Abstract
Objective
To review and highlight the historical and recent advances of imaging in spine surgery and to discuss current applications and future directions.
Methods
A PubMed review of the current literature was performed on all relevant articles that examined historical and recent imaging techniques used in spine surgery. Studies were examined for their thoroughness in description of various modalities and applications in current and future management.
Results
We reviewed 97 articles that discussed past, present, and future applications for imaging in spine surgery. Although most historical approaches relied heavily upon basic radiography, more recent advances have begun to expand upon advanced modalities, including the integration of more sophisticated equipment and artificial intelligence.
Conclusions
Since the days of conventional radiography, various modalities have emerged and become integral components of the spinal surgeon's diagnostic armamentarium. As such, it behooves the practitioner to remain informed on the current trends and potential developments in spinal imaging, as rapid adoption and interpretation of new techniques may make significant differences in patient management and outcomes. Future directions will likely become increasingly sophisticated as the implementation of machine learning, and artificial intelligence has become more commonplace in clinical practice.
Keywords: imaging, magnetic resonance, computed tomography, radiograph, spine surgery, guided navigation, machine learning, artificial intelligence
Introduction
Before 1895, the knowledge about imaging of the spine was very limited. However, the mid-1980s hosted a few pre-existing imaging techniques, starting with plain films and tomography1). However, these techniques were insufficient in the complete diagnosis of spinal pathologies, thus encouraging the need for supplemental and more precise imaging. This was due to the complex anatomical configuration of the cervical, thoracic, lumbar, and sacral vertebrae along with variability in their individual structure through irregular contours and shapes. Inevitably, this posed a special challenge when imaging the spine2). To address these issues, various new modalities including computed tomography (CT), magnetic resonance imaging (MRI), angiography, and myelography as well as multiple dimensional views of the spine were introduced to obtain an improved image quality. However, these modalities do have certain limitations when it comes to multiplanar visualization of spinal anatomy.
Using the correct imaging modality during the diagnostic workup can play a crucial role in the evaluation of the spine and may subsequently aid in preoperative planning. Many previous studies have documented the use of different modalities but do not discuss developing trends and innovations that may relate to future applications. The following narrative review highlights the current advancement of various spine imaging modalities and their future use and limitations to optimize their usage in intraoperative spine surgeries. Therefore, highlighting their potential to lead to a rapid diagnostic workup and a safer and more prompt treatment plan3).
History of Imaging in the Spine
On November 8, 1895, Wilhelm Conrad Roentgen demonstrated the existence and application of X-rays, ultimately ushering in an era where imaging of osseous anatomy became a medical reality. Though initially used as a form of novel photography, it was not long before society quickly realized the medical utility of Roentgen's discovery. By the early 1900s, X-rays became an invaluable diagnostic tool and was rapidly adopted for use in imaging of the spine4).
Though initial images were undoubtedly limited by technology at the time, within decades of Roentgen's discovery, investigators managed to refine his X-ray technique by incorporating synchronous motion into image acquisition. At the time, by rotating the X-ray and film about a patient, the radiologist was able to obtain multiple images of an area of interest and minimize issues involved with superimposition of structures on conventional films. This practice became known as tomography and served as an invaluable tool in the evaluation of vertebral fractures, allowing evaluation for complications such as presence of bony debris in the spinal canal2).
As fascinations with radiography grew, in the 1920s, practitioners soon realized that manipulation of surrounding tissue densities would allow for differentiation between adjacent structures. In one case, Sicard and Forestier3) inadvertently introduced an iodine-based solution into the epidural space when attempting to target a patient's lumbar muscles―a technique that at the time, was considered effective management for sciatica. The duo was known to occasionally take radiographs after injections to evaluate for other pathologies and, as such, fortuitously discovered that iodine enhanced the anatomy of the spinal cord and subarachnoid space. Not long thereafter, various improvements were made to ensure the safety of these iodine-based contrast agents, and practitioners began to regularly inject these solutions into the dural sac, intervertebral discs, and various arteries, leading to what is now known as myelography, discography, and angiography, respectively2).
Because of limitations imposed by computing power, it would not be until 1973, when modern CT would emerge. Reportedly, Dr. Godfrey Hounsfield conceived of CT in a thought experiment, where he reasoned it would be possible to identify the contents of a box by taking X-rays at every possible angle around it5). Extrapolating further, he believed this concept may have some degree of medical utility and could identify the contents of a human skull. Within the year, Hounsfield had successfully produced the first CT images of a patient's brain6). Hounsfield's original CT scanner was quickly adapted for full-body use, and with it came modern CT images of the spine.
Also in 1973, Paul Lauterbur7), a chemist who heavily worked with nuclear magnetic resonance (NMR) spectroscopy, conceived that the application of a magnetic field on a large object could produce images based on its chemical structure. This theory, in part, laid the foundation for modern MRI. MRI evolved during the early 1970s, producing images with low spatial resolution that advanced the discrimination of soft tissue proving superiority to CT, allowing earlier diagnoses. In his initial experiment, Lauterbur imposed a magnetic field on a clam at two 45° angles, captured the NMR signal, and collated the images to produce a rough two-dimensional depiction of the shellfish. Furthermore, unlike CT, MRI had the advantage of not requiring ionizing radiation to produce high-resolution images. This accomplishment would ultimately be recognized with the Nobel Prize in Physiology and Medicine8). Throughout the late 1980-1990s, as technology improved, this modality was adapted for use in humans and led to high-resolution descriptions of spinal anatomy and pathology9-11).
Current Applications
Despite this, in the modern era, imaging techniques continue to evolve and supplant its predecessors in evaluation of the spine. Because of the improved resolution of modern CT-scanners, techniques such as myelography have largely fallen out of favor. Also, discography has fallen out of favor and no longer recommended because of increased rates of pain postoperatively induced during a discogram12).
Similarly, with the introduction of various MRI pulse sequences and an increase in electromagnetic fields to provide greater accuracy, resolution of present-day T1- and T2-weighted images has dramatically improved, making possible diagnoses of spinal pathology that was invisible at the turn of the century. The following sections will serve as a discussion highlighting the current indications, strengths, and limitations of various modern imaging techniques in the setting of spine surgery.
Plain radiographs
Plain radiographs remain one of the most commonly used imaging techniques in the evaluation of pain, numbness, weakness, or any other symptoms localizing to the spine. It is the first diagnostic test of choice in the setting of trauma, malignancy, infection, deformity, and degenerative spine disease and offers many benefits due to ease in acquisition and relatively low cost. Limitations of radiographs are evident when attempting to identify soft tissue, three-dimensional characteristics, and high image resolutions. For instance, bone loss is typically not appreciable on conventional radiographs until over 30% to 40% loss has occurred13). However, when used in conjunction with other indicated advanced imaging techniques, there are very few reasons why the diagnostician should completely avoid plain radiographs in the workup of spinal pathology.
A particular strength of plain radiographs includes the ability to implement specialized views to evaluate stability and flexibility. Used in conjunction with standard anteroposterior and lateral views of the spine, flexion-extension, sitting, and bending views help the spine surgeon appreciate changes in the anatomy that occur with movement. These views have become critical in the management of spinal pathology as instability invariably warrants some type of fusion, whereas flexibility frequently guides correction of deformity14-18).
Radiographs also frequently serve as the canvas for measuring various parameters of the spine and pelvis, including assessment of coronal and sagittal balance, measurement of Cobb angles, pelvic incidence (PI), pelvic tilt (PT), lordosis, and kyphosis (Fig. 1). The utility of these measures has been well-validated in a number of studies since their conception and have critical roles in the surgical management of deformity and spinopelvic relationships. In the past decade, particular attention has been paid to the relationship between PI and lumbar lordosis (LL) in spinal surgery. Namely, studies suggest that PI and LL exist in some degree of equilibrium between 0° and 10°19,20). When PI-LL difference falls outside these values, either from spinal pathology or as a result of surgery, studies report that patients have worse clinical and radiographic outcomes19,20).
Figure 1.
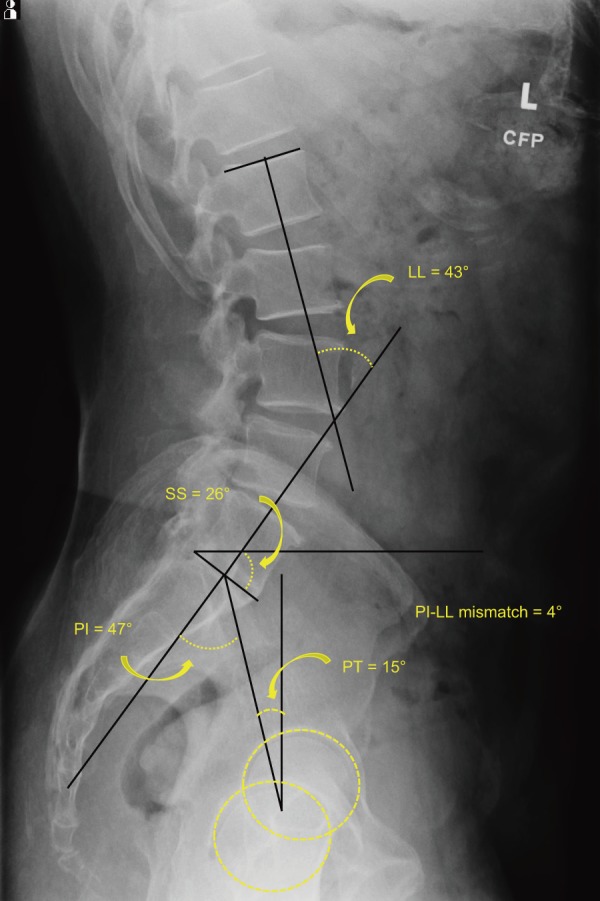
Spinopelvic Radiographic Parameters.
LL=lumbar lordosis;
SS=sacral slope;
PI=pelvic incidence;
PT=pelvic tilt
Recently, new studies have found that radiographic evaluation of the spine may also prove useful in the management of hip arthroplasty. In a series of experiments by Buckland et al.21-23), lumbar fusion and the associated radiographic changes in PT led to an increased rate of dislocation in primary total hip arthroplasty patients. They go on to show that risk of dislocation was inversely related to the pelvis's capacity for retroversion when going from standing to seated positions and could be quantified on respective lateral radiographic views of the lumbar spine and sacrum. Moreover, these studies have implications that the spine may have a larger role in influencing other parts of the appendicular skeleton, and radiographs may continue to serve as a crux for evaluating this relationship.
Regarding intraoperative use of X-rays, fluoroscopy has become a pivotal instrument in spine surgery due to the introduction of the portable “C-arm.” At the time of writing, nearly all spine procedures currently rely on fluoroscopic images to assist with placement of hardware and intraoperative assessment of radiographic parameters. This has become especially important in the setting of instrumented fusion and interbody placement as malpositioning of these devices has been associated with complications such as dural tears, nerve injury, and reoperation24-26). Despite the advantages fluoroscopy has introduced, there is a continued need to improve upon this technology. Some studies currently report that use of fluoroscopy has only led to accurate pedicle screw placement in 28%-85% of cases, a distribution that roughly matches the accuracy of traditional freehand techniques27-29).
CT
CT has exceptional utility in evaluation of the osseous structures of the spine, largely due to its ability to reconstruct three-dimensional and multiplanar images (Fig. 2). As a result, CT is often ordered in the setting of trauma to closely examine fracture characteristics or in cases where a high-resolution depiction of the patient's anatomy is required. However, outside of these scenarios, CT tends to be used sparingly because of the risks of higher radiation exposure and is less frequently used in the outpatient setting than conventional radiographs and MRI.
Figure 2.
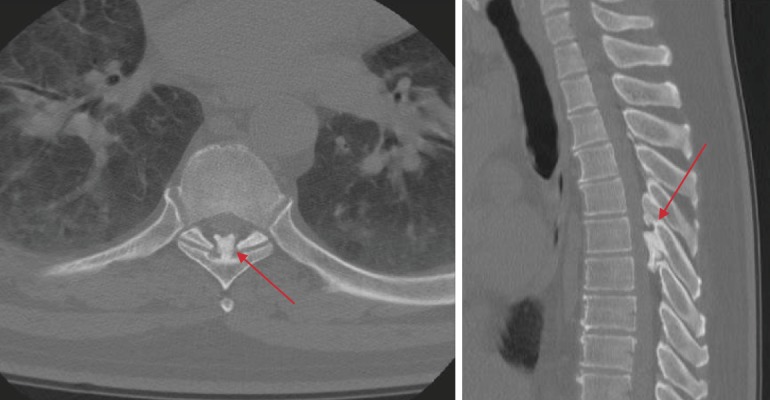
Spinal Pathology on Axial and Sagittal Computed Tomography (CT).
Demonstrates ossification of the ligamentum flavum at T5-T6 with intrusion into the spinal canal (red arrows).
Nonetheless, CT has demonstrated significant utility in the setting of preoperative and intraoperative evaluation of the spine. For instance, in the setting of instrumented fusion, a CT may be ordered to assess pedicle anatomy at the projected operative levels, providing the surgeon with the information required to plan placement of screws. Though these images are often used in conjunction with intraoperative fluoroscopy, some centers routinely elect to use more sophisticated imaging devices (i.e., the “O-arm”). The “O-arm” is a portable adaptation of CT, where axial plane images can be acquired through a 360° projection and capture of X-rays. Though this technology may provide greater stereotactic information, current literature is mixed regarding improvements in hardware placement and raises additional concerns related to operative time and radiation exposure30-32). In a retrospective study, Laudato et al.30) noted that the use of the “O-arm” offered no significant improvements in pedicle screw placement when compared with freehand techniques. Conversely, when comparing the “O-arm” to the “C-arm,” Verma et al. noted that screw misplacement rates were 0.9% and 8.8%, respectively32). Given these findings, the general consensus is that no particular intraoperative imaging technique is superior to another and that the use of a particular modality should be surgeon and institution-dependent.
CT myelography
Comparatively, CT myelography has experienced only marginal advances since originally described by Sicard and Forestier. Though modern contrast agents are now water-soluble and less hazardous than the iodized oils used in the 1920s, advances in MRI and risks associated with radiation exposure have led to fewer CT myelography procedures being performed in the modern era. CT myelography, however, does continue to have some utility as it can improve diagnosis of foraminal/lateral recess stenosis and disk lesions. Bartynski and Lin33) demonstrated that CT myelography is superior to MRI in these circumstances as MRI tended to miss lateral recess nerve root compression in 30% of cases. Similarly, MRI tends to underestimate the width of the spinal canal and foramina, exaggerating the severity of stenosis when compared with CT myelography34). Also, CT myelography remains the diagnostic test of choice in patients unable to receive an MRI (e.g., pacemaker and claustrophobia).
Discography
Similarly, discography has largely fallen out of favor in the modern era because of controversial evidence and risks associated with the procedure. In 1968, Holt35) performed discographies on 30 volunteers and reported a 37% false-positive rate. Though a number of studies have subsequently pointed out flaws in Holt's original analysis and study design, advances in other imaging modalities have essentially made discography obsolete. Further, Carragee and Alamin12) highlighted additional shortcomings of this procedure by demonstrating increased rates of iatrogenically induced disc degeneration with this technique. Because of this, there are strict indications for the use of discography; patients must have persistent nonradicular pain suspicious of a discogenic etiology without causative imaging findings and for preoperative evaluation before spinal fusion36).
Angiography
Angiography is perhaps the only contrast-based procedure that is still routinely used in imaging of the spine. Combined with CT or MRI, angiography allows clear visualization of specific structures due to variations in vascular flow. This is particularly useful for the characterization of many spinal tumors and/or in the setting where information about vertebral artery anatomy is required preoperatively (e.g., upper cervical spine procedures and concern for vertebral artery injury). Doing so allows the surgeon to plan his/her surgical approach and limit the risk of vascular injury. With recent growth in popularity of anterior lumbar approaches to the spine, some surgeons may also elect for angiography of the great vessels due to common problematic variants that may impede placement of interbodies37-39). In one study, Datta et al.37) noted that the use of CT angiography in anterior lumbar surgery affected surgical decision making in 21% of cases and 11.8% of patients had anomalies in prevertebral vascular anatomy.
MRI
Presently, because of its ability to acquire high-resolution multiplanar images of vertebral and soft tissue anatomy without risks associated with radiation exposure, MRI is the diagnostic procedure of choice for most spinal pathologies. As such, MRI is routinely ordered in the clinic setting and offers relatively high sensitivity and specificity for infections, tumors, disc degeneration, pathologic fractures, and herniations. In certain circumstances, however, MRI is limited, as it is relatively expensive and has varying degrees of utility in obese, claustrophobic, and pacemaker-dependent patients.
The imaging quality of the traditional MRI was initially limited because of limitations imposed by weaker magnetic fields and its associated dependencies on an electrical current40). As technology evolved, introduction of superconducting magnets has led to a progressive growth in field strength from 0.15 T to as high as 9.4 T in some settings40). Such advances ultimately resulted in modern high-resolution images and has since allowed the characterization and identification of various degenerative spinal phenotypes, such as disc degeneration, disc displacement, disc space narrowing, structural and non-structural endplate abnormalities, high-intensity zones, and osteophytes (Fig. 3, 4, 5)9,41-48). These phenotypes have since been heavily associated with concomitant symptomatology and are thought to contribute to axial neck and/or back pain, worse surgical outcomes, and decreased health-related quality of life scores44,49-53).
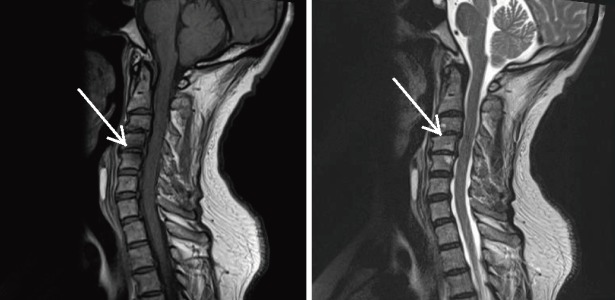
Figure 3.
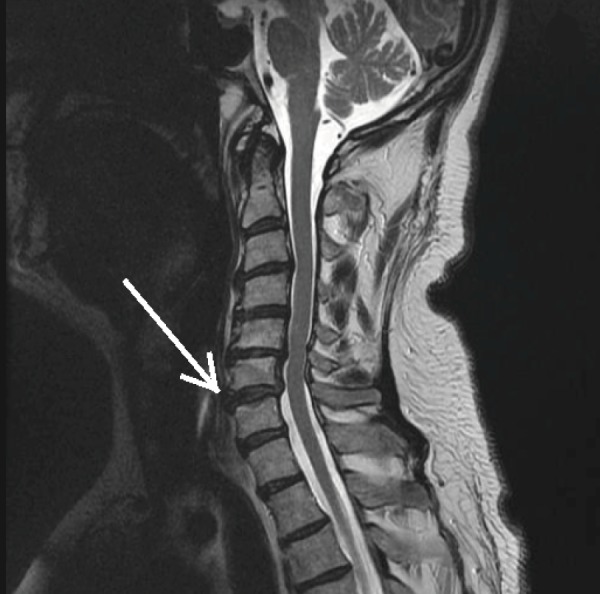
T2-Weighted MRI of the Cervical Spine Demonstrating Anterior Disc Displacement (white arrow).
Figure 4.
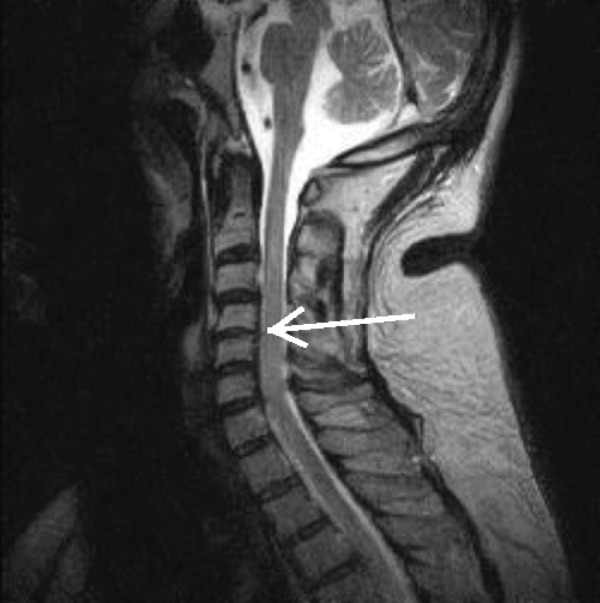
T2-Weighted MRI of the Cervical Spine Demonstrating Disc Space Narrowing (white arrow).
Figure 5.
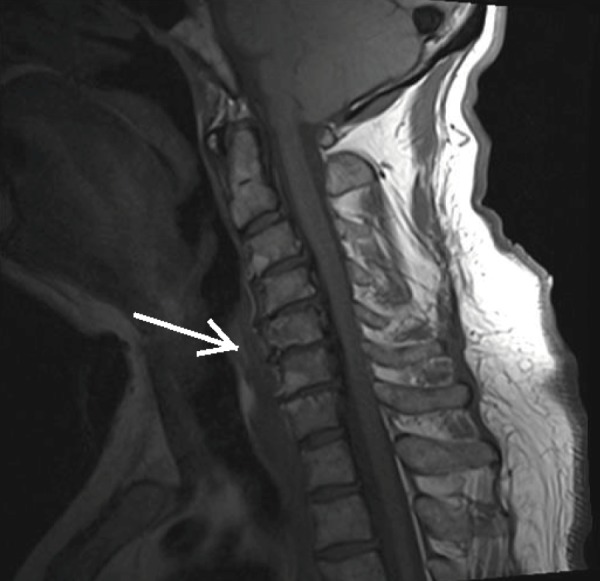
T1-Weighted MRI of the Cervical Spine Demonstrating Anterior Osteophyte Formation (white arrow).
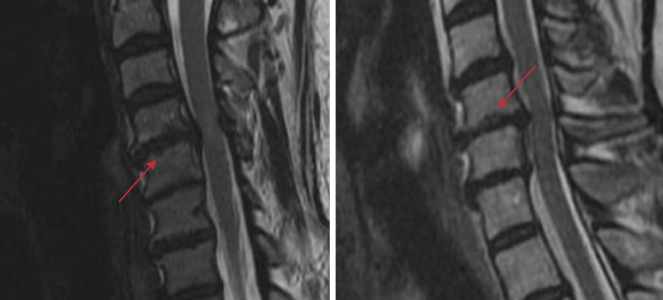
Notably, MRI has allowed for particularly detailed characterization of intervertebral discs and endplates, leading to the development of validated classification schemes as proposed by Pfirrmann and Modic. Pfirrmann grade is obtained by evaluating the intensity of intervertebral discs on T2-weighted images and is a reliable five-tiered classification scheme for characterization of disk degeneration41). Grade I disks are considered healthy and typically appear hyperintense on T2 MRI. Loss of intensity occurs with progression of degeneration, until Grade V is realized (i.e., a “black disk”) (Fig. 6). Similarly, Modic changes are vertebral endplate anomalies and have been strongly linked to clinical symptoms such as low back pain. They occur in three types (I, II, and III) and were defined using a combination of T1- and T2-weighted sequences. Type I changes appear as hypointense on T1 and hyperintense on T2 and have the strongest associations with instability and pain54). Conversely, type II changes are hyperintense on both T1 and T2 sequences and represent fatty marrow changes around the endplates55). Lastly, type III Modic changes are hypointense on both T1 and T2, represent more advanced degeneration and are associated with significant endplate sclerosis56). Despite this, presence of Modic changes on MRI has yet to lead to concrete therapeutic value and currently does little to influence clinical decision making. Realizing this, recent efforts from investigators have attempted to discern more about the pathogenesis and associations of Modic changes with other degenerative features of the spine, hoping that distinctive patterns may emerge to aid in this dilemma (Fig. 7, 8, 9)44,50).
Figure 6.

T2-Weighted MRI of the Cervical Spine Demonstrating a Degenerated “Black Disc” (white arrow).
Figure 7.
T1- (Left) and T2-weighted (Right) MRI of a Type I Modic Change (white arrows).
Figure 8.
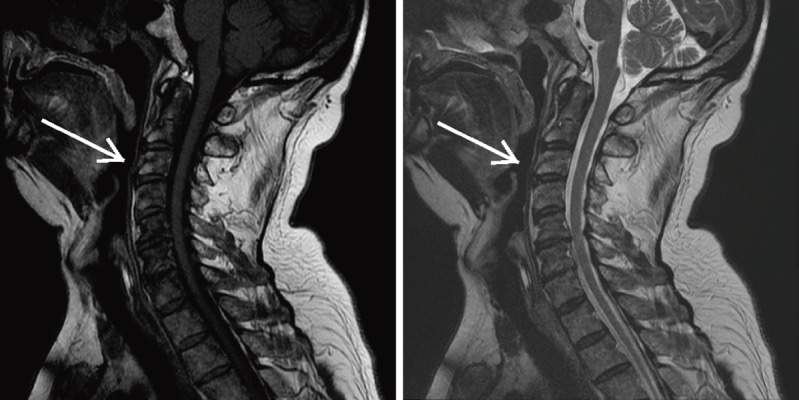
T1- (Left) and T2-weighted (Right) MRI of a Type II Modic Change (white arrows).
Figure 9.

T1- (Left) and T2-weighted MRI of a Type III Modic Change (white arrow) and a Typical Endplate Abnormality (red arrow).
These shortcomings, however, extend beyond Modic changes, as many other image-based findings lack clear etiology and clinical utility. An example of this is a hyperintense T2-signal found in the posterior annulus of intervertebral discs, known as high-intensity zones (HIZs). Originally conceived of as a diagnostic indicator of high-grade annular tears, HIZs have since fallen into mystery and are now only recognized as being linked to symptoms of back pain (Fig. 10)57). Though some studies have shown that a patient's pain symptoms were reproducible with discography, no features of a patient's clinical history or examination have been able to predict the presence of HIZs58). As such, the culpability of HIZs in producing clinical symptoms remains unknown. Similarly, endplate abnormalities (“Schmorl's nodes”) are gross MRI findings that are believed to represent herniation of an intervertebral disc through the endplate of an adjacent vertebral body. Though primarily asymptomatic in most cases, like Modic changes and HIZs, no clear consensus exists regarding their pathogenesis and contribution to clinical decision making. In a recent MRI study by Samartzis et al.45), Schmorl's nodes of the lumbar spine, when phenotypically grouped based on their size and shape, were found to map differentially to the severity of disk degeneration. These findings suggest the possibility that spinal phenotypes such as HIZs, Modic changes, and Schmorl's nodes may largely be components of larger degenerative spine disease, and future investigation on their significance is warranted (Fig. 11).
Figure 10.
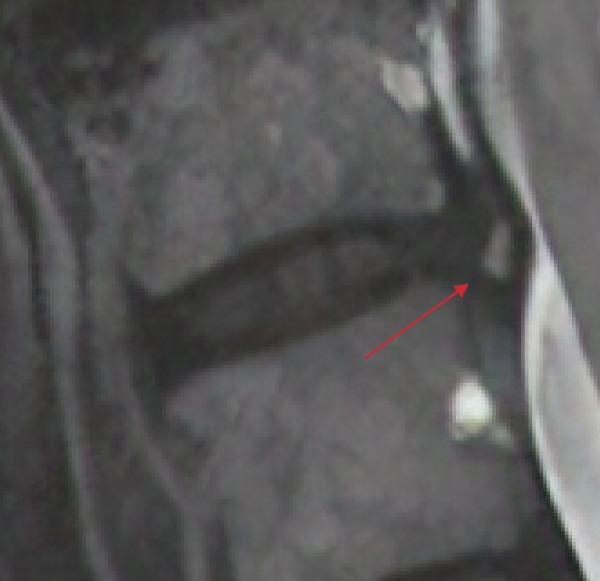
T2-weighted MRI of a High-intensity Zone (HIZ) (red arrow).
Figure 11.
T2-weighted MRI of Typical (Left) and Atypical (Right) Endplate Abnormalities (red arrows).
Given the abundance of these described pathological findings, concern regarding their implications in surgical outcomes has amassed, with growing evidence suggesting patients with a large confluence of degenerative MRI findings portends worse outcomes. For example, in one prospective study examining 134 patients, patients with lumbar disk herniations were far more likely to fail conservative therapy if the affected disc demonstrated a greater degree of degenerative findings on MRI59). Also, degenerative pathology located at the level adjacent to a fusion construct is largely thought to contribute to outcomes of adjacent segment degeneration and disease60-64). This, combined with associations with pain and disability, has demonstrated that MRI is not only a convenience but a necessity in the delivery of standard of care44,49-53).
Despite these strengths, traditional T1- and T2-weighted MRI has notable limitations. Specifically, MRI has been criticized for its inability to quantify the current tissue status, such that the observed imaging findings are likely representative of biochemical processes that occurred months to years prior65). Furthermore, assessment of traditional MRI is ultimately a subjective process, where differences in signal intensity are open to interpretation by the observer. Mulconrey et al.66) demonstrated that MRI assessments of various degenerative lumbar spine findings had an interobserver reliability ranging from 0.67 to 0.77. Although such findings are respectable, a more quantitative measure would eliminate the need for subjective interpretation. Lastly, conventional T1 and T2 sequences are limited in detailed imaging of musculoskeletal tissues such as cartilage and bone marrow and occasionally have difficulties distinguishing between fat and other soft tissues. These shortcomings have subsequently fueled innovations in MRI, where new sequences have been developed to address these criticisms. Quantitative MRI was developed to assign numerical measures of signal intensity on T1 and T2 sequences, eliminating some need for subjective interpretation67). Similarly, sodium MRI was developed to take advantage of the biochemical function of active membranous sodium-potassium pumps, to better identify the current viability of tissues68). On a deeper level, MR spectroscopy MRI can achieve similar results as sodium MRI, as it can be used to assess for specific metabolites in target tissues69). To address limited signal intensities in cortical bone, ultrashort echo time (UTE) sequences were developed and are capable of eliciting hyperintense signals in osseous structures67). Other methodologies have also been used to address echo time to varying degrees of success and include short tau inversion recovery protocols, allowing the suppression of adipose tissue70). New techniques continue to be developed in MRI and include other applications that include manipulation of magnetic field directionality. T1-rho MRI is but one example of this technique and is currently being developed for use in cartilage imaging71).
Collectively, the proliferation of MR innovations has proven instrumental in the field of spine surgery, allowing increases in diagnostic yield for various pathologies and assists in the prediction of outcomes after a given procedure. Studies have demonstrated that quantitative MRI can differentiate between signals in herniated discs and annular tears when compared with disks without gross abnormalities72-74). Similarly, Samartzis et al.75) demonstrated the potential applicability of sodium MRI in identification of intervertebral discs associated with low back pain. More recently, evidence surrounding other sequences has gained additional clinical utility, as UTE MRI has led to the description of the “UTE Disk Sign,” a finding represented by a hyperintense or hypointense band across a degenerative disk associated with chronic low back pain and disability76). Such findings not only highlight the utility of these added sequences but also suggest the potential for MRI to contribute to the prognostication of outcomes after spine surgery.
Bone scintigraphy
Despite the strengths of the aforementioned imaging techniques, they are unable to provide information about the metabolic activity of bone. Conversely, bone scintigraphy is a nuclear medicine-based modality where osteoclastic activity can be visualized through intravenous injection of technetium-99 m and/or other agents that emit gamma rays. During this exam, following the injection of one of these agents, the patient is placed in front of a scintillation camera to detect gamma ray emissions at three scheduled phases: (1) angiographic, (2) blood pool, and (3) bone. The angiographic phase refers to when the agent is primarily located in the major vessels of the body and occurs shortly after injection. Similarly, as time passes, the blood pool phase visualizes soft tissues and other areas of hypervascularity, whereas the bone phase occurs last, and will demonstrate signal intensity in areas with increased bone turnover. As a result, in the spine, scintigraphy has utility in diagnosing osseous neoplastic growth, osteomyelitis, metabolic disease of the bone, Spondylolysis, and compression fractures77).
Future Trends
EOS biplanar X-ray
EOS imaging allows a holistic rendering of the entirety of a patient's skeletal anatomy at significantly lower doses of radiation when compared with conventional X-rays and CT (Fig. 12). Studies have testified to a radiation reduction as significant as 50%-80% less than conventional radiography78). This is an especially advantageous option for patients suffering from spinal deformities that require frequent imaging such as scoliosis. Furthermore, as the patient is imaged upright using EOS, the obtained three-dimensional weight-bearing images of the spine arguably provide the most optimal view of the spine. However, the associated decreases in radiation exposure and size of the films produced have significant drawbacks in imaging resolution. As an example, EOS is not calibrated for use in the management of osseous fractures given a less-detailed depiction of cortical structures. As such, targeted radiographic imaging is largely preferred should assessment of a particular spinal region be required. Nonetheless, EOS is currently being used in many centers across the United States and is actively used to affect management decisions (Fig. 13).
Figure 12.
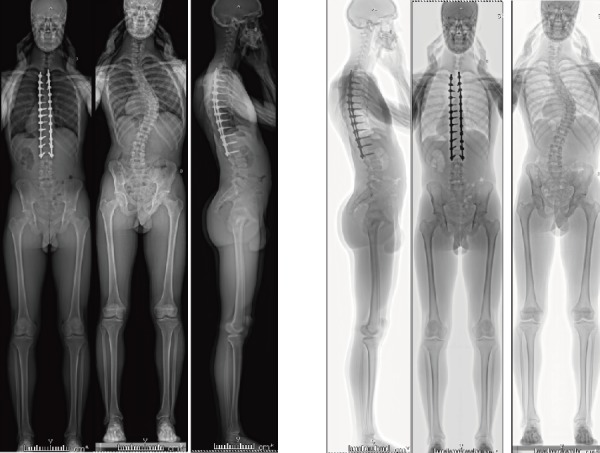
Full-Length Images Obtained using EOS Biplanar Radiographs.
Figure 13.

EOS Biplanar X-Ray Imaging Device.
Image-guided-therapy (robotics)
Image-guided navigational technology is now available in many centers worldwide, though indications for use in spine surgery continue to be debated. These techniques include pedicle screw placement, disk replacement, spinal tumor resection, and anterior approaches to the spine. Image-guided approaches rely on virtual reconstructions of either fluoroscopic and/or CT scans and are derived from a number of different algorithms that rely either on intraoperative or preoperative registration of anatomical images. In intraoperative registration, three or more anatomical landmarks are identified and paired with the navigational algorithm to generate a three-dimensional reconstruction of the spinal anatomy79). Conversely, preoperative algorithms utilize intraoperative surface recognition using a specialized probe to match bony contours to preoperatively acquired CT images79). At present, there are no preoperative algorithms that utilize fluoroscopic images. Some studies support that the combination of both intraoperative and preoperative techniques may be used efficaciously to minimize registration errors, though this may not necessarily translate to significant navigational benefit80). Fluoroscopy-based techniques, however, also rely on a stationary reference device for intraoperative images and may be accidentally disturbed during the procedure, forcing the surgeon to recalibrate the device and increase operating time. As such, Holly et al.80) reasoned that preoperative CT algorithms may be used with sufficient success.
Depending on the operative level of the spine, image-guided surgery has demonstrated acceptable to excellent results in pedicle screw placement. Though favorable screw placement has been realized in the cervical and lumbar spine, in thoracic vertebrae, there is little data to support the use of these techniques. This is likely due to a combination of understudy and small anatomical margins for error. However, in the upper cervical spine, where pedicle size is generally smaller than thoracic levels, some investigators concluded that image-guided approaches were not only beneficial but mandatory for safe fixation81,82). Success in these scenarios is believed to be highly dependent upon user familiarity with navigation systems and precision of algorithmic reconstructions by minimizing motion artifact83,84). In comparison, the lumbar spine has forgiving anatomy due to larger pedicle size, and studies estimate screw malpositioning in only 5%-8% of cases85,86). However, these rates are roughly comparable with traditional freehand techniques in the lumbar spine and thus does not necessarily warrant routine use of navigation technology in posterior instrumentation87).
Outside of pedicle screw instrumentation, image-guided techniques are in the process of being optimized for indications such as disk replacement, oncologic resection, and anterior approaches to the spine. Disk replacement surgery is becoming increasingly more common in the cervical and lumbar spine and is known to rely heavily on the correct positioning of the disk implant. One study by Smith et al. found that there were no significant differences in disk prosthesis positioning between image-guided techniques and standard fluoroscopy, whereas in comparison, others report significant improvements88,89). Similarly, reports on successful application of image guidance in spinal tumors and anterior approaches are currently limited to case reports and warrant further study90,91).
As improvements in these navigational systems are being made, concerns about radiation exposure are also becoming more prevalent. Interestingly, radiation exposure to patients and operating room staff alike has been shown to be lower relative to standard fluoroscopy92). This is especially pronounced in three-dimensional fluoroscopic techniques as Gebhard et al. found a near tenfold reduction in radiation levels92). This particular benefit of image-guided approaches is arguably one of the most powerful drivers for consistent incorporation of these techniques in routine clinical practice, as spinal surgery is perhaps one of the most radio-intensive operating room procedures93).
Machine learning, artificial intelligence
The implications of artificial intelligence (AI) in medical imaging continue to be studied and may have a significant impact on spine surgery in the coming years. Machine learning is a type of programming algorithm where a computer can be trained to perform various tasks based on provided data. This is typically performed by utilizing a set of “training data” where a large sample of input and output data is directly provided to the algorithm. This subsequently allows the program to apply these findings to input “testing data” to generate results based on pattern recognition. These data can subsequently be examined for accuracy and consistency by the investigator.
In the setting of spinal imaging, a number of algorithms that are capable of varying degrees of complexity, ranging from simple tasks such as labeling of vertebral levels to AI-guided pedicle screw placement, have been created. In 2017, Forsberg et al.94) queried MRI data containing sagittal projections of the cervical and lumbar spine to create an AI capable of identifying vertebral levels and intervertebral discs in 96%-97% of cases. In a separate study, Galbusera et al.95) created an algorithm capable of enhancing radiographic and MRI files for increased image resolution and included the automated conversion of various imaging modalities (e.g., X-ray to MRI and MRI to CT) with some degree of success. They noted that their AI demonstrated excellent reliability when converting between T2-weighted MRI sequences, though fared poorly when switching between different imaging techniques. In another example, Burström et al.96) demonstrated a pedicle screw navigational system that could properly suggest screw entry points with 95.4% accuracy in patients undergoing deformity correction at Cobb angles less than 75%. Notably, their entire algorithm was able to create these projections at a mean of 11 s. In all instances, the authors suggest that as the amount of available data for these machine learning algorithms increases, their capabilities would exponentially increase, possibly leading to applications in suggesting surgical procedure for sagittal alignment, predicting patient outcomes, and more97).
Conclusion
Throughout history imaging techniques have steadily improved, and with it, the capabilities of surgical management of the spine. Although once crude radiographic images were considered a remarkable achievement, current efforts now aim to incorporate computer-based intelligence that can reproduce physician diagnostic and therapeutic tendencies. Though traditional imaging techniques will likely still have a role in healthcare for years to come, there too, exists a future where management of the spine may be entirely governed by the machine.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Acknowledgement
We would like to acknowledge Ms. Soraya Barchi & Dr. Stefan Parent for their assistance in acquiring key images required for the completion of this manuscript.
References
- 1.Nimsky C, Carl B. Historical, current, and future intraoperative imaging modalities. Neurosurg Clin N Am. 2017;28(4):453-64. [DOI] [PubMed] [Google Scholar]
- 2.Hesselink JR. Spine imaging: history, achievements, remaining frontiers. A.J.R. Am J Roentgenol. 1988;150(6):1223-9. [DOI] [PubMed] [Google Scholar]
- 3.Hoeffner EG, Mukherji SK, Srinivasan A, et al. Neuroradiology back to the future: spine imaging. A.J.N.R. Am J Neuroradiol. 2012;33(6):999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewing SB. Modern Radiology in Historical Perspective. Academy Med. 1963;38:779. [Google Scholar]
- 5.Isherwood I. Sir Godfrey Hounsfield. Radiology. 2005;234(3):975-6. [Google Scholar]
- 6.Hounsfield GN. Computerized transverse axial scanning (tomography):Part 1. Description of system. B.J.R. Suppl. 1973;46(552):1016-22. [DOI] [PubMed] [Google Scholar]
- 7.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242(5394):190-1. [PubMed] [Google Scholar]
- 8.Bradley WG. History of medical imaging. Proc Am Philos Soc. 2008;152(3):349-61. [PubMed] [Google Scholar]
- 9.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1):193-9. [DOI] [PubMed] [Google Scholar]
- 10.Edelman RR, Shoukimas GM, Stark DD, et al. High-resolution surface-coil imaging of lumbar disk disease. A.J.R. Am J Roentgenol. 1985;144(6):1123-9. [DOI] [PubMed] [Google Scholar]
- 11.Smoker WR, Godersky JC, Knutzon RK, et al. The role of MR imaging in evaluating metastatic spinal disease. A.J.R. Am J Roentgenol. 1987;149(6):1241-8. [DOI] [PubMed] [Google Scholar]
- 12.Carragee EJ, Alamin TF. Discography. A review. Spine J. 2001;1(5):364-72. [DOI] [PubMed] [Google Scholar]
- 13.Berquist TH. Imaging of the postoperative spine. Radiol Clin North Am. 2006;44(3):407-18. [DOI] [PubMed] [Google Scholar]
- 14.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19(11):1824-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janjua MB, Tishelman JC, Vasquez-Montes D, et al. The value of sitting radiographs: analysis of spine flexibility and its utility in preoperative planning for adult spinal deformity surgery. J Neurosurg Spine. 2018;29(4):414-21. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama Y. Surgical treatment for adult spinal deformity: conceptual approach and surgical strategy. Spine Surg Relat Res. 2017;1(2):56-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood KB, Popp CA, Transfeldt EE, et al. Radiographic evaluation of instability in spondylolisthesis. Spine (Phila Pa 1976). 1994;19(15):1697-703. [DOI] [PubMed] [Google Scholar]
- 18.Yao G, Cheung JPY, Shigematsu H, et al. Characterization and predictive value of segmental curve flexibility in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976). 2017;42(21):1622-8. [DOI] [PubMed] [Google Scholar]
- 19.Protopsaltis TS, Lafage R, Smith JS, et al. The lumbar pelvic angle, the lumbar component of the T1 pelvic angle, correlates With HRQOL, PI-LL mismatch, and it predicts global alignment. Spine (Phila Pa 1976). 2018;43(10):681-7. [DOI] [PubMed] [Google Scholar]
- 20.Rothenfluh DA, Mueller DA, Rothenfluh E, et al. Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur Spine J. 2015;24(6):1251-8. [DOI] [PubMed] [Google Scholar]
- 21.Buckland AJ, Puvanesarajah V, Jain A, et al. Dislocation of primary total hip arthroplasty is more common in patients with lumbar spinal fusion. Spine J. 2016;16(10):S263-4. [DOI] [PubMed] [Google Scholar]
- 22.Buckland AJ, Vigdorchik J, Schwab FJ, et al. Acetabular anteversion changes due to spinal deformity correction: bridging the gap Between hip and spine surgeons. J Bone Joint Surg Am. 2015;97(23):1913-20. [DOI] [PubMed] [Google Scholar]
- 23.Buckland AJ, Zhou PL, Moon JY, et al. Sitting-standing lumbopelvic mechanics are altered by spinal degeneration and fusion. Spine J. 2017;17(10):S154. [Google Scholar]
- 24.Feng B, Shen J, Zhang J, et al. How to deal with cerebrospinal fluid leak during pedicle screw fixation in spinal deformities surgery with intraoperative neuromonitoring change. Spine (Phila Pa 1976). 2014;39(1):E20-5. [DOI] [PubMed] [Google Scholar]
- 25.Esses SI, Sachs BL, Dreyzin V. Complications Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine (Phila Pa 1976). 1993;18(15):2231-8. [DOI] [PubMed] [Google Scholar]
- 26.Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4(4):304-9. [DOI] [PubMed] [Google Scholar]
- 27.Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J. 2012;21(2):247-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro WH, Halm H, Jerosch J, et al. Accuracy of pedicle screw placement in lumbar vertebrae. Spine (Phila Pa 1976). 1996;21(11):1320-4. [DOI] [PubMed] [Google Scholar]
- 29.Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976). 1990;15(1):11-4. [DOI] [PubMed] [Google Scholar]
- 30.Laudato PA, Pierzchala K, Schizas C. Pedicle screw insertion accuracy using O-arm, robotic guidance, or freehand technique: A comparative study. Spine (Phila Pa 1976). 2018;43(6):E373-8. [DOI] [PubMed] [Google Scholar]
- 31.Pitteloud N, Gamulin A, Barea C, et al. Radiation exposure using the O-armⓇ surgical imaging system. Eur Spine J. 2017;26(3):651-7. [DOI] [PubMed] [Google Scholar]
- 32.Verma SK, Singh PK, Agrawal D, et al. O-arm with navigation versus C-arm: a review of screw placement over 3 years at a major trauma center. Br J Neurosurg. 2016;30(6):658-61. [DOI] [PubMed] [Google Scholar]
- 33.Bartynski WS, Lin L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. A.J.N.R. Am J Neuroradiol. 2003;24(3):348-60. [PMC free article] [PubMed] [Google Scholar]
- 34.Grams AE, Gempt J, Förschler A. Comparison of spinal anatomy between 3-Tesla MRI and CT-myelography under healthy and pathological conditions. Surg Radiol Anat. 2010;32(6):581-5. [DOI] [PubMed] [Google Scholar]
- 35.Holt EP Jr. The question of lumbar discography. J Bone Joint Surg Am. 1968;50(4):720-6. [DOI] [PubMed] [Google Scholar]
- 36.Guyer RD, Ohnmeiss DD. Lumbar discography. Position statement from the North American Spine Society Diagnostic and Therapeutic Committee. Spine (Phila Pa 1976). 1995;20(18):2048-59. [PubMed] [Google Scholar]
- 37.Datta JC, Janssen ME, Beckham R, et al. The use of computed tomography angiography to define the prevertebral vascular anatomy prior to anterior lumbar procedures. Spine (Phila Pa 1976). 2007;32(1):113-9. [DOI] [PubMed] [Google Scholar]
- 38.Ebraheim NA, Xu R, Farooq A, et al. The quantitative anatomy of the iliac vessels and their relation to anterior lumbosacral approach. J Spinal Disord. 1996;9(5):414-7. [PubMed] [Google Scholar]
- 39.Kleeman TJ, Michael Ahn U, Clutterbuck WB, et al. Laparoscopic anterior lumbar interbody fusion at L4-L5: an anatomic evaluation and approach classification. Spine (Phila Pa 1976). 2002;27(13):1390-5. [DOI] [PubMed] [Google Scholar]
- 40.Edelman RR. The history of MR imaging as seen through the pages of radiology. Radiology. 2014;273(2):S181-200. [DOI] [PubMed] [Google Scholar]
- 41.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873-8. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976). 1990;15(5):411-5. [DOI] [PubMed] [Google Scholar]
- 43.Mok FPS, Samartzis D, Karppinen J, et al. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976). 2010;35(21):1944-52. [DOI] [PubMed] [Google Scholar]
- 44.Määttä JH, Karppinen JI, Luk KDK, et al. Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale population-based study. Spine J. 2015;15(9):1933-42. [DOI] [PubMed] [Google Scholar]
- 45.Samartzis D, Mok FPS, Karppinen J, et al. Classification of Schmorl's nodes of the lumbar spine and association with disc degeneration: a large-scale population-based MRI study. Osteoarthr Cartil. 2016;24(10):1753-60. [DOI] [PubMed] [Google Scholar]
- 46.Schmorl G. The human spine in health and disease. Hum Spine Health Dis. 1971. [Google Scholar]
- 47.Jha SC, Takata Y, Abe M, et al. High intensity zone in lumbar spine and its correlation with disc degeneration. J Med Invest. 2017;64(1):39-42. [DOI] [PubMed] [Google Scholar]
- 48.Walraevens J, Liu B, Meersschaert J, et al. Qualitative and quantitative assessment of degeneration of cervical intervertebral discs and facet joints. Eur Spine J. 2009;18(3):358-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karki DB, Gurung G, Adhikary KP, et al. Magnetic resonance imaging findings in degenerative disc disease of cervical spine in symptomatic patients. J Nepal Health Res Counc. 2015;13(31):196-200. [PubMed] [Google Scholar]
- 50.Määttä JH, Karppinen J, Paananen M, et al. Refined phenotyping of Modic changes: imaging biomarkers of prolonged severe low back pain and disability. Medicine. 2016;95(22):e3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teraguchi M, Yim R, Cheung JP-Y, et al. The association of high-intensity zones on MRI and low back pain: a systematic review. Scoliosis Spinal Disord. 2018;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams FMK, Manek NJ, Sambrook PN, et al. Schmorl's nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007;57(5):855-60. [DOI] [PubMed] [Google Scholar]
- 53.Zanoli G, Jönsson B, Strömqvist B. SF-36 scores in degenerative lumbar spine disorders: analysis of prospective data from 451 patients. Acta Orthop. 2006;77(2):298-306. [DOI] [PubMed] [Google Scholar]
- 54.Ghodsi SM, Rouhani R, Abdollahzade S, et al. Frequency of vertebral endplate Modic changes in patients with unstable lumbar spine and its effect on surgical outcome. Asian Spine J. 2015;9(5):737-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fayad F, Lefevre-Colau MM, Drapé JL, et al. Reliability of a modified Modic classification of bone marrow changes in lumbar spine MRI. Joint Bone Spine. 2009;76(3):286-9. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Huang B, Hao L, et al. Association between Modic changes and endplate sclerosis: evidence from a clinical radiology study and a rabbit model. J Orthop Translat. 2019;16:71-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361-9. [DOI] [PubMed] [Google Scholar]
- 58.Rankine JJ, Gill KP, Hutchinson CE, et al. The clinical significance of the high-intensity zone on lumbar spine magnetic resonance imaging. Spine (Phila Pa 1976). 1999;24(18):1913-9; discussion 1920. [DOI] [PubMed] [Google Scholar]
- 59.Motiei-Langroudi R, Sadeghian H, Seddighi AS. Clinical and magnetic resonance imaging factors which may predict the need for surgery in lumbar disc herniation. Asian Spine J. 2014;8(4):446-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Ma L, Yang D, et al. Incidence and risk factors of postoperative adjacent segment degeneration following anterior decompression and instrumented fusion for degenerative disorders of the cervical spine. World Neurosurg. 2017;105:78-85. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto K, Aizawa T, Kanno H, et al. Adjacent segment degeneration after fusion spinal surgery-a systematic review. Int Orthop. 2019;43(4):987-93. [DOI] [PubMed] [Google Scholar]
- 62.Zhang C, Berven SH, Fortin M, et al. Adjacent segment degeneration Versus disease After lumbar spine fusion for degenerative pathology: A systematic review With meta-analysis of the literature. Clin Spine Surg. 2016;29(1):21-9. [DOI] [PubMed] [Google Scholar]
- 63.Li C, He Q, Tang Y, et al. The fate of adjacent segments with pre-existing degeneration after lumbar posterolateral fusion: the influence of degenerative grading. Eur Spine J. 2015;24(11):2468-73. [DOI] [PubMed] [Google Scholar]
- 64.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6) (Suppl.):190S-4S. [DOI] [PubMed] [Google Scholar]
- 65.Urban JPG, Winlove CP. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging. 2007;25(2):419-32. [DOI] [PubMed] [Google Scholar]
- 66.Mulconrey DS, Knight RQ, Bramble JD, et al. Interobserver reliability in the interpretation of diagnostic lumbar MRI and nuclear imaging. Spine J. 2006;6(2):177-84. [DOI] [PubMed] [Google Scholar]
- 67.Bae WC, Chen PC, Chung CB, et al. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27(4):848-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madelin G, Lee JS, Regatte RR, et al. Sodium MRI: methods and applications. Prog Nucl Magn Reson Spectrosc. 2014;79:14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yabuki S, Konno S-I, Kikuchi S-I. Assessment of pain due to lumbar spine diseases using MR spectroscopy: a preliminary report. J Orthop Sci. 2013;18(3):363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connell MJ, Hargaden G, Powell T, et al. Whole-body turbo short tau inversion recovery MR imaging using a moving tabletop. A.J.R. Am J Roentgenol. 2002;179(4):866-8. [DOI] [PubMed] [Google Scholar]
- 71.Regatte RR, Akella SVS, Borthakur A, et al. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229(1):269-74. [DOI] [PubMed] [Google Scholar]
- 72.Cui YZ, Yang XH, Liu PF, et al. Preliminary study on diagnosis of lumbar disc degeneration with magnetic resonance T1p, T2 mapping and DWI quantitative detection technologies. Eur Rev Med Pharmacol Sci. 2016;20(16):3344-50. [PubMed] [Google Scholar]
- 73.Chokan K, Murakami H, Endo H, et al. Evaluation of water retention in lumbar intervertebral disks Before and After exercise stress With T2 mapping. Spine (Phila Pa 1976). 2016;41(7):E430-6. [DOI] [PubMed] [Google Scholar]
- 74.Trattnig S, Stelzeneder D, Goed S, et al. Lumbar intervertebral disc abnormalities: comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur Radiol. 2010;20(11):2715-22. [DOI] [PubMed] [Google Scholar]
- 75.Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J. 2015;15(9):1919-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pang H, Bow C, Cheung JPY, et al. The UTE disc sign on MRI: A novel imaging biomarker associated With degenerative spine changes, low back pain, and disability. Spine (Phila Pa 1976). 2018;43(7):503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook GJR, Gnanasegaran G, Chua S. Miscellaneous indications in bone scintigraphy: metabolic bone diseases and malignant bone tumors. Semin Nucl Med. 2010;40(1):52-61. [DOI] [PubMed] [Google Scholar]
- 78.Melhem E, Assi A, El Rachkidi R, et al. EOSⓇ biplanar X-ray imaging: concept, developments, benefits, and limitations. J Child Orthop. 2016;10(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tjardes T, Shafizadeh S, Rixen D, et al. Image-guided spine surgery: state of the art and future directions. Eur Spine J. 2010;19(1):25-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holly LT, Bloch O, Johnson JP. Evaluation of registration techniques for spinal image guidance. J Neurosurg Spine. 2006;4(4):323-8. [DOI] [PubMed] [Google Scholar]
- 81.Acosta FL Jr., Quinones-Hinojosa A, Gadkary CA, et al. Frameless stereotactic image-guided C1-C2 transarticular screw fixation for atlantoaxial instability: review of 20 patients. J Spinal Disord Tech. 2005;18(5):385-91. [DOI] [PubMed] [Google Scholar]
- 82.Paramore CG, Dickman CA, Sonntag VKH. The anatomical suitability of the C1-2 complex for transarticular screw fixation. J Neurosurg. 1996;85(2):221-4. [DOI] [PubMed] [Google Scholar]
- 83.Arand M, Hartwig E, Kinzl L, et al. Spinal navigation in cervical fractures―A preliminary clinical study on Judet-osteosynthesis of the axis. Comput Aided Surg. 2001;6(3):170-5. [DOI] [PubMed] [Google Scholar]
- 84.Seichi A, Takeshita K, Nakajima S, et al. Revision cervical spine surgery using transarticular or pedicle screws under a computer-assisted image-guidance system. J Orthop Sci. 2005;10(4):385-90. [DOI] [PubMed] [Google Scholar]
- 85.Sagi HC, Manos R, Benz R, et al. Electromagnetic field-based image-guided spine surgery Part One: Results of a Cadaveric Study Evaluating Lumbar Pedicle Screw Placement. Spine (Phila Pa 1976). 2003;28(17):2013-8. [DOI] [PubMed] [Google Scholar]
- 86.Fu TS, Chen LH, Wong CB, et al. Computer-assisted fluoroscopic navigation of pedicle screw insertion: an in vivo feasibility study. Acta Orthop Scand. 2004;75(6):730-5. [DOI] [PubMed] [Google Scholar]
- 87.Parker SL, McGirt MJ, Farber SH, et al. Accuracy of free-hand pedicle screws in the thoracic and lumbar spine: analysis of 6816 consecutive screws. Neurosurgery. 2011;68(1):170-8; discussion 178. [DOI] [PubMed] [Google Scholar]
- 88.Smith HE, Vaccaro AR, Yuan PS, et al. The use of computerized image guidance in lumbar disk arthroplasty. J Spinal Disord Tech. 2006;19(1):22-7. [DOI] [PubMed] [Google Scholar]
- 89.Marshman LAG, Friesem T, Rampersaud YR, et al. Significantly improved lumbar arthroplasty placement using image guidance: technical note. Spine (Phila Pa 1976). 2007;32(18):2027-30. [DOI] [PubMed] [Google Scholar]
- 90.Rajasekaran S, Kamath V, Shetty AP. Intraoperative Iso-C three-dimensional navigation in excision of spinal osteoid osteomas. Spine (Phila Pa 1976). 2008;33(1):E25-9. [DOI] [PubMed] [Google Scholar]
- 91.Ohmori K, Kawaguchi Y, Kanamori M, et al. Image-guided anterior thoracolumbar corpectomy: a report of three cases. Spine (Phila Pa 1976). 2001;26(10):1197-201. [DOI] [PubMed] [Google Scholar]
- 92.Gebhard FT, Kraus MD, Schneider E, et al. Does computer-assisted spine surgery reduce intraoperative radiation doses? Spine (Phila Pa 1976). 2006;31(17):2024-7; discussion 2028. [DOI] [PubMed] [Google Scholar]
- 93.Theocharopoulos N, Perisinakis K, Damilakis J, et al. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg Am. 2003;85(9):1698-703. [DOI] [PubMed] [Google Scholar]
- 94.Forsberg D, Sjöblom E, Sunshine JL. Detection and labeling of vertebrae in MR images using deep learning with clinical annotations as training data. J Digit Imaging. 2017;30(4):406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galbusera F, Bassani T, Casaroli G, et al. Generative models: an upcoming innovation in musculoskeletal radiology? A preliminary test in spine imaging. Eur Radiol Exp. 2018;2(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burström G, Buerger C, Hoppenbrouwers J, et al. Machine learning for automated 3-dimensional segmentation of the spine and suggested placement of pedicle screws based on intraoperative cone-beam computer tomography. J Neurosurg Spine. 2019;31(1):147-54. [DOI] [PubMed] [Google Scholar]
- 97.Lafage R, Pesenti S, Lafage V, et al. Self-learning computers for surgical planning and prediction of postoperative alignment. Eur Spine J. 2018;27(Suppl 1):123-8. [DOI] [PubMed] [Google Scholar]