Abstract
Objective:
Investigate the temporal effects of focused ultrasound (FUS)-induced blood–brain barrier (BBB) opening in post-radiotherapy mouse brains.
Methods and materials:
C57B6 mice without tumors were used to simulate the scenario after gross total resection (GTR) of brain tumor. Radiation dose of 6 Gy x 5 was delivered to one-hemisphere of the mouse brain. FUS-induced BBB-opening was delivered to the irradiated and non-irradiated brain and was confirmed with MRI. Dynamic MRI was performed to evaluate blood vessel permeability. Two time points were selected: acute (2 days after radiation) and chronic (31 days after radiation).
Results:
BBB opening was achieved after FUS in the irradiated field as compared to the contralateral non-irradiated brain without any decrease in permeability. In the acute group, a trend for higher gadolinium concentration was observed in radiated field.
Conclusion:
Localized BBB-opening can be successfully achieved without loss of efficacy by FUS as early as 2 days after radiotherapy.
Advances in knowledge:
Adjuvant radiation after GTR is commonly used for brain tumors. Focused ultrasound facilitated BBB-opening can be achieved without loss of efficacy in the post-irradiated brain as early as 2 days after radiation therapy. This allows for further studies on early application of FUS-mediated BBB-opening.
Introduction
Glioblastoma (GBM) is a devastating primary cancer of the brain in which the outcomes are poor with a mean survival rate of 8–15 months.1 The primary treatment is maximal safe resection of the tumor, in which the goal is to achieve a gross total resection (GTR). The difficulty with GBM is that the disease is infiltrative and adjuvant therapies play a vital role including radiation and chemotherapy.
There is a clear dichotomy between the treatment of translational mice tumor models vs clinical treatment of GBM. Despite multiple beneficial treatments in mice, there has been no advancement in systemic treatments for patients with GBM over the past 14 years since Stupp and colleagues demonstrated that the addition of adjuvant temozolomide with radiation improved survival benefit.1 Unlike many of the preclinical GBM models that present with a destructive solitary mass, patients with GBM typically presents with two components: (1) a large destructive solid component with necrosis that enhances with T1-contrast and (2) a diffusely infiltrative component that extends extensively into the brain in regions of edema that does not enhance with contrast.1–3 Typically, maximal safe resection removes the destructive component and the challenge for treatment is the residual microscopic disease in regions of the brain in which the blood–brain barrier (BBB) remains relatively intact. This is evident as gadolinium does not permeate into the brain parenchyma. Adjuvant radiation treatment is used to treat the resection cavity and the radiated region often extends up to 2 cm beyond the radiographic regions of concern for microscopic disease which includes large regions of normal brain.
The BBB regulates the transcellular transport and prevents any large molecules (>400 Da) from entering the brain.4 Systemic therapies employed in primary brain tumors such as GBM or secondary brain tumors in the setting of brain metastases have a wide range in size up to kilodalton (kDa) range: temozolomide (TMZ) is approximately 194 Da, carboplatin is approximately 371 Da, and antibody-based therapies are approximately 150 kDa..5,6 Even with small molecular weight drugs, preclinical studies showed that focused ultrasound (FUS)-mediated BBB-opening enhanced TMZ delivery into GBM tumors.7 Thus, in the setting tumor microscopic disease residing in regions of the brain that lack of gadolinium enhancement, it suggests that the BBB limits bioavailability of systemically administered drugs to the brain.8
Recent advances in FUS research opens new avenues for non-invasive, targeted drug delivery to the brain. FUS generates a directed acoustic field in which systemically administered microbubbles oscillate and exert mechanical forces on the endothelial cell walls. As a result, the BBB can be transiently opened in a controlled manner, allowing therapeutic agents of various sizes to enter the brain parenchyma.9 Recent Phase 1/2a clinical trial, showed that repeated ultrasound-induced BBB opening with an implantable transducer was well-tolerated by GBM patients receiving concurrent carboplatin.10,11 In addition, the intracranial MRI-guided FUS device, ExAblate, was FDA approved for ablative use for patients with essential tremors. Multiple Phase I trials have demonstrated the feasibility of FUS-mediated BBB-opening.12,13 To this date, very little is known whether the timing of FUS-mediated BBB-opening is affected by prior-irradiation of the brain. Here, we hypothesized that post-irradiation brain changes may affect the BBB-opening induced by FUS, and that the timing in which FUS-mediated BBB-opening occurs, in relationship to the end of radiation treatment, will affect BBB-opening.
In order to simulate the post-operative and post-irradiation setting, where microscopic disease occur in areas of the brain in which the BBB-remains relatively intact, we elected to analyze intact C57BL6 mouse brains with no gross disease. 30 Gy was delivered in 5 fractions to a single hemisphere of the brain (irradiated field) while sparing the contralateral side (non-irradiated field). BBB-opening was performed on Day 7 (2 days after end of radiation) (acute) and Day 36 (30 days after end of radiation) (chronic). The BBB opening was performed in the bilateral striatum. This is the first study to examine whether the degree of BBB-opening using FUS is affected by prior radiotherapy and its timing.
Methods and materials
Animal model
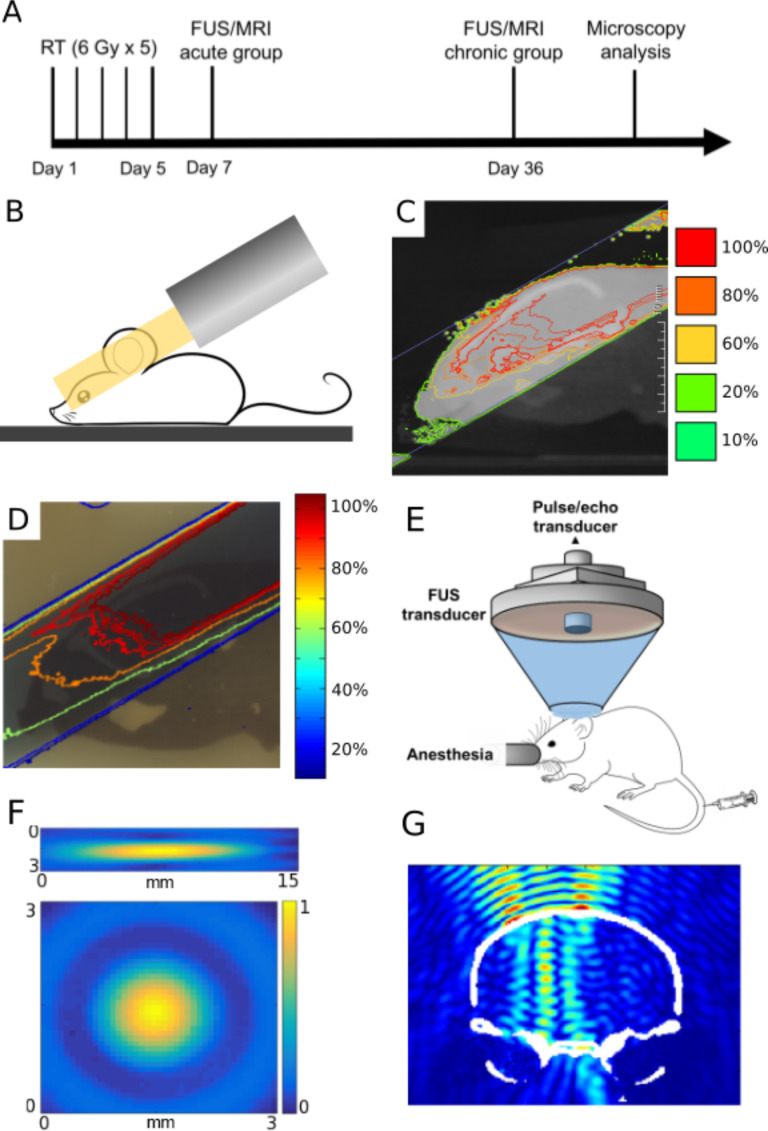
All in vivo experiments were conducted in accordance to our Institutional Animal Care and Use Committee. 7-week-old Male C57BL6 mice were divided into an acute and a chronic group (n = 4–5 per group). Experimental design is depicted in Figure 1A.
Figure 1.
Radiation and FUS experimental setup and timeline. (A) Experimental timeline of treatments and analyses. (B) Schematic of half brain radiation with a single beam using SARRP system. (C) Simulated dose distribution of radiation treatment plan in MuriPlan of the mouse brain. (D) Representative isodose lines measured with radiochromic film from irradiated mouse brain phantom. Both (C, D) are normalized to the maximum dose value in the brain. (E) Experimental setup of the FUS system used in all sonications. (F) The FUS focus was calibrated using an acoustic hydrophone to be 7.5 × 1 × 1 mm3. (G) Simulation of normalized FUS beam profile in the targeted half brain (coronal view). FUS,focused ultrasound; SARRP, Small Animal Radiation Research Platform.
Radiation and mouse-like phantom
Half brain radiation was delivered using the Small Animal Radiation Research Platform system by Xstrahl (Camberley, Surrey, UK). Cone beam CT (CBCT) was obtained and a single hemisphere was contoured as clinical target volume (CTV) and a single beam was designed in the sagittal arrangement to deliver 6 Gy radiation through a 10 × 10 mm2 collimator prescribed to the isocenter. A coverage of 90% dose to 95% vol was achieved. The medial beam edge was placed midline to the brain to spare the contralateral hemisphere. Each animal received 30 Gy in 5 fractions radiation daily. Figure 1B,C illustrates the simulated radiation dose distribution in the mouse brain. Two mouse-like phantoms (axial and sagittal planes) were used in this study to validate the radiation delivery accuracy.14 Radiochromic film, EBT3 (Ashland Advanced Materials, Niagara Falls, NY), was analyzed as previously described.14 (Figure 1D).
Focused ultrasound
The FUS system was composed of a single element focused ultrasound transducer (Imasonic, France). (Figure 1E) The acoustic parameters used in this study include: the free field (i.e. in water) peak-rarefactional pressure of 0.72 MPa; pulse repetition frequency of 5 Hz; pulse length of 5 ms; and a total duration of 30 s per sonication. Striatum was the target in this study where four (2 × 2 with 1 mm grid) sonications were performed on the irradiated and non-irradiated sides.15
MRI imaging and analysis
A 9.4 T small animal MRI system was used for brain imaging throughout this study (Bruker Medical, Boston, MA). After FUS, dynamic contrast enhanced (DCE) MR images were collected before and after an intraperitoneal injection of 0.2 ml gadodiamide (Gd) (Omniscan®, GE Healthcare, NJ). The permeability (Ktrans) of the BBB-opened region was analyzed based on the DCE MR images using the general kinetic model.15
Microscopy and analysis
Approximately 2 h after the sonication, mice were transcardially injected with fluorescently labeled Lycopersicon esculentum (Tomato) Lectin (Vector Laboratories, Burlingame, CA) to label the vasculature. Histological slides of the brain were analyzed with a confocal microscope (Nikon, Melville, NY) and vascular analyses were based on signals from the lectin channel using a custom-written program (MATLAB, Natick, MA). In addition, Hematoxylin and Eosin staining was performed on frozen brain sections with a slice thickness of 6 µm and were examined by an independent neuropathologist. Full details for material and methods can be seen in Supplementary Material 1.
Statistics
All statistical analyses were performed using GraphPad software (GraphPad Software, Inc., La Jolla, CA). Paired two-tailed Student’s t-test was used for intragroup comparison and a p-value < 0.05 was considered statistically significant.
Results
In this study, single brain hemisphere radiation was delivered. Films from the mouse phantom experiments illustrate qualitatively agreement between predicted dose vs the actual dose delivered (Figure 1C–D) with 90% isodose line covering the 95% of the unilateral brain. The FUS focus size was 7.5 × 1 × 1 mm3(Figure 1F). In addition, transcranial FUS beam simulation demonstrated well localized energy distribution within the targeted brain hemisphere (Figure 1G).
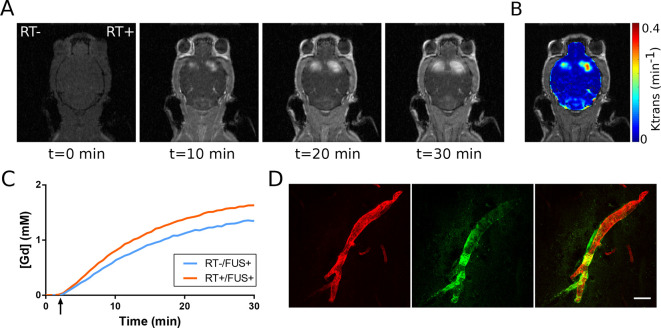
The BBB-opening was confirmed with DCE MR images. Representative images of a mouse in the acute radiation therapy (RT) group over a course of 30 min are shown in Figure 2A. The permeability Ktrans is overlaid on the MR image (Figure 2B), and the Gd concentrations from both sides are plotted (Figure 2C). To assess whether BBB-opening can achieve antibody permeation, fluorescently labelled IgG antibody isotype control (green) was observed leaking out of a blood vessel (red) at the BBB opened the region (Figure 2D).
Figure 2.
Confirmation of BBB opening and vascular permeability. (A) Contrast-enhanced dynamic T1 MRI scans illustrate gadolinium penetration into the brain parenchyma. (B) Brain permeability (Ktrans) was calculated based on serial dynamic scans. (C) Representative Gd concentration was traced over the entire scanning duration (30 min). (D) Fluorescence imaging demonstrated IgG antibody isotype control (green) leaking out from the blood vessel (red) after FUS-induced BBB opening. BBB, blood–brain barrier; FUS, focused ultrasound; Gd, gadolinium.
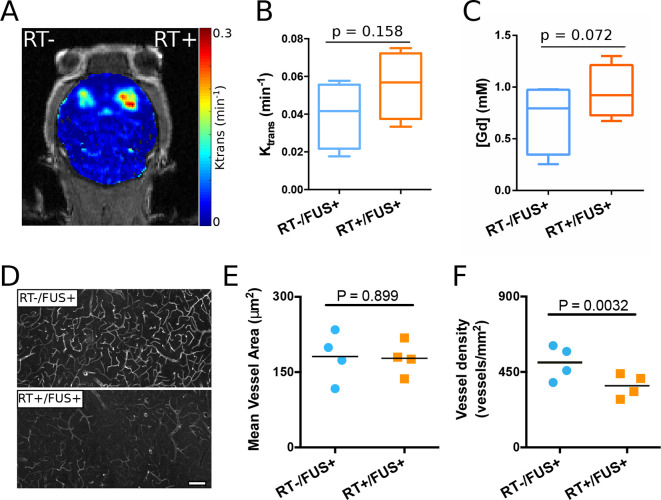
BBB-opening 2 days post-irradiation (acute group) showed no significant loss of permeability and Gd penetrance concentration (Figure 3A–C). Interestingly, there was a trend for higher Gd penetrance concentration measurements (p = 0.072) in the irradiated (RT) side (0.954 ± 0.261 mM) compared to that of the non-irradiated (non-RT) side (0.705 ± 0.342 mM). The vasculature of the BBB opened region was imaged and analyzed, as shown in Figure 3D–F. Qualitatively, the RT side revealed less lectin labeling and many more lectin labeled blood vessels were seen on the contralateral, non-RT side. This observation was confirmed with quantitative analysis, where vessel density (number of blood vessels per image plane) on the non-RT side was significantly higher than the RT side (137.6%, p = 0.0032). The mean vessel area (i.e. the average size of each blood vessel) did not differ between the two hemispheres (p = 0.899) for this group.
Figure 3.
Acute effects of radiation (2 days after RT) on FUS-induced BBB opening (n = 4). (A) Representative brain permeability map in the striatum. (B) The permeability (Ktrans) and (C) calculated gadolinium concentration showed an increased trend on the RT side, although there are no statistically significant differences. (D) Representative vascular staining (lectin) images from sections with and without radiation treatment. (E) Measurements of mean vessel area showed no significant difference. (F) Measurements of blood vessel density indicated a significant decrease on the hemisphere received both radiation and FUS (p = 0.0032). FUS, focused ultrasound; RT, radiation therapy.
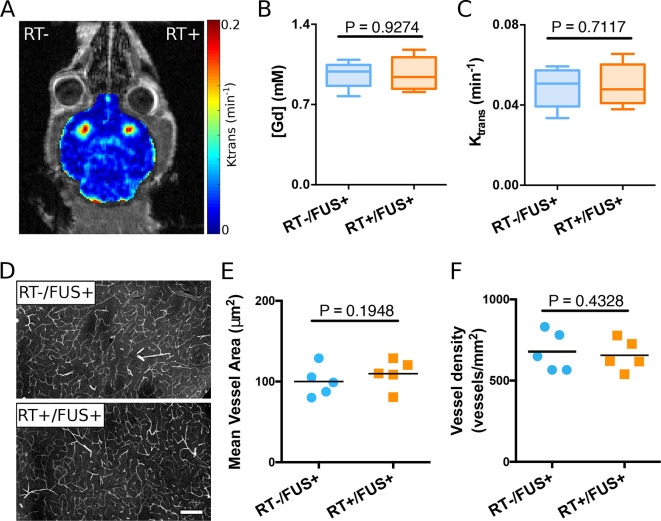
To investigate the delayed effects of RT on the efficiency of FUS-induced the BBB opening, a separate group underwent radiation treatment and then delayed bilateral sonication approximately 4 weeks after radiation. The permeability at the BBB opening and the Gd penetrance concentration were the same in the two hemispheres (Figure 4A–C). Figure 4D–F illustrates the vasculature in the post-BBB opened striatum regions. The mean vessel areas of the two hemispheres and the vessel density did not differ statistically. Hematoxylin and Eosin images of the brains from both the acute and chronic RT groups were examined by a neuropathologist, revealing no evidence of gross or microscopic inflammation or necrosis. There were no morbidity or mortality from FUS delivery.
Figure 4.
Delayed effects of radiation on FUS-induced BBB opening (n = 5). (A) Representative brain permeability map demonstrating bilateral BBB opening in the striatum. (B) Calculated Gd concentration and (C) permeability (Ktrans) showed no statistically significant difference between the left and right hemispheres. (D) Representative vascular staining (lectin) images from sections with and without radiation treatment. (E) Quantitative analysis of mean vessel area and (F) vessel density also demonstrated no significant difference between the two hemispheres. BBB, blood–brain barrier; FUS, focused ultrasound; Gd, gadolinium.
Discussion
FUS-mediated BBB-opening offers an exciting and novel treatment delivery strategy for patients with brain tumors. The limitation of knowledge to translating this technology is the understanding of how prior therapies, such as radiation treatment, alters the efficiency of BBB-opening. This is the first study to examine the temporal effects of radiation in relationship to FUS-mediated BBB-opening.
The challenges associated with drug delivery into the brain have been longlasting and identifying innovative solutions are of high interest. FUS offers an exciting potential for non-invasive and spatially controlled delivery system that can transiently and safely open the BBB. Recent clinical trials demonstrated feasibility and safety of ultrasound-induced BBB-opening in patients with GBM.10–12 Patients treated included those with recurrent GBM suggesting they had prior radiation treatment. The question remains whether BBB-opening is safe and feasible in a radiated field and whether the chronicity of FUS delivery, in relationship to radiation treatment, affects BBB-opening and whether chronicity of BBB-opening affects outcomes.
GBM is a devastating disease where unlike preclinical tumor models, the grossly visible tumor is often resected. The challenge for current treatment regimens is the microscopic disease in the infiltrating that extend into the regions of the brain where the BBB remains relatively intact. This is typically in the regions of the brain that is contrast-non-enhancing but T2/FLAIR hyperintense, and is at high risk of recurrence. Standard of care post-operative radiation treatment typically involves the resection cavity and regions concerning for microscopic disease plus a 1.5–2 cm additional margin. This targeted irradiation region often extends to regions of normal brain. In strategies to enhance drug delivery, we postulate that similar areas will require BBB-opening, thus it is important to understand how prior radiation treatment affects BBB-opening. To model the post-operative setting for GBM, we used post-radiation normal mouse brain as a surrogate to study the effectiveness of FUS-induced BBB opening in the brain tissue around the primary tumor. Results from this study for the first time demonstrated that FUS-facilitated BBB opening can be safely and efficiently achieved in both acute and chronic post-RT brains. The radiation course used here is 30 Gy in 5 fractions similar to that used in a recent short-course RT trial in elderly glioma patients. Although not statistically significant, both the vascular permeability and Gd penetrance concentration on the RT side increased in the acute group. This observation together with acute decreased vascular density (27.3%, as labeled by intravenous lectin) may be explained by the vascular modifying effect of RT. Interestingly, the differences in vascular permeability, Gd penetrance concentration and mean vessel density diminished 4 weeks after the RT in the chronic group (not statistically different). These results indicate the relatively transient effects of RT-induced vascular modifications.
Conclusion
This is the first in vivo study to show that FUS-induced BBB opening in the brain was not compromised with prior RT. Lastly, RT-induced vascular modifying effects should be taken into consideration when performing FUS treatments.
Footnotes
The authors Shutao Wang and Cheng-Chia Wu contributed equally to the work.
Funding: This study was supported by the ASTRO 2016 Residents/ Fellows in Radiation Oncology Research Seed Grant, the Irving Institute Imaging Pilot Award, Louis V. Gerstner, Jr. Scholar Award, an unrestricted research donation from Barry Neustein, and the NIH Biomedical Research Support Shared Instrumentation Grant 1S10OD010631-01A1.
Disclosure: S.K.C. reports personal fees and financial support from AbbVie, and financial support from Sanofi.
Contributor Information
Shutao Wang, Email: shutao.wang@icahn.mssm.edu.
Cheng-Chia Wu, Email: cw2666@cumc.columbia.edu.
Hairong Zhang, Email: hz2440@columbia.edu.
Maria Eleni Karakatsani, Email: mek2204@columbia.edu.
Yi-Fang Wang, Email: yw2762@cumc.columbia.edu.
Yang Han, Email: yh2583@columbia.edu.
Kunal R Chaudhary, Email: kc2849@cumc.columbia.edu.
Cheng-Shie Wuu, Email: csw6@cumc.columbia.edu.
Elisa Konofagou, Email: ek2191@columbia.edu.
Simon K Cheng, Email: sc3225@cumc.columbia.edu.
REFERENCES
- 1.Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah SAA, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: a review. Biomed Pharmacother 2017; 92: 681–9. doi: 10.1016/j.biopha.2017.05.125 [DOI] [PubMed] [Google Scholar]
- 2.Bianco J, Bastiancich C, Joudiou N, Gallez B, des Rieux A, Danhier F. Novel model of orthotopic U-87 Mg glioblastoma resection in athymic nude mice. J Neurosci Methods 2017; 284: 96–102. doi: 10.1016/j.jneumeth.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 3.Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol 2018; 20: 184–91. doi: 10.1093/neuonc/nox175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM. Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin Pharmacol Ther 2015; 97: 347–61. doi: 10.1002/cpt.18 [DOI] [PubMed] [Google Scholar]
- 5.Agarwala SS, Kirkwood JM, Temozolomide KJM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 2000; 5: 144–51. doi: 10.1634/theoncologist.5-2-144 [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006; 103: 11719–23. doi: 10.1073/pnas.0604318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei K-C, Chu P-C, Wang H-YJ, Huang C-Y, Chen P-Y, Tsai H-C, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One 2013; 8: e58995. doi: 10.1371/journal.pone.0058995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samiotaki G, Vlachos F, Tung Y-S, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med 2012; 67: 769–77. doi: 10.1002/mrm.23063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother 2015; 15: 477–91. doi: 10.1586/14737175.2015.1028369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 2016; 8: 343re2. doi: 10.1126/scitranslmed.aaf6086 [DOI] [PubMed] [Google Scholar]
- 11.Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res 2019; 25: 3793–801. doi: 10.1158/1078-0432.CCR-18-3643 [DOI] [PubMed] [Google Scholar]
- 12.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-Brain barrier opening in primary brain tumors with non-invasive MR-Guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 2019; 9: 321. doi: 10.1038/s41598-018-36340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, et al. Blood-Brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun 2018; 9: 2336. doi: 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C-C, Chaudhary KR, Na YH, Welch D, Black PJ, Sonabend AM, et al. Quality assessment of stereotactic radiosurgery of a melanoma brain metastases model using a Mouselike phantom and the small animal radiation research platform. Int J Radiat Oncol Biol Phys 2017; 99: 191–201. doi: 10.1016/j.ijrobp.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlachos F, Tung Y-S, Konofagou EE. Permeability assessment of the focused ultrasound-induced blood-brain barrier opening using dynamic contrast-enhanced MRI. Phys Med Biol 2010; 55: 5451–66. doi: 10.1088/0031-9155/55/18/012 [DOI] [PMC free article] [PubMed] [Google Scholar]