Abstract
For over a century, ionising radiation has been used to treat cancer based on its cytotoxic effects on tumour cells. Technical progress has enabled more precise targeting of the tumour to reduce normal tissue toxicity while delivering higher radiation doses per fraction of treatment.
In 1953, unexpected regression in lesions outside of the irradiated field were noted by an observant physician, RH Mole, who named such phenomenon “abscopal effect” from the Latin ab (position away from) and scopus (mark or target), in an article published in this journal. Clinical abscopal responses have been reported over the years but because of their very rare occurrence they could not be methodically studied, remaining akin to a curiosity. Nevertheless, their occurrence has ignited interest in studying the systemic effects of radiotherapy. Progress in dissecting the mechanisms that govern the function of the immune system in cancer has enabled to study the implication of immunity in the abscopal effect of radiation. It has become clear that ionising radiation activates canonical pathways of response to viral infections, and can stimulate antitumour immunity. These immune stimulatory effects of radiation have become clinically relevant in the current era of cancer immunotherapy, rendering abscopal responses in patients an attainable aim. Here, we will briefly review the parallel evolutions of two separate fields of medicine, radiation therapy and cancer immunology, and discuss their therapeutic partnership.
Introduction
The specialty of radiation oncology was born from the convergence of physics, chemistry, biology and medicine,1 and has continued to evolve through the progress of each of these disciplines. A cancer cell-centric view of the problem2 drove efforts to improving cancer cell kill, which in the case of radiation therapy focused on safely enhancing tumour dose and interference with DNA damage repair.3 During the past two decades, a paradigm shift that recognises the essential role of the immune system in cancer development and progression has become broadly accepted,4 reflecting the extraordinary progress in cancer immunology and immunotherapy.5 The recognition of the necessity to also target the immune system when treating cancer to achieve long-term tumour regression and possibly cure, has changed the global strategy of oncology.6 Whereas cytotoxic agents, including chemotherapy and radiation therapy, remain a mainstay of treatment, there is a growing appreciation for their direct and indirect effects on cancer immunity as key determinants for clinical success or failure.7,8 This insight is especially important to design combinatorial therapies that can recruit into response tumours refractory to immunotherapy. In this new landscape radiation has emerged as a promising modality because of its potential for enhancing responses to immunotherapy,9 extending its role from a local treatment to one that can be used to activate effective immune responses and tackle systemic disease, by inducing systemic, abscopal effects. Here, we will review the history and mechanisms of the abscopal effect of radiation and discuss some of the barriers to achieving abscopal responses. Finally, we will propose future experimental questions to optimise the induction of abscopal responses in the clinic.
A brief history of the abscopal effect of radiation
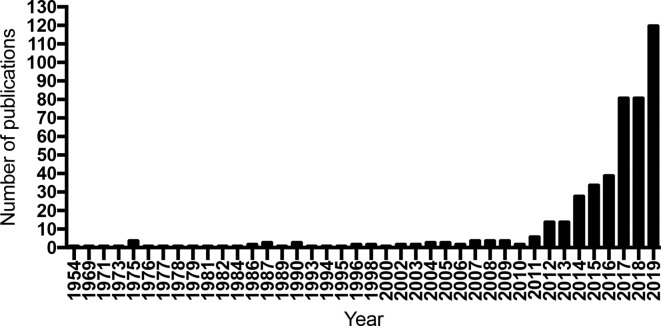
Abscopal responses were first described in 1953 in an article published in this journal by RH Mole,10 who had noticed that tumour regression was sometimes observed in non-treated lesions when one specific tumour area was focally irradiated. The definition of abscopal responses is based on the concurrent presence of metastatic sites that will regress after focal radiotherapy to one. This phenomenon has been repeatedly reported over the years in the radiation oncology and radiology literature,11–15 but it remained confined to be a curiosity due to its rare occurrence. A systematic review of the literature between 1969 and 2014 identified only 46 reported cases of abscopal responses.16 It is only with the introduction of immune checkpoint blockade (ICB) therapy in clinical practice that the interest in abscopal effects of radiation has soared, with a continuous increase in the number of publications since 2011, the year when the first immune checkpoint blocking antibody, ipilimumab, was approved for patients treatment (Figure 1). This increase reflects the occurrence of abscopal effects when radiotherapy is used in patients treated with ICB, in cancers otherwise unresponsive to ICB. It also demonstrates the emergence of a new strategy in oncology that of investigating a personalised approach to immunising patients against their individual tumour.17,18
Figure 1.
Number of publications per year identified doing a search on Pubmed for the term “Abscopal effect” from 1954 to 2019.
Pitfalls in defining abscopal effects
Unfortunately, over the years the term “ abscopal’ has been often misused, both in preclinical and clinical setting. For instance, it has been applied to define responses in some preclinical experimental settings that do not satisfy the requirements to measure an abscopal effect. Tumour control that is achieved after vaccination of mice with ex vivo irradiated cancer cells is not an abscopal response.19. In experiments designed to demonstrate abscopal responses, rejection of concurrent established tumours is studied. The importance of a rigorous definition stems from the different requirements for development of an immune response after injection of irradiated cancer cells vs irradiation of a tumour that has developed an immune suppressive tumour microenvironment (TME), as described in the next sessions. Thus, preclinical models of synchronous development of multiple cancers better mimic clinical metastatic disease, permitting to measure abscopal effects. Importantly, the results of these preclinical experiments in poorly immunogenic tumours have translated to the clinic, as discussed later.
A misuse of the concept of abscopal responses can also occur in clinical trials. To correctly assess abscopal responses of focal radiation and immunotherapy, prospective randomised trials must be designed with a control arm of immunotherapy alone vs the experimental arm that combines the same systemic immunotherapy with focal radiation. This design prevents misinterpreting as abscopal a response induced by the systemic effects of the immunotherapy tested. Another approach consists of pilot, single arm prospective studies that combine focal radiation with immunotherapies that have already failed to demonstrate activity when used alone, a strategy we tested in several trials.20–22 In the latter situation, radiotherapy can “re-position” immunotherapy agents, by synergising with them to induce an effective immune rejection of cancer that the drug alone could not achieve.
Finally, the term “abscopal” has been employed to define responses in non-treated lesions in mice and patients treated with various types of intratumoral immunotherapy that does not include focal radiotherapy. We agree with the consensus statement from an expert panel on intratumoral immunotherapy that this is not an appropriate use of “abscopal” and the term “anenestic tumour responses” should be used instead to describe tumour responses in non-injected lesions.23
From bench to bedside: modelling the immune mechanisms of radiation
The implantation of the tumour in immunodeficient mice has been a conventional experimental system to test the efficacy of cancer therapeutics against human tumours for a long time, which obscured the role of adaptive immunity in the response to the treatment tested. While a systemic effect of focal radiotherapy was already forseen by Dr Joseph Shohan,24 it is only in 1979 that original experiments by Stone and colleagues reported that the dose of focal radiotherapy needed to cure mice bearing syngeneic tumours was about twice as large in the absence of T cells than in their presence,25 demonstrating the key role of T cells in achieving tumour elimination by focal radiotherapy. In the following years, much progress was made in understanding the function, specificity and mechanisms of activation of T cells. However, the role of the immune system in tumour control and the potential therapeutic value of adaptive immunity remained controversial until the early 2000s. Among the many discoveries that moved the field forward was the experimental demonstration that the elimination of neoplastic cells by the immune system results in selection of tumours that are able to escape immune control.26 This work formed the basis of the “immunoediting” theory.27 Immunoediting, together with initial evidence that targeting an inhibitory receptor hindering T cell activation leads to tumour rejection,28,29 fostered investigations into the mechanisms of tumour immune escape.
In parallel, increased understanding of the biology of dendritic cells (DCs) and their role in activating T cells raised interest for their use as cancer vaccines, although several challenges were identified, including which tumour antigens to load DCs with.30 Taking advantage of the availability of Flt3 ligand (Flt3L), a growth factor capable of expanding DCs in mice,31 Chakravarty et al32 hypothesised that radiation, by inducing cancer cell death, could promote uptake of tumour antigens by DCs33 in mice treated with Flt3L. Using a highly metastatic variant of mouse Lewis lung carcinoma they showed that a tumour curative single dose of 60 Gy focal radiation did not extend the survival of the mice, which died of lung metastases. However, focal radiation combined with Flt3L markedly improved survival, which was T cell-dependent, suggesting that the combined treatment was able to prime antitumour T cells.32
In the early 2000s, we set out to test the hypothesis that the abscopal effect was mediated by the activation of antitumour T cell responses in a mouse model of breast cancer. To this end, we used 67NR, a non-metastatic tumour, and an experimental system where the tumour was implanted in both flanks of an immunecompetent mouse and only one tumour irradiated, while the other was followed to measure abscopal responses. Radiation given at a dose of 2 or 6 Gy caused growth delay of the treated tumour but had no effect on the untreated one. We reasoned that a tumour could grow in immunocompetent syngeneic mice only if it suppressed immune recognition, as postulated by the immunoediting hypothesis, and radiation alone was insufficient to overcome the barriers hindering antitumour immune responses. Defective DC function had been identified as a mechanism of immune evasion in breast cancer,34 so we treated mice with Flt3L to restore a functional DC compartment. While Flt3L alone had no effect on tumour growth, when used with radiation it promoted abscopal responses, as demonstrated by regression of the unirradiated tumour, that were tumour-specific and mediated by activation of antitumour T cells.35 These results demonstrated a new paradigm, whereby barriers to an immunological intervention that by itself has no demonstrated therapeutic effect could be overcome by focal radiotherapy, and induce systemic tumour responses. It also provided unequivocal evidence that the abscopal effect of radiation is immune-mediated, and can be induced if radiation is combined with strategies that address established, tumour-associated immunological dysfunctions.
This concept was confirmed in the following years in many preclinical studies testing combinations of radiation with immune modulators for their ability to induce local and systemic antitumour T cell responses.36,37 Importantly, some of these combinations were translated into clinical trials. For instance, we conducted a proof of principle clinical trial, which tested the combination of focal radiation with a DC growth factor available for clinical use, granulocyte-macrophage colony-stimulating factor (GM-CSF), in patients with advanced metastatic disease (NCT02474186). GM-CSF alone has no therapeutic effect in solid tumours. However, in this trial of 41 patients, in combination with focal radiotherapy it resulted in a rate of 27% abscopal responses. Interestingly, patients achieving an abscopal response had a protracted survival compared to the ones who did not.20 Similarly, Brody et al38 tested the combination of focal radiation and a Toll-like Receptor 9 (TLR9) agonist injected into the irradiated tumour, in 15 patients with low-grade B-cell lymphoma. One patient achieved a complete clinical response, and three reached a partial response, associated with development of tumour-reactive CD8 T cells.
Inspired by the growing evidence from Allison’s lab that CTLA-4 was a critical inhibitory receptor limiting T cell activation in tumours, and that CTLA-4 blockade was effective when combined with a tumour vaccine in inducing the rejection of poorly immunogenic tumours,29,39 we tested if an in situ vaccination strategy by focal radiation could induce responses to CTLA-4 blockade in resistant tumours. Results obtained in different mouse tumour models showed that focal radiation and CTLA-4 blockade elicited antitumour CD8 T cells capable of controlling distant micrometastatic disease,40 and mediate abscopal responses.41 The clinical relevance of these findings became evident a few years later when abscopal responses were observed in a melanoma patient. This patient initially had responded to ipilimumab (the FDA-approved human anti-CTLA-4 antibody) but eventually progressed in multiple visceral sites. Maintained on ipilimumab therapy despite progression, she received palliative radiation to one metastasis, with measurable responses at multiple abscopal sites.42 This case report fostered several retrospective investigations in melanoma patients who had received radiation while on treatment with ipilimumab, as well as prospective clinical studies testing radiation and ICB in melanoma and other cancers.21,43–48 Although the results of many of these studies have yet to be reported, current evidence indicates that the combination of radiation with ICB is generally safe. The persistent unpredictability of abscopal responses however, highlights the hurdles and pitfalls that need to be considered in moving the field forward,49 as will be discussed below.
Mechanisms of in situ vaccination by radiation
Activation of naïve T cells requires their interaction with a DC that cross-presents tumour antigens and provides co-stimulatory signals to the T cell. Conventional DCs Type 1 (cDC1) are very efficient at activating CD8 T cells and have been shown to play a central role in cancer.50 Radiation promotes tumour antigen cross-presentation by enhancing the translocation to the cell surface of “eat me signals” like calreticulin, that stimulate the phagocytosis of the cancer cells by DCs, and the release of damage-associated molecular pattern (DAMP) molecules that lead to DC activation and expression of co-stimulatory molecules.51 Among the various DAMP induced by radiation, a critical role is played by DNA fragments, which as part of the DNA-damage response (DDR) to radiation, gain access to the cytosol of irradiated cells. In the cytosol, DNA leads to activation of canonical viral defense pathways via cyclic GMP-AMP synthase (cGAS)/stimulator of Interferon genes (STING), culminating in the production of interferon type I (IFN-I) and IFN-stimulated genes (ISG) including cytokines and chemokines that recruit innate and adaptive immune cells to the tumour.52 The DNA that gains access to the cytosol of the irradiated cancer cells has been demonstrated to activate the cGAS/STING pathway in the cancer cells themselves, as well as in innate immune cells present in the TME, including DCs.19,53,54 The mechanisms responsible for transfer of the IFN-stimulatory cytosolic DNA from cancer cells to DCs in the irradiated tumour remain incompletely characterised: in addition to phagocytosis, exosomes produced by the irradiated cancer cells may contribute to this process.55
The IFN-I produced in the TME was demonstrated to be critical for the recruitment of cDC1 to the tumour and for the development of spontaneous and radiation-induced antitumour CD8 T cell responses in experimental models.56–58 Once loaded with tumour antigens and activated by DAMPs in the irradiated tumour, cDC1 migrate to the draining lymph node (dLN) where they activate naïve CD8 T cells.59 Therefore, the functionality of the dLN is critical for priming of antitumour T cell responses by radiation: consistently, inclusion of the dLNs in the irradiated field and their damage have been shown to hinder this process,60 a clinically relevant finding.
Once activated in the dLN tumour-specific CD8 T cells migrate to the tumour, guided by inflammatory cytokines and chemokines that are upregulated by radiation.61,62 Their ability to extravasate and infiltrate the tumour is enhanced by radiation-induced adhesion molecules on the vascular endothelium.59,63 Recognition and killing of the cancer cells by cytotoxic CD8 T cells (CTLs) is also enhanced by radiation-induced upregulation of major histocompatibility class I antigens (MHC-I), NKG2D ligands and death receptors on the cancer cells.64–67 Conversely, radiation-induced upregulation of programmed death ligand-1 (PDL-1) on the cancer and myeloid cells can inhibit CTL-mediated tumour rejection.68,69
Enhanced presentation of some tumour antigens by MHC-I expressed on the cancer cells following radiation has been shown to improve their recognition by CTL.65,66 Reits et al66 investigated in more details the mechanisms whereby irradiated cancer cells increase their expression of surface MHC-I. They demonstrated by mass spectrometry analysis of the peptides eluted from surface MHC-I of the melanoma MelJuSo cell line that peptides derived from enzymes involved in DNA repair and protein catabolism, which are upregulated in expression following radiation, were uniquely presented by the irradiated cells. We have provided the first evidence of development of CD8 T cells specific for a mutated neoantigen encoded in KPNA2, a gene upregulated in expression by radiation, in a patient with chemotherapy-refractory metastatic non-small cell lung cancer (NSCLC) that was treated with ipilimumab and focal radiotherapy to one metastasis.21 These tumour-specific T cell clones appeared in the peripheral blood shortly after completion of radiation and the first cycle of ipilimumab, and remained elevated while the patient achieved a complete response in all of the non-irradiated lesions. Together with an increase in IFN-I that was detectable in the circulation following radiation, these data support the interpretation that in situ vaccination was achieved in this patient, and that the observed abscopal effects were mediated by the neoantigen-specific T cells. This patient was part of a clinical trial of combined ipilimumab and focal radiation in refractory, metastatic NSCLC. In this disease setting, ipilimumab alone has demonstrated lack of significant activity, however in 18% of the 39 patients accrued objective abscopal responses were detected with the addition of focal radiotherapy to a single lesion. Of notice, patients with objective responses had a significant elevation of IFN-I when measured in the blood 3 weeks post-radiation and compared to pre-radiation levels.21
Overall, current data support a model whereby radiation, as a direct consequence of DNA damage, elicits cellular responses that mimic a viral infection. The cytosolic DNA stimulates IFN-I and downstream ISGs to recruit and activate DCs. Immunogenic mutations that are expressed in rapidly induced genes involved in DNA repair and stress responses are preferentially presented in the MHC-I pathway, similarly to the rapid synthesis and preferential presentation of viral genome-encoded proteins in infected cells.70 The lower antigen levels required at the effector phase as compared to the priming phase of the antitumour immune response, may explain the abililty of T cells specific for immunogenic mutations upregulated by radiation to mediated abscopal responses. Morevover, both in mice and cancer patients, CTL-mediated killing of tumour cells has been shown to induce an antigen cascade or spread, i.e. the activation of T cells recognising additional tumour antigens different from the antigen recognised by the CTL.71,72 Thus, efficient killing at the irradiated tumour site could prime T cells to multiple tumour antigens that are shared with the non-irradiated metastases.
Barriers to the abscopal effect
Despite the progress made in understanding the immunological effects of ionising radiation, and the emerging promise of the combination of immunotherapy and radiation, responses remain unpredictable. Additional studies are needed to understand why in some patients focal radiotherapy with ICB elicits abscopal responses while in others it fails to. Table 1 lists some of the potential mechanisms to explain these failures, by schematically grouping them as associated with three main sources, host, tumour and treatment. Naturally, multiple causes may converge to generate therapeutic failure, and are listed separately to simply ease their description. Examples of host’s characteristics that may preclude the abscopal effect include hematological impairment at the time of combined treatment, with a neutrophil to lymphocytic ratio >4,20 limited tolerance to ICB, hindering adequate blockade,45 and the presence of a microbiome that is unfavourable to response to ICB.73
Table 1.
Examples of mechanisms associated with failure to achieve an abscopal response to radiation and immunotherapy
| Host | Tumour | Treatment |
|---|---|---|
| Advanced immune-suppression | Cytosolic DNA sensors and/or IFN-I genes methylation | Inadequate immunotherapy to overcome established cancer immunosuppression |
| Patient microbiome | Induction of multiple immune suppressive mediators (TGF β, adenosine, PDL-1) | Suboptimal dose and fractionation of radiation |
| Host toxicity after immunotherapy | Cancer heterogeneity | Suboptimal targeting (need to treat all tumour sites) |
Among the possible causes that are tumour-specific are the downregulation of the molecular machinery required for the IFN-I pathway activation in response to radiation (for instance, through the process of methylation),74 the induction of multiple immunosuppressive mediators, including PDL-1, TGFβ, adenosine,68,69,75 and the reality of antigenic heterogeneity among different metastases.76 Evidence is rapidly emerging for the need to individually define and then strategically address multiple immunosuppressive mechanisms that characterise patients’ established metastasis, to achieve therapeutic success of radiation and immunotherapy combinations.77
With regard to treatment-related barriers, much debate exists on how to optimise the application of classical radiotherapy when combined with immunotherapy and particularly ICB. Our group has compared fractionated (3–5 fractions of 6 to 8 Gy each) vs single dose regimens of radiotherapy, and shown the superiority of the former in inducing abscopal responses with ICB in preclinical models.41 Similarly, a fractionated treatment with 2 doses of 7.5 Gy/fraction was shown to be more effective at inducing tumour control and tumour immunity than a single dose of 15 Gy by Schaue et al.78 Although data from trials comparing prospectively different radiation regimens for their efficacy in inducing abscopal responses with ICB in patients are not available, a recent retrospective review of patients with brain metastasis treated with ICB and either single dose or fractionated stereotactic radiosurgery (SRS) supports the superiority of the hypo-fractionated regimen of 9 Gy in three fractions (total dose 27 Gy) vs single dose SRS.79 A similar fractionation regimen (8 Gy in 3 fractions, total dose 24 Gy) was tested by Theelen et al80 in a prospective trial of metastatic NSCLC patients, comparing radiotherapy plus pembrolizumab to drug alone and demonstrating doubling of objective response rate and median survival by the combinatorial approach. More studies are warranted to establish the optimal fractionated regimen and whether ablative doses (BED >100) are required81–83
Finally, as mentioned above, genomic and immune heterogeneity among metastatic sites is common and has been shown to affect antigenic composition and influence the response to immunotherapy.76,84,85 While targeting a single site and monitoring response outside the field is a practical way to assess a successful systemic immune response, in a setting of advanced metastatic disease it is likely to be limited by the fact that different metastases may not share common antigens. An approach of irradiating each metastatic site, as tested in a pilot study by Palma et al86 in the setting of oligometastatic disease (up to five sites) has shown to significantly enhance survival when compared to best supportive care, and offers promise for combination with ICB, and future immunotherapies.
Conclusions
The original intuitions for a systemic effect of ionising radiation have been confirmed by preclinical work on the viral mimicry of ionising radiation and substantiated a new therapeutic paradigm that applies focal radiotherapy as a partner to immunotherapy. Both modalities have the potential to benefit from the partnership, but much research is still required to refine this approach and enhance its potential to successfully immunise patients against their cancers.
The path so perceptively opened by Mole in his original publication in this journal is now followed by many investigators. Furthermore, growing numbers of patients are surviving their cancer because of the abscopal effects of radiation.
Interest in the abscopal effect has catalysed efforts to understand the immune effects of radiation and has enabled its combination with modern immunotherapy, opening a novel application for one of the oldest cancer treatments.
Author Note
We apologize to the many investigators whose work contributed to the understanding of the immune effects of radiation and to the advancement of radiation and immunotherapy combinations for not citing their work due to space limitation.
Footnotes
Funding: S.D. is supported by NIH (R01 CA198533 and R01CA201246), The Chemotherapy Foundation, the Breast Cancer Research Foundation (BCRF-19–053), and by DOD BC180595P1. SCF is supported by DOD BC180476, DOD BC180595 and the Breast Cancer Research Foundation (BCRF-19–053).
Competing Interests: The authors declare that they have no competing interests related to this work. However, Dr Demaria has received research support from Lytix Biopharma and Nanobiotix, consultant fees from EMD Serono, Mersana Therapeutics, and Lytix Biophrama Dr Formenti has received prior honorarium for consulting/speaker from AstraZeneca, Merck, Regeneron, Bayer, Serono, and research funding from Varian, Merck, Bristol Meyer Squibb.
Contributor Information
Sandra Demaria, Email: szd3005@med.cornell.edu.
Silvia C Formenti, Email: formenti@med.cornell.edu.
REFERENCES
- 1.Lenz M. The early workers in clinical radiotherapy of cancer at the radium Institute of the Curie Foundation, Paris, France. Cancer 1973; 32: 519–23. doi: [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. doi: 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 3.Biau J, Chautard E, Verrelle P, Dutreix M. Altering DNA repair to improve radiation therapy: specific and multiple pathway targeting. Front Oncol 2019; 9: 1009. doi: 10.3389/fonc.2019.01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161: 205–14. doi: 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–5. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28: 690–714. doi: 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 8.Burnette B, Weichselbaum RR. The immunology of ablative radiation. Semin Radiat Oncol 2015; 25: 40–5. doi: 10.1016/j.semradonc.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015; 1: 1325–32. doi: 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 10.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26: 234–41. doi: 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 11.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol 1975; 48: 863–6. doi: 10.1259/0007-1285-48-574-863 [DOI] [PubMed] [Google Scholar]
- 12.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol 1973; 46: 220–2. doi: 10.1259/0007-1285-46-543-220 [DOI] [PubMed] [Google Scholar]
- 13.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology 1969; 93: 410–2. doi: 10.1148/93.2.410 [DOI] [PubMed] [Google Scholar]
- 14.Rees GJ, Ross CM. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol 1983; 56: 63–6. doi: 10.1259/0007-1285-56-661-63 [DOI] [PubMed] [Google Scholar]
- 15.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys 1977; 2(1-2): 141–7. doi: 10.1016/0360-3016(77)90020-7 [DOI] [PubMed] [Google Scholar]
- 16.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016; 40: 25–37. doi: 10.1016/j.currproblcancer.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 17.Pierce RH, Campbell JS, Pai SI, Brody JD, Kohrt HEK. In-Situ tumor vaccination: bringing the fight to the tumor. Hum Vaccin Immunother 2015; 11: 1901–9. doi: 10.1080/21645515.2015.1049779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012; 84: 879–80. doi: 10.1016/j.ijrobp.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017; 548: 466–70. doi: 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and GM-CSF in patients with metastatic solid tumors: a proof of principle trial to generate abscopal responses. Lancet Oncol 2015; 16: 795–803. [DOI] [PubMed] [Google Scholar]
- 21.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018; 24: 1845–51. doi: 10.1038/s41591-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancer. Clin Cancer Res 2018; 24: 2493–504. doi: 10.1158/1078-0432.CCR-17-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marabelle A, Andtbacka R, Harrington K, Melero I, Leidner R, de Baere T, et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT-IT. Ann Oncol 2018; 29: 2163–74. doi: 10.1093/annonc/mdy423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shohan J. Some theoretical considerations on the present status of roentgen therapy. The Boston Medical and Surgical Journal 1916; 175: 321–7. doi: 10.1056/NEJM191609071751001 [DOI] [Google Scholar]
- 25.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 1979; 63: 1229–35. [PubMed] [Google Scholar]
- 26.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. Ifngamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410: 1107–11. doi: 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–8. doi: 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 28.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 2011; 241: 104–18. doi: 10.1111/j.1600-065X.2011.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–6. doi: 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 30.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003; 15: 138–47. doi: 10.1016/S0952-7915(03)00015-3 [DOI] [PubMed] [Google Scholar]
- 31.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in FLT3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996; 184: 1953–62. doi: 10.1084/jem.184.5.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 1999; 59: 6028–32. [PubMed] [Google Scholar]
- 33.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86–9. doi: 10.1038/32183 [DOI] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 1997; 3: 483–90. [PubMed] [Google Scholar]
- 35.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–70. doi: 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 36.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105: 256–65. doi: 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol 2012; 2: 104. doi: 10.3389/fonc.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28: 4324–32. doi: 10.1200/JCO.2010.28.9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999; 190: 355–66. doi: 10.1084/jem.190.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-Mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11(2 Pt 1): 728–34. [PubMed] [Google Scholar]
- 41.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15: 5379–88. doi: 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–31. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014; 3: e28780. doi: 10.4161/onci.28780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015; 4: e1046028. doi: 10.1080/2162402X.2015.1046028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–7. doi: 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiniker SM, Reddy SA, Maecker HT, Subrahmanyam PB, Rosenberg-Hasson Y, Swetter SM, et al. A Prospective Clinical Trial Combining Radiation Therapy With Systemic Immunotherapy in Metastatic Melanoma. Int J Radiat Oncol Biol Phys 2016; 96: 578–88. doi: 10.1016/j.ijrobp.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016; 4: 51. doi: 10.1186/s40425-016-0156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–12. doi: 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2016; 2: 286–94. doi: 10.1016/j.trecan.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böttcher JP, Reis E Sousa C, Reis ESC. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 2018; 4: 784–92. doi: 10.1016/j.trecan.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol 2015; 25: 11–17. doi: 10.1016/j.semradonc.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced Abscopal effect. Trends Immunol 2018; 39: 644–55. doi: 10.1016/j.it.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. Sting-Dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41: 843–52. doi: 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackenzie KJ, Carroll P, Martin C-A, Murina O, Fluteau A, Simpson DJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017; 548: 461–5. doi: 10.1038/nature23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamond JM, Vanpouille-Box C, Spada S, Rudqvist N-P, Chapman JR, Ueberheide BM, et al. Exosomes shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res 2018; 6: 910–20. doi: 10.1158/2326-6066.CIR-17-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208: 1989–2003. doi: 10.1084/jem.20101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 2011; 208: 2005–16. doi: 10.1084/jem.20101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 2011; 71: 2488–96. doi: 10.1158/0008-5472.CAN-10-2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–23. doi: 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 60.Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res 2018; 24: 5058–71. doi: 10.1158/1078-0432.CCR-17-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-Induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181: 3099–107. doi: 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-Induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008; 180: 3132–9. doi: 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 63.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-Dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24: 589–602. doi: 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003; 170: 6338–47. doi: 10.4049/jimmunol.170.12.6338 [DOI] [PubMed] [Google Scholar]
- 65.Garnett CT, Palena C, Chakraborty M, Chakarborty M, Tsang K-Y, Schlom J, Hodge JW, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004; 64: 7985–94. doi: 10.1158/0008-5472.CAN-04-1525 [DOI] [PubMed] [Google Scholar]
- 66.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–71. doi: 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest 2012; 122: 3718–30. doi: 10.1172/JCI61931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–95. doi: 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014; 74: 5458–68. doi: 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- 70.Lhuillier C, Rudqvist N-P, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med 2019; 11: 40. doi: 10.1186/s13073-019-0653-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005; 11: 3353–62. doi: 10.1158/1078-0432.CCR-04-2062 [DOI] [PubMed] [Google Scholar]
- 72.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004; 64: 4328–37. doi: 10.1158/0008-5472.CAN-04-0073 [DOI] [PubMed] [Google Scholar]
- 73.Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer 2019; 7: 108. doi: 10.1186/s40425-019-0574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konno H, Yamauchi S, Berglund A, Putney RM, Mulé JJ, Barber GN. Suppression of sting signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 2018; 37: 2037–51. doi: 10.1038/s41388-017-0120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, García-Martínez E, Rudqvist N-P, et al. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front Immunol 2017; 8: 229. doi: 10.3389/fimmu.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Mattos-Arruda L, Sammut S-J, Ross EM, Bashford-Rogers R, Greenstein E, Markus H, et al. The genomic and immune landscapes of lethal metastatic breast cancer. Cell Rep 2019; 27: 2690–708e10. doi: 10.1016/j.celrep.2019.04.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018; 18: 313–22. doi: 10.1038/nrc.2018.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012; 83: 1306–10. doi: 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer 2019; 7: 102. doi: 10.1186/s40425-019-0588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Noort vander V, Aerts J, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019;11 Jul 2019. doi: 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989; 62: 679–94. doi: 10.1259/0007-1285-62-740-679 [DOI] [PubMed] [Google Scholar]
- 82.Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-Dose factors in radiotherapy: a review of the human data. Radiother Oncol 1990; 19: 219–35. doi: 10.1016/0167-8140(90)90149-Q [DOI] [PubMed] [Google Scholar]
- 83.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2(7 Suppl 3): S94–100. doi: 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 84.Reuben A, Spencer CN, Prieto PA, Gopalakrishnan V, Reddy SM, Miller JP, et al. Genomic and immune heterogeneity are associated with differential responses to therapy in melanoma. NPJ Genom Med 2017; 207 04 2017. doi: 10.1038/s41525-017-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, et al. Evolution of metastases in space and time under immune selection. Cell 2018; 175: 751–65e16. doi: 10.1016/j.cell.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 86.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019; 393: 2051–8. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]