Abstract
Objective
To study the immunomodulatory effect of dimethyl fumarate (DF) on granulocyte macrophage colony-stimulating factor (GM-CSF) production in CD4+ T cells in experimental autoimmune encephalomyelitis (EAE) and human peripheral blood mononuclear cells (PBMCs).
Methods
We collected splenocytes and CD4+ T cells from C57BL/6 wild-type and interferon (IFN)-γ–deficient mice. For human PBMCs, venous blood was collected from healthy donors, and PBMCs were collected using the Percoll gradient method. Cells were cultured with anti-CD3/28 in the presence/absence of DF for 3 to 5 days. Cells were stained and analyzed by flow cytometry. Cytokines were measured by ELISA in cell supernatants. For in vivo experiments, EAE was induced by myelin oligodendrocyte glycoprotein35–55 and mice were treated with oral DF or vehicle daily.
Results
DF acts directly on CD4+ T cells and suppresses GM-CSF–producing Th1 not Th17 or single GM-CSF+ T cells in EAE. In addition, GM-CSF suppression depends on the IFN-γ pathway. We also show that DF specifically suppresses Th1 and GM-CSF–producing Th1 cells in PBMCs from healthy donors.
Conclusions
We suggest that DF exclusively suppresses GM-CSF–producing Th1 cells in both animal and human CD4+ T cells through an IFN-γ–dependent pathway. These findings indicate that DF has a better therapeutic effect on patients with Th1-dominant immunophenotype. However, future longitudinal study to validate this finding in MS is needed.
MS, the leading cause of disability in young adults, is an inflammatory demyelinating disease with axonal injury in the CNS.1 The rapidly growing number of diagnosed cases and available immune-modifying therapies in recent years is motivating scientists and clinicians to further study the mechanism of action of currently approved medications to discover novel underlying pathways that can be targeted to develop new and more efficient treatments for MS.
Patients with relapsing-remitting MS (RRMS) patients are treated with oral dimethyl fumarate (DF) since 2013 in the United States,2 and DF is added to the armamentarium of disease-modifying therapies for MS. In terms of an underlying mechanism of action, DF inhibits interleukin (IL)-12p35 and IL-23p19 transcription in dendritic cells. This leads to the generation of type-2 dendritic cells, which indirectly increases IL-4+ Th2 cells in both experimental autoimmune encephalomyelitis (EAE) and human cells and ameliorates inflammatory responses.3,4 In addition, EAE mice treated with DF show a significant reduction in the total number of interferon (IFN)-γ–, IL-17–, and granulocyte macrophage colony-stimulating factor (GM-CSF)–producing CD4+ T cells among CNS-infiltrating cells.5 These findings add to the role of DF in decreasing the inflammatory profile of T cells indirectly by changing the phenotype of antigen presenting cells, but the direct effect of DF on pathogenic T-cell subtypes has not been adequately studied.
Among the various types of immune cells involved in the pathogenesis of EAE, T cells have been the main focus of research because of their pathogenic role in animal models of demyelination and the abundance of T cells in active demyelinating brain lesions in patients with MS.6,7
Th1 and Th17 cells are considered the main culprits in EAE pathogenicity,8,9 and their signature cytokines, IFN-γ and IL-17, play a role in disease pathogenesis.10,11 Lack of IFN-γ leads to more severe EAE, and the absence of IL-17 does not affect EAE development.12,13 Given that neither Th1 (IFN-γ) nor Th17 (IL-17) signature cytokines are required for the development of EAE,14 we and others have shown that GM-CSF is an essential cytokine for EAE induction. GM-CSF–producing CD4+ T cells can effectively induce EAE by passive transfer, and lack of GM-CSF in Th1 or Th17 cells abrogates their encephalitogenicity. In addition, GM-CSF–deficient mice are resistant to EAE induction.15,16
Here, we studied the direct effect of DF on CD4+ T cells and their cytokine profiles. We demonstrate that DF significantly decreases GM-CSF in CD4+ T cells in vitro and in vivo. Further evaluation showed that the decrease in GM-CSF is more prominent in Th1 than that in Th17 or single GM-CSF+CD4+ T cells. In addition, the suppressive effect of DF on GM-CSF was abrogated by the lack of IFN-γ. We also evaluated the effect of DF on human PBMCs and confirmed that DF significantly decreases GM-CSF in Th1 cells.
Methods
Mice
Female C57BL/6 mice, 7–9 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed at animal facility at Thomas Jefferson University with water and food ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
EAE induction and clinical evaluation
Mice were immunized subcutaneously with 200 μg of myelin oligodendrocyte glycoprotein (MOG)35–55 (GenScript, Piscataway, NJ) emulsified in complete Freund's adjuvant (DIFCO Laboratories) containing Mycobacterium tuberculosis H37Ra (5 mg/mL; DIFCO Laboratories, Detroit, MI). In addition, mice were intraperitoneally injected with 200 ng of pertussis toxin at 0 and 48 hours after immunization. Clinical EAE was assessed daily in a blind fashion using the clinical scoring system from 0: normal to 5: death as described previously.17
DF treatment
DF (Sigma-Aldrich, St. Louis, MO) was used in an emulsion of 0.8% methylcellulose. For in vivo treatment, DF solution was administered by oral gavage twice daily (750 μg for 25 g mice in 200 μL volume), starting the day of EAE induction until termination (day 23 post immunization). The solution was prepared early everyday and stored at 4°C. Mice receiving vehicle were used as controls.
Isolation of CNS-infiltrating mononuclear cells and splenocytes
To collect mononuclear cells (MNCs), mice were extensively perfused at day 23 post immunization with ice-cold phosphate buffered saline. For spleen cells, spleens were collected and disrupted in 70 μm cell strainer before RBC lysis. To collect CNS-infiltrating cells, brains and spinal cords were removed, thinly minced in Liberase TL (Roche, Indianapolis, IN), and incubated at 37°C for 30 minutes. Then, the CNS was strained through a 70 μm cell strainer, and MNCs were enriched by centrifugation on a 70/30 Percoll gradient for 30 minutes at 2,000 rpm.17
Splenocyte and lymphocyte culture and ELISA experiments
Spleen cells were cultured in Iscove Modified Dulbecco medium (Gibco, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Gibco), 5% l-glutamine (Gibco), 5% penicillin/streptomycin (Gibco), and β-mercaptoethanol (Sigma). Spleen cells were stimulated with 1 μg/mL anti-CD3/CD28 agonistic antibodies for 72 hours at 37°C, 5% CO2. For antigen-specific recall response, CNS-infiltrating cells from EAE mice, at a concentration of 1 × 106 cells/mL, were stimulated with 10 μg/mL MOG35–55 at 37°C, 5% CO2. At the indicated time points, supernatants were collected and centrifuged to eliminate cellular debris. Cytokine levels in supernatants were measured by ELISA. We analyzed the following cytokines: GM-CSF (R&D Systems, Minneapolis, MN, DY415), human GM-CSF (R&D Systems DY215), IFN-γ (R&D Systems DY485), human IFN-γ (R&D Systems DY285), IL-17 (R&D Systems DY421), and human IL-17 (R&D Systems DY317).
Purification of CD4+ T cells
MNCs from spleens were subjected to positive selection according to the manufacturer's recommendation (CD4 microbeads; Miltenyi Biotec, Auburn, CA, 130-049-201).
Proliferation assay
Splenocytes were cultured in 96-well plates at a concentration of 1 × 106 cells/mL and stimulated with anti-CD3/28 (1 μg/mL each) for 48 hours. Cells were pulsed with 0.5 μCi of 3H-thymidine for the last 18 hours. Thymidine incorporation was measured using a scintillation counter.
Human blood samples and cell culture
All subjects provided informed consent before their participation in the current study. All human studies were approved by the Thomas Jefferson University Institutional Review Board. Blood was obtained from 8 healthy donors at the Department of Neurology, Thomas Jefferson University. PBMCs were collected by Ficoll-Paque Plus density gradient centrifugation. PBMCs were washed and cultured at a density of 1 × 106 cells/mL in X-VIVO 15 serum-free medium. Cells were stimulated with 1 μg/mL of anti-CD3 (HIT3a; BD Biosciences, San Jose, CA) and 1 μg/mL anti-CD28 (CD28.2; BD Biosciences) for 120 hours in the presence of 0.1 μg/mL of DF (Sigma-Aldrich).
Flow cytometry experiments
For analysis of surface markers, MNCs were surface stained in flow cytometry (FACS) buffer (phosphate buffered saline containing 3% fetal bovine serum and 0.02% NaN3) with fluorescence antibodies for 20 minutes at 4°C. For analysis of cytokine production, MNCs were activated with phorbol 12-myristate 13-acetate (50 ng/mL), ionomycin (500 ng/mL), and Golgi-Stop (1 μg/mL) for 4 hours. Then, cells were surface stained as mentioned previously, before being fixed and permeabilized with buffers (Fix/Perm; ThermoFisher, Frederick, MD). Finally, cells were incubated with cytokine-specific antibodies for 30 minutes at 4°C. All antibodies and reagents used in this section were purchased from BD Biosciences, except for phorbol 12-myristate 13-acetate and ionomycin (both from Sigma-Aldrich). Samples were acquired on an FACS Aria equipment (BD Biosciences). Analyses were performed with FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical analysis was performed using one-way analysis of variance or the Kruskal-Wallis test (all analyses performed with GraphPad Prism software, San Diego, CA). A probability level (p value) of *: p < 0.05, **: p < 0.01, and ***: p < 0.001 was statistically significant for all tests. All error bars represent standard error of the mean. For human results, we used pretreatment and posttreatment paired t test analysis.
Data availability
Raw FACS and ELISA files are not included in this article. Any unpublished and anonymized data will be shared upon request from a qualified investigator.
Results
DF acts directly on CD4+ T cells and decreases GM-CSF production
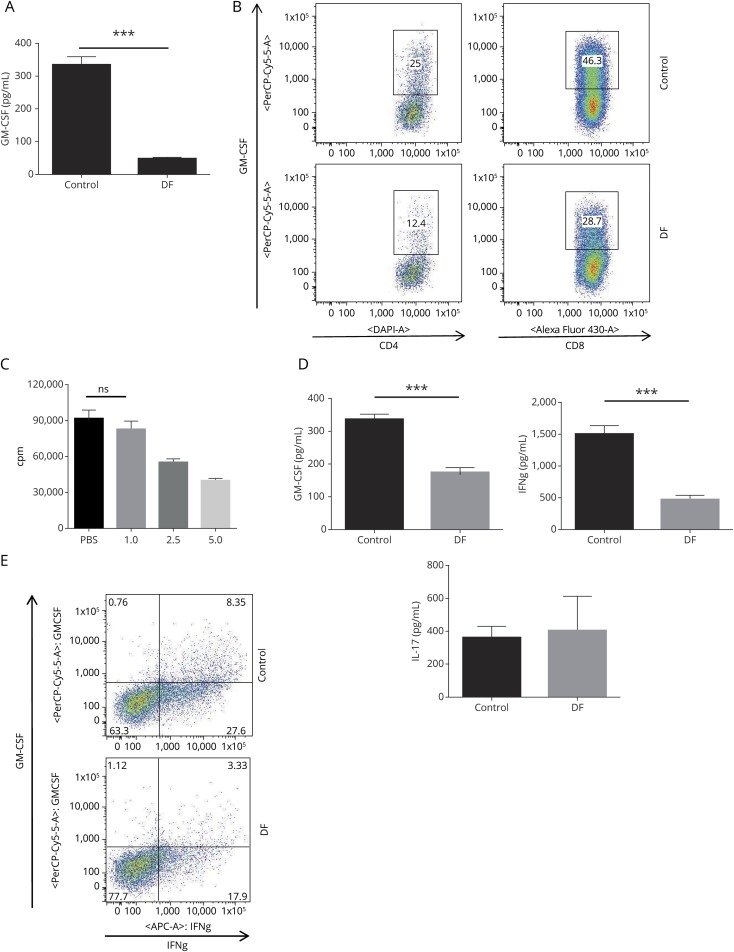
To evaluate the role of DF on GM-CSF production in T cells, we first collected splenocytes from B6 wild-type (WT) mice and cultured them with anti-CD3/CD28 in the presence and absence of DF (1 μg/mL) for 72 hours. We found that GM-CSF in culture supernatants was significantly lower in DF-treated cells compared with controls (figure 1A). FACS analysis of the same cells showed a significant reduction of GM-CSF in both CD4+ and CD8+ T cells (figure 1B). As previously shown in several studies, DF induces T-cell lymphopenia in patients with MS.18 To confirm that the effect of DF on the T-cell cytokine profile is not dependent on its lymphopenic effect, we performed a proliferation assay on activated splenocytes with anti-CD3/CD28 in the presence or absence of varying doses of DF. Administration of DF at a dose of 1 μg/mL, which we used in all our in vitro experiments, did not show a significant suppressive effect on splenocyte proliferation (figure 1C).
Figure 1. DF decreases GM-CSF production and GM-CSF+Th1 cells in mouse splenocytes.
Splenocytes from wild-type (WT) mice were stimulated with 1 μg/mL of anti-CD3/28 antibody in the presence or absence of DF (1 μg/mL) for 72 hours. (A) GM-CSF levels in culture supernatants were determined by ELISA. Data are representative of 3 experiments (mean ± SEM; n = 4 replicates per group). (B) Cells were stimulated with ionomycin/PMA and GolgiPlug in the last 4 hours of culture. After intracellular staining for GM-CSF, flow cytometry analysis of gated CD4+ and CD8+ T cells showed that DF administration decreased GM-CSF production in both CD4+ and CD8+ T cells. (C) Proliferation assay on splenocytes treated with different DF doses. DF did not affect proliferation of splenocytes at a dosage of 1 μg/mL. (D) CD4+ T cells were purified from WT spleen cells and stimulated with anti-CD3/28 in the presence or absence of DF. IL-17, IFN-γ, and GM-CSF were measured in cell supernatants. DF decreased IFN-γ and GM-CSF, but not IL-17. (E) Flow cytometry analysis demonstrated that DF significantly reduced GM-CSF production in IFN-γ–producing CD4+ (Th1) cells. DF = dimethyl fumarate; GM-CSF = granulocyte macrophage colony-stimulating factor; IFN = interferon; IL = interleukin; PMA = phorbol 12-myristate 13-acetate; SEM = standard error of the mean. ***p < 0.001.
Following the above-mentioned experiment, we studied how DF directly affects purified CD4+ T cells from mice. CD4+ T cells were isolated and cultured with anti-CD3/CD28 Abs with or without DF for 72 hours. We measured IFN-γ, GM-CSF, and IL-17 in the supernatant after the incubation period. We found that DF significantly decreased GM-CSF and IFN-γ, but not IL-17, in purified CD4+ T cells compared with controls (figure 1D). In addition, FACS analysis of the same cells demonstrated that DF decreased GM-CSF in IFN-γ+CD4+ T (Th1) cells (figure 1E).
DF treatment significantly decreased GM-CSF+ Th1 cells in CNS-infiltrating cells
To analyze the immunomodulatory effect of DF treatment in EAE, we first immunized all mice and then treated half of them with oral DF in an emulsion of 0.8% methylcellulose (DF group) and the other half with a similar volume of 0.8% methylcellulose vehicle (control group). Mice in the control group developed EAE, with an average severity of around 2.5 in scoring (score range from 1.25 to 4 with a median score of 2.625). By contrast, DF-treated mice showed very mild clinical signs with an average clinical score of ≤1 (score range from 0 to 1.5 with a median score of 0.75) (figure 2A).
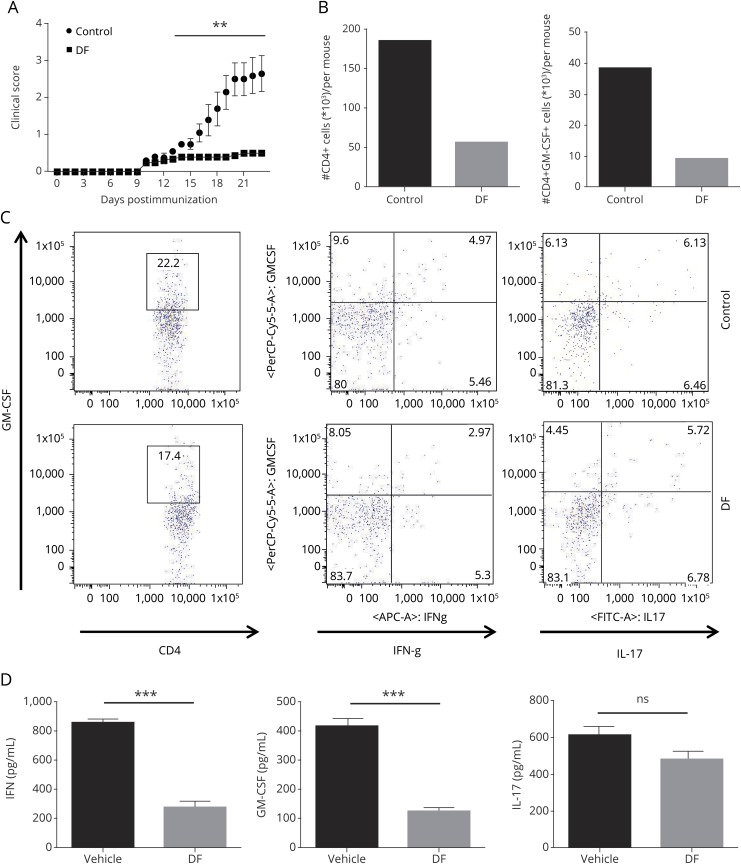
Figure 2. DF ameliorates experimental autoimmune encephalomyelitis and suppresses GM-CSF–producing Th1 cells in the CNS.
(A) Wild-type B6 mice were immunized with 200 μg of MOG35–55 and were treated with DF (treatment group) or placebo (control group) by oral administration starting on the day of immunization. Clinical signs were scored daily following a 0–5 scale. Data represent 1 of 3 experiments and the mean clinical scores ± SEM (n = 5 each group). (B) CNS-infiltrating cells from DF-treated and control mice were isolated on day 23 of disease. DF treatment significantly decreased total CD4+ T cells and GM-CSF–producing CD4+ T cells among CNS-infiltrating cells. (C) Cells were stained with GM-CSF, IFN-γ, and IL-17A antibodies and analyzed by flow cytometry. We found that GM-CSF was decreased in Th1 cells, but not Th17 cells, after DF treatment. (D) Splenocytes from both treated and control groups were collected and cultured/stimulated with 25 μg/mL MOG35–55 for 72 hours. Concentrations of GM-CSF, IFN-γ, and IL-17A in culture supernatants were measured by ELISA. There was significantly less GM-CSF and IFN-γ in treated mice compared with controls. **p < 0.01; and ***p < 0.001. One of 2 experiments is shown. DF = dimethyl fumarate; GM-CSF = granulocyte macrophage colony-stimulating factor; IL = interleukin; MOG = myelin oligodendrocyte glycoprotein; SEM = standard error of the mean.
We killed the mice at disease peak (day 23) and assessed the number of infiltrating MNCs in the CNS of both DF-treated and control groups. In addition to counting the total number of infiltrating cells, we evaluated the immunophenotype of those cells by flow cytometry. The total number of CD4+ T cells and GM-CSF+CD4+ T cells was dramatically decreased in the CNS of the DF-treated group (figure 2B). We also looked at IFN-γ and IL-17 in CNS-infiltrating CD4+ T cells and, interestingly, found that DF decreases GM-CSF+CD4+ cells that coproduce IFN-γ, but not IL-17 (figure 2C), given that DF has a greater suppressive effect in vivo on GM-CSF–producing Th1 cells than that on Th17 cells.
Splenocytes from treated and control mice were isolated, cultured, and stimulated with MOG33–35 for 72 hours. GM-CSF, IFN-γ, and IL-17 were measured in culture supernatants by ELISA. As shown in figure 2D, splenocytes from DF-treated mice produced a significantly decreased amount of GM-CSF and IFN-γ, but not IL-17.
DF requires an intact IFN-γ pathway to suppress GM-CSF in CD4+ T cells
Based on the observation that DF suppresses GM-CSF predominantly in Th1 cells, and to further evaluate the underlying mechanism of the suppressive effect of DF on GM-CSF, we investigated whether IFN-γ plays a role in this phenomenon.
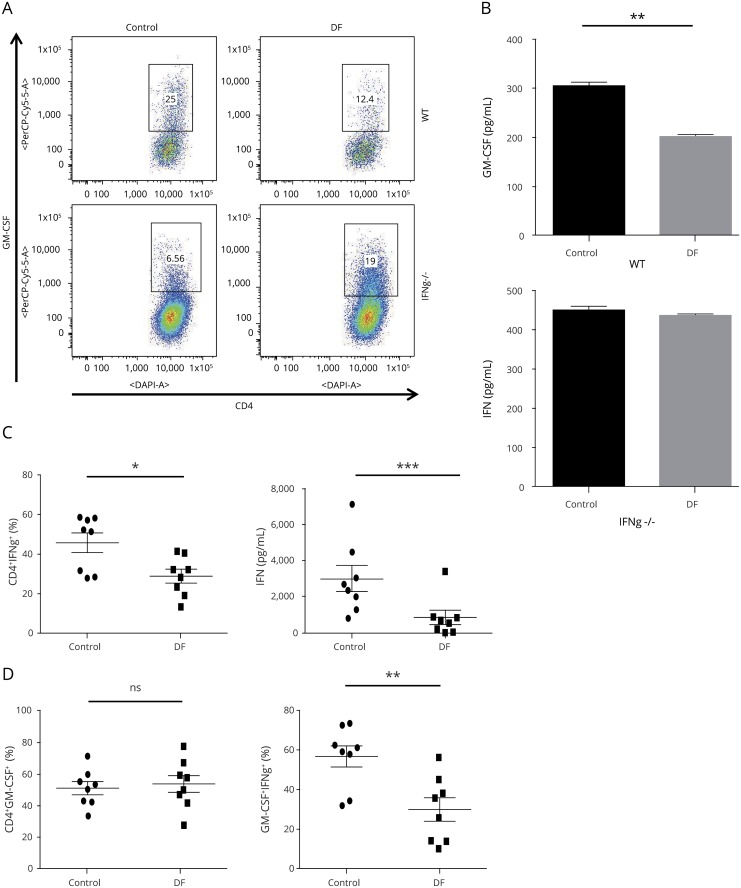
WT and IFN-γ–deficient splenocytes were cultured and stimulated with anti-CD3/28 in the presence or absence of DF. Although DF suppressed GM-CSF–producing CD4+ T cells and GM-CSF cytokines in the supernatant of WT splenocytes, the lack of IFN-γ abrogated the effect of DF on GM-CSF in CD4+ T cells. This finding implies that IFN-γ plays a crucial role in the suppressive effect of DF on GM-CSF (figure 3, A and B).
Figure 3. The suppressive effect of DF on GM-CSF depends on IFN-γ in murine cells.
(A) Splenocytes were isolated from wild-type (WT) and IFN-γ−/− mouse and stimulated with 1 μg/mL of anti-CD3/28 antibodies in the presence or absence of DF (1 μg/mL) for 72 hours. DF treatment significantly decreased GM-CSF in CD4+ T cells from WT but not CD4+ T cells from IFN-γ−/− mice. (B) Measurement of GM-CSF in cell supernatants by ELISA confirmed the same finding. Data are mean ± SEM and representative of 1 of 3 experiments. DF reduced GM-CSF production and GM-CSF+Th1 cells in human PBMCs. PBMCs from healthy donors (n = 8) were stimulated with 1 μg/mL of anti-CD3/anti-CD28 antibody in the presence or absence of DF (0.1 μg/mL) for 120 hours. (C) Cells were stimulated with PMA/ionomycin and GolgiPlug in the last 4 hours of culture. Flow cytometry analysis showed that DF treatment decreased IFN-γ+CD4+ (Th1) cells. Measurement of IFN-γ in cell supernatants confirmed that DF reduced IFN-γ in human PBMC culture. (D) DF treatment did not affect the total percentage of GMCSF+CD4+ T cells but significantly decreased GM-CSF–producing Th1 cells. A paired t test was used for the statistical analysis of human PBMC results before and after treatment. DF = dimethyl fumarate; GM-CSF = granulocyte macrophage colony-stimulating factor; PMA = phorbol 12-myristate 13-acetate; SEM = standard error of the mean. *p < 0.05; **p < 0.01, and ***p < 0.001.
DF decreases Th1 and GM-CSF–producing Th1 cells in human PBMCs
To determine the best dosage of DF in human in vitro experiments, we performed a proliferation assay and treated cells with 10, 1, and 0.1 μg/mL of DF as described previously. Based on our result, 0.1 μg/mL DF had the least toxic effects on human PBMCs (data not shown). To evaluate the effect of DF on human PBMCs, we cultured and stimulated PBMCs from healthy donors with human anti-CD3/28 with or without DF (0.1 μg/mL) for 120 hours. Cells were analyzed with flow cytometry after 5 days, and multiple cytokines were measured by ELISA in cell supernatants. For statistical analysis, we used the paired t test to compare pretreatment and posttreatment results.
DF treatment significantly reduced IFN-γ+CD4+ (Th1) T cells, and ELISA analysis of PBMC culture supernatants demonstrated that DF significantly decreased IFN-γ production (figure 3C). In terms of GM-CSF–producing CD4+ T cells, DF significantly decreased GM-CSF+ Th1 cells with no effect on total GM-CSF–producing CD4+ T cells, implying that the suppressive effect of DF on GM-CSF is mostly on Th1 cells (figure 3D).
Discussion
DF was approved by the Food and Drug Administration for the treatment of MS in 2013, and its efficacy has been well demonstrated. During 2 years of treatment, DF reduced clinical relapse and lesion frequency while improving health-related quality of life in adults with RRMS.19,20 However, the underlying therapeutic mechanism of DF is still under investigation. DF has neuroprotective effects,4,21,22 including reducing spinal cord inflammation and protecting myelin and neurons.4,23 Also, researchers who have studied the Nrf2 transcriptional pathway and oxidative stress that DF modulates4,21,24 found that both DF and its active metabolite, monomethyl fumarate, induce the reactive oxygen species scavenger glutathione in oligodendrocytes, astrocytes, and hippocampal cells.22,25,26 Other pathways have also been reported, including the hydroxycarboxylic acid receptor 2 pathway and nuclear factor kappa-light-chain-enhancer of activated B cells.23,27,28 DF has been shown to promote Th2 cytokine profiles,3,22,29 and transferring DF-induced IL‐17AlowIFN‐γlowIL‐4+CD4+ T cells has demonstrated its therapeutic effect in EAE.30
In patients with RRMS after 6 months of DF treatment, the proportion of Th2 cells was increased among memory T cells but with a decrease in the proportion of Th1 and with no change in Th17 cell proportion among memory T cells.31 Another study, however, showed a decrease in CD4+ T cells producing IFN-γ, IL-17, and GM-CSF, with no significant change in IL-10 and IL-4 after 12 months.32 We also found that direct administration of DF decreased proinflammatory cytokine production in the supernatant of human PBMCs obtained from healthy subjects. When we took a closer look at CD4+ T-cell subsets, DF suppressed only GM-CSF+Th1 cells, but not GM-CSF+Th17 or GM-CSFonly CD4+ T cells. One limitation of our study is that the human data are based on very small number of samples from healthy donors. Further studies are necessary to confirm the effect of DF on GM-CSF–producing cells from patients with MS.
Although IL-17 and IFN-γ are implicated in MS, mice still develop EAE even in the absence of these cytokines.33,34 Previous studies have shown that GM-CSF secreted by CNS-infiltrating T helper cells is essential for EAE, even more so than IL-17 or IFN-γ. Lack of GM-CSF or GM-CSF receptor abrogates EAE development, and adoptively transferred GM-CSF–deficient Th1 or Th17 cells do not induce EAE.15 We showed that DF caused a decrease in GM-CSF+ Th1 cells in both the periphery and CNS-infiltrating cells during EAE, and given the fact that GM-CSF–expressing cells are the crucial determinant of EAE susceptibility and progression, one of the underlying therapeutic mechanisms of DF appears to be through suppression of GM-CSF+ Th1 cells.
In a variety of T helper cell populations, different transcription factors, including bhlhe40, RORγt, T-bet, GATA-3, and STAT5,35–38 are active at multiple time points. It has been reported that IL-7–activated STAT5 promotes GM-CSF generation of CD4+ T cells, which differ from RORγt-, T-bet–, and GATA-3–promoted GM-CSF–producing CD4+ T cells (Sheng et al., 2014). Assay for transposase-accessible chromatin using sequencing analysis of GM-CSF–expressing cells showed an open IFN-γ locus in both GM-CSF– and ex-GM-CSF–expressing cells, and single-cell RNA-sequencing showed that pathogenic Th17 cells gain Th1-like characteristics once they enter the mouse-inflamed CNS.34,39 On the epigenetic basis, GM-CSF–producing T cells are also more related to IFN-γ+ T cells. Here, we show that DF affects only GM-CSF+IFN-γ+CD4+ T cells, and its GM-CSF suppressive effect relies on an intact IFN-γ pathway. Determining whether DF affects one or several transcription factors in this pathogenic T-cell subset could provide insights into its precise therapeutic mechanisms in MS. Future research on DF should focus on the molecular pathway of GM-CSF and IFN-γ generation after DF treatment.
In conclusion, we evaluated the role of DF on CD4+ T cells and its effects on GM-CSF production. For mouse splenocyte experiments, GM-CSF–producing Th1 T cells were reduced, but other GM-CSF–producing T cells were not. For EAE model experiments, DF decreased the percentage of GM-CSF+ Th1 cells in the CNS. In human ex vivo experiments, GM-CSF+ Th1 cells were reduced in healthy donor PBMCs after DF treatment; however, a larger study in patients before and after DF treatment is needed to validate current findings in MS. We also demonstrated that the suppressive effect of GM-CSF is through the IFN-γ pathway. The molecular basis of the effects of DF on GM-CSF–producing T cells is unknown and merits further study.
Acknowledgment
The authors thank Katherine Regan for substantive editing of the manuscript.
Glossary
- DF
dimethyl fumarate
- EAE
experimental autoimmune encephalomyelitis
- GM-CSF
granulocyte macrophage colony-stimulating factor
- MNC
mononuclear cell
- RRMS
relapsing-remitting MS
- WT
wild type
Appendix. Authors
Study funding
This work was supported by grants R01AI106026 and R01NS0088729 from the NIH.
Disclosure
F. Safavi, R. Thome, Z. Li, G.-X. Zhang, and A. Rostami report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Herrero-Herranz E, Pardo LA, Gold R, Linker RA. Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol Dis 2008;30:162–173. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Gold R, Miller DH, et al. Effect of BG-12 on contrast-enhanced lesions in patients with relapsing–remitting multiple sclerosis: subgroup analyses from the phase 2b study. Mult Scler 2012;18:314–321. [DOI] [PubMed] [Google Scholar]
- 3.Ghoreschi K, Bruck J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 2011;208:2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678–692. [DOI] [PubMed] [Google Scholar]
- 5.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci USA 2016;113:4777–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 7.Constantinescu CS, Gran B. The essential role of T cells in multiple sclerosis: a reappraisal. Biomed J 2014;37:34–40. [DOI] [PubMed] [Google Scholar]
- 8.Lalor SJ, Segal BM. Th1-mediated experimental autoimmune encephalomyelitis is CXCR3 independent. Eur J Immunol 2013;43:2866–2874. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald DC, Ciric B, Touil T, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 2007;179:3268–3275. [DOI] [PubMed] [Google Scholar]
- 10.Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, McFarland HF. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity 1993;15:137–143. [DOI] [PubMed] [Google Scholar]
- 11.Edwards LJ, Robins RA, Constantinescu CS. Th17/Th1 phenotype in demyelinating disease. Cytokine 2010;50:19–23. [DOI] [PubMed] [Google Scholar]
- 12.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med 2000;192:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haak S, Croxford AL, Kreymborg K, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest 2009;119:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur J Immunol 2010;40:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 2011;12:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McQualter JL, Darwiche R, Ewing C, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 2001;194:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Li Z, Ciric B, Safavi F, Zhang GX, Rostami A. Selective depletion of CD11c+ CD11b+ dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE. Eur J Immunol 2016;46:2454–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 2015;21:796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 20.Havrdova E, Hutchinson M, Kurukulasuriya NC, et al. Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of: Gold R, Kappos L, Arnold D, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107; and Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97. Expert Opin Pharmacother 2013;14:2145–2156. [DOI] [PubMed] [Google Scholar]
- 21.Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012;341:274–284. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht P, Bouchachia I, Goebels N, et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflamm 2012;9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. J Clin Invest 2014;124:2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Chuikov S, Taitano S, et al. Dimethyl fumarate protects neural stem/progenitor cells and neurons from oxidative damage through Nrf2-ERK1/2 MAPK pathway. Int J Mol Sci 2015;16:13885–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H, Taraboletti A, Shriver LP. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol 2015;5:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SX, Lisi L, Dello Russo C, et al. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN neuro 2011;3:e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loewe R, Holnthoner W, Groger M, et al. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol 2002;168:4781–4787. [DOI] [PubMed] [Google Scholar]
- 28.Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 2015;130:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng H, Guerau-de-Arellano M, Mehta VB, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor kappaB (NF-kappaB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem 2012;287:28017–28026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruck J, Glocova I, Geisel J, Kellerer C, Rocken M, Ghoreschi K. Dimethyl fumarate-induced IL-17(low) IFN-gamma(low) IL-4(+) Th cells protect mice from severe encephalomyelitis. Eur J Immunol 2018;48:1588–1591. [DOI] [PubMed] [Google Scholar]
- 31.Gross CC, Schulte-Mecklenbeck A, Klinsing S, Posevitz-Fejfar A, Wiendl H, Klotz L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e183 doi: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montes Diaz G, Fraussen J, Van Wijmeersch B, Hupperts R, Somers V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep 2018;8:8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 1996;156:5–7. [PubMed] [Google Scholar]
- 34.Komuczki J, Tuzlak S, Friebel E, et al. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1beta. Immunity 2019;50:1289–1304.e6. [DOI] [PubMed] [Google Scholar]
- 35.Lin CC, Bradstreet TR, Schwarzkopf EA, et al. Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun 2014;5:3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovett-Racke AE, Rocchini AE, Choy J, et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity 2004;21:719–731. [DOI] [PubMed] [Google Scholar]
- 37.Sheng W, Yang F, Zhou Y, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res 2014;24:1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura Y, Christodoulopoulos P, Cameron L, et al. Upregulation of the transcription factor GATA-3 in upper airway mucosa after in vivo and in vitro allergen challenge. J Allergy Clin Immunol 2000;105:1146–1152. [DOI] [PubMed] [Google Scholar]
- 39.Gaublomme JT, Yosef N, Lee Y, et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 2015;163:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw FACS and ELISA files are not included in this article. Any unpublished and anonymized data will be shared upon request from a qualified investigator.