Abstract
Case series and registry data suggest that diabetic retinopathy requiring treatment is rare in youth with T1D prior to 18 years of age. We evaluated this question in the standardized clinical trial setting by retrospectively reviewing diabetic retinopathy examinations from participants in the Diabetes Control and Complications Trial (DCCT) who were 13 to <18 years of age at randomization. Standardized stereoscopic 7-field fundus photographs were obtained every 6 months during DCCT (1983–93). Photographs were graded centrally using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. Transitions in diabetic retinopathy status over time were described. 195 participants with median baseline HbA1c of 9.3% (103 in the conventional and 92 in the intensive treatment groups) had an average of 5.3 diabetic retinopathy assessments during 2.3 years of follow-up (range 1–11) while under 18 years of age during the DCCT. No participant developed severe non-proliferative diabetic retinopathy or proliferative diabetic retinopathy and only 1 participant (in the intensive group) reached clinically significant macular edema (CSME) while less than 18 years of age. In this incident case, baseline characteristics included diabetes duration 9.3 years, HbA1c 10.3%, LDL 131 mg/dl, and mild NPDR (35/35 ETDRS scale); CSME resolved without treatment. Similar analyses using age cut-offs of < 19, 20, or 21 years demonstrated a slight rise in diabetic retinopathy requiring treatment over late adolescence. Clinical trial evidence suggests that frequent eye exams may not be universally necessary in youth <18 years of age with T1D.
Keywords: Diabetic retinopathy, screening, adolescents, type 1 diabetes
INTRODUCTION
The guidelines of the International Society of Pediatric and Adolescent Diabetes (ISPAD) recommend diabetic retinopathy screening to start at age 11 years with a type 1 diabetes (T1D) duration of 2–5 years.1 Similarly, the American Diabetes Association (ADA) recommends retinopathy screening for youth with diabetes using an initial comprehensive, dilated eye exam at age ≥ 10 years or after puberty has started, whichever is earlier, once the youth has had T1D for 3–5 years.2 Recommendations on the time interval between the initial screening eye exam and subsequent rescreening examinations are evolving for both children and adults, taking into account many factors, especially prior diabetic retinopathy stage as well as history of glycemic control and diabetes duration. In adults, subsequent annual eye exams had been standard practice, yet the 2017 ADA position statement suggests that in adult patients, less frequent eye exams can be considered when prior examinations have demonstrated no evidence of retinopathy and the patient has a history of good glycemic control.3 The consideration of previous eye exam grading and glycemia are supported by data from cohort studies of adult patients, including a recent recommendation based on over three decades of monitoring participants enrolled in the Diabetes Control and Complications Trial and its follow-up study, the Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC).4
Less evidence is available to guide the follow-up eye exam schedule for the pediatric population. Several case report series in the pediatric population describe no or mild non-proliferative diabetic retinopathy with only rare advanced disease, including proliferative diabetic retinopathy or clinically significant macular edema, requiring intervention prior to 18 years of age. However, these reports vary in the methodologies used, including the number of eye examinations per subject, duration of monitoring, duration of diabetes, and examination technique (e.g., ophthalmologist’s direct ophthalmoscopy, centrally graded digital fundus photos, and/or self-report of treatment).5–9 In the absence of large clinical trial outcomes, the recent ADA guidelines includes an expert recommendation to consider less frequent exams, perhaps every two years, for pediatric patients, with the advice of an eye professional and based on risk factor assessement.2 Similarly, the ISPAD guidelines recommend rescreening every two years for those with diabetes duration less than 10 years, mild non-proliferative diabetic retinopathy and good glycemic control.1
To further inform the development of screening guidelines for youth with type 1 diabetes (T1D), we analyzed the development of diabetic retinopathy in the DCCT/EDIC cohort, focusing on participants who were adolescents (aged 13 to <18 years) on enrollment into the DCCT. In the context of this multicenter clinical investigation with frequent scheduled eye exams, centrally graded fundus photographs, and well-documented history of glycemic control, we determined the frequency of development of proliferative diabetic retinopathy and clinically-significant macular edema prior to 18 years of age. We reviewed transitions among the various retinopathy states (no diabetic retinopathy (no DR); mild, moderate, and severe non-proliferative diabetic retinopathy (NPDR); proliferative diabetic retinopathy (PDR); and clinically significant macular edema (CSME)) over time based on baseline age at entry into the DCCT.
METHODS
Subjects
This manuscript analyzes data from DCCT subjects who were adolescent (i.e., <18 years of age) at the time of DCCT randomization. Secondary analyses then used the following age cut-offs: <19 years, <20 years, and <21 years. The eye exams were performed during the DCCT years and through EDIC year 4 (i.e., up to 1998). The DCCT and EDIC protocols were approved by the institutional review boards of all participating centers and all participants provided written informed consent and assent, as appropriate. The detailed protocol and methods of the DCCT and EDIC studies have been previously described.10,11 Briefly, during 1983 – 1989, the DCCT randomly assigned a total of 1441 participants to receive intensive (n=711) or conventional (n=730) treatment, with the former aimed at achieving glycemic control as close to the non-diabetic range as safely possible. Two cohorts were included in DCCT: the primary prevention cohort (n=726 participants with diabetes duration of 1 to 5 years, albumin excretion rate (AER) <40 mg/24hr, and no diabetic retinopathy) and the secondary intervention cohort (n=715 participants with diabetes duration of 1 to 15 years, AER ≤200 mg/24hr, and at least one microaneurysm in either eye, but no more than moderate non-proliferative diabetic retinopathy). Of the 1441 DCCT participants, 195 were adolescents (13 to < 18 years of age) on entry, 92 of whom were assigned to intensive therapy and 103 to conventional therapy, with 125 in the primary cohort and 70 in the secondary cohort.
At the end of the DCCT, after an average follow-up of 6.5 years, all participants were taught intensive therapy and were referred back to their healthcare providers for subsequent diabetes care. Starting in 1994, EDIC enrolled 97% of the surviving DCCT cohort, and 94% of the cohort survivors still participate actively after more than 20 years of additional follow-up.
Retinopathy Assessments
Standardized stereoscopic seven-field fundus photographs were obtained every 6 months during the DCCT and every fourth year (i.e., 1/4 of the cohort examined each year) during EDIC. In addition, in EDIC year 4, fundus photographs were completed on the entire cohort. The photographs were graded centrally using the final Early Treatment of Diabetic Retinopathy Study (ETDRS) grading scale12, and graders were masked to treatment assignment, age, duration of diabetes, glycemic control, and other risk factors. Annual quality control measures included masked duplicate re-grades of photographs with consistent achievement of target threshold levels of 70% agreement within 1-step on the grading scale and 90% agreement within 2-steps on the grading scale.
Outcomes
Based on the ETDRS score, several retinopathy states were defined: no diabetic retinopathy (no DR, 10/10 ETDRS), mild non-proliferative diabetic retinopathy (mild NPDR, 20/<20 to 35/35), moderate NPDR (43/<43 to 47/47), severe NPDR (53/<53 and 53/53), and PDR (61/<61 or greater on the ETDRS scale or pan-retinal photocoagulation). In the 1990s during the time of the study data collection, the presence and severity of macular edema was assessed from fundus photographs using the ETDRS definition: retinal thickening and/or obvious hard exudates within 1 disc diameter (1500 JLm) of the center of the macula. Clinically significant macular edema was defined as retinal thickening (or hard exudates adjacent to retinal thickening) located within 500 JLm of the center of the macula, or an area of retinal thickening at least 1 disc area in size with some within 1 disc diameter of the center of the macula.13 The analytical definition of CSME included CSME on fundus photography or focal photocoagulation. All outcomes are reported as the subject level retinopathy severity (i.e., severity of the eye with more advanced disease determines retinopathy state).
Statistical Analysis
Discrete variables were described using count (percent), while continuous variables used median (first and third quartiles). The incidence of retinopathy outcomes (PDR and CSME) was described separately for each age cut-off (i.e., <18, <19 years, <20 years, and <21 years) using all retinopathy assessments in the participants below that age cut-off (i.e., all diabetic retinopathy screening examinations until ages 18, 19, 20 or 21 years, respectively). Rates and 95% upper confidence limits (UCL) were computed under Poisson models.14
The size of the adolescent cohort and the extremely low rates of PDR and CSME did not allow analyses of risk factors for progression of diabetic retinopathy.
RESULTS
Baseline characteristics of the 195 adolescents are reported in Table 1. All 195 adolescents in the DCCT were between 13 and 17 years of age on enrollment. Of the 195 adolescents, 51% were females. The median age was 15 years, with a median duration of diabetes of 3.9 years (range 1–15 years) and 168 adolescents (86%) had diabetes for ≥ 2 years. The median HbA1c was 9.3%, the median systolic blood pressure (BP) was 110 mmHg, the median diastolic BP was 70 mmHg, and the median AER was 13 mg/24 hour. Only 3% of the adolescents reported smoking. Forty-seven percent of the adolescents were from the original DCCT intensive treatment group.
Table 1.
Baseline characteristics* of the 195 adolescent DCCT/EDIC participants (age 13 to < 18 years) at DCCT enrollment.
| Age<18y (n=195) | |
|---|---|
| Group (intensive treatment) | 92 (47.2%) |
| Cohort (primary) | 125 (64%) |
| Age (years) | 15 (14,16) |
| HbA1c (%) | 9.3 (8.2,10.9) |
| Sex (females) | 100 (51.3%) |
| BMI (kg/m2) | 21.7 (20,23.7) |
| Smokers (yes) | 6 (3%) |
| Duration of diabetes (years) | 3.9 (2.5,6.5) |
| Diastolic blood pressure (mmHg) | 70 (64,78) |
| Systolic blood pressure (mmHg) | 110 (102,120) |
| Total cholesterol (mg/dL) | 161 (143,182) |
| HDL (mg/dL) | 45 (41,52) |
| Triglycerides (mg/dL) | 71 (56.5,96) |
| AER (mg/24 h) | 13 (7.2,22.3) |
| Estimated GFR (mL/min per 1.73 m2) | 144.8 (137.6,152.2) |
Data are presented as median (first quartile, third quartile) for continuous variables and n (%) for discrete variables.
There were 1031 retinopathy assessments during the DCCT among the 195 participants while they were still under 18 years of age, for an average of 5.3 retinopathy assessments over an average of 2.34 years of follow-up per participant. Table 2 presents the 836 observed transitions from the 1031 retinopathy assessments. There were 116 (14%) transitions to more severe diabetic retinopathy (105 from no DR to mild NPDR, 1 from no DR to moderate NPDR, 9 from mild to moderate NPDR, and 1 from mild NPDR to CSME) and 92 (11%) transitions were to less severe diabetic retinopathy. Indeed, participants remained in the same diabetic retinopathy state from one visit to the next 75% of the time. While still less than 18 years of age, none of the 195 participants reached PDR (rate of 0 per year per 1,000 individuals at risk with 95% upper confidence limit UCL=6.6), and only one participant reached CSME (rate of 2.2 per year per 1,000 individuals at risk with 95% UCL=10.4).
Table 2.
Observed number of transitions of the retinopathy status from one visit to the next visit among the 195 adolescent participants while still less than 18 years of age*.
| From→To | No DR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | CSME |
|---|---|---|---|---|---|---|
| 1 No DR | 393 | 105 | 1 | 0 | 0 | 0 |
| 2 Mild NPDR | 83 | 230 | 9 | 0 | 0 | 1 |
| 3 Moderate NPDR | 0 | 9 | 3 | 0 | 0 | 0 |
| 4 Severe NPDR | 0 | 0 | 0 | 0 | 0 | 0 |
There were 1031 retinopathy assessment visits in the 195 participants from 13 to <18 years of age.
The participant who progressed to CSME was a 16.5 year old female in the secondary intervention cohort who was assigned to receive intensive treatment. At baseline, she already had mild diabetic retinopathy (20/<20 to 35/35 score on the ETDRS scale), a duration of diabetes of 9.3 years, a BMI of 24 kg/m2, an HbA1c of 10.3%, systolic blood pressure (BP) of 110 mmHg, diastolic BP of 74 mmHg, LDL of 131 mg/dl, HDL of 44 mg/dl, total cholesterol of 209 mg/dl, triglycerides of 168 mg/dl, AER=60 mg/24 hour, and eGFR=156 mL/min per 1.73 m2. She developed CSME by 6 months after her baseline visit (Figure 1), at which time her HbA1c had dropped to 8.46%, BMI was unchanged from baseline, and SBP and DBP increased to 120 and 78 mmHg, respectively. She did not smoke and she was not pregnant at any point during the evaluation period. By 12 months after her baseline visit, the CSME improved without intervention (Figure 1). By the end of the DCCT, her HbA1c was 6%; her HbA1c has remained between 6–7% without ocular interventions and with normal GFR throughout EDIC.
Figure 1.
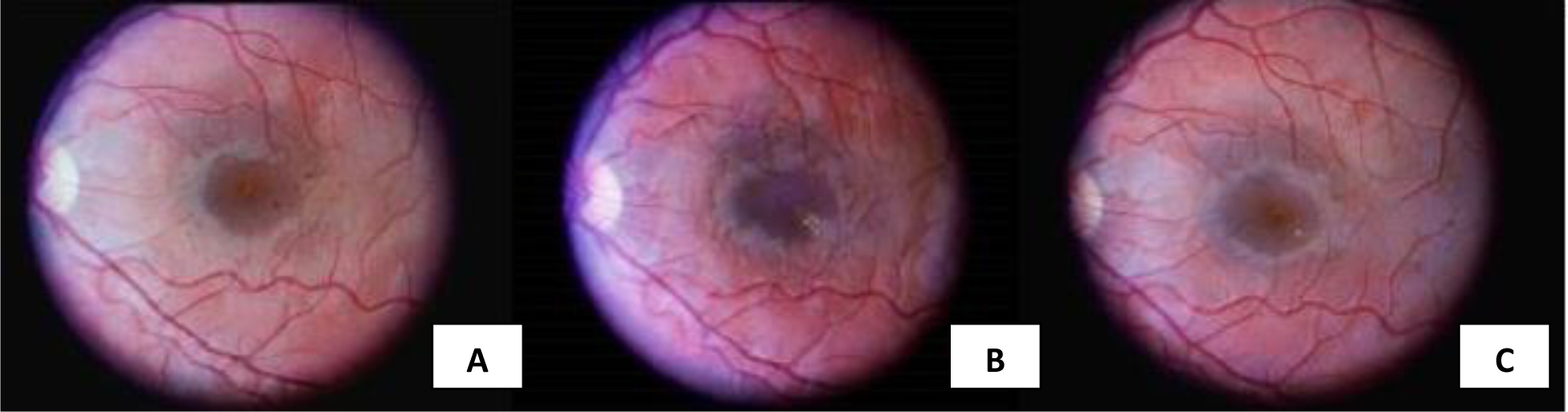
Seven-field fundus photography of incident case at (A) baseline (i.e., time of randomization,1983), b) 6 months after randomization to intensive treatment, and c) after 12 months of trial duration. Image (B) demonstrates an increase in macula hard exudate as compared to baseline with exudate from near the macular center progressing infero-temporally, supporting the diagnosis of CSME at that time. Image (C) shows substantial resolution of the exudates after 12 months of the study and without ophthalmologic intervention.
We then extended our investigations to the period of late adolescence to young adulthood using age cut-offs of < 19, <20, and < 21 years. The baseline characteristics of participants by age cut-off are presented in Supplementary Table 1 and transitions among retinopathy states are presented in Table 3. The number of cases of more advanced diabetic retinopathy (i.e., PDR and CSME) increased from age under 18 to under 21 years, with a more pronounced rise as participants approached 21 years. In each case of PDR or CSME prior to age 21, baseline HbA1c was >9% with diabetes duration >4 years and mild or moderate NPDR was present at DCCT entry.
Table 3.
| Table 3A. Transitions of the retinopathy status among the 222 participants <19 years of age. | |||||
|---|---|---|---|---|---|
| From→To | None | Mild NPDR | Moderate NPDR | Severe NPDR | PDR/CSME |
| 1 No DR | 504 | 161 | 1 | 0 | 0 |
| 2 Mild NPDR | 113 | 377 | 13 | 0 | 2 |
| 3 Moderate NPDR | 0 | 15 | 5 | 0 | 0 |
| 4 Severe NPDR | 0 | 0 | 0 | 0 | 0 |
| Table 3B. Transitions of the retinopathy status among the 244 participants <20 years of age. | |||||
| From→To | None | Mild NPDR | Moderate NPDR | Severe NPDR | PDR/CSME |
| 1 No DR | 604 | 214 | 2 | 0 | 0 |
| 2 Mild NPDR | 143 | 548 | 29 | 0 | 3 |
| 3 Moderate NPDR | 0 | 22 | 12 | 1 | 0 |
| 4 Severe NPDR | 0 | 0 | 0 | 0 | 0 |
| Table 3C. Transitions of the retinopathy status among 290 participants <21 years of age. | |||||
| From→To | None | Mild NPDR | Moderate NPDR | Severe NPDR | PDR/CSME |
| 1 No DR | 697 | 245 | 2 | 0 | 0 |
| 2 Mild NPDR | 170 | 751 | 40 | 0 | 4 |
| 3 Moderate NPDR | 0 | 32 | 23 | 2 | 5 |
| 4 Severe NPDR | 0 | 0 | 0 | 0 | 1 |
DISCUSSION
In our setting of a longitudinal clinical trial with expert grading of scheduled fundus images and detailed clinical characterization of diabetes management and co-morbidities, we demonstrate that the development of treatable retinal lesions prior to 18 years of age is rare in adolescents with type 1 diabetes with characteristics similar to our cohort. Among the 195 DCCT participants who were <18 years of age at DCCT enrollment, while they were still under 18 years of age, only one case of CSME and no cases of PDR were observed over an average of 2.3 years of follow-up and an average of 5.3 retinopathy assessments. The single CSME case was detected at the six-month visit in a female participant who had an HbA1c of 10.3%, microalbuminuria, and mild NPDR at baseline. As previously documented in the study as a whole, this case may represent transient worsening of early retinopathy that was observed following institution of intensive therapy and rapid lowering of HbA1c in the secondary cohort.15 The development of CSME, unlike NPDR and PDR, can also occur without changes in HbA1c and may improve without intervention.16 Regardless of the mechanism, this case of CSME was followed by improvement or regression in diabetic retinopathy over time without retinal therapeutic intervention.
We did observe a rise in rates of PDR/CSME events among the DCCT/EDIC participants under 19 and 20 years of age (no PDR cases in either group, 2 CSME cases among those <19, and 3 CSME cases among those <20 years of age), and a further increase was observed in those <21 years of age (3 PDR and 7 CSME cases). (See supplementary table 2 for rates of diabetic retinopathy by age cut-off.) No ocular procedures were performed while these participants were under 20 years of age; there was one scatter laser surgery performed 0.5 years after reaching PDR for participants under 21 years of age. These findings are consistent with our prior work4 in the entire DCCT/EDIC cohort over more than 25 years of follow-up, which showed that annual or more frequent retinopathy screening for PDR/CSME is only necessary for high-risk individuals with advanced diabetic retinopathy (such as severe NPDR) and/or high glycemic exposure.
Thus, as a reference for rescreenings, we suggest consideration of an initial screening eye exam for youth as per current guidelines followed by rescreenings starting either 1) at age 18 years if no DR or mild DR is found on initial screening, or 2) earlier than age 18 years and with an increased frequency between rescreens if moderate NPDR or more severe retinopathy stage is found on the initial screen.3,17 While this report represents too few cases to determine risk factors and definitive laboratory cut-offs, historic mean HbA1c levels (for example, whether patients meet ADA targets, or perhaps are consistently less than 8%) might be additionally considered to help identify low risk youth who do not require frequent ophthalmic rescreens. (See supplementary materials in which additional cases of progression to treatable lesions include individuals prior to 21 years of age with HbA1c 8.5% or higher.) More investigations in adolescents are needed to identify additional factors by which to stratify high risk youth, and may include micro/macroalbuminuria, blood pressure, diabetes duration, lipid levels (especially triglyceride levels), and potentially other factors that have yet to be determined, such as lesions in the peripheral retina (i.e., peripherally predominant lesions detected on ultrawide field fundus photography) which, in adults, forewarn retinopathy progression.18,19 Such investigations should include current modalities such as optical coherence tomography, which has advanced detection of CSME20, 21 and was not available during the DCCT.
Reducing the number of retinal screening exams would reduce the burden of type 1 diabetes for patients and families of youth and decrease healthcare expenditures. According to the Juvenile Diabetes Research Foundation (JRDF), there are currently approximately 200,000 people under the age of 20 with type 1 diabetes in the United States.22 The 2009 SEARCH study estimated that roughly 58,000 and 75,000 adolescents between the ages of 10–14 and 15–19, respectively, had type 1 diabetes in the United States in 2009.23 Using the same estimated annual 1.2% and 1.8% increase in incidence reported over 2003–2012 for 10–14 year olds and 15–19 year olds, respectively24, and assuming similar prevalence across years within each age group, we estimate that approximately 78,000 adolescents ages 13 to <18 are living with type 1 diabetes in the US in 2018. Assuming that approximately 20% meet current treatment goals25, our recommendation of testing adolescents ages 13 to <18 with controlled glycemia and no other complications only once in this 5-year time period would result in approximately 80% savings among this subset of adolescents. Since digital fundus photography is commonly included at retinopathy screening visits and costs approximately $2004 per evaluation, this recommendation would result in approximately $2.5 million in annual savings compared to annual testing, for a total savings of approximately $50 million over 20 years.24 or, if compared to biennial testing, approximately $25 million dollars over 20 years.
It should be noted that there are limitations to our study. Given the recruitment criteria for the DCCT, our adolescent cohort does not include the full age-range of pediatric patients with diabetes; very few were diagnosed under 6 years of age and thus had limited disease duration during adolescence and participants enrolled were 96.5% Caucasian lacking racial and ethnic diversity. Hence, our data tells us little about the prepubertal years. However, international registries have suggested that the prepubertal years contribute less than the pubertal/post-pubertal years to the long-term risk of microvascular complications.26–28 While mean HbA1c levels for our adolescent cohort pre-DCCT are not known, they were potentially higher than mean HbA1c levels in most youth today with a similar duration of diabetes. This would suggest that currently it would be even less likely to find retinal lesions requiring treatment prior to age 18. Additionally, intrinsic characteristics and risk factors from the single individual who reached CSME before 18 years of age may not be representative of other adolescents. Overall, our findings pertain to adolescents with similar characteristics to those evaluated in this study; additional studies with a broader representation of the pediatric population and varying HbA1c levels should be conducted.
For now, our data are reassuring that retinal lesions requiring treatment rarely develop prior to age 18 years in youth with type 1 diabetes, and, for the majority of youth with an initial screen of no DR or mild NPDR, a single screening with fundus photography may be enough before 18 years of age.
Supplementary Material
ACKNOWLEDGEMENTS.
David M. Nathan is acknowledged as the editor for DCCT/EDIC publications.
Funding: The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
Footnotes
A complete list of the participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2017;376:1507-16.
Duality of Interest: Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater NJ).
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
REFERENCES
- 1.Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatric Diabetes 2018; 19 (Suppl 27): 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 12. Children and adolescents. ADA Standards of Medical Care in Diabetes. Diabetes Care 2018; 41(Suppl 1): S126–136 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, Chew E, Duh EJ, et al. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017;40:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Bebu I, Hainsworth D, et al. Frequency of Evidence-Based Screening for Retinopathy in Type 1 Diabetes. The New England Journal of Medicine 2017;376:1507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geloneck MM, Forbes BJ, Shaffer J, Ying G, Binenbaum G. Ocular Complications in Children with Diabetes Mellitus. Ophthalmology 2015;122:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon N, Karthikeyan A, Castle A, et al. Natural history of retinopathy in children and young people with type 1 diabetes. Eye (London, England) 2016;30:987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchamp G, Boyle CT, Tamborlane WV, et al. Treatable Diabetic Retinopathy Is Extremely Rare Among Pediatric T1D Exchange Clinic Registry Participants. Diabetes Care 2016;39:e218–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo B, Steffen AT, Swan K, Sikes K, Weinzimer SA, Tamborlane WV. Clinical outcomes and cost-effectiveness of retinopathy screening in youth with type 1 diabetes. Diabetes Care 2007;30:362–3. [DOI] [PubMed] [Google Scholar]
- 9.Lueder GT, Pradhan S, White NH. Risk of Retinopathy in Children With Type 1 Diabetes Mellitus Before 2 Years of Age. American Journal of Ophthalmology 2005;140:930–1. [DOI] [PubMed] [Google Scholar]
- 10.The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT). Diabetes 1986;35:530–545. [PubMed] [Google Scholar]
- 11.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC): Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98 Suppl 5:786–806. [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985; 103: 1796–806 [PubMed] [Google Scholar]
- 14.Fay MP. Confidence intervals that match Fisher’s exact or Blaker’s exact tests. Biostatistics 2010;11:373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The DCCT Research Group. The Effect of Intensive Diabetes Treatment on the Progression of Diabetic Retinopathy in Insulin-Dependent Diabetes Mellitus. Arch Ophthalmol 1995;113:36–51. [DOI] [PubMed] [Google Scholar]
- 16.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision (London, England) 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keenum Z, McGwin G, Witherspoon CD, Haller JA, Clark ME, Owsley C. Patients’ Adherence to Recommended Follow-up Eye Care After Diabetic Retinopathy Screening in a Publicly Funded County Clinic and Factors Associated With Follow-up Eye Care Use. JAMA Ophthalmology 2016;134:1221–8. [DOI] [PubMed] [Google Scholar]
- 18.Silva PS, Cavallerano JD, Haddad NMN, et al. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015;122:949–56. [DOI] [PubMed] [Google Scholar]
- 19.Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015;122:2465–72. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proceedings of the National Academy of Sciences 2015;112(18):E2395–E2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina 2015;35(11):2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Type 1 Diabetes Facts 2018. Juvenile Diabetes Research Foundation. (Accessed 18 July, 2018, at http://www.jdrf.org/about/what-is-t1d/facts/).
- 23.Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of Diabetes in U.S. Youth in 2009: The SEARCH for Diabetes in Youth Study. Diabetes Care 2014;37:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. The New England Journal of Medicine 2017;376:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood JR, Miller KM, Maahs DM, et al. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care 2013;36:2035–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porta M, Schellino F, Montanaro M, et al. Prevalence of retinopathy in patients with type 1 diabetes diagnosed before and after puberty. Acta Diabetol 2014;51:1049–54. [DOI] [PubMed] [Google Scholar]
- 27.Falck AA, Käär ML, Laatikainen LT. Prevalence and risk factors of retinopathy in children with diabetes. A population-based study on Finnish children. Acta Ophthalmology 1993;71:801–9. [DOI] [PubMed] [Google Scholar]
- 28.Olsen BS, Sjølie AK, Hougaard P, et al. The significance of the prepubertal diabetes duration for the development of retinopathy and nephropathy in patients with type 1 diabetes. Journal of Diabetes and its Complications 2004;18:160–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


