Summary
Background
The prevalence and prognosis of digestive system involvement, including gastrointestinal symptoms and liver injury, in patients with COVID-19 remains largely unknown. We aimed to quantify the effects of COVID-19 on the digestive system.
Methods
In this systematic review and meta-analysis, we systematically searched PubMed, Embase, and Web of Science for studies published between Jan 1, 2020, and April 4, 2020. The websites of WHO, CDC, and major journals were also searched. We included studies that reported the epidemiological and clinical features of COVID-19 and the prevalence of gastrointestinal findings in infected patients, and excluded preprints, duplicate publications, reviews, editorials, single case reports, studies pertaining to other coronavirus-related illnesses, and small case series (<10 cases). Extracted data included author; date; study design; country; patient demographics; number of participants in severe and non-severe disease groups; prevalence of clinical gastrointestinal symptoms such as vomiting, nausea, diarrhoea, loss of appetite, abdominal pain, and belching; and digestive system comorbidities including liver disease and gastrointestinal diseases. Raw data from studies were pooled to determine effect estimates.
Findings
We analysed findings from 35 studies, including 6686 patients with COVID-19, that met inclusion criteria. 29 studies (n=6064) reported gastrointestinal symptoms in patients with COVID-19 at diagnosis, and the pooled prevalence of digestive system comorbidities was 4% (95% CI 2–5; range 0–15; I2=74%). The pooled prevalence of digestive symptoms was 15% (10–21; range: 2–57; I2=96%) with nausea or vomiting, diarrhoea, and loss of appetite being the three most common symptoms. The pooled prevalence of abnormal liver functions (12 studies, n=1267) was 19% (9–32; range 1–53; I2=96%). Subgroup analysis showed patients with severe COVID-19 had higher rates of abdominal pain (odds ratio [OR] 7·10 [95% CI 1·93–26·07]; p=0·003; I2=0%) and abnormal liver function including increased ALT (1·89 [1·30–2·76]; p=0·0009; I2=10%) and increased AST (3·08 [2·14–4·42]; p<0·00001; I2=0%) compared with those with non-severe disease. Patients in Hubei province, where the initial COVID-19 outbreak occurred, were more likely to present with abnormal liver functions (p<0·0001) compared with those outside of Hubei. Paediatric patients with COVID-19 had a similar prevalence of gastrointestinal symptoms to those of adult patients. 10% (95% CI 4–19; range 3–23; I2=97%) of patients presented with gastrointestinal symptoms alone without respiratory features. Patients who presented with gastrointestinal system involvement had delayed diagnosis (standardised mean difference 2·85 [95% CI 0·22–5·48]; p=0·030; I2=73%). Patients with gastrointestinal involvement tended to have a poorer disease course (eg, acute respiratory distress syndrome OR 2·96 [95% CI 1·17–7·48]; p=0·02; I2=0%).
Interpretation
Our study showed that digestive symptoms and liver injury are not uncommon in patients with COVID-19. Increased attention should be paid to the care of this unique group of patients.
Funding
None.
Introduction
The emergence and spread of COVID-19 since December, 2019, has brought great challenges to global public health. As of April 23, 2020, more than 2·5 million confirmed cases and more than 175 000 deaths had been reported globally.1
Respiratory tract manifestations such as fever and cough are the most commonly reported symptoms in patients with COVID-19.2 Evidence of digestive system involvement in patients with COVID-19 was first reported by a group in China.3 Emerging data showed that the gastrointestinal tract and liver might also represent target organs of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the basis of the findings that angiotensin-converting enzyme 2 (ACE2), the major receptor of SARS-CoV-2, is expressed in the gastrointestinal tract as well as liver cells.4 The detection of SARS-CoV-2 viral RNA in patients' stool and the potential for faecal–oral transmission has raised great concern and could pose a challenge for the control and prevention of COVID-19.5, 6, 7
Research in context.
Evidence before this study
The emergence and spread of coronavirus disease 2019 (COVID-19) has brought great challenges to global public health. An increasing number of studies have reported gastrointestinal symptoms and liver injury in patients with COVID-19, and patients with severe disease tend to have a higher risk of developing gastrointestinal symptoms and abnormal liver function. However, results have been inconsistent, with high heterogeneity among studies, and the exact magnitude of gastrointestinal and liver involvement is uncertain. Detailed pooled estimates of the prevalence of gastrointestinal and liver involvement in COVID-19 are needed. Whether this unique group of patients has a poor disease course remains unclear. We searched the PubMed, Embase, Web of Science, and WHO databases of publications, The Lancet COVID-19 Resource Centre, New England Journal of Medicine, JAMA, BMJ, Gastroenterology, Gut, American Journal of Gastroenterology, and the US Centers for Disease Control and Prevention for COVID-19-related publications, published between Jan 1 and April 10, 2020, using the keywords “coronavirus”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “novel coronavirus”, “nCoV”, “2019-nCoV”, and “COVID-19”. Eligible studies reporting the prevalence of gastrointestinal findings in infected patients were included, and preprints, duplicate publications, reviews, editorials and small case reports (<10 cases) were excluded.
Added value of this study
We have provided a pooled estimate of the prevalence of gastrointestinal symptoms and liver injury in patients with COVID-19. Furthermore, we did subgroup analyses of patients with severe versus non-severe disease, patients in Hubei province versus outside of Hubei, and paediatric versus adult patients. Features of patients with pre-existing digestive diseases and those initially presenting with gastrointestinal symptoms were summarised. The disease course of patients with digestive system involvement was further analysed. We included the first subgroup analyses of patients in Hubei province versus outside of Hubei, and paediatric versus adult patients.
Implications of all the available evidence
Digestive symptoms and liver injury are not uncommon in patients with COVID-19. Compared with patients with non-severe disease, patients with severe COVID-19 had a higher risk of developing gastrointestinal symptoms and liver injury. Children with COVID-19 had a similar prevalence of gastrointestinal symptoms as did adults with COVID-19. Patients with COVID-19 in Hubei had a higher prevalence of abnormal liver functions than those outside of Hubei. Approximately 10% of patients with COVID-19 might present with gastrointestinal symptoms only, without respiratory symptoms. Patients with digestive system involvement as initial symptoms have delayed diagnosis of COVID-19, and those with digestive involvement have a tendency to progress to severe or critical disease and a poor disease course. Increased attention should be paid to the early identification of these patients.
An increasing number of studies have reported gastrointestinal symptoms and liver injury in patients with COVID-19, and patients with severe disease tend to have an increased risk of developing gastrointestinal symptoms and abnormal liver function.2 However, results have been inconsistent, with high heterogeneity among studies, and the exact magnitude of gastrointestinal and liver involvement remains uncertain. Compared with adult patients, paediatric patients (aged <18 years) seem to have clinically milder symptoms and show less severe alterations in radiological and laboratory testing parameters.8 Whether paediatric patients have a lower risk of gastrointestinal and liver involvement remains unclear. Moreover, several studies have provided information on the epidemiology and clinical manifestation of the disease outside of Hubei province, China. Whether gastrointestinal manifestations and liver injury in patients in Hubei differ from those outside Hubei has seldom been investigated.9, 10
More importantly, the prognosis of patients with COVID-19 with gastrointestinal symptoms is still largely unknown. Studies11, 12 implied that patients with COVID-19 with digestive symptoms might have a worse clinical outcome than those without digestive symptoms, emphasising the importance of including symptoms such as diarrhoea to diagnose COVID-19 early. In one study,11 the rate of severe or critical disease was also markedly increased in patients with COVID-19 with gastrointestinal symptoms compared with in those without gastrointestinal symptoms. Moreover, patients with COVID-19 with gastrointestinal symptoms had significantly higher rates of complications of acute respiratory distress syndrome and liver injury than did those without these symptoms.11 Pan and colleagues12 also showed that as the severity of the disease increased, digestive symptoms became more pronounced.
We did a systematic review and meta-analysis of emerging studies reporting gastrointestinal symptoms and liver injury in patients with COVID-19 on the basis of disease severity, age group, and geographical region. We also explored the disease course of patients with gastrointestinal symptoms.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched PubMed, Embase, and Web of Science databases on April 4, 2020 (updated April 10, 2020) for articles published from Jan 1, 2020, using the keywords “coronavirus”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “novel coronavirus”, “nCoV”, “2019-nCoV”, and “COVID-19.” Considering the urgency of the topic and the need to increase the sensitivity of the search, a grey literature search was done using the same keywords on Google Scholar to capture the most recently published articles. Furthermore, COVID-19 publications in the WHO database of publications, The Lancet COVID-19 Resource Centre, New England Journal of Medicine, JAMA, BMJ, Gastroenterology, Gut, American Journal of Gastroenterology, and the US Centers for Disease Control and Prevention were screened for potentially relevant publications, included accepted pre-proof publications. Additional articles were retrieved by screening the reference lists of included studies and from the archives of the reviewers. The literature search was restricted to articles published in English. One of the reviewers (YQ) with experience in database searches designed the search strategy, which was subsequently revised by other authors. Because of the large number of records identified from the grey literature, the Google Scholar search was limited to titles. However, no additional limits were applied in the PubMed, Embase, or Web of Science searches. Records were managed with EndNote (version X9.0) to exclude duplicates.
Eligible studies reported the epidemiological and clinical features of COVID-19 and the prevalence of gastrointestinal findings in infected patients. Given that preprint papers in databases such as bioRvix and medRvix were not peer-reviewed, we did not include papers found in such databases in our analysis to avoid any potential misinformation being disseminated. The following studies were excluded: duplicate publications; reviews; editorials; single case reports; studies pertaining to other coronavirus-related illnesses, such as Middle East respiratory syndrome (MERS); and small case series (<10 cases). Two reviewers (YQ, J-SH) independently screened the titles and abstracts according to these eligibility criteria. Disagreement was discussed with another author (RM) and subsequently resolved via consensus.
Two reviewers (J-SH, J-YT) independently rated the quality of included studies using the National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies.13 Any disagreement was resolved by the third senior reviewer (RM).
Data extraction and definitions
The two investigators (QY and J-SH) who did the literature search also independently extracted the data from included studies. Disagreements were resolved by a third investigator (RM) or by consensus. We extracted the following variables: author; date; study design; country; patient demographics; number of participants in severe and non-severe disease groups; and prevalence of clinical gastrointestinal symptoms such as vomiting, nausea, diarrhoea, loss of appetite, abdominal pain, and belching, together with prevalence of digestive system comorbidities including liver disease and gastrointestinal diseases. Liver injury was defined according to the studies. Patients with abnormal liver test results, such as increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin concentrations, were also classified as having liver injury. Disease severity was defined according to the studies, mainly on the basis of the symptoms present at diagnosis—eg, patients with pulse oxygen saturation (SpO2) less than 90%,14 or need of intensive care unit (ICU) care,15 or with acute respiratory distress syndrome16 were classified as having severe disease. COVID-19 was diagnosed on the basis of the study criteria, with reference to WHO guidance.17 This study was done in accordance with PRISMA guidelines.
Data synthesis and statistical analysis
To estimate standardised mean difference (SMD) or weighted mean differences, we used two simple formulas proposed by Hozo and colleagues18 to estimate the mean using the values of the median, the low and high ends of the range, and the sample size. Odds ratios (OR) were used to describe the ratio of the probability of events occurring in patients with severe versus non-severe COVID-19. Owing to heterogeneity within and between studies, a random-effects model was used to estimate the average effect and its precision, which would give a more conservative estimate of the 95% CI. We used the I 2 statistic and Cochran's Q test to assess statistical heterogeneity. A meta-analysis was planned to assess the association of gastrointestinal symptoms and liver injury with demographic data, outcomes, and disease characteristics. The meta-analysis was done with the metaprop command of the meta package in R (version 3.2.0) for pooling single-armed rates. We used Stata (version 12.1) with the command metareg (for meta-regression) for the assessment of publication bias, and Review Manager (version 5.3) for all other analyses.
Funnel-plot asymmetry as proposed by Egger and colleagues19 was used to investigate the possibility of publication bias.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
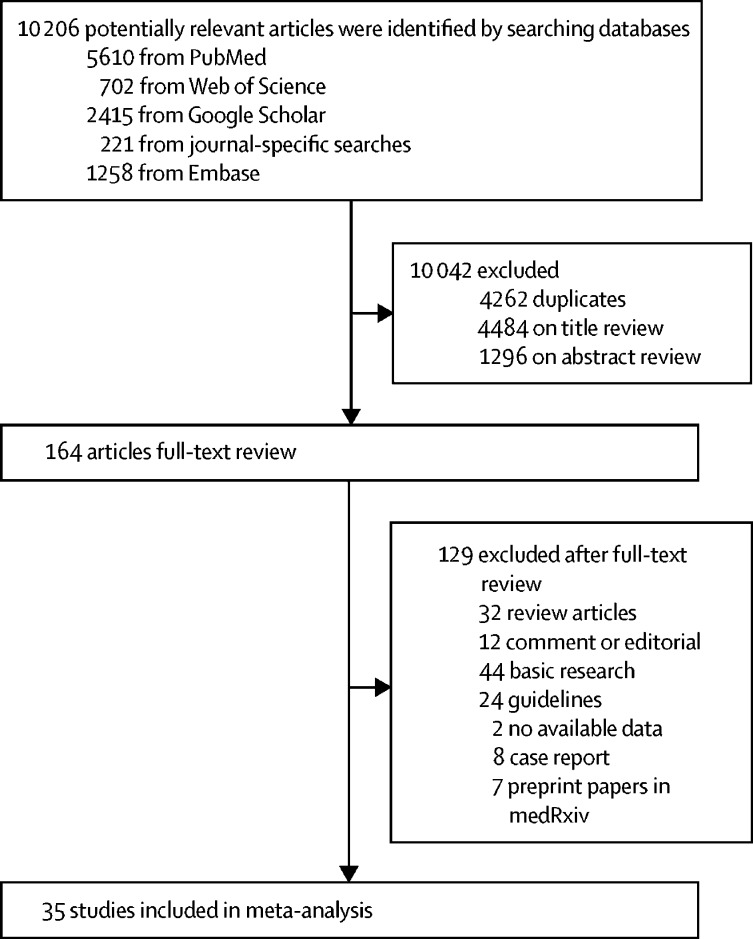
10 206 records were initially identified by our searches. After removal of duplicates, 5944 remained. After review of titles and abstracts, 164 articles were deemed to meet criteria for full-text review. After exclusion of review articles, editorials, guidelines, and basic research, 52 potentially relevant reports remained for detailed assessment. Of these 52 studies, two20, 21 were excluded for having no absolute numbers for outcomes, eight22, 23, 24, 25, 26, 27, 28, 29 were excluded for being case series with fewer than ten patients, and seven studies were further excluded for being pre-print papers; thus 35 studies,2, 8, 9, 12, 14, 15, 16, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 including 6686 patients with COVID-19, met the inclusion criteria and were included in our analyses (figure 1 ).
Figure 1.
Study selection
The main characteristics of patients and studies included in the meta-analysis are shown in the appendix (pp 1–3). Most studies were from China, except for one from Singapore31 and one from the USA,30 and one worldwide.53 The studies included mainly adult patients, except for four paediatric studies8, 37, 43, 54 and six studies2, 9, 38, 45, 46, 53 with a small group of children (36 patients in total). All the included studies were rated fair for quality according to the NIH Quality Assessment Tool for Case Series Studies (appendix pp 4, 5).
By combining 21 studies reporting gastrointestinal data of patients with COVID-19 at diagnosis, the pooled estimate of the prevalence of digestive system comorbidities (ie, underlying gastrointestinal disease and liver disease) was 4% (95% CI 2–5; range 0–15; I 2=74%; appendix p 13). The pooled prevalence of liver comorbidities was 3% (2–4; range 0–25; I 2=57%). The most reported digestive system comorbidities included chronic hepatitis or liver cirrhosis and peptic ulcer.
Several case series have reported gastrointestinal symptoms being the initial symptoms of COVID-19.22, 23, 25, 26, 27, 28, 29, 40 COVID-19-induced diarrhoea at onset was first reported in a patient with COVID-19 in China,58 and subsequently confirmed in patients in Singapore31 and Japan.59 The pooled estimate of gastrointestinal symptoms as presenting symptoms was 10% (95% CI 4–19; range 3–23; I 2=97%; appendix p 14). Patients presenting with gastrointestinal symptoms had longer duration from illness onset to hospital admission (standardised mean difference [SMD] 2·85 [95% CI 0·22–5·48]; p=0·030; I 2=73%).
Results regarding the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in stool or rectal swab are summarised in the appendix (p 6).5, 8, 23, 31, 37, 49, 59, 60, 61, 62, 63 The pooled estimate of SARS-CoV-2 viral RNA positivity in faecal samples was 54% (95% CI 44–64; I 2=28%), with positivity persisting for up to 47 days after symptom onset (appendix p 6).61
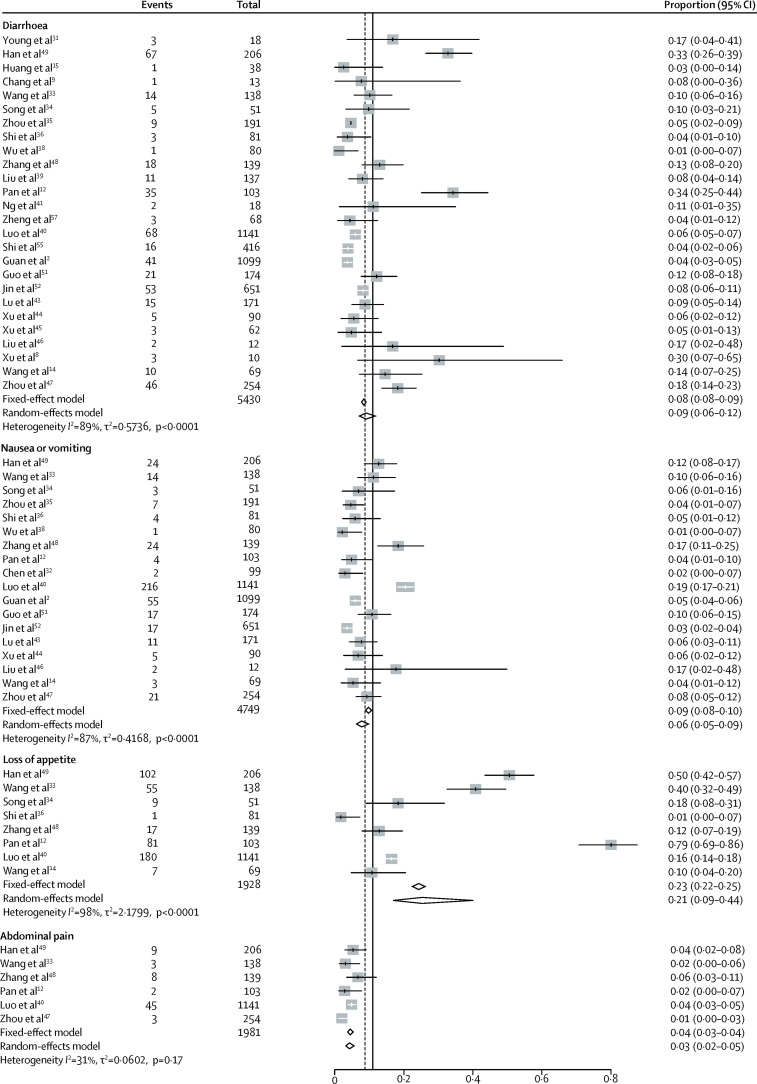
Gastrointestinal symptoms and abnormal liver function in patients with COVID-19 are summarised in the appendix (pp 7–10). Combining all 29 studies (n=6064) reporting gastrointestinal symptoms in patients with COVID-19 at diagnosis, the pooled prevalence of digestive symptoms was 15% (95% CI 10–21; range 2–57; I 2=96%; appendix p 15). Nausea or vomiting, diarrhoea, and loss of appetite were the main gastrointestinal symptoms. The pooled prevalence of diarrhoea was 9% (95% CI 6–12; range 1–34; I 2=89%), nausea or vomiting 6% (5–9; range 1–19; I 2=87%), loss of appetite 21% (9–44; range 1–79; I 2=98), and abdominal pain 3% (2–5; range 1–4; I 2=31%; figure 2 ).
Figure 2.
Pooled estimate of the prevalence of gastrointestinal symptoms in patients with COVID-19
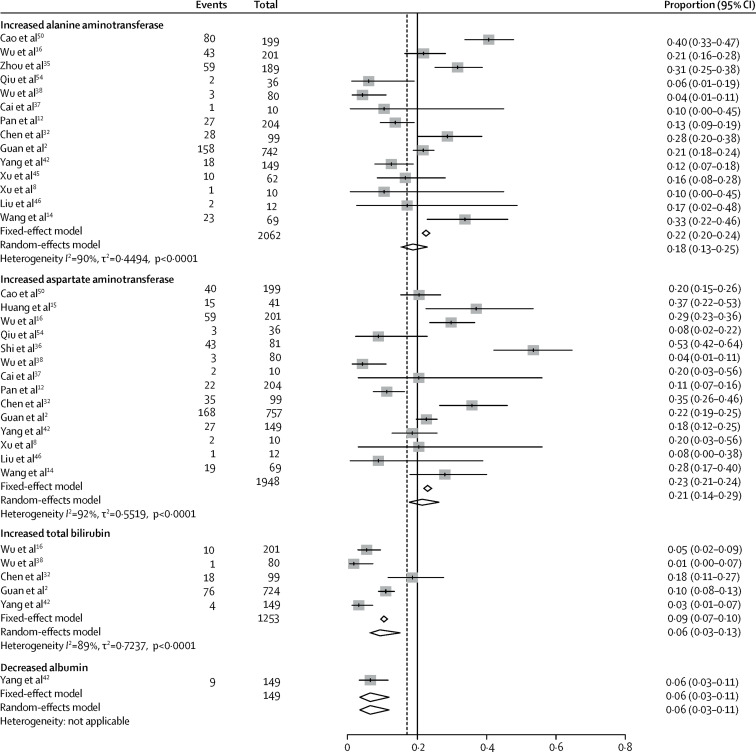
The pooled prevalence of liver injury, from 12 studies (n=1267), was 19% (95% CI 9–32; range 1–53; I 2=96%; appendix p 16). The pooled prevalence of increased ALT was 18% (13–25; range 4–40; I 2=90%), increased AST was 21% (14–29; range 4–53; I 2=92%), and increased total bilirubin was 6% (3–13; range 1–18%; I 2=89%; figure 3 ). The pooled prevalence of decreased albumin was 6% (3–11; figure 3).
Figure 3.
Pooled estimate of the prevalence of abnormal liver chemistry in patients with COVID-19
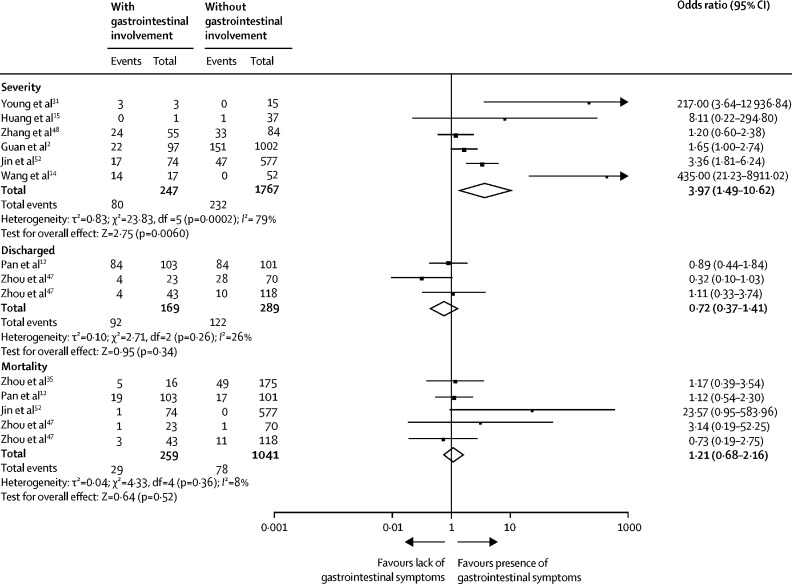
The proportion of patients with severe or critical COVID-19 was markedly increased in patients with gastrointestinal symptoms compared with those without gastrointestinal symptoms (OR 3·97 [95% CI 1·49–10·62]; p=0·0060, I 2=79%; figure 4 ). However, the risk of severe disease was not increased among patients with digestive comorbidities compared with patients without these comorbidities (OR 0·57 [95% CI 0·15–2·18]; p=0·41; I 2=44%; appendix p 17).
Figure 4.
Prognosis of patients with COVID-19
Patients stratified by digestive system involvement. Odds ratio calculated with Mantel-Haenszel random-effects model.
Patients with gastrointestinal symptoms had an increased risk of acute respiratory distress syndrome (OR 2·96 [95% CI 1·17–7·48]; p=0·020) and liver injury (2·71 [1·52–4·83]; p=0·0007; appendix p 18). However, the pooled rates of discharge (OR 0·72 [95% CI 0·37–1·41]; p=0·34; figure 4), length of hospital stay (SMD 0·22 [95% CI 0·14–0 ·58]; p=0·11), and mortality (OR 1·21 [95% CI 0·68–2·16]; p=0·52; n=1300 patients and n=107 deaths; I 2=8%; figure 4) were similar between patients with and without gastrointestinal symptoms.
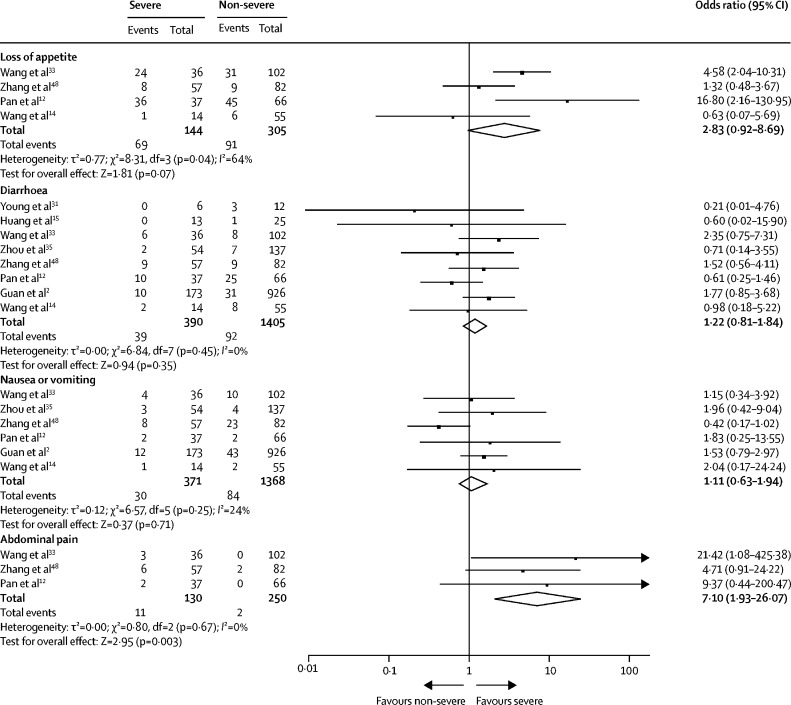
We analysed the difference in gastrointestinal symptoms between patients with severe and non-severe COVID-19 (figure 5 ). Patients with severe COVID-19 were more likely to present with abdominal pain (OR 7·10 [95% CI 1·93–26·07]; p=0·003; I 2=0%) compared with those with non-severe disease. However, we found no significant difference between patients with severe and non-severe disease in loss of appetite (2·83 [0·92–8·69]; p=0·07; I 2=64%), diarrhoea (1·22 [0·81–1·84]; p=0·35; I 2=0%), or nausea or vomiting (1·11 [0·63–1·94]; p=0·71; I 2=24%).
Figure 5.
Gastrointestinal symptoms according to COVID-19 severity (severe vs non-severe)
Odds ratio calculated with Mantel-Haenszel random-effects model.
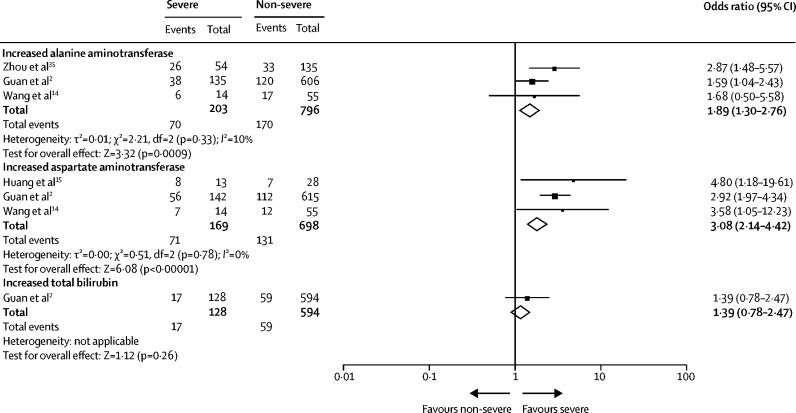
We found a higher risk of abnormal liver chemistry including increased ALT (OR 1·89 [95% CI 1·30–2·76]; p=0·0009; I 2=10%) and AST (3·08 [2·14–4·42]; p<0·00001, I 2=0%; figure 6 ) in patients with severe COVID-19 than in those with non-severe disease. Liver damage indices, including ALT, and AST, and total bilirubin concentrations were significantly higher in patients with severe disease than in those with non-severe disease (appendix p 19). However, the pooled analysis did not show a significant difference in albumin concentrations between the two groups (appendix p 19).
Figure 6.
Liver chemistry according to COVID-19 severity (severe vs non-severe)
Odds ratio calculated with Mantel-Haenszel random-effects model.
We further analysed the differences between patients in Hubei (the place of the initial COVID-19 outbreak in China, n=4009) versus those outside of Hubei province (n=2677). The incidence of overall gastrointestinal symptoms at diagnosis (17% [95% CI 10–28] vs 9% [6–14]; p=0·078) was similar between the two groups. We found a higher proportion of patients in Hubei presenting with nausea or vomiting compared with those outside of Hubei (appendix p 11). Other symptoms including diarrhoea and loss of appetite were similar between patients in Hubei and outside Hubei (appendix p 11).
However, we found a higher risk of liver injury in patients in Hubei compared with those outside of Hubei (21% [95% CI 4–59] vs 10% [4–25]; p<0·0001). This trend was further confirmed by the finding that a larger proportion of patients in Hubei had increased total bilirubin concentrations compared with those of patients outside of Hubei (appendix p 11).
We further analysed whether digestive symptoms varied between adult (n=6420) and paediatric patients (n=266). Gastrointestinal symptoms, including diarrhoea and nausea or vomiting were similar between the two groups (appendix p 11). Similarly, children with COVID-19 had a similar risk of liver injury to that of adult patients (9% [95% CI 3–21] vs 21% [9–43]; p=0·0516). However, children were less likely to present with increased ALT and AST compared with adult patients (appendix p 11).
Significant publication bias was found by both funnel plot and Egger test (p<0·0001) for gastrointestinal symptoms (appendix p 20) but not for liver injury (p=0·18; data not shown).
Discussion
An increasing number of studies have reported the involvement of the digestive system in patients with COVID-19. We aimed to investigate the pooled prevalence of gastrointestinal symptoms and liver injury in patients with COVID-19. Overall, gastrointestinal symptoms were reported in 15% of patients with COVID-19 and liver injury in 19% of patients. As the severity of the disease increases, digestive symptoms and liver injury become more pronounced. About 10% of patients presented with gastrointestinal symptoms alone without respiratory features; these patients have delayed diagnosis of COVID-19. Patients with gastrointestinal symptoms have increased risk of severe or critical disease, and development of acute respiratory distress syndrome.
Over the course of the COVID-19 pandemic, some patients have initially presented with abdominal symptoms without fever or respiratory manifestations.58 In a large multicentre study12 of 204 patients with COVID-19 in three heavily affected hospitals during the initial outbreak in China, 103 (50%) patients presented with digestive symptoms as their chief complaint. Six (3%) patients presented with digestive symptoms but no respiratory symptoms. In a large case series40 (n=1141) of patients admitted to hospital with COVID-19, 183 (16%) presented with gastrointestinal symptoms only. Wang and colleagues33 also found that around 10% of patients initially presented with diarrhoea and nausea 1–2 days before the development of fever and dyspnoea.
Patients with digestive symptoms had a variety of manifestations, such as loss of appetite, diarrhoea, vomiting, and abdominal pain.12 Autopsy studies are important to help investigate histopathological changes in the gastrointestinal tract in patients with COVID-19. Only one autopsy report with details of gastrointestinal pathology has been published in an 85-year-old man with COVID-19, which showed segmental dilatation and stenosis in the small intestine.64 Further studies are needed to clarify whether this finding is secondary to COVID-19 or a pre-existing gastrointestinal comorbidity.
In addition to digestive symptoms, patients with COVID-19 are also at risk of developing liver injury. Studies have shown that patients had varying degrees of liver function abnormalities—the incidence ranged from 1% to 53%—mainly indicated by abnormal ALT and AST concentrations, accompanied by slightly increased bilirubin concentrations. Albumin was decreased in severe cases (around 26·3–30·9 g/L).32 Our findings indicate that one in five patients will develop liver function abnormalities, especially in patients with severe disease, thus close attention should be paid to liver dysfunction when treating patients with COVID-19 over the hospitalisation period. Liver injury was characterised by slight increases in hepatocyte-related enzymes, including ALT and AST. Cholangiocyte-related enzymes, such as alkaline phosphatase and γ-glutamyl transpeptidase, were also reported to be slightly increased in a few patients.65 Studies on the exact mechanism of COVID-19-related liver injury are scarce. Liver abnormalities of patients with COVID-19 may be due to viral infection in liver cells or other causes such as drug toxicity and systemic inflammation.66 A post-mortem biopsy study in a patient with COVID-19 showed moderate microvascular steatosis and mild lobular and portal activity, indicating that the injury could have been caused by either COVID-19 or drug-induced liver injury.67, 68 Similar to the situation in patients with SARS, drugs such as antivirals might cause liver injury in patients with COVID-19.69 However, one study reported that no significant difference in the prevalence of liver injury existed between patients on medication versus those who were not, when stratified by pre-hospital medications, including antibacterial drugs, antiviral drugs (abidol, oseltamivir, acyclovir), and antipyretic drugs such as paracetamol.68 Further studies are warranted in this setting.
A link between gastrointestinal involvement and disease severity of COVID-19 has been proposed. In a multicentre study, Pan and colleagues12 investigated the prevalence and outcomes of patients with COVID-19 with digestive symptoms. In 99 patients who presented with digestive symptoms as their chief complaint, a longer time from onset to admission was observed compared with patients without digestive symptoms (9·0 days vs 7·3 days). As the severity of the disease increased, digestive symptoms became more numerous. Patients without digestive symptoms were more likely to be cured and discharged than were patients with digestive symptoms (60% vs 34%).12 This finding was consistent with the study from Wang and colleagues,33 who found that patients admitted to the ICU were more likely to have abdominal pain and loss of appetite compared with non-ICU patients.33 We did subgroup analyses to investigate the difference in gastrointestinal symptoms between patients with severe and non-severe COVID-19. We found a higher prevalence of abdominal pain in patients with severe COVID-19 than in those with non-severe disease. Cai and colleagues37 showed that liver injury occurred more frequently in patients with severe disease than in patients with non-severe disease. We also found a significantly higher rate of liver function abnormalities in those with severe disease than in those with non-severe disease. In patients with COVID-19 who died, the incidence of liver injury might reach as high as 58%70 or 78%.71 One study reported that serum ALT increased up to 7590 U/L and AST up to 1445 U/L in a patient with severe COVID-19.32
We further investigated the disease course and outcomes in subgroups of patients with digestive system involvement. We found that patients presenting with initial gastrointestinal symptoms only had longer duration from illness onset to hospital admission. The rate of severe or critical illness was markedly increased in patients with gastrointestinal symptoms than in those without. Patients with gastrointestinal symptoms also had an increased risk of acute respiratory distress syndrome. No significant difference was seen when considering pooled rates of discharge, length of hospital stay, and rates of death between patients with and without gastrointestinal symptoms. This finding might be due to the somewhat low incidence of each event. Moreover, the risk of severe disease was not increased among patients with COVID-19 with existing gastrointestinal or liver-related comorbidities compared with patients without such comorbidities. Thus, newly presenting gastrointestinal symptoms rather the existing digestive comorbidities were predictive of severe disease course. Altogether, our findings support the importance of early inclusion of symptoms such as diarrhoea in the diagnosis of COVID-19.
The characteristics of patients with COVID-19 outside of Hubei might differ from those of patients in Hubei. An early study including 80 cases of COVID-19 in Jiangsu province showed that patients with COVID-19 exhibited milder or more moderate symptoms and a lower proportion of liver dysfunction compared with patients in Wuhan.38 Our subgroup analysis suggested that no difference existed in the rate of overall gastrointestinal symptoms between patients within Hubei and those outside. Patients in Hubei had a higher risk of presenting with abnormal liver function than did those outside of Hubei. One explanation is that Hubei might have had more patients with severe disease compared with outside of Hubei, which might result in the higher percentage of patients with abnormal liver function in Hubei. However, the studies included in our analysis did not show significant differences in the proportion of patients with severe disease between Hubei and outside of Hubei (data not shown). Thus this geographic difference needs to be further investigated in future studies.
A few paediatric cases of COVID-19 have been reported and associated clinical features have yet to be fully investigated. In an investigation8 of ten children (median age 80 months [IQR 2–188]) with COVID-19 in China, these patients had clinically milder symptoms and showed fewer alterations in radiological and laboratory testing parameters, compared with adult patients. We included four paediatric studies with 227 patients.8, 37, 43, 54 According to our subgroup analysis, children with COVID-19 had a lower risk of increased ALT concentrations compared with adults. However, gastrointestinal symptoms were similar between children and adults.
Emerging data suggest the prolonged presence of SARS-CoV-2 RNA in stool samples or rectal swabs even after the patients' respiratory specimens become negative.8, 60 Much attention has been paid to the possibility of viral shedding from the gastrointestinal tract and faecal–oral transmission. In an investigation8 of ten paediatric patients, eight (80%) persistently tested positive on rectal swabs even after nasopharyngeal tests became negative.8 Our pooled estimate of the prevalence of SARS-CoV-2 viral RNA positivity in faecal samples was 54% (95% CI 44–64). Viral positivity can persist for as long as 47 days after symptom onset (appendix p 6).61 Data from Wu and colleagues60 suggest the possibility of extended duration of viral shedding in faeces, for nearly 5 weeks after the patients' respiratory samples tested negative for SARS-CoV-2. However, the clinical implications of prolonged viral excretion in faeces, including the association with disease course, severity, and even recurrence of COVID-19, remains unclear. More studies are needed to show the virus' replication competence, abundance in stool, and stability in the environment.72, 73
This meta-analysis has several potential limitations. First, an assessment of the methodological quality showed deficiencies in the studies assessed—all 35 studies included were considered to be of low quality. Second, because of insufficient data reported in the original publications, we could not assess the effects of other factors, such as sex, age, and comorbidities, on the rate of gastrointestinal symptoms at diagnosis and risk of liver injury. Third, the criteria for severe COVID-19 differed among studies, which might have contributed to the heterogeneity of the meta-analysis. Last, significant heterogeneity and publication bias were observed in our study for estimating the prevalence of digestive system symptoms. After reviewing each study, the non-specific symptom of appetite loss and two studies40, 49 that were focused on patients with gastrointestinal symptoms contributed to most of the heterogeneity. When these studies were excluded from the analysis, the pooled prevalence of digestive symptoms was with modest heterogeneity. The pooled rates of the other three symptoms (nausea or vomiting, diarrhoea, and abdominal pain) were similar to our original results, with only mild or modest heterogeneity.
In conclusion, our results suggest that digestive symptoms and liver injury are not uncommon in patients with COVID-19. Compared with patients with non-severe disease, those with severe COVID-19 had a higher risk of developing gastrointestinal symptoms and liver injury. Patients in Hubei had a similar risk of developing gastrointestinal symptoms but higher risk of liver injury than did those outside of Hubei. Children with COVID-19 had a similar risk of gastrointestinal symptoms to that of adult patients. A tenth of patients with COVID-19 might present only with gastrointestinal symptoms without respiratory symptoms; such patients could have delayed diagnosis. Patients with gastrointestinal symptoms have a tendency to develop severe or critical disease and have a poor disease course. Increased attention should be paid to the care of this unique group of patients.
This online publication has been corrected. The corrected version first appeared at thelancet.com/gastrohep on June 15, 2020
Contributors
RM and M-HC conceived the study. SG, M-HC, and RM supervised the overall study. RM and YQ wrote the manuscript. RM, YQ, J-SH, J-YT, and X-HL analysed the data. JS, JL, L-RZ, YC, SCN, and MI critically revised the manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Ren Mao, Email: maoren2023@gmail.com.
Min-Hu Chen, Email: chenminhu@mail.sysu.edu.cn.
Supplementary Material
References
- 1.WHO Coronavirus disease 2019 (COVID-19) Situation Report – 94. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200423-sitrep-94-covid-19.pdf?sfvrsn=b8304bf0_4
- 2.Guan WJ, Ni Z, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao F, Tang M, Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi F, Qian S, Zhang S. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Comm. 2020 doi: 10.1016/j.bbrc.2020.03.044. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holshue ML, DeBolt C, Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R, Corman VM, Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. published online April 1. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, Li T, He Z. A pathological report of three COVID-19 cases by minimally invasive autopsies. Chin J Pathol. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Li X, Zhu B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang D, Lin M, Wei L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Gao Y, Lou L. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020 doi: 10.1183/13993003.00398-2020. published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X, Lian J, Hu J. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020 doi: 10.1136/gutjnl-2020-320926. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan L, Mu M, Ren H. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000620. published April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute Study quality assessment tools. http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 14.Wang Z, Yang B, Li Q. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020 doi: 10.1001/jamainternmed.2020.0994. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y, Zhang D, Yang P. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Quan B, Li X. A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25755. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JF, Yuan S, Kok K. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Chen X, Lu Y. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Bioscience Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Li X, Zhang W. Clinical features and treatment of 2019-nCov pneumonia patients in Wuhan: report of a couple cases. Virol Sin. 2020 doi: 10.1007/s12250-020-00203-8. published Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lescure F, Bouadma L, Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo C, Yao L, Zhang L. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai'an, Jiangsu Province, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu N, Li W, Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng L, Xia S, Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young BE, Ong S, Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song F, Shi N, Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai J, Xu J, Lin D. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa198. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Liu J, Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. published online Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu K, Fang Y, Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020 doi: 10.1097/CM9.0000000000000744. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.03.043. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng M, Lee E, Yang J. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothoracic Imaging. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang W, Cao Q, Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–439. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu X, Zhang L, Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Yu C, Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X, Wu X, Jiang X. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Yang Y, Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Z, Zhao N, Shu Y. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.020. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Dong X, Cao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 49.Han C, Duan C, Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000664. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo W, Li M, Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin X, Lian J, Hu J. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020 doi: 10.1136/gutjnl-2020-320926. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du M, Cai G, Chen F. Multi-omics evaluation of gastrointestinal and other clinical characteristics of SARS-CoV-2 and COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.045. published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu H, Wu J, Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi S, Qin M, Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu P, Duan F, Luo C. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 doi: 10.1001/jamaophthalmol.2020.1291. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng M, Gao Y, Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y, Liu P, Shi X. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-320891. published online March 5. [DOI] [PubMed] [Google Scholar]
- 59.Hosoda T, Sakamoto M, Shimizu H. SARS-CoV-2 enterocolitis with persisting to excrete the virus for about two weeks after recovering from diarrhea: a case report. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.87. published online March 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghinai I, McPherson TD, Hunter JC. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y, Guo C, Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carvalho A, Alqusairi R, Adams A. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000667. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C, Gao G, Xu Y. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Int Med. 2020 doi: 10.7326/M20-0991. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Wang R, Qu G. Macroscopic autopsy findings in a patient with COVID-19. J Forensic Med. 2020;36:1–3. (in Chinese). [Google Scholar]
- 65.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chai X, Hu L, Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 67.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan Z, Chen L, Li J. Clinical features of COVID-19 related liver damage. medRxiv. 2020 doi: 10.1101/2020.02.26.20026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y, Yang R, Xu Y. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.27.20029009. (preprint). [DOI] [Google Scholar]
- 71.Zhang B, Zhou X, Qiu Y. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.02.26.20028191. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.