||||||
| Physical signs | |||||||||
| - Abdominal tenderness | 86 (10.0) | 16 (17.8) | 102 (10.8) | ||||||
| - Jaundice | 84 (9.8) | 7 (7.8) | 91 (9.6) | ||||||
| - Basal lung crepitations | 66 (7.7) | 7 (7.8) | 73 (7.7) | ||||||
| - Hepatomegaly | 46 (5.4) | 7 (7.8) | 53 (5.6) | ||||||
| - Ascites | 30 (3.5) | 2 (2.2) | 32 (3.4) | ||||||
| - Neck stiffness | 20 (2.3) | 2 (2.2) | 22 (2.3) | ||||||
| - Cardiac murmur | 15 (1.8) | 4 (4.4) | 19 (2.0) | ||||||
| Severity assessment | |||||||||
| - Respiratory rate ≥22 rate per minute | 178 (20.8) | 25 (27.8) | 203 (21.4) | ||||||
| - Glasgow coma scale <15 | 122 (14.2) | 15 (16.7) | 137 (14.5) | ||||||
| - Systolic blood pressure ≤100 mmHg | 100 (11.7) | 14 (15.6) | 114 (12.0) | ||||||
| - qSOFA score ≥2 | 55 (6.4) | 9 (10.0) | 64 (6.8) | ||||||
| Comorbid conditions | |||||||||
| - Hypertension | 112 (13.1) | 10 (11.1) | 122 (12.9) | ||||||
| - Tuberculosis | 91 (10.6) | 1 (1.1) | 92 (9.7) | ||||||
| - Diabetes mellitus | 72 (8.4) | 8 (8.9) | 80 (8.4) | ||||||
| - Cardiovascular diseases* | 31 (3.6) | 3 (3.3) | 34 (3.6) | ||||||
| - Chronic liver disease† | 15 (1.8) | 4 (4.4) | 19 (2.0) | ||||||
| - Malignancy | 17 (2.0) | 1 (1.1) | 18 (1.9) | ||||||
| - Hematological malignancy | 10 (1.2) | 1 (1.1) | 11 (1.2) | ||||||
| - Solid organ tumors | 7 (0.8) | 0 (0.0) | 7 (0.7) | ||||||
| - HIV | 14 (1.6) | 0 (0.0) | 14 (1.5) | ||||||
| - Renal disease | 10 (1.2) | 1 (1.1) | 11 (1.2) | ||||||
| - Respiratory diseases‡ | 6 (0.7) | 3 (3.3) | 6 (0.6) | ||||||
| - Neurological diseases | 5 (0.6) | 1 (1.1) | 9 (1.0) | ||||||
| Recent exposure to antimicrobial agents | |||||||||
| - No | 591 (69.0) | 29 (32.2) | 620 (65.5) | ||||||
| - Yes | 148 (17.3) | 22 (24.4) | 170 (17.9) | ||||||
| - Did not know | 118 (13.8) | 39 (43.3) | 157 (16.6) | ||||||
| Admission diagnosis | |||||||||
| - Unspecified fever | 293 (34.2) | 36 (40.0) | 329 (34.7) | ||||||
| - Enteric fever | 88 (10.3) | 12 (13.3) | 100 (10.6) | ||||||
| - Lower respiratory tract infection | 58 (6.8) | 4 (4.4) | 62 (6.5) | ||||||
| - Meningitis or encephalitis | 47 (5.5) | 2 (2.2) | 49 (5.2) | ||||||
| - Septicemia | 30 (3.5) | 5 (5.6) | 35 (3.7) | ||||||
| - Cerebrovascular disease | 34 (4.0) | 0 (0.0) | 34 (3.6) | ||||||
| - Urinary tract infection | 26 (3.0) | 6 (6.7) | 32 (3.4) | ||||||
| - Pulmonary tuberculosis | 29 (3.4) | 1 (1.1) | 30 (3.2) | ||||||
| - Cirrhosis of liver | 27 (3.2) | 1 (1.1) | 28 (3.0) | ||||||
| - Acute viral infection | 24 (2.8) | 3 (3.3) | 27 (2.9) | ||||||
| - Dengue fever/Dengue hemorrhagic fever | 18 (2.1) | 2 (2.2) | 20 (2.1) | ||||||
| - Acute gastroenteritis | 14 (1.6) | 3 (3.3) | 17 (1.8) | ||||||
| - Infective endocarditis | 12 (1.4) | 4 (4.4) | 16 (1.7) | ||||||
| - HIV infection | 13 (1.5) | 1 (1.1) | 14 (1.5) | ||||||
| - Acute viral hepatitis A, B or C | 5 (0.6) | 6 (6.6) | 11 (1.2) | ||||||
| - Leptospirosis | 4 (0.5) | 1 (1.1) | 5 (0.5) | ||||||
| - Malaria | 4 (0.5) | 1 (1.1) | 5 (0.5) | ||||||
| - Leprosy | 3 (0.4) | 0 (0.0) | 3 (0.3) | ||||||
| YGH admission status | |||||||||
| - Admitted | 763 (89.0) | 87 (96.0) | 850 (89.8) | ||||||
| - Treated as outpatient | 94 (11.0) | 3 (3.3) | 97 (10.2) | ||||||
BSI, Bloodstream infection; qSOFA, quick sequential (sepsis-related) organ failure assessment; HIV, human immunodeficiency virus; YGH, Yangon General Hospital.
*Cardiovascular diseases include heart failure, ischemic heart disease and valvular heart disease;
†Chronic liver diseases include alcoholic hepatitis, chronic viral hepatitis B or C, and cirrhosis of liver;
‡Respiratory diseases include asthma, chronic obstructive pulmonary disease, and pneumonia.
Bloodstream infections, assessment of severity, and the vital status
Of 947 participants, 90 (9.5%) had BSI. The median (range) age of participants with BSI was 33 (13, 94) years and 37 (41.1%) were female (Table 1). Of 90 participants with BSI, 82 (91.1%) had CO BSI including 76 (84.4%) CA BSI and 6 (6.7%) HCA BSI. Eight (8.9%) had HA BSI.
Of all participants, 64 (6.8%) had a qSOFA score ≥2 (Table 1). Bloodstream infection was detected in 9 (14.0%) of 64 participants with qSOFA score ≥2 compared to 81 (9.2%) of 883 with score <2 (OR 1.62; 95% CI 0.77–3.40; p = 0.202). Vital status at discharge was assessed in 470 (49.6%) participants of which 64 (13.6%) died. Of 44 participants with known vital status and qSOFA score ≥2, 21 (47.7%) died compared to 45 (10.6%) of 426 with known vital status and qSOFA score <2 (OR 7.73; 95% CI 3.97–15.07; p<0.001). Thirteen (24.1%) of 54 participants with known vital status and BSI died compared to 53 (12.7%) of 416 participants with known vital status and who did not have BSI (OR 2.17; 95% CI 1.09–4.32; p = 0.027). BSI was found in 12 (12.0%) of 100 participants clinically diagnosed with enteric fever and 5 (16.7%) of 30 participants diagnosed to have septicemia on admission.
Blood culture
Blood culture volume adequacy and pathogen isolation
Of 947 blood cultures, 90 (9.5%) yielded pathogens, 44 (4.6%) grew likely contaminants, and 15 (1.6%) flagged positive but did not grow on subculture. Among 888 bottles that neither grew contaminants nor showed growth on subculture, 84 (10.5%) of 799 adequately filled blood culture bottles grew pathogens compared to 6 (6.7%) of 89 underfilled bottles (OR = 1.63; 95% CI 0.69–4.69, p = 0.354). No bottles were over-filled.
Bloodstream isolates
From 90 positive blood cultures, 91 pathogens were isolated. On one occasion two pathogens, Escherichia coli and Klebsiella pneumoniae, were isolated from a single bottle. Among 91 pathogens isolated, 76 (83.5%) were gram-negative bacteria, 11 (12.1%) were gram-positive bacteria, and 4 (4.4%) were yeasts (Table 2). We confirmed 1 (33.3%) B. pseudomallei among 3 Burkholderia spp. by latex agglutination assay. Of 91 pathogens, 77 (84.6%) were isolated from CA BSI, 8 (8.8%) from HA BSI, and 6 (6.6%) from HCA BSI. All 43 (100%) S. enterica were isolated from participants with CA BSI.
Table 2. Bloodstream pathogenic isolates recovered from febrile patients admittd to Yangon General Hospital, 2015–2016.
| Pathogens isolated | n (%) |
|---|---|
| Gram-negative bacteria | 76 (83.5) |
| Enterobacteriaceae | 70 (76.9) |
| Salmonella enterica spp. | 43 (47.3) |
| S. enterica Typhi | 33 (36.3) |
| S. enterica Paratyphi A | 10 (11.0) |
| Escherichia coli | 20 (22.0) |
| Klebsiella pneumoniae | 7 (7.7) |
| Non-Enterobacteriaceae | 6 (6.6) |
| Acinetobacter spp. | 2 (2.2) |
| Burkholderia cepacia complex | 2 (2.2) |
| Burkholderia pseudomallei | 1 (1.1) |
| Pseudomonas aeruginosa | 1 (1.1) |
| Gram-positive bacteria | 11 (12.1) |
| Staphylococcus aureus | 6 (6.6) |
| Beta-hemolytic Streptococcus* | 2 (2.2) |
| Enterococcus faecalis | 2 (2.2) |
| Streptococcus anginosus | 1 (1.1) |
| Yeast | 4 (4.4) |
| Candida tropicalis | 2 (2.2) |
| Candida albicans | 1 (1.1) |
| Candida famata | 1 (1.1) |
| Total | 91 (100.0) |
*Beta-hemolytic streptococci included: Streptococcus agalactiae (n = 1) and Streptococcus dysgalactiae (n = 1).
Blood cultures with no growth on subculture
Among 15 blood culture bottles that flagged positive on the BacT/ALERT system but did not yield isolates on subculture, 1 (6.7%) tested positive for pneumococcal antigen. Gram-positive cocci in pairs were observed in the gram-stained smear of the pneumococcal antigen-positive blood-broth mixture whereas the remainder had negative gram-stains.
Clinical data of participants with bloodstream infection
Among 100 participants with clinically suspected enteric fever, 11 (11.0%) had blood cultures positive for S. enterica Typhi or Paratyphi A. Thirty-two (74.4%) of 43 participants with blood culture-positive S. enterica were not identified as having enteric fever clinically. Among 43 participants with blood culture-confirmed S. enterica BSI, typhoid fever complications were observed in none. S. agalactiae was isolated from a 22 year-old previously healthy participant presenting with fever, headache, and neck stiffness.
Seasonal pattern of bloodstream infections
Of 947 blood cultures, pathogens were isolated in 43 (10.2%) of 423 collected during the dry season whereas 47 (9.0%) were isolated from 524 in the wet season (OR 1.15; 95% CI 0.74–1.77; p = 0.533). Enterobacteriaceae were isolated from 34 (79.1%) of 43 positive blood cultures during the dry season and 35 (74.5%) of 47 positive blood cultures during the wet season (OR 1.29; 95% CI 0.848–3.47; p = 0.606). S. enterica was isolated from 24 (51.1%) of 47 positive blood cultures in the wet season whereas 19 (44.2%) were identified in 43 positive blood cultures during the dry season (OR 1.32; 95% CI 0.57–3.02; p = 0.514).
Antimicrobial susceptibility of bloodstream isolates
The AST patterns of gram-negative pathogens isolated from blood cultures from febrile patients are shown in Table 3. Of 76 gram-negative bacteria, 22 (28.9%) were MDR and 2 (2.6%) were XDR (Table 4). All MDR and XDR bacteria were susceptible to colistin (Table 3). We have recently reported the AST pattern of S. enterica isolates [28]. Among gram-positive bloodstream isolates, no S. aureus were methicillin-resistant (MRSA). The Enterococcus faecalis and pathogenic streptococci were susceptible to all antimicrobial agents tested.
Table 3. Antimicrobial susceptibility pattern of gram-negative bloodstream isolates from febrile patients at Yangon General Hospital, 2015–2016.
| Pathogens, number (%) susceptible | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antimicrobial agents | S. enterica Typhi*, n = 33 |
S. enterica Paratyphi A*, n = 10 |
E. coli, n = 20 | K. pneumoniae, n = 7 |
Acinetobacter spp, n = 2 |
B. cepacia complex †, n = 2 |
B. pseudomallei †, n = 1 | P. aeruginosa, n = 1 |
| Ampicillin | 33 (100) | 10 (100) | 1 (5) | 0 (0) | NT | NT | 1 (100) | NT |
| Amoxicillin-clavulanate | 33 (100) | 10 (100) | 1 (5) | 4 (57) | NT | NT | NT | NT |
| Piperacillin-tazobactam | 33 (100) | 10 (100) | 14 (70) | 5 (71) | 0 (0) | 2 (100) | 1 (100) | 1 (100) |
| Ceftriaxone | 33 (100) | 10 (100) | 3 (15) | 4 (57) | 0 (0)† | NT | 1 (100) | NT |
| Ceftazidime | 33 (100) | 10 (100) | 4 (20) | 4 (57) | 0 (0) † | 2 (100) | 1 (100) | 1 (100) |
| Cefepime | 33 (100) | 10 (100) | 3 (15) | 5 (71) | 2 (100)† | 2 (100) | 1 (100) | 1 (100) |
| Aztreonam | 33 (100) | 10 (100) | 3 (15) | 4 (57) | NT | NT | NT | NT |
| Ertapenem | 33 (100) | 10 (100) | 19 (95) | 7 (100) | NT | NT | NT | NT |
| Imipenem | 33 (100) | 10 (100) | 19 (95) | 7 (100) | 2 (100) | NT | NT | 1 (100) |
| Meropenem | 33 (100) | 10 (100) | 19 (95) | 7 (100) | 2 (100) | 2 (100) | 1 (100) | 1 (100) |
| Amikacin | 0 (0) ‡ | 0 (0) ‡ | 18 (90) | 6 (86) | 2 (100) | NT | NT | 1 (100) |
| Gentamicin | 0 (0) ‡ | 0 (0) ‡ | 10 (50) | 5 (71) | 2 (100) | NT | NT | 0 (100) |
| Tobramycin | 0 (0) ‡ | 0 (0) ‡ | 9 (45) | 5 (71) | 2 (100) | NT | NT | NT |
| Nalidixic acid | 0 (0) | 0 (0) | NT | NT | NT | NT | NT | NT |
| Ciprofloxacin | 0 (0) | 0 (0) | 4 (20) | 4 (57) | 2 (100) | 0 (0) | 1 (100) | 1 (100) |
| Azithromycin | 33 (100) | 10 (100) | NT | NT | NT | NT | NT | NT |
| Tigecycline | 33 (100) | 10 (100) | 20 (100) | 3 (43) | NT | NT | NT | NT |
| Tetracycline | 33 (100) | 10 (100) | 4 (20) | 4 (57) | 1 (50) § | NT | 1 (100) | NT |
| Trimethoprim-Sulfamethoxazole | 33 (100) | 10 (100) | 5 (25) | 4 (57) | 2 (100) | NT | 1 (100) | NT |
| Chloramphenicol | 33 (100) | 10 (100) | 11 (55) | 5 (71) | NT | NT ¶ | NT | NT |
| Colistin | 33 (100) | 10 (100) | 20 (100) | 7 (100) | 2 (100) | NT | NT | NT |
NT: Not tested.
*Results have recently been reported in a separate paper [28];
†AST interpretation according to CLSI guidelines [19];
‡Aminoglycosides (amikacin, gentamicin, tobramycin) are not effective clinically against Salmonella spp. and are recommended to be reported as resistant [19];
§Tested with doxycycline.
¶AST not tested since disk diffusion test is not reliable.
Table 4. Prevalence of phenotypic antimicrobial resistance patterns among gram-negative bloodstream pathogens isolated from febrile patients at Yangon General Hospital, 2015–2016.
| Phenotypic resistance pattern | E. coli | K. pneumoniae | Acinetobacter spp | S. enterica* | Total |
|---|---|---|---|---|---|
| MDR | (n = 20) | (n = 7) | (n = 2) | (n = 43) | |
| XDR | n (%) | n (%) | n (%) | n (%) | n |
| MDR | 17 (85) | 3 (43) | 2 (100) | 0 (0) | 22 |
| XDR | 2†(10) | 0 (0) | 0 (0) | 0 (0) | 2 |
| ESBL | 15 (75) | 3 (43) | 0 (0) | 0 (0) | 18 |
MDR, Multi-drug resistance; XDR, extensive drug resistance; ESBL, extended-spectrum beta-lactamase.
*Results reported in a separate paper [28];
†One ESBL-producer and one carbapenemase-producer.
Phenotypic confirmation of extended-spectrum beta-lactamase, AmpC beta-lactamase, and carbapenemase production among Enterobacteriaceae
Of 70 Enterobacteriaceae, phenotypic testing identified ESBL-production in 18 (25.7%), AmpC beta-lactamase production in 1 (1.4%), and carbapenamase-production in 1 (1.4%) (Table 4). Of 17 MDR E. coli, ESBL-production was found in 14 (82.4%) and AmpC beta-lactamase in 1 (5.9%), while 2 XDR isolates produced an ESBL (n = 1) or a carbapenemase enzyme (n = 1). All 3 (100.0%) MDR K. pneumoniae produced an ESBL. No S. enterica produced an ESBL, AmpC, or carbapenemase.
Among non-Salmonella Enterobacteriaceae, ESBL-production was detected in 12 (63.2%) of 19 CA BSI, 2 (66.7%) of 3 HCA BSI, and 4 (80.0%) of 5 HA BSI. We identified an AmpC beta-lactamase-producing E. coli (n = 1) in a CA BSI. An E. coli isolate which produced a carbapenemase (n = 1) was recovered from a diabetic patient with HCA BSI who was previously admitted to a private healthcare center in Yangon for <48 hours.
Antimicrobial resistance mechanisms among Escherichia coli and Klebsiella pneumoniae bloodstream isolates
We have recently reported the genomic analysis of the S. enterica isolates [28]. Here we report the genomic analysis of the E. coli and K. pneumoniae isolates. Of 27 E. coli and K. pneumoniae isolates, ESBL genes were identified in 19 (70.4%), including 18 with phenotypic evidence of ESBL production. An ESBL gene was also identified in the single carbapenemase-producing E. coli (Table 5). Of 19 E. coli and K. pneumoniae isolates that harbored an ESBL gene, blaCTX-M 15 (group 1 CTX-M) was detected in 17 (89.5%) while the remaining 2 (10.5%), both E. coli, harbored a group 9 CTX-M gene (1 blaCTX-M 14 and 1 blaCTX-M 27). We identified blaCMY-42 in the AmpC producer and blaNDM-5 in the carbapenemase-producing E. coli. (Table 5).
Table 5. Beta-lactamase genes identified in Escherichia coli and Klebsiella pneumoniae bloodstream isolates from febrile patients at Yangon General Hospital, 2015–2016.
| Resistance genes | E. coli | K. pneumoniae | Total |
|---|---|---|---|
| (n = 20) | (n = 7) | (n = 70) | |
| n (%) | n (%) | n (%) | |
| ESBL | 19 (27.1) | ||
| blaCTX-M-15 | 14* (70.0) | 3 (42.9) | 17 (24.3) |
| blaCTX-M-14 | 1 (5.0) | 0 (0.0) | 1 (1.4) |
| blaCTX-M-27 | 1 (5.0) | 0 (0.0) | 1 (1.4) |
| Carbapenemase | |||
| blaNDM-5 | 1 (5.0) | 0 (0.0) | 1 (1.4) |
| AmpC | |||
| blaCMY-42 | 1 (5.0) | 0 (0.0) | 1 (1.4) |
ESBL, extended spectrum beta lactamase.
*One isolate co-produced blaNDM-5
ESBL, extended spectrum beta lactamase. *An isolate co-produced blaNDM-5.
Of 19 ciprofloxacin-resistant E. coli and K. pneumoniae isolates, mutations in QRDR were found in 16 (84.2%) while 14 (73.7%) had plasmid-mediated quinolone resistance (PMQR) genes. Among 16 isolates that possessed QRDR mutations, three types of mutations (two in gyrA and one in parC) were seen in 15 (93.8%) E. coli (S2 Table). Among E. coli, 11 (68.8%) of 16 ciprofloxacin-resistant isolates encoded PMQR genes, aac-6’-Ib-cr being the most prevalent (S2 Table). Only the PMQR gene qnrB was found in all 3 (100.0%) ciprofloxacin-resistant K. pneumoniae (S2 Table).
Sequence type determination and phylogenetic analysis
Using WGS data, we confirmed that 6 (30.0%) of 20 E. coli were sequence type (ST) 131 and all harbored blaCTX-M-15. Phylogenetic analysis of the E. coli ST131 strains identified that our bloodstream isolates were not clonally related to each other (S1 Fig). Other E. coli sequence types included ST405 and ST648 and there were 3 (15.0%) isolates of each. Two each of E. coli ST405 and ST648 also carried blaCTX-M-15. One (50.0%) of 2 E. coli ST648 isolates harboring blaCTXM-15 also bore blaNDM-5. Among 7 K. pneumoniae isolates, we identified sequence types as 2 (28.6%) ST23, and 1 (14.3%) each of ST20, ST35, and ST307. We also identified that the single isolate of S. agalactiae belonged to the sequence type 283.
Discussion
We found that S. enterica Typhi and Paratyphi A were collectively the most common cause of CA BSI at a tertiary referral hospital in Yangon, Myanmar. As previously reported, all S. enterica isolates were resistant to both nalidixic acid and ciprofloxacin, but were susceptible to all other antimicrobial classes tested [28]. ESBL-production was common among E. coli and K. pneumoniae. We also identified one isolate of carbapenemase-producing E. coli that was also XDR. One patient had a blood culture positive for B. pseudomallei and one for S. agalactiae ST283. Methicillin resistant S. aureus was not found in our study. S. pneumoniae antigen was detected in one of 15 positive blood culture bottles with no growth on subsequent subculture.
Among participants, 9.5% had blood cultures positive for pathogens. Previously, pathogen-positive blood cultures were detected in 28 (34.6%) of 81 patients who attended a military hospital in Yangon [34] and 111 (34.3%) of 324 hospitalized patients in Mandalay, situated in central Myanmar [35]. The lower prevalence of BSI in our study compared to previous studies is likely due to the inclusion of all febrile patients in this prospective study, independent of disease severity or clinical discretion.
The finding of S. enterica Typhi and Paratyphi A as the leading cause of BSI in Yangon is consistent with similar studies from other parts of South and South-East Asia [5, 36–38]. In a study of patients suspected to have enteric fever in Mandalay in central Myanmar during 2012–2013, S. enterica Typhi was isolated from 4.5% of blood cultures [35]. A study at Yangon Children’s Hospital (YCH) in 1998–1999 identified S. enterica Typhi as the most common cause of pediatric BSI, accounting for 43.1% bloodstream pathogens [6]. In high typhoid fever incidence settings, typhoid incidence tends to be highest among infants and young children [37, 39]. Therefore, the YCH finding is not surprising and consistent with other work suggesting that typhoid fever incidence is high in Yangon [14].
Fluoroquinolones have become the mainstay of enteric fever management in South and South-East Asia, including in Myanmar, in response to the emergence of MDR S. enterica Typhi [40]. MDR S. enterica Typhi was identified in Myanmar more than a decade ago [6], prompting a switch to the fluoroquinolone ciprofloxacin for the treatment of suspected enteric fever. We demonstrate that contemporary S. enterica isolates were resistant to nalidixic acid and ciprofloxacin, yet susceptible to ampicillin, chloramphenicol, and trimethoprim-sulphamethoxazole [28]. Notably, neither nalidixic acid nor ciprofloxacin resistance was detected in S. enterica isolates from the YCH study in 1998 [6]. However, by 2012 a quarter of S. enterica bloodstream isolates from Mandalay were nalidixic acid resistant [35] and by 2014 half of S. enterica isolates at YGH were ciprofloxacin-resistant [41]. Nalidixic acid susceptibility testing was not done in a 2014 YGH study [42]. As supported by our recent genomic analysis [28], these changes are likely due to the recent emergence and spread of fluoroquinolone-resistant typhoidal S. enterica in Myanmar, and possibly due to lack of ascertainment of unrecognized nalidixic acid-resistance and changes in fluoroquinolone susceptibility interpretive criteria [43].
Azithromycin is an alternative option for the treatment of uncomplicated MDR and fluoroquinolone-resistant S. enterica Typhi infections [44]. Reports of azithromycin treatment failure have been noted between 2004 and 2005 in Vietnam [45] and during 2016 in India [46] associated with azithromycin MICs 2–8 μg/mL. The range of azithromycin MIC for S. enterica isolates in our study was 2–12 μg/mL. YGH treatment guidelines recommend the use of azithromycin or an extended-spectrum cephalosporin for the management of enterica fever due to ciprofloxacin-resistant S. enterica. Since we could not access the antimicrobial treatment given at YGH for all participants, we could not correlate treatment outcome among study participants with azithromycin MIC. Likewise, ESBL-producing, ceftriaxone-resistant S. enterica Typhi is the cause of an ongoing outbreak of typhoid fever in Pakistan that began in 2016 [9]. Although ESBL production has not been reported in S. enterica Typhi isolates from Myanmar, its emergence might be curtailed by judicious use of antimicrobials in the community and its spread by early identification and intervention with vaccine.
We identified CO BSI due to ESBL-producing Enterobacteriaceae, as well as MDR and XDR phenotypes. Unsurprisingly, the proportion of isolates producing an ESBL and with a XDR phenotype was smaller than our previous retrospective study that included HA BSI [41] and lower than other studies including HA BSI from YGH performed in early 2015 [47] and from North Okkalapa Hospital (NOGH), Yangon, in 2016 [48]. Nonetheless, the availability of antimicrobial agents without prescription over the counter [49] and use of antimicrobial agents in food animals [50] may contribute to the development of AMR among pathogens acquired in the community.
We identified ESBL genes in nearly three quarters of E. coli and K. pneumoniae bloodstream isolates, most commonly blaCTX-M-15. Previously, we reported blaCTX-M-15 in just over 70% of ESBL-producing Enterobacteriaceae isolated from adult inpatients with BSI at YGH in 2014 [41]. Our findings are consistent with the global dissemination of blaCTX-M-15 among gram-negative bacteria [51]. This gene is frequently detected among E. coli and K. pneumoniae isolated from other Asian countries [12, 52]. In addition, E. coli ST131 commonly encodes blaCTX-M-15 and has been associated with community-onset infections [51]. The detection of the globally disseminated blaCTX-M-15 gene among ESBL-producers, including the pandemic E. coli ST131, in our study warrants further investigations in other areas of Myanmar.
We identified blaNDM-5 in a single E. coli isolate that phenotypically produced a carbapenemase. Notably, blaNDM-5 was not detected in our previous retrospective study at YGH in 2014 [41]. Our finding in the present study is consistent with those from two Myanmar studies done at YGH in early 2015 [53] and at NOGH in Yangon in 2016 [48] in which blaNDM-5 was detected among the majority of E. coli investigated. Isolates bearing blaNDM-5 have been detected increasingly in Asian countries [54–57] and among travelers to the Indian subcontinent [58, 59]. Identification of blaNDM-5 from an HCA BSI associated with a private hospital, in our prospective study raises concerns that blaNDM-5 E. coli strains may be widespread both in public and private hospitals in Myanmar. Of concern, NDM-5 producing E. coli also bore blaCTX-M-15 and other antimicrobial resistance genes. This suggests possible transfer of plasmids with multiple resistance genes or presence of multiple plasmids carrying AMR genes.
The pandemic sequence type E. coli ST131 was predominant among community-onset E. coli BSI in our study. E. coli ST131 has been reported in several Asian and South-East Asian countries [52]. Such strains often express blaCTX-M-15 [51, 52], are more likely to be multidrug resistant than other ESBL-producing E. coli [51], and are more likely to be associated with community-acquired infections [51]. Although less common, carriage of blaCTX-M-15 has also been identified in E. coli ST38, ST405, and ST648 [51]. Similarly, we identified that all six E. coli ST131 acquired in the community carried the blaCTX-M-15 gene and were MDR, and blaCTX-M-15 was also carried by E. coli with STs 405 and 648. Consistent with other research [54, 58, 59], one of our E. coli ST648 isolates that carried blaCTXM-15 also bore blaNDM-5. Notably, E. coli ST648 is an emerging lineage associated with MDR [60]. This suggests that newly emerged E. coli associated with MDR is a cause of BSI in Myanmar.
Mutations in the QRDR, most commonly in the form of the Ser83Phe mutation, was often associated with fluoroquinolone resistance among Enterobacteriaceae in our study. This mutation was common in E. coli isolates, and as we have previously reported, was found in all S. enterica [28]. Some ciprofloxacin-resistant E. coli harbored aac-6’-Ib-cr, the most prevalent PMQR, in addition to QRDR mutations. The contemporary Myanmar study performed at the NOGH also detected aac-6’-Ib-cr as the major PMQR found in ciprofloxacin-resistant E. coli [48]. The NOGH study did not investigate QRDR mutations [48]. The plasmid-mediated aac-6’-Ib-cr is a variant of aac-6’-Ib that encodes aminoglycoside acetyltransferase enzyme and possess the ability to reduce the activities of both aminoglycosides and fluoroquinolones [61]. The gene has been widely identified among ciprofloxacin-resistant E. coli [61–63]. Our findings point to a need to avoid extensive use of fluoroquinolones to prevent further spread of resistance in the community and to consider alternative drug regimens.
Few gram-positive pathogens were isolated in our study. No MRSA was found. All Streptococcus spp. and E. faecalis were susceptible to all antimicrobial agents tested. S. pneumoniae were not isolated from blood cultures, although one positive blood culture that failed to grow on subculture was positive by the Binax antigen test. This might suggest that S. pneumoniae is an uncommon cause of BSI in Yangon. No published studies on invasive pneumococcal disease in Myanmar are available. Based on evidence that pneumococcal disease is common in children <5 years of age [64], pneumococcal conjugate vaccine was first introduced into the Myanmar national expanded programme of immunization in 2016. Our inability to immediately subculture blood culture bottles that flagged positive overnight or on weekends may have hindered our ability to isolate S. pneumoniae because of autolysis [65]. This is a common problem in laboratories with limited staff and resources [17]. Furthermore, 17.9% of participants reported antimicrobial use prior to enrollment, potentially lowering blood culture sensitivity. One blood culture, which signaled positive by the BacT/ALERT and in which gram-positive cocci were observed by microscopy, was positive for S. pneumoniae cell wall antigen by the Binax antigen test. This test has been suggested as an alternative means of detecting S. pneumoniae when there is no growth on subculture [17]. We recognize that the use of blood culture alone for the diagnosis of pneumococcal diseases underestimates its prevalence. As such, we recommend the future use of antigen detection as a bed-side test on urine samples when pneumococcal disease is clinically suspected or as an adjunct for hemolyzed blood cultures in clinical laboratories.
We isolated and identified two beta-hemolytic streptococci, including one isolate of S. agalactiae or Lancefield group B streptococcus (GBS). GBS is the leading cause of neonatal sepsis and meningitis in babies born to mothers with genital tract colonization [66], and is associated with puerperal sepsis in pregnant mothers [67]. It also causes invasive diseases in the elderly [68] and the immunocompromised [69]. Specific serotypes of S. agalactiae have been identified to cause serious infections in non-pregnant and otherwise healthy adults in Asia [70–72]. S. agalactiae ST283 was found to be associated with severe infections after consumption of raw freshwater fish in Singapore [71] and Lao PDR [72]. The GBS isolate from our study belonged to ST283. To our knowledge, detection of S. agalactiae ST283 has not been reported from either humans or aquatic animals in Myanmar (Kay Lwin Tun, pers comm, April 2019). Consumption of raw fish is uncommon in Myanmar [73, 74]. However, farmed fish is a growing source of protein in Asia [75] and the consumption of raw fish is increasingly popular in some areas [76, 77] including Myanmar [78]. The isolation of S. agalactiae ST283 in our study warrants greater attention to this pathogen in both people and fish in Myanmar.
Our study had a number of limitations. First, study participants seeking healthcare at a tertiary hospital in a large city were enrolled. Therefore, findings may not represent BSI among patients admitted to district level hospitals in areas other than Yangon. Second, since participants transferred from hospitals other than YGH were included in the study not all BSIs met the definition of community-acquired or community-onset. Third, nearly 20% of participants had taken antimicrobial agents before seeking healthcare at YGH, potentially reducing blood culture sensitivity. Fourth, our study did not include pediatric patients. Similar research is warranted among patients <12 years of age. Fifth, performing single aerobic blood culture lacks sensitivity for detection of bacteremia and will miss anaerobic bacteremia [79, 80]. Sixth, the lack of longitudinal clinical data and additional blood cultures meant that we may have classified some organisms as contaminants that were causing bacteremia. Finally, participants did not systematically undergo HIV testing or a test for blood parasites, so we were unable to examine potentially important associations between these co-infections and bacteremia.
In summary, we found that typhoidal S. enterica was the most common cause of CA BSI among febrile patients attending a large tertiary referral hospital in Yangon, Myanmar. The absence of MDR typhoidal Salmonella and the high prevalence of fluoroquinolone resistance is notable. This finding has important implications for the empiric management of enteric fever in Yangon and underscores the value of CA BSI surveillance to monitor the pattern of infecting organisms, serovars, and antimicrobial susceptibility. The emergence of ESBL- and carbapenemase-producing gram-negative bacteria carrying globally prevalent drug resistance genes among CO BSI highlights the need not only to redouble efforts to control antimicrobial-resistant organisms in healthcare facilities, but also to prevent their emergence and spread in the community. Isolation of ESBL- and NDM-producing Enterobacteriaceae from CO BSI also highlights the need to better understand the acquisition, carriage, and mode of transmission of antimicrobial resistant organisms outside the hospital setting. We recommend extending surveillance of AMR to include other common locations for healthcare seeking among all age groups in Myanmar, including primary healthcare centers and private hospitals. In so doing, we will gain a better understanding of antimicrobial resistance in the community setting which may help in the development of the national antimicrobial resistance plan.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
The presence or absence of mutations in the QRDR, PMQR genes, and the extended-spectrum beta-lactamase gene blaCTXM-15 are shown on the right. 3044 core genome SNPs were identified. A maximum likelihood tree was inferred from core genome SNPs, and rooted using the minimal ancestor deviation method [30]. The scale bars represent the phylogenetic distance of 10 SNPs. MIC, minimum inhibitory concentration; QRDR, quinolone resistance-determining region; PMQR, plasmid-mediated quinolone resistance; ESBL, extended-spectrum beta-lactamase; SNP, single nucleotide polymorphism.
(TIF)
Acknowledgments
We would like to thank all participants of this study. We thank team members of the YGH febrile illness study: specifically to Dr. Khine Mar Htun, Dr, Khin Khin Kyourk, Dr. Su Hnin Aung, Dr. Su Htet Aung, Dr. Su Myat Aye, Dr. Sai Nay Linn Htet, Dr. Si Thu Sein Win, Dr. Htet Htet Lin, Dr. Htet Htet Lwin, Dr. Win Thit Lwin, Dr. Lin Thit Lwin, and Dr. Hein Htet Aung for sample collection; staff of the Clinical Microbiology Laboratory Section, Yangon General Hospital. We thank Dr. Khwar Nyo Zin, Consultant Microbiologist, Clinical Microbiology Laboratory, YGH for her kind offer to let us use her laboratory facilities. We thank laboratory staff from Southern Community Laboratories, Dunedin, New Zealand for their kind assistance and sharing their expertise with confirmatory laboratory work.
Data Availability
All relevant data and links to data are provided within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the New Zealand Health Research Council through an e-ASIA Joint Research Programme (grant 16/697), and an Otago Medical School Collaborative Research Grant, University of Otago, New Zealand. TOM received support through University of Otago Doctoral Scholarship. JAC also received support from Bill & Melinda Gates Foundation grant OPP1151153. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Archibald LK, McDonald LC, Rheanpumikankit S, Tansuphaswadikul S, Chaovanich A, Eampokalap B, et al. Fever and human immunodeficiency virus infection as sentinels for emerging mycobacterial and fungal bloodstream infections in hospitalized patients ≥ 15 years old, Bangkok. J Infect Dis. 1999;180(1):87–92. Epub 1999/06/03. 10.1086/314836 . [DOI] [PubMed] [Google Scholar]
- 2.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86(2):246–53. 10.4269/ajtmh.2012.11-0409 PubMed PMID: PMC3269275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One. 2015;10(6):e0127962 10.1371/journal.pone.0127962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244(5):379–86. Epub 1998/12/10. PubMed 10.1046/j.1365-2796.1998.00379.x . [DOI] [PubMed] [Google Scholar]
- 5.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community-acquired bacterial bloodstream infections in developing countries in South and South-East Asia: a systematic review. Lancet Infect Dis. 2012;12(6):480–7. Epub 2012/05/29. 10.1016/S1473-3099(12)70028-2 . [DOI] [PubMed] [Google Scholar]
- 6.Shwe TN, Nyein MM, Yi W, Mon A. Blood culture isolates from children admitted to Medical Unit III, Yangon Children's Hospital, 1998. Southeast Asian J Trop Med Public Health. 2002;33(4):764–71. Epub 2003/05/22. . [PubMed] [Google Scholar]
- 7.Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis. 2001;7(2):302–5. PubMed PMID: PMC2631738. 10.3201/eid0702.010232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Fact sheets: antimicrobial resistance: World Health Organization; 2018 [cited 2019 23 April]. Available from: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- 9.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio. 2018;9(1):e00105–18. Epub 2018/02/22. 10.1128/mBio.00105-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, et al. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56(5):641–8. Epub 11/13. 10.1093/cid/cis942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlieghe E, Phe T, De Smet B, Chhun Veng H, Kham C, Lim K, et al. Bloodstream infection among adults in Phnom Penh, Cambodia: key pathogens and resistance patterns. PLoS One. 2013;8(3):e59775 10.1371/journal.pone.0059775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan J, Zhao D, Liu L, Chen Y, Zhou J, Jiang Y, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. 2016;72(1):273–80. 10.1093/jac/dkw372 [DOI] [PubMed] [Google Scholar]
- 13.Department of Population, Ministry of Immigration and Population, The Republic of the Union of Myanmar. The 2014 Myanmar population and housing census: Yangon Region, census report Volume 3—L. Office No. 48, Nay Pyi Taw: 2016.
- 14.Oo WT, Myat TO, Htike WW, Ussher JE, Murdoch DR, Lwin KT, et al. Incidence of typhoid and paratyphoid fevers among adolescents and adults in Yangon, Myanmar. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2019;68 (suppl 2):S124–S9. 10.1093/cid/ciy1109 PubMed Central PMCID: PMC30845332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–74. 10.1001/jama.2016.0288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. International statistical classification of diseases and related health problems 10th revision [Internet]. Geneva: World Health Organization; 2016. [cited 2016]. 5th Edition:[Available from: https://icd.who.int/browse10/2016/en. [Google Scholar]
- 17.Petti CA, Woods CW, Reller LB. Streptococcus pneumoniae antigen test using positive blood culture bottles as an alternative method to diagnose pneumococcal bacteremia. Journal of clinical microbiology. 2005;43(5):2510–2. 10.1128/JCM.43.5.2510-2512.2005 PubMed PMID: PMC1153727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement M100-S25. 2015;35(3). [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. CLSI supplement M100 S26 26th ed. Clincal and Laboratory Standards Institute: Wayne PA; 2016.
- 20.Duval BD, Elrod MG, Gee JE, Chantratita N, Tandhavanant S, Limmathurotsakul D, et al. Evaluation of a latex agglutination assay for the identification of Burkholderia pseudomallei and Burkholderia mallei. Am J Trop Med Hyg. 2014;90(6):1043–6. 10.4269/ajtmh.14-0025 PubMed PMID: PMC4047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Healthcare Safety Network, United States Centres for Disease Control and Prevention. Common commensals list.xls [cited 2018]. Available from: www.cdc.gov/nhsn/XLS/master-organism-Com-Commensals-Lists.xlsx.
- 22.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters version 7.1 2017 [cited 2017 20 June]. Available from: http://www.eucast.org/clinical_breakpoints.
- 23.The European Committee on Antimicrobial Susceptibility Testing. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance version 2.0 2017 [cited 2017 30 July]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf.
- 24.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(3):268–81. Epub 2011/07/29. 10.1111/j.1469-0691.2011.03570.x . [DOI] [PubMed] [Google Scholar]
- 25.Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Current opinion in infectious diseases. 2008;21(5):531–8. Epub 2008/08/30. 10.1097/QCO.0b013e32830f453a . [DOI] [PubMed] [Google Scholar]
- 26.Macherey-Nagel. Genomic DNA from tissue: user manual 2017 [cited 2017 23 June]. Available from: http://www.mn-net.com/Portals/8/attachments/Redakteure_Bio/Protocols/Genomic%20DNA/UM_gDNATissueXS.pdf.
- 27.Seeman T, Goncalves da Silva A, Bulach DM, Schultz MB, Kwong JC, Howden BP. Nullarbor [software]. 2016 [20 August 2018]. Available from: https://github.com/tseemann/nullarbor.
- 28.Oo KM, Myat TO, Htike WW, Biswas A, Hannaway RF, Murdoch DR, et al. Molecular mechanisms of antimicrobial resistance and phylogenetic relationships of Salmonella enterica isolates from febrile patients in Yangon, Myanmar. Trans R Soc Trop Med Hyg. 2019;113:641–8. Epub 2019/06/22. 10.1093/trstmh/trz053 . [DOI] [PubMed] [Google Scholar]
- 29.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43(3):e15 10.1093/nar/gku1196 PubMed Central PMCID: PMC25414349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tria FDK, Landan G, Dagan T. Phylogenetic rooting using minimal ancestor deviation. Nat Ecol Evol. 2017;1(7):0193 10.1038/s41559-017-0193 [DOI] [PubMed] [Google Scholar]
- 31.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–64. 10.1128/CMR.00002-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–8. 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 33.World Weather and Climate Information. Climate and average weather in Myanmar (Burma) [18 September 2018]. Available from: https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine-in-Myanmar-Burma.
- 34.Mar Mar Nyein Malar Than, Tin Mar Lwin Ni Ni Aung. Isolation of bacterial pathogens from blood samples collected from in-patients, Medical Ward, Defence Service General Hospital, Mingalardon during 1998–1999. Myanmar Health Sciences Res J. 2001;13(1):21–5. [Google Scholar]
- 35.Thwe SM, Kyaw AK, Lwin MM, Htet KKK, Han TM, Thida, et al. Initiation of hospital-based active typhoid fever sentinel surveillance among suspected cases in Mandalay. Myanmar Health Sciences Research J. 2016;28(1):1–4. [Google Scholar]
- 36.Phetsouvanh R, Phongmany S, Soukaloun D, Rasachak B, Soukhaseum V, Soukhaseum S, et al. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am J Trop Med Hyg. 2006;75(5):978–85. [PMC free article] [PubMed] [Google Scholar]
- 37.Antillon M, Warren JL, Crawford FW, Weinberger DM, Kurum E, Pak GD, et al. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Neglect Trop Dise. 2017;11(2):e0005376 10.1371/journal.pntd.0005376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchello CS, Dale AP, Pisharody S, Rubach MP, Crump JA. A systematic review and meta-analysis of the prevalence of community-onset bloodstream infections among hospitalized patients in Africa and Asia. Antimicrob Agents Chemother. 2020;64:e01974–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckle GC, Walker CLF, Black RE. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Global Health. 2012;2(1). 10.7189/jogh.02.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhutta ZA. Current concepts in the diagnosis and treatment of typhoid fever. BMJ. 2006;333(7558):78–82. PubMed PMID: PMC1489205. 10.1136/bmj.333.7558.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myat TO, Hannaway RF, Zin KN, Htike WW, Win KK, Crump JA, et al. ESBL- and carbapenemase-producing Enterobacteriaceae in patients with bacteremia, Yangon, Myanmar, 2014. Emerg Infect Dis. 2017;23(5):857–9. 10.3201/eid2305.161100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myat TO, Prasad N, Thinn KK, Win KK, Htike WW, Zin KN, et al. Bloodstream infections at a tertiary referral hospital in Yangon, Myanmar. Trans R Soc Trop Med Hyg. 2014;108(11):692–8. Epub 2014/09/26. 10.1093/trstmh/tru151 . [DOI] [PubMed] [Google Scholar]
- 43.Humphries RM, Fang FC, Aarestrup FM, Hindler JA. In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(8):1107–13. 10.1093/cid/cis600 [DOI] [PubMed] [Google Scholar]
- 44.Effa EE, Bukirwa H. Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev. 2008;(4):Art.No.: CD006083. 10.1002/14651858.CD006083.pub2 PubMed PMID: CD006083. [DOI] [PubMed] [Google Scholar]
- 45.Dolecek C, Phi La Tran T, Rang Nguyen N, Le T, Ha V, Tuan Phung Q, et al. A multi-center randomised controlled trial of gatifloxacin versus azithromycin for the treatment of uncomplicated typhoid fever in children and adults in Vietnam. PLoS One. 2008;3:e2188 10.1371/journal.pone.0002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manesh A, Balaji V, Kumar DRN, Rupali P. A case of clinical and microbiological failure of azithromycin therapy in Salmonella enterica serotype Typhi despite low azithromycin MIC. Int J Infect Dis. 2017;54(C):62–3. 10.1016/j.ijid.2016.11.409 [DOI] [PubMed] [Google Scholar]
- 47.Mon M, Mendes AJ, Silva D, Zin KN, Htike WW, Da Costa PM. Study of multidrug-resistant gram-negative bacteria isolates obtained from hospitalized patients in Yangon, Myanmar. Myanmar Health Sciences Research J. 2017;29(2):151–7. [Google Scholar]
- 48.Aung MS, San N, Maw WW, San T, Urushibara N, Kawaguchiya M, et al. Prevalence of extended-spectrum beta-lactamase and carbapenemase genes in clinical isolates of Escherichia coli in Myanmar: dominance of blaNDM-5 and emergence of blaOXA-181. Microb Drug Resist. 2018;00(00). 10.1089/mdr.2017.0387 [DOI] [PubMed] [Google Scholar]
- 49.Sakeena MHF, Bennett AA, McLachlan AJ. Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: a systematic review. International journal of antimicrobial agents. 2018;52(6):771–82. doi: 10.1016/j.ijantimicag.2018.09.022. 10.1016/j.ijantimicag.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 50.Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: A developing country-perspective. Front Microbiol. 2016;7:1881 10.3389/fmicb.2016.01881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitout J. Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol. 2012;3(9). 10.3389/fmicb.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyeong CM, Cheol-In K, Hyun KS, Visanu T, Man-kit ST, Eun HY, et al. Emergence and dissemination of ST131 Escherichia coli isolates among patients with hospital-acquired pneumonia in Asian countries. Microb Drug Resist. 2017;23(1):79–82. 10.1089/mdr.2016.0009 . [DOI] [PubMed] [Google Scholar]
- 53.Sugawara Y, Akeda Y, Sakamoto N, Takeuchi D, Motooka D, Nakamura S, et al. Genetic characterization of blaNDM-harboring plasmids in carbapenem-resistant Escherichia coli from Myanmar. PLoS One. 2017;12(9):e0184720 10.1371/journal.pone.0184720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano R, Nakano A, Hikosaka K, Kawakami S, Matsunaga N, Asahara M, et al. First report of metallo-beta-lactamase NDM-5-producing Escherichia coli in Japan. Antimicrob Agents Chemother. 2014;58(12):7611–2. 10.1128/AAC.04265-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman M, Shukla SK, Prasad KN, Ovejero CM, Pati BK, Tripathi A, et al. Prevalence and molecular characterisation of New Delhi metallo-beta-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. International journal of antimicrobial agents. 2014;44(1):30–7. Epub 2014/05/17. 10.1016/j.ijantimicag.2014.03.003 . [DOI] [PubMed] [Google Scholar]
- 56.Chen D, Gong L, Walsh TR, Lan R, Wang T, Zhang J, et al. Infection by and dissemination of NDM-5-producing Escherichia coli in China. J Antimicrob Chemother. 2016;71(2):563–5. 10.1093/jac/dkv352 [DOI] [PubMed] [Google Scholar]
- 57.Ranjan A, Shaik S, Mondal A, Nandanwar N, Hussain A, Semmler T, et al. Molecular epidemiology and genome dynamics of New Delhi metallo-beta-lactamase-producing extraintestinal pathogenic Escherichia coli strains from India. Antimicrob Agents Chemother. 2016;60(11):6795–805. 10.1128/AAC.01345-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hornsey M, Phee L, Wareham DW. A Novel variant, NDM-5, of the New Delhi metallo beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother. 2011;55(12):5952–4. 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wailan AM, Paterson DL, Caffery M, Sowden D, Sidjabat HE. Draft genome sequence of NDM-5-producing Escherichia coli sequence type 648 and genetic context of NDM-5 in Australia. Genome Announc. 2015;3(2):e00194–15. 10.1128/genomeA.00194-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson JR, Johnston BD, Gordon DM. Rapid and specific detection of the Escherichia coli sequence type 648 Complex within phylogroup F. Journal of clinical microbiology. 2017;55(4):1116–21. Epub 2017/01/20. 10.1128/JCM.01949-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, et al. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nature medicine. 2006;12(1):83–8. Epub 2005/12/22. 10.1038/nm1347 . [DOI] [PubMed] [Google Scholar]
- 62.Kim ES, Jeong J- Y, Jun J- B, Choi S- H, Lee S- O, Kim M- N, et al. Prevalence of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme among Enterobacteriaceae blood isolates in Korea. Antimicrob Agents Chemother. 2009;53(6):2643–5. 10.1128/AAC.01534-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frasson I, Cavallaro A, Bergo C, Richter SN, Palù G. Prevalence of aac(6')-Ib-cr plasmid-mediated and chromosome-encoded fluoroquinolone resistance in Enterobacteriaceae in Italy. Gut Pathog. 2011;3(1):12 10.1186/1757-4749-3-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar R, Arora N, Santosham M. South Asia symposium on pneumococcal disease and the promise of vaccines: meeting report. Vaccine. 2016;34(23):2622–6. 10.1016/j.vaccine.2016.03.071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrol KC, Hobden J.A. The streptococci, enterococci, and related genera. In: Carrol KC, Butel J.S., Morse S.A., Mietzner T., editor. Jawetz Melnick & Adelberg's Medical Microbiology 27 ed: McGraw-Hill Education; 2016. p. 213–27. [Google Scholar]
- 66.Fluegge K, Siedler A, Heinrich B, Schulte-Moenting J, Moennig MJ, Bartels DB, et al. Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics. 2006;117(6):e1139–45. Epub 2006/05/10. 10.1542/peds.2005-2481 . [DOI] [PubMed] [Google Scholar]
- 67.Deutscher M, Lewis M, Zell ER, Taylor TH, Jr., Van Beneden C, Schrag S. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(2):114–23. Epub 2011/06/22. 10.1093/cid/cir325 . [DOI] [PubMed] [Google Scholar]
- 68.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;41(6):839–47. Epub 2005/08/19. 10.1086/432804 . [DOI] [PubMed] [Google Scholar]
- 69.Pimentel BA, Martins CA, Mendonca JC, Miranda PS, Sanches GF, Mattos-Guaraldi AL, et al. Streptococcus agalactiae infection in cancer patients: a five-year study. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2016;35(6):927–33. Epub 2016/03/20. 10.1007/s10096-016-2617-9 . [DOI] [PubMed] [Google Scholar]
- 70.Morozumi M, Wajima T, Takata M, Iwata S, Ubukata K. Molecular characteristics of group B streptococci isolated from adults with invasive infections in Japan. Journal of clinical microbiology. 2016;54(11):2695–700. 10.1128/JCM.01183-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalimuddin S, Chen SL, Lim CTK, Koh TH, Tan TY, Kam M, et al. 2015 epidemic of severe Streptococcus agalactiae sequence type 283 infections in Singapore associated with the consumption of raw freshwater fish: a detailed analysis of clinical, epidemiological, and bacterial sequencing data. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;64 (suppl 2):S145–52. Epub 2017/05/06. 10.1093/cid/cix021 . [DOI] [PubMed] [Google Scholar]
- 72.Barkham T, Dance DAB, Vongsouvath M, Newton P, Chen S. Streptococcus agalactiae ST283 has been present in Laos for over 18 years (Conference abstract). Int J Infect Dis. 2018;73 (Supplement):189. doi: 10.1016/j.ijid.2018.04.3843. [DOI] [Google Scholar]
- 73.Khin Maung Win. Eating raw fish is not a Myanmar food habit. Health Digest Myanmar. 2004.
- 74.Downs SM, Glass S, Linn KK, Fanzo J. The interface between consumers and their food environment in Myanmar: an exploratory mixed-methods study. Public health nutrition. 2018:1–14. Epub 2018/12/19. 10.1017/s1368980018003427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tidwell JH, Allan GL. Fish as food: aquaculture's contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries. EMBO Rep. 2001;2(11):958–63. 10.1093/embo-reports/kve236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macpherson CNL. Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol. 2005;35(11):1319–31. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Van Thi Phan Annette Kjær Ersbøll, Dung Trung Do Anders Dalsgaard. Raw-fish-eating behavior and fishborne zoonotic trematode infection in people of northern Vietnam. Foodborne Pathog Dis. 2011;8(2):255–60. 10.1089/fpd.2010.0670 . [DOI] [PubMed] [Google Scholar]
- 78.Aung Phyo Wai, Win Win Maw, Aye Chan Moe, Boonmars T, Nawa Y. Human gnathostomiasis in Myanmar: a review of local literature. Southeast Asian J Trop Med Public Health. 2018;49(4):543–8. [Google Scholar]
- 79.Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123(5):1615–24. doi: 10.1378/chest.123.5.1615. 10.1378/chest.123.5.1615 [DOI] [PubMed] [Google Scholar]
- 80.Hung M, Chen S, Wang J, Chang S, Hsueh P-R, Liao C-H, et al. Community-acquired anaerobic bacteremia in adults: one-year experience in a medical center. J Microbiol Immunol Infect. 2005;38:436–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
The presence or absence of mutations in the QRDR, PMQR genes, and the extended-spectrum beta-lactamase gene blaCTXM-15 are shown on the right. 3044 core genome SNPs were identified. A maximum likelihood tree was inferred from core genome SNPs, and rooted using the minimal ancestor deviation method [30]. The scale bars represent the phylogenetic distance of 10 SNPs. MIC, minimum inhibitory concentration; QRDR, quinolone resistance-determining region; PMQR, plasmid-mediated quinolone resistance; ESBL, extended-spectrum beta-lactamase; SNP, single nucleotide polymorphism.
(TIF)
Data Availability Statement
All relevant data and links to data are provided within the manuscript and its Supporting Information files.