Abstract
Objective
To assess the impact of APOE polymorphisms on cognitive performance in patients newly diagnosed with clinically isolated syndrome (CIS) or relapsing-remitting MS (RRMS).
Methods
This multicenter cohort study included 552 untreated patients recently diagnosed with CIS or RRMS according to the 2005 revised McDonald criteria. The single nucleotide polymorphisms rs429358 (ε4) and rs7412 (ε2) of the APOE haplotype were assessed by allelic discrimination assays. Cognitive performance was evaluated using the 3-second paced auditory serial addition test and the Multiple Sclerosis Inventory Cognition (MUSIC). Sum scores were calculated to approximate the overall cognitive performance and memory-centered cognitive functions. The impact of the APOE carrier status on cognitive performance was assessed using multiple linear regression models, also including demographic, clinical, MRI, and lifestyle factors.
Results
APOE ε4 homozygosity was associated with lower overall cognitive performance, whereas no relevant association was observed for APOE ε4 heterozygosity or APOE ε2 carrier status. Furthermore, higher disability levels, MRI lesion load, and depressive symptoms were associated with lower cognitive performance. Patients consuming alcohol had higher test scores than patients not consuming alcohol. Female sex, lower disability, and alcohol consumption were associated with better performance in the memory-centered subtests of MUSIC, whereas no relevant association was observed for APOE carrier status.
Conclusion
Along with parameters of a higher disease burden, APOE ε4 homozygosity was identified as a potential predictor of cognitive performance in this large cohort of patients with CIS and early RRMS.
MS is a chronic neuroinflammatory disease, which mostly affects young adults. Apart from physical impairment, decline of cognitive functions is one of its most disabling aspects. A meta-analysis of data acquired by genome-wide association studies identified a total of 234 significant associations and a further 416 variants potentially associated with MS.1 However, so far, little is known about the contribution of genetic risk factors to the development of cognitive impairment in MS.
The APOE gene locus has been discussed as a possible mediator of cognitive impairment as it is associated with the evolution of dementias like Alzheimer disease (AD).2 It may encode 3 different isoforms of apolipoprotein E (APOE2, APOE3, and APOE4), which are defined by the haplotype combination of common single nucleotide polymorphisms (SNPs) at 2 nearby loci on the APOE gene. The SNPs are labeled rs429358 (base exchange from cytosine to thymine [C>T] leading to the haplotype APOE ε4) and rs7412 (base exchange C>T resulting in the haplotype APOE ε2). The APOE ε4 haplotype leads to an amino acid exchange from cysteine to arginine at position 112 of the APOE protein resulting in the isoform APOE4, whereas the haplotype APOE ε2 leads to an amino acid exchange from arginine to cysteine at position 158 of the APOE protein resulting in the isoform APOE2. APOE ε3 is the common variant.3 APOE ε4 is associated with faster memory decline over the adult life course4 and is a major risk factor for AD, with an 8- to 12-fold increase in APOE ε4 homozygotes.3 Although it was shown in a sufficiently powered study that APOE variants have no effect on MS susceptibility,5 reports on the influence of APOE variants on cognitive performance in patients with MS have been contradictory.6,7 Therefore, this study aims to assess the potential impact of APOE polymorphisms on parameters of cognitive function in a large multicenter, prospectively collected German data set of untreated patients with clinically isolated syndrome (CIS) and early relapsing-remitting MS (RRMS). As a number of demographic, clinical, MRI, and lifestyle risk factors have been shown to enhance cognitive decline in MS and to adversely influence disease progression,8–10 these were also included in the analyses.
Methods
Standard protocol approvals, registrations, and patient consents
This multicenter prospective longitudinal observational cohort study (German National MS Cohort) was approved by the ethics committee of Ruhr-University Bochum (registration no. 3714-10) and consecutively all local committees of the participating centers (22 centers in Germany). All patients provided written informed consent.
The German National MS cohort and clinical data
A total of 552 participants from the German National MS cohort, a multicenter, prospective, and observational study, were included. This study was approved by the ethics committee of Ruhr-University Bochum (registration no. 3714-10) as described previously.11 All participants were aged at least 18 years, untreated regarding disease-modifying therapies, and diagnosed with either CIS with first symptoms within the previous 6 months and fulfilling at least 3 Barkhof criteria12 or RRMS according to the 2005 revised McDonald criteria13 with first symptoms not more than 3 years before study enrollment. For inclusion, patients must not have received a steroid pulse due to a relapse in the 4 weeks before study enrollment. All participants provided written informed consent.
Assessments included clinical, demographic, MRI, and lifestyle variables and screening tests for cognitive function and blood sampling.11 At the point of study enrollment, patients were asked to assess their current drinking and smoking habits via questionnaire. In response to the question “Do you currently drink alcohol?”, patients could select from 3 categories: (0) no, (1) occasionally, and (2) regularly. Based on this, we dichotomized the participants into current no alcohol consumers (category 0) and current alcohol consumers (categories 1 and 2). Similarly, in response to the question “Do you currently smoke?”, patients could select from 6 categories: (0) no, (1) occasionally, but not on a daily basis, (2) up to 5 cigarettes daily, (3) 6–10 cigarettes daily, (4) 11–20 cigarettes daily, and (5) >20 cigarettes daily. We dichotomized the participants into current nonsmokers (category 0) and current smokers (all other categories). Body weight and height were physically measured on site at the time of study enrollment. Body mass index (BMI) was then calculated as BMI = weight (kg)/height (m)2. School-level education was categorized according to the highest school leaving qualification (level 1: lower-level secondary school [German Hauptschule]; level 2: higher-level secondary school; level 3: higher education entrance qualification [German Abitur]). Depressive symptoms were assessed by the 21-item Beck Depression Inventory II (BDI-II),14 and severity of fatigue was evaluated by the Fatigue Scale for Motor and Cognitive Functions (FSMC).15
Cognitive assessment
Cognitive assessment included the 3-second paced auditory serial addition test (PASAT 3) and the Multiple Sclerosis Inventory Cognition (MUSIC) cognitive screening tests.
The PASAT 3 is a measure of cognitive function that assesses auditory information processing speed, working memory, divided attention, and calculation ability. PASAT 3 data were extracted from the Multiple Sclerosis Functional Composite.16 Individual PASAT 3 test scores were z-standardized, stratified for age and education based on normative data from a German sample of n = 241 healthy controls.17,18
MUSIC is a brief multiple-domain cognitive screening test, which is widely used in German-speaking countries and was developed for the rapid assessment of the most frequently impaired cognitive domains in patients with MS. It consists of 5 cognitive subtests. In the subtests (1) and (2), the patient is asked to remember as many words as possible out of 2 consecutive word lists, each consisting of 10 different words to evaluate working memory. In subtest (3), the patient is given 2 alternating word categories, for which they are asked to find as many associated terms as possible within 1 minute. This subtest was designed to test verbal fluency. Subtest (4) is a modified Stroop Task and assesses susceptibility to interference. In subtest (5), the patient is asked to recall the terms of the first given list of words to asses memory consolidation.19 Individual test scores were z-standardized based on normative data from n = 158 German-speaking healthy young adults.17,19 All tests were taken for the first time at study enrollment so that results were not expected to be biased by learning effects.
Biosamples and genotyping
The SNPs rs429358 (ε4) and rs7412 (ε2) in APOE and the Y chromosome marker rs2032598 (for sampling and handling control) were analyzed using allelic discrimination assays based on TaqMan chemistry according to the manufacturer's protocol (Applied Biosystems, Inc.). Genotyping was performed on 96-well plates with approximately 5% controls run in duplicates across plates. Genotyping efficiency was ≥99.6% for all SNPs. Deviation of the genotypes from Hardy-Weinberg equilibrium (HWE) as a potential marker for genotyping quality was assessed using the Pearson χ2 test. The genotype distribution of rs429358 and rs7412 did not deviate from HWE.
MRI analysis
MRI scans of all patients with CIS and MS included a T1-weighted sequence, a fluid-attenuated inversion recovery sequence, and contrast-enhanced T1-weighted images and were analyzed by a neuroradiologist with regard to lesion number, size, and location and to contrast-enhancing lesions. The neuroradiologist was blinded to clinical data.
Statistical analysis
Statistical analyses were performed using SPSS 23.0 software (IBM Corp.). Continuous variables are described by their median and interquartile range, and categorical variables by numbers and percentages.
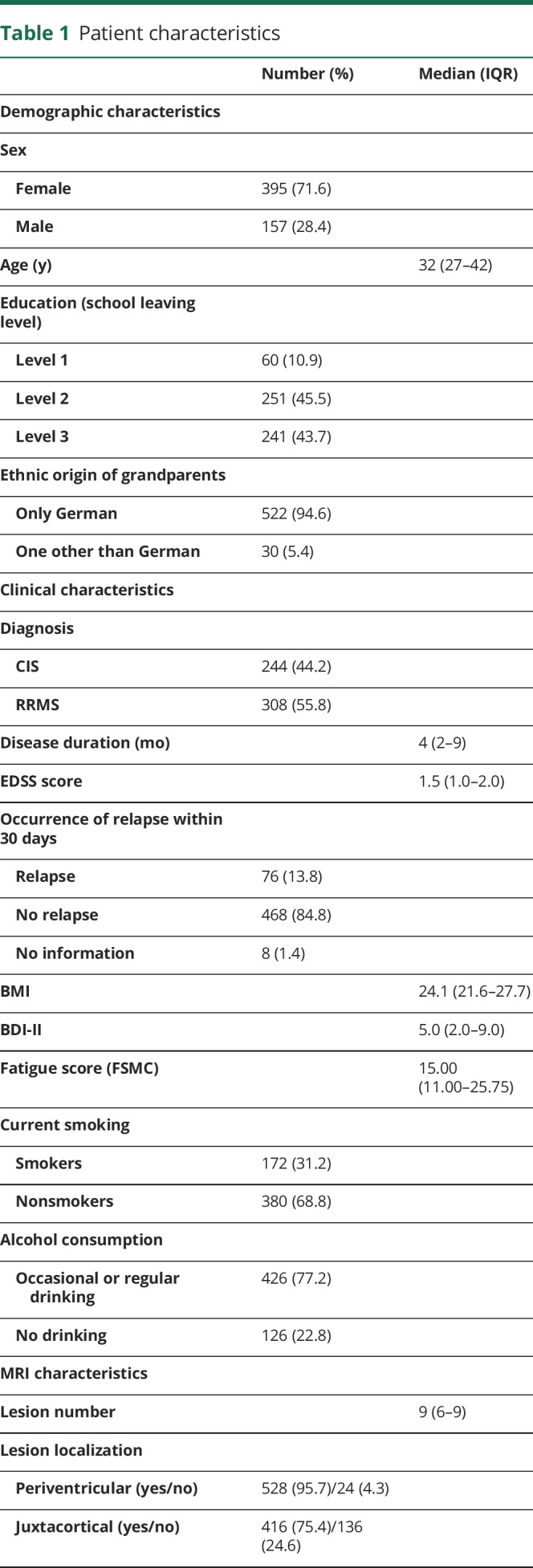
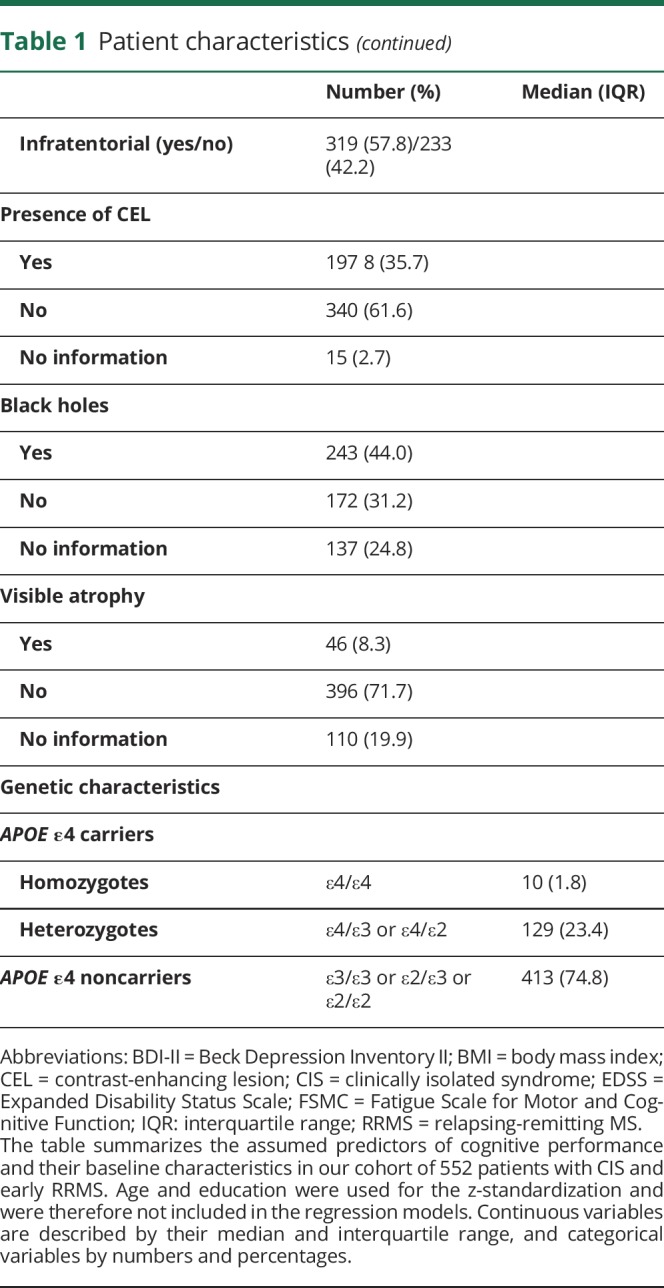
A variety of general sociodemographic factors known to influence cognitive status and previously discussed disease-specific risk factors for cognitive impairment in MS20 were assessed. The list of the potential predictors and their baseline characteristics are summarized in table 1. Age and education were not included in the further analyses as cognitive test results had already been corrected for age and education by z-standardization.
Table 1.
Patient characteristics
To approximate the overall cognitive performance of each patient, we calculated an unweighted mean z-score:
Mean z-score = (z-score of PASAT 3 + z-score of MUSIC total test score)/2.
In addition, the results of the memory-centered MUSIC subtests 1, 2, and 5 were added up to form a memory-centered sum score:
Memory-centered sum score = (z-score of verbal learning list A + z-score of verbal learning list B + z-score of verbal recall)/3.
To extract those factors, which contributed most to cognitive performance in our cohort, variables were preselected by performing univariate linear regression analyses of each potential predictor with the cognitive outcome parameter under investigation. Variables with p values of regression coefficients <0.1 were subsequently selected for inclusion to a multiple linear regression model for the respective outcome. Dichotomous variables were dummy coded.
All our analyses are exploratory. Hence, p values are only given for descriptive reasons. However, we consider an association as statistically relevant in case of p < 0.05.
Data availability
The raw data used in preparation of the figures and tables will be shared in anonymized format on request of a qualified investigator to the corresponding author for purposes of replicating procedures and results.
Results
Characteristics of the 552 patients included in this study are reported in table 1. Of note, 25.2% of the patients were carriers of the APOE ε4 allele. Ten of these (1.8%) were homozygotes, which is in line with the reported prevalence in healthy control populations of Caucasians21 and with the prevalence in a larger German cohort of patients with MS.22 Ethnicity of our cohort was homogeneous. Of note, 94.6% of the patients had grandparents of German origin only. The others (5.4%) had 1 grandparent with origin other than German. There were no patients with more than 1 grandparent with origin other than German enrolled in this study.
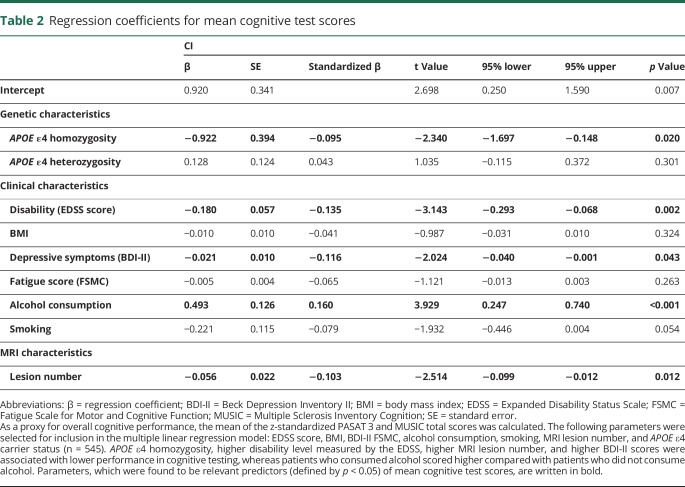
After preselection as described above, the parameters Expanded Disability Status Scale (EDSS) score, BMI, BDI-II, FSMC, alcohol consumption, smoking, MRI lesion number, and APOE ε4 carrier status were included in a multiple linear regression model for the prediction of the overall cognitive performance evaluated by the mean score of the z-standardized PASAT 3 and MUSIC test scores. It was found that APOE ε4 homozygosity, higher disability level measured by the EDSS, higher MRI lesion number, and higher BDI-II scores were associated with lower performance in cognitive testing, whereas patients who consumed alcohol scored higher compared with patients who did not consume alcohol (table 2). The R2 of the overall model was 0.133 (adjusted R2 = 0.118). APOE ε4 heterozygosity was not associated with the overall cognitive performance.
Table 2.
Regression coefficients for mean cognitive test scores
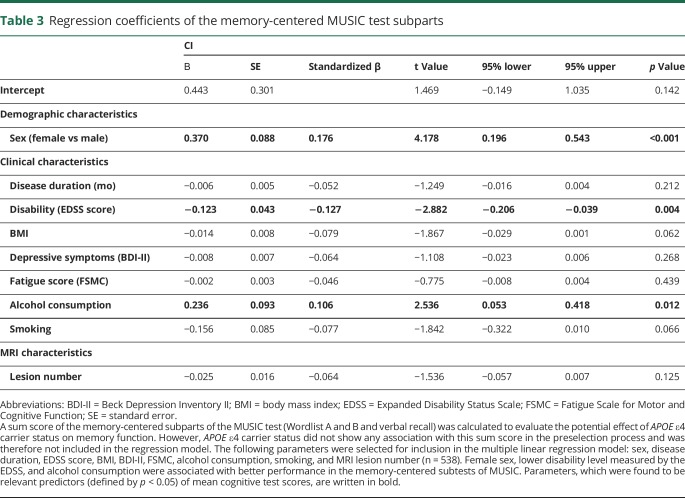
To evaluate whether the effect of APOE ε4 carrier status was more pronounced in memory-mediated cognitive domains resembling its effects in AD, a sum score of the memory-centered subparts of the MUSIC test was investigated in a second multiple linear regression model. However, we found no relevant association of APOE ε4 homo- or heterozygosity with the memory-centered sum score in the univariate regression analysis. Therefore, APOE ε4 carrier status was not included in the multiple regression model for the memory-centered sum score. We found that male sex and higher EDSS scores were associated with worse performance in these subtests. In line with the results of the mean score of overall cognitive performance, alcohol consumption was associated with better test results again (table 3). The R2 of the overall model was 0.109 (adjusted R2 = 0.094).
Table 3.
Regression coefficients of the memory-centered MUSIC test subparts
The findings concerning the effect of alcohol consumption are limited by the fact that they were no longer detectable after variation of dichotomization into nondrinkers and occassional drinkers vs regular drinkers.
We observed no association of the APOE ε2 carrier status with any of the cognitive outcome parameters in the univariate regression analyses. Therefore, APOE ε2 carrier status was not included in any of the multiple linear regression models.
Discussion
Cognitive impairment is one of the most difficult challenges for young adults faced with a diagnosis of MS because neuropsychological symptoms may already be experienced early on23,24 and are among the main reasons for unemployment and reduced quality of life.25 We here assessed the putative role of APOE polymorphisms on the cognitive outcome parameters PASAT 3 and MUSIC test scores in patients with CIS and early RRMS of a homogenous cohort in terms of origin, short disease duration, and treatment-naive state.
Neither APOE ε2 carrier status nor APOE ε4 heterozygosity showed an influence on the evaluated cognitive outcome parameters. However, we observed a relevant association of APOE ε4 homozygosity with a lower overall cognitive performance.
In AD, APOE ε4 carriers have a higher risk of developing AD and show decreased APOE plasma levels compared with APOE ε2 and APOE ε3 carriers. Among APOE ε4 carriers, lower plasma levels are associated with an even greater risk of developing AD. Therefore, it was suggested that a decrease of APOE protein function mediates the evolution of AD and that the risk increases dose dependently.3 As APOE is thought to be involved in repairing neuronal injury, synapse formation, and scavenging of toxins,26,27 we hypothesized that impaired repair mechanisms in MS lesions could mediate more pronounced neurodegenerative processes in APOE ε4 carriers compared with noncarriers.
Our current finding that cognitive performance was impaired in homozygous APOE ε4 carriers only might indicate that a dose-dependent decrease of APOE function mediates cognitive decline in MS, as it does in AD, and that a prolonged follow-up would reveal more pronounced effects of APOE later in the course of MS. Supporting this, previous studies in smaller cohorts of patients with MS with mean disease durations of 8.328 and 13 years26 reported an association of APOE ε4 with dysfunction in some cognitive domains including verbal fluency and memory.
To evaluate whether the observed negative impact of APOE ε4 homozygosity on cognitive performance was mainly caused by impaired memory functions, resembling the assumed APOE ε4-mediated effects in AD,29 additional analysis of a memory-centered sum score was performed. However, we found no relevant association of APOE ε4 homo- or heterozygosity with this memory-centered sum score. This might indicate an AD-independent APOE ε4-mediated effect on cognitive performance in patients with MS. This hypothesis is also supported by the young median age of our cohort as APOE ε4-dependent progression of formerly cognitive unimpaired people to mild cognitive impairment (MCI) and AD was found to be most pronounced in older people aged 70–75 years.30 Furthermore, a recent study using PET imaging biomarkers of AD even suggested that some aspects of MS pathobiology retard the accumulation of β-amyloid, which is one of the main pathologic correlates of AD.31 Nevertheless, we cannot exclude the possibility that patients with APOE ε4 homozygosity performed worse in the cognitive tests because of an APOE ε4-mediated increased risk of developing MCI or AD.
In an effort to correct for potential confounders, we included a range of parameters known or assumed to influence cognitive performance in patients with MS in our analyses. Apart from the putative impact of APOE ε4 homozygosity, we observed a relevant association of markers of the disease burden with the overall cognitive performance. Patients with a higher disability level as assessed by the EDSS and with a higher number of T2 lesions in MRI performed worse in cognitive testing, which is in line with previous reports.17,23,32 Higher scores of depressive symptoms in BDI-II were also associated with an impaired performance. Depression is known to be associated with reduced attention and processing speed in patients with MS.33 As PASAT 3 and MUSIC tests both include an assessment of these cognitive domains, our current finding seems plausible. Surprisingly, we observed a positive influence of alcohol consumption on the cognitive outcome, shedding light on recent reports associating alcohol consumption with lower neurologic disability in MS,34 and a reduced risk of developing MS.35 However, this finding has to be interpreted with care as it may be attributed to the dichotomization of drinking habits and as the questionnaires used in this study were not laid out for accurate quantification of alcohol consumption (e.g., units/month).
Two additional limitations of this study have to be addressed. First, the observed effect of APOE ε4 is based on a very low number of APOE ε4 homozygotes, which makes our findings sensitive to potential confounders, not accounted for. As the estimated prevalence of APOE ε4 homozygosity in Caucasian MS populations is only 1.8%, an even larger cohort than ours would be needed to improve the statistical power. Second, the tests used to assess cognitive performance in this study pose another potential limitation of our observations. PASAT 3 and MUSIC are both screening tests for cognitive performance, which offer the advantage that they may be incorporated into routine diagnostics comparatively easily. However, they lack the sensitivity and reliability to detect MS-specific cognitive impairment of extended test batteries, like for instance the Symbol Digit Modalities Test.36
Besides markers of disease burden, depression, and lifestyle habits, this study identified APOE ε4 homozygosity as a potential predictor of cognitive performance in this cohort of patients with CIS and early RRMS. This indicates a role of APOE as a genetic risk factor for cognitive impairment in MS and might even suggest an APOE ε4 effect unrelated to concomitant AD. Therefore, future work confirming the current findings in young homozygous APOE ε4 patients in a larger and independent MS cohort would be valuable.
Acknowledgment
This work was supported by the German Ministry for Education and Research (BMBF) German Competence Network Multiple Sclerosis (KKNMS). The authors thank Andreas Zymny for technical assistance and Cheryl Ernest for proofreading the manuscript.
Glossary
- AD
Alzheimer disease
- BDI-II
Beck Depression Inventory II
- BMI
body mass index
- CIS
clinically isolated syndrome
- EDSS
Expanded Disability Status Scale
- FSMC
Fatigue Scale for Motor and Cognitive Function
- HWE
Hardy-Weinberg equilibrium
- MCI
mild cognitive impairment
- MUSIC
Multiple Sclerosis Inventory Cognition
- PASAT 3
3-second paced auditory serial addition test
- RRMS
relapsing-remitting MS
- SNP
single nucleotide polymorphism
Appendix 1. Authors
Appendix 2. Coinvestigators
Study funding
No targeted funding reported.
Disclosure
S. Engel, C. Graetz, M. Muthuraman, G. Toenges, G. Antony, and S. Groppa report no disclosures. A. Salmen received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme, none related to this work. B. Ambrosius received travel grants from Novartis, not related to this work. A. Bayas received personal compensation from Merck, Biogen, Bayer Vital, Novartis, Teva, Roche, and Sanofi/Genzyme and grants for congress trips and participation from Biogen, Teva, Novartis, Sanofi/Genzyme, and Merck. A. Berthele reports grants from Bayer HealthCare, personal fees from Biogen, Merck Serono, Teva, Novartis, and Genzyme, and compensations for clinical trials from Biogen, Novartis, Genzyme, Roche, and Alexion Pharmaceuticals. C. Heesen received research grants and speaker honoraria from Biogen, Genzyme, Roche, and Merck, none related to this work. L. Klotz received honoraria for lecturing and serving on advisory boards, as well as travel expenses for attending meetings and financial research support from Novartis, Biogen, Sanofi Genzyme, and the Deutsche Forschungsgemeinschaft (DFG; German Research Society). T. Kümpfel received travel expenses and personal compensations from Bayer HealthCare, Teva Pharma, Merck Serono, Novartis, Sanofi-Aventis/Genzyme, Roche, and Biogen and grant support from Bayer Schering AG, Novartis, and Chugai Pharma, none related to this work. R.A. Linker received research support and/or personal compensation for activities with Bayer HealthCare, Biogen, Genzyme/Sanofi, Merck, Novartis Pharma, Roche, and Teva Pharma. S.G. Meuth received honoraria for lecturing, travel expenses for attending meetings, and/or financial research support from Almirall, Bayer HealthCare, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, and Teva. F. Paul serves on the scientific advisory board for Novartis; received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor for PLoS One; is an associate editor for Neurology® Neuroimmunology & Neuroinflammation; consulted for Sanofi Genzyme, Biogen Idec, MedImmune, Shire, and Alexion; and received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy-Jackson Charitable Foundation, and National Multiple Sclerosis of the USA. M. Stangel received honoraria for scientific lectures or consultancy from Bayer HealthCare, Biogen, Baxter/Baxalta, CSL Behring, Euroimmun, Grifols, Merck Serono, Novartis, Roche, Sanofi-Aventis, and Teva. His institution received research support from Bayer HealthCare, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva. B. Tackenberg received personal speaker honoraria and consultancy fees as a speaker and advisor from Bayer HealthCare, Biogen, CSL Behring, Grifols, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, Teva, und UCB Pharma. His University received unrestricted research grants from Biogen Idec, Novartis, Teva, Bayer HealthCare, CSL Behring, Grifols, Octapharma, Sanofi Genzyme, and UCB Pharma. F. Then Bergh received travel support to attend scientific meetings, personal speaker honoraria, and consultancy fees as a speaker and advisor from Bayer HealthCare, Biogen, Merck Serono, Novartis, Roche, Sanofi Genzyme, and Teva. He received, through his institution, unrestricted research grants from Novartis, Teva, Bayer HealthCare, and Actelion. He received funding from the DFG and, through TRM Leipzig, from the BMBF. H. Tumani received speaker honoraria from Bayer, Biogen, Fresenius, Genzyme, Merck, Novartis, Roche, Siemens, and Teva; serves as section editor for the Journal of Neurology, Psychiatry, and Brain Research; and receives research support from Fresenius, Genzyme, Merck, and Novartis. Frank Weber received honoraria from Genzyme, Novartis, Teva, and Biogen for speaking or for serving on a scientific advisory board, a travel grant for the attention of a scientific meeting from Merck Serono and Novartis, and grant support from Merck Serono, Novartis, and from the Federal Ministry of Education and Research (BMBF, Projects Biobanking and Omics in ControlMS as part of the Competence Network Multiple Sclerosis). B. Wildemann received grants from the German Ministry of Education and Research, Dietmar Hopp Foundation, and Klaus Tschira Foundation, grants and personal fees from Biogen, Merck Serono, Sanofi Genzyme, Novartis Pharmaceuticals, and Teva Pharma, and personal fees from Bayer HealthCare, none related to this work. U.K. Zettl received speaker fees from Aventis, Almirall, Biogen, Bayer, Merck, Novartis, Roche, and Teva. B. Hemmer served on scientific advisory boards for F. Hoffmann-La Roche Ltd, Novartis, Bayer AG, and Genentech; he has served as DMSC member for AllergyCare; he or his institution has received speaker honoraria from Biogen Idec, Teva Neuroscience, Merck Serono, MedImmune, Novartis, Desitin, and F. Hoffmann-La Roche Ltd; his institution has received research support from Chugai Pharmaceuticals and holds part of two patents, one for the detection of antibodies and T cells against KIR4.1 in a subpopulation of MS patients and one for genetic determinants of neutralizing antibodies to interferon β during the last 3 years. H. Wiendl receives honoraria for acting as a member of scientific advisory boards and as a consultant for Biogen, Evgen, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi Genzyme, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Sanofi Genzyme, Teva, and WebMD Global. Prof. Wiendl is acting as a paid consultant for AbbVie, Actelion, Biogen, IGES, Novartis, Roche, Sanofi Genzyme, and the Swiss Multiple Sclerosis Society. His research is funded by the BMBF, DFG, Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children's Foundation, Biogen GmbH, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi Genzyme. Ralf Gold serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases and on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis. F. Zipp has recently received research grants and/or consultation funds from the DFG, Genzyme, Merck Serono, Roche, Novartis, Sanofi-Aventis, Celgene, ONO, and Octapharma. C.M. Lill reports no disclosures. F. Luessi served on the advisory board of Roche and received travel funding from Teva. Go to Neurology.org/NN for full disclosures.
References
- 1.Patsopoulos NA. Genetics of multiple sclerosis: an overview and new directions. Cold Spring Harb Perspect Med 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffernan AL, Chidgey C, Peng P, Masters CL, Roberts BR. The neurobiology and age-related prevalence of the epsilon4 allele of apolipoprotein E in Alzheimer's disease cohorts. J Mol Neurosci 2016;60:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron 2019;101:820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) epsilon4 and cognitive decline over the adult life course. Transl Psychiatry 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lill CM, Liu T, Schjeide BM, et al. Closing the case of APOE in multiple sclerosis: no association with disease risk in over 29 000 subjects. J Med Genet 2012;49:558–562. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Zhao CB, Vollmer TL, Tyry TM, Kuniyoshi SM. APOE epsilon 4 allele is associated with cognitive impairment in patients with multiple sclerosis. Neurology 2008;70:185–190. [DOI] [PubMed] [Google Scholar]
- 7.van der Walt A, Stankovich J, Bahlo M, et al. Apolipoprotein genotype does not influence MS severity, cognition, or brain atrophy. Neurology 2009;73:1018–1025. [DOI] [PubMed] [Google Scholar]
- 8.Ramanujam R, Hedstrom AK, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol 2015;72:1117–1123. [DOI] [PubMed] [Google Scholar]
- 9.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 2014;85:67–75. [DOI] [PubMed] [Google Scholar]
- 10.Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One 2015;10:e0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Bismarck O, Dankowski T, Ambrosius B, et al. Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurol Neuroimmunol Neuroinflamm 2018;5:e446 doi: 10.1212/NXI.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120 (pt 11):2059–2069. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 15.Penner IK, Raselli C, Stocklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler 2009;15:1509–1517. [DOI] [PubMed] [Google Scholar]
- 16.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999;122 (pt 5):871–882. [DOI] [PubMed] [Google Scholar]
- 17.Johnen A, Burkner PC, Landmeyer NC, et al. Can we predict cognitive decline after initial diagnosis of multiple sclerosis? Results from the German National early MS cohort (KKNMS). J Neurol 2019;266:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer P, Baum K, Bauer H, Gohler H, Miltenburger C. Normalization of the Brief Repeatable Battery of Neuropsychological tests (BRB-N) for German-speaking regions. Application in relapsing-remitting and secondary progressive multiple sclerosis patients [in German]. Nervenarzt 2004;75:984–990. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese P, Kalbe E, Kessler J. Das multiple sklerose inventarium cognition (MUSIC). Psychoneuro 2004:30;384–388. [Google Scholar]
- 20.Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 2011;7:332–342. [DOI] [PubMed] [Google Scholar]
- 21.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–1356. [PubMed] [Google Scholar]
- 22.Lill CM, Liu T, Schjeid BM, et al. Closing the case of APOE in multiple sclerosis: no association with disease risk in over 29,000 subjects. J Med Genet 2012;46:558–562. [DOI] [PubMed] [Google Scholar]
- 23.Patti F, Amato MP, Trojano M, et al. Cognitive impairment and its relation with disease measures in mildly disabled patients with relapsing-remitting multiple sclerosis: baseline results from the Cognitive Impairment in Multiple Sclerosis (COGIMUS) study. Mult Scler 2009;15:779–788. [DOI] [PubMed] [Google Scholar]
- 24.Strober L, Englert J, Munschauer F, Weinstock-Guttman B, Rao S, Benedict RH. Sensitivity of conventional memory tests in multiple sclerosis: comparing the Rao brief Repeatable neuropsychological battery and the minimal assessment of cognitive function in MS. Mult Scler 2009;15:1077–1084. [DOI] [PubMed] [Google Scholar]
- 25.Campbell J, Rashid W, Cercignani M, Langdon D. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J 2017;93:143–147. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Tu JL, Gale SD, et al. APOE epsilon4 is associated with exacerbation of cognitive decline in patients with multiple sclerosis. Cogn Behav Neurol 2011;24:128–133. [DOI] [PubMed] [Google Scholar]
- 27.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 1994;264:850–852. [DOI] [PubMed] [Google Scholar]
- 28.Koutsis G, Panas M, Giogkaraki E, et al. APOE epsilon4 is associated with impaired verbal learning in patients with MS. Neurology 2007;68:546–549. [DOI] [PubMed] [Google Scholar]
- 29.El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer's disease: a review. Ageing Res Rev 2016;27:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonham LW, Geier EG, Fan CC, et al. Age-dependent effects of APOE epsilon4 in preclinical Alzheimer's disease. Ann Clin Transl Neurol 2016;3:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeydan B, Lowe VJ, Reichard RR, et al. Imaging biomarkers of Alzheimer disease in multiple sclerosis. Ann Neurol 2020;87:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler 2017;23:1258–1267. [DOI] [PubMed] [Google Scholar]
- 33.Leavitt VM, Brandstadter R, Fabian M, et al. Dissociable cognitive patterns related to depression and anxiety in multiple sclerosis. Mult Scler Epub 2019 Jun 25. [DOI] [PMC free article] [PubMed]
- 34.Diaz-Cruz C, Chua AS, Malik MT, et al. The effect of alcohol and red wine consumption on clinical and MRI outcomes in multiple sclerosis. Mult Scler Relat Disord 2017;17:47–53. [DOI] [PubMed] [Google Scholar]
- 35.Andersen C, Sondergaard HB, Bang Oturai D, et al. Alcohol consumption in adolescence is associated with a lower risk of multiple sclerosis in a Danish cohort. Mult Scler 2019;25:1572–1579. [DOI] [PubMed] [Google Scholar]
- 36.Sonder JM, Burggraaff J, Knol DL, Polman CH, Uitdehaag BM. Comparing long-term results of PASAT and SDMT scores in relation to neuropsychological testing in multiple sclerosis. Mult Scler 2014;20:481–488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used in preparation of the figures and tables will be shared in anonymized format on request of a qualified investigator to the corresponding author for purposes of replicating procedures and results.