Abstract
The current communication seeks to provide an updated narrative review on latest methods of reducing implant contaminations used during spine surgery. Recent literature review has shown that both preoperative reprocessing and intraoperative handling of implants seem to contaminate implants. In brief, during preoperative phase, the implants undergo repeated bulk cleaning with dirty instruments from the OR, leading to residue buildup at the interfaces and possibly on the surfaces too. This, due to its concealed nature, remains unnoticed by the SPD (sterile processing department) or other hospital staff. Nevertheless, these can be avoided by using individually prepackaged presterilized implants. In the intraoperative phase, the implants (in the sterile field) are directly touched by the scrub tech with soiled (assisting the surgeon dispose the tissues from the instruments in use) gloves for loading onto an insertion device. It is then kept exposed on the working table (either separately or next to the used instruments as the pedicles hole are being prepared). Latest investigation has shown that by the time it is implanted in the patient, it can harbor up to 10e7 bacterial colony-forming units. The same implants were devoid of such colony-forming units, when sheathed by an impermeable sterile sheath around the sterile implant.
Keywords: Surgical site infection, contamination, bioburden, orthopedic implants, asepsis, pedicle screws, biofilm, reprocessing, intraoperative handling, implant prophylaxis, occult infection
Prevention of surgical site infection (SSI) in spine surgery is a major thrust area among the clinicians, researchers, and other healthcare professionals. SSIs add enormous burden to individuals and society in terms of medications, reoperations, extended stays at the hospital, lost productivity and wages, and emotional and physical trauma afflicted upon patients and their families. Recent randomized controlled trial by McClelland et al. demonstrated that SSIs occur at the higher end of 2%-13% and are egregiously underestimated largely based on retrospective data not subjected to the inclusivity of SSI as defined by the Centers for Disease Control and Prevention (CDC)1). In addition to its clinical presentation, there have been several studies performed on occult forms of SSI2-5). According to literature data, these occult SSIs were present in at least 10%-30% of patients with chronic pain and were detected only during revision spine surgery2-5). In some studies, researchers collected periprosthetic tissue from the area around each screw or implant for gram staining, histopathological analysis, and long cultures to identify any attribute that would indicate chronic infection3). Propionibacterium acnes, a low virulent bacterium which is quite common in late onset SSI, was present in at least 50%-70%, while the remaining were staphylococcus2-5). Furthermore, many of these patients with chronic pain and occult infection had hardware loosening and pseudarthrosis2-5).
In 2017, Anderson et al. presented a thorough review on various preventative techniques being employed or advocated in the field of spine surgery, with varying level of evidences for each6). Their narrative review recommended systematic approach from proper patient selection and optimization of medical conditions, particularly reducing smoking and glycemic control, to screening for staphylococcus organisms, and subsequent decolonization is a promising method to reduce endogenous bacterial burden6). They categorized preoperative measures to further include warming of patients, skin preparation using chlorhexidine and alcohol solutions, and timely administration of antibiotics6). Following this was meticulous surgical technique and maintenance of “standard” sterile techniques. Postoperative methods included tissue oxygenation, glycemic control, and proper wound closure. However, it should be noted that the only foreign body which remains in the patient, i.e., the implants, was deemed “sterile” as received after reprocessing and was handled freely inside “sterile field.” A subsequent review, published in early 2018, tried addressing this gap by focusing on implant contamination, both preoperatively and intraoperatively7). They concluded that the evidences for failures with preoperative reprocessing in hospitals and the associated risks are well published, with few countries already issuing a ban on reprocessing of implants used for orthopedic surgery7). In Scotland, for example, the deadline for conversion of all orthopedic units to prepackaged and presterilized implants was on December 31, 2007. They further emphasized that the failure mode here is not only the poor compliance by SPD but the impracticality of repeated cleaning and sterilization of hundreds of small implants with multiple components, each with interface clearances of less than a fraction of millimeter7-9). The second source of contamination they identified was intraoperative, i.e., the physical handling, and other exposure (air, surfaces, accessory, instruments, etc.) of implants inside the “sterile field” constituted another challenge7). All the studies identified in the review demonstrated that surgical gloves which handle the implants, either during loading implants on to insertion device or while doing other maneuvers, have a high rate of contamination, potentially from the patient's own skin flora7). The contamination of implants then facilitates the transfer of contaminants deeper into the tissue, well below the accessible surgical site open to irrigation. Although very few studies recorded SSI rates as their endpoint, the ones that did also showed reduction in the SSI rates with better implant handling7).
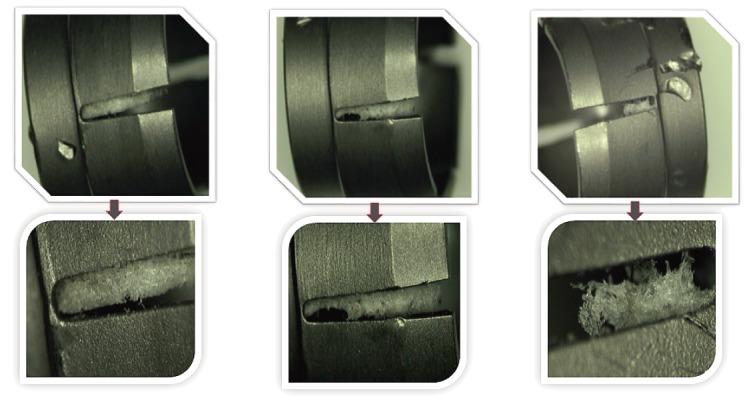
Lately, newer evidences have been published demonstrating high contamination rates of implants10-22). A study looking at preoperative contaminants identified three types of contaminants: corrosion, saccharide of unknown origin (biofilm, endotoxins, fatty tissue), and soap residue mixed with fat, each occupying isolated diametrical areas of 1.4 mm, 1.5 mm, and 3.4 mm, respectively (Fig. 1, 2, 3) (used with permission)10). In addition, salt residues were also found at interfaces between the tulip head and shaft of pedicle screws10). The corrosion stains were present on the outer surfaces of the implants, whereas an active corrosion with material erosion was seen at the inner rim of the pedicle screw head (tulip) and in some parts of the washer10). The saccharides and soap were present in the interfaces with low permeability (interior region of the multipiece assembled device)10). This result led to surveillance of the hospital processes to identify modes of failure and its comparison against the manufacture's guidelines. The failure mode identified with this process was the impracticality of cleaning and sterilizing small implants with intricate features. In the study, addressing the intraoperative contaminants, a multicenter trial comparing the standard and a standalone method of preventing microbial contamination of implants, during spinal fusion, was compared11-22). The crucial information unmasked here was the presence of bacterial contaminants on implants when handled using currently accepted “sterile” techniques (Fig. 4, 5)11-22). The study demonstrated that using a functional (something that allows the scrub tech to attach insertion device without exposure or touching), impermeable, sterile sheath around the sterile implant, which guards the implants intraoperatively until it is implanted into the patient, significantly decreases bacterial count and growth (Fig. 4, 5)11-22). The data they presented were binary between the groups despite varied hospitals, operating theaters, surgeons, and hospital staffs11-22). This strongly supports the effectiveness of using a guard to protect implants intraoperatively. Furthermore, it doesn't change the surgical flow nor does it require additional compliance of the surgical staff member. The source of intraoperative contamination may be from the flora of the patients and personnel(s) or from the environment itself. However, the author emphasized that their study demonstrates that implants without guard act as vehicle for transmittance of some of these contaminants (unknown sources) deep inside of surgical sites4).
Figure 1.
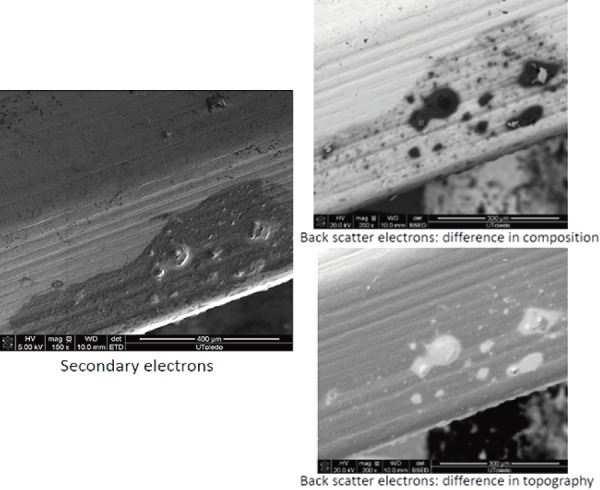
Corrosion on the tulip interface10) (used with permission).
Figure 2.
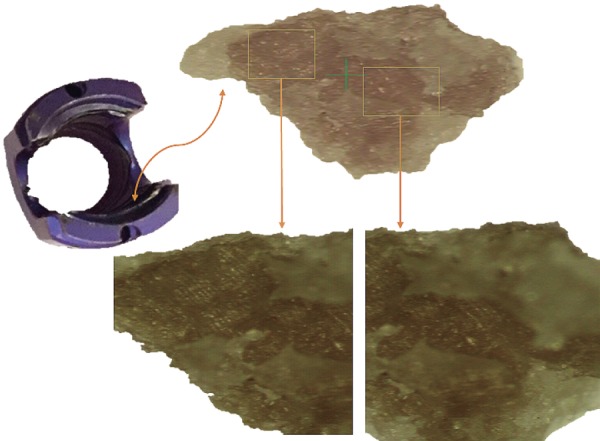
Saccharide of unknown origin10) (used with permission).
Figure 3.
Soap residue mixed with fat10) (used with permission).
Figure 4.
[A] Group 2: Intraoperative picture of scrub tech touching pedicle screws while loading. [B] Group 2: Intraoperative picture of exposed pedicle screws to open airflow and surfaces, therefore making them prone to intentional or unintentional contact/contamination. [C] Group 1: Intraoperative picture of guarded pedicle screws.
Figure 5.
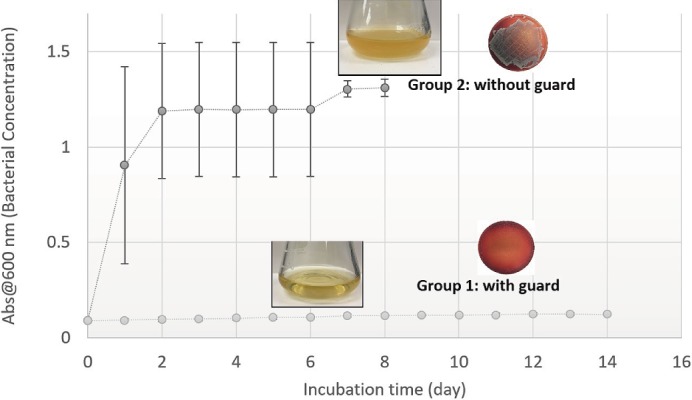
Quantitative spectroscopy and pictorial depiction (flask turbidity) showing saturated levels of growth within 24-48 hours in group 2 versus no growth for 14 days in group 1. Next to each are the representative culture plate samples from group 1 and 2, after 7 days and 1 day, respectively.
SSI is multifactorial; however, the key constituents that define the pathogenesis of SSI are the virulence, host-site immunity, and dosage15). The virulence is the microorganism's ability to infect the host. Although many bacterial species have been identified to cause SSI, the most common ones, Staphylococcus epidermidis and Staphylococcus aureus, are always present at the vicinity as part of a patient's own flora. In addition, they have the potential to form biofilms, secluding itself from macrophages or other immune responses at the host site. The host sites in spine surgery are the pedicles of the vertebrae. This in combination with availability of metal surface provides a conducive environment for the bacteria to grow. Lastly, the dose dictates how much bacterial bioburden the “sterile” implant carries, after handling and at implantation. All the latest research reviewed here focuses on the reducing the constituent of dose and thus the overall pathogenesis of SSI.
Conflicts of Interest: JW reports royalties from Biomet, SeaSpine, Amedica, and Synthes; investment/options from Bone Biologics, PearlDiver, electroCore, and Surgitech; nonfinancial support from North American Spine Society, Cervical Spine Research Society, Spine, The Spine Journal, Clinical Spine Surgery, Global Spine Journal, Society for Brain Mapping and Therapeutics, American Orthopaedic Association, board of directors from AO Foundation, and Society for Brain Mapping and Therapeutics; and grants from AOSpine North America, outside the submitted work. AA reports royalties from Paradigm Spine and Joimax, consultancy from Spinal Balance, and editorial board membership from Clinical Spine Surgery and Spine, outside the submitted work. SG reports consultancy from DePuy (Johnson & Johnson), Magnifi Group, SI-Bone, Spinal Kinetics, Spinal Balance, institutional support from DePuy (Johnson & Johnson), Globus, royalties from DePuy (Johnson & Johnson), and stock from Spinal Kinetics, Spinal Balance, and SI-Bone outside the submitted work. AKA and VKG report royalties from Paradigm Spine, Joimax and investment/options from OsteoNovus and Spinal Balance, outside the submitted work. CK reports consultancy from Medtronic, SeaSpine, Intellirod, Spinal Balance, and Camber, outside the submitted work. NA reports consultancy from Medtronic, Globus, GYS Tech, and Spinal Balance; speakership from DePuy Synthes and Stryker; stocks from Bonovo, Medtronic, Globus, GYS Tech, Spinal Balance, AF Cell, Theracell, Atlas Spine, and Paradigm Spine; royalties from Elsevier, Medtronic, and Globus; SAB from GYS Tech, Globus, and Spinal Balance; and editorial board membership from Gray's Anatomy, outside the submitted work. The rest of the authors (BL, CS, HE, and VS) have nothing to disclose.
Sources of Funding: Partial funding for the support of this study was received from a National Science Foundation collaboration group (Center for Disruptive Musculoskeletal Innovation, I/UCRC Collaborative Award #s: 1361975/L & 1361977).
Author Contributions: Each coauthor satisfied the four criteria as defined by ICMJE.
References
- 1.McClelland S, Takemoto RC, Lonner BS, et al. Analysis of postoperative thoracolumbar spine infections in a prospective randomized controlled trial using the Centers for Disease Control surgical site infection criteria. Int J Spine Surg. 2016;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu X, Lieberman IH. Revision spine surgery in patients without clinical signs of infection: How often are there occult infections in removed hardware? Eur Spine J. 2018;27(10):2491-5. [DOI] [PubMed] [Google Scholar]
- 3.Callanan TC, Lebl DR, Cammisa FP, et al. Occult infection in patients who have undergone spinal surgery with instrumentation. Spine J. 2016;16(10):S132-3. [Google Scholar]
- 4.Leitner L, Malaj I, Sadoghi P, et al. Pedicle screw loosening is correlated to chronic subclinical deep implant infection: a retrospective database analysis. Eur Spine J. 2018;27(10):2529-35. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman IH, Hu X. Revision spine surgery in patients without clinical signs of infection: How often are there occult infections in removed hardware? Spine J. 2017;17(10):S187. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PA, Savage JW, Vaccaro AR, et al. Prevention of surgical site infection in spine surgery. Neurosurgery. 2017;80(3S):S114-23. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Schultz C, Goel VK, et al. Implant prophylaxis: the next best practice toward asepsis in spine surgery. Global Spine J. 2018;8(7):761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell M. Hospital uses team approach to improve processes, reduce costs. AORN J. 1998;68(1):68-72. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, MacMillan A, Goel V, et al. A paradigm shift toward terminally sterilized devices. Clin Spine Surg. 2018;31(7):308-11. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Schultz C, Agarwal AK, et al. Harboring contaminants in repeatedly reprocessed pedicle screws. Global Spine J. 2018:2192568218784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Lin B, Wang JC, et al. Efficacy of intraoperative implant prophylaxis in reducing intraoperative microbial contamination. Global Spine J. 2019;9(1):62-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal A, Lin B, Wang JC, et al. Reducing bacterial dose during instrumented spine surgery: A clinical study on a novel and effortless method, NASS, Los Angeles. Sept. 26-29, 2018. [Google Scholar]
- 13.Agarwal A, Lin B, Garfin S, et al. Avoiding unnecessary contamination of implant-bone interface: A multi-center study, SMISS, Las Vegas. Sept. 6-8, 2018. [Google Scholar]
- 14.Agarwal A, Lin B, Anand N, et al. Can we reduce the infection rates associated with high implant density in deformity surgeries? SRS, Montréal, Canada, Sept 18-21, 2019. [Google Scholar]
- 15.Agarwal A, Lin B, Karas C, et al. Avoiding infection and screw loosening due to biofilm at bone-screw interface: Periop measures, ISSLS, Kyoto, Japan, June 3-7, 2019. [Google Scholar]
- 16.Agarwal A, Lin B, Wang JC, et al. Multi-center trial revealing the presence of bacterial contamination on pedicle screws during spinal fusion and prevention method, JSSR, Yokohama, Japan, April 18-20, 2019. [Google Scholar]
- 17.Agarwal A, Lin B, Elgafy H, et al. Evidence of bacterial biofilm initiation at the screw-bone interface and the method of avoiding such, GSC, Toronto, Canada, May 15-18, 2019. [Google Scholar]
- 18.Agarwal A, Lin B, Wang JC, et al. Reducing risks of deep bone infection during instrumented spinal fusion. ISASS, Anaheim California, April 3-5, 2019. [Google Scholar]
- 19.Agarwal A, Lin B, Anand N, et al. Initiatives to reduce infection rates and long term screw loosening due to biofilm formation must start at the time of surgery. Safety in Spine Surgery Summit, New York City NY, April 26, 2019. [Google Scholar]
- 20.Agarwal A, Lin B, Elgafy H, et al. Intraoperative guard to avoid biofilm and infection at pedicle screw & bone interface. ICORS, Montreal, Canada, June 19-22, 2019. [Google Scholar]
- 21.Agarwal A, Lin B, Schultz C, et al. Stopping bacterial transmission to patients through pedicle screws at the bone-screw interface. A clinical study with high size of effect and binary data between the groups. ORS, Austin, TX, Feb 2-5, 2019. [Google Scholar]
- 22.Agarwal A, Lin B, Garfin S, et al. “Sterile Implant” really “Sterile” after intraoperative handling? Safety in Spine Surgery Summit, New York City NY, April 20, 2018. [Google Scholar]


