Abstract
Cytochrome P450 (P450) 2E1 is the major P450 enzyme involved in ethanol metabolism. That role is shared with two other enzymes that oxidize ethanol, alcohol dehydrogenase and catalase. P450 2E1 is also involved in the bioactivation of a number of low molecular weight cancer suspects, as validated in vivo in mouse models where cancers could be attenuated by deletion of Cyp2e1. P450 2E1 does not have a role in global production of reactive oxygen species but localized roles are possible, e.g. in mitochondria. The structures, conformations, and catalytic mechanisms of P450 2E1 have some unusual features among P450s. The concentration of hepatic P450 varies ≥ 10-fold among humans, possibly in part due to single nucleotide variants. The level of P450 2E1 may have relevance in the rates of oxidation of drugs, particularly acetaminophen and anesthetics.
Keywords: Cytochrome P450, P450 2E1, CYP2E1, ethanol, nitrosamines, kinetic isotope effects, SNP, SNV
1. Introduction to cytochrome P450 enzymes
Cytochrome P450 (P450, CYP) enzymes are responsible for ~ 95% of the oxidation and reduction of chemicals, based on all literature citations [1]. These enzymes were first studied because they are the main catalysts involved in the metabolism of drugs, steroids, fat-soluble vitamins, chemical carcinogens, industrial chemicals, and other entities [2]. In humans there are 57 P450, or CYP, genes, but the more than 380,000 P450 genes are found throughout nature and are responsible for many biosynthetic reactions [3, 4].
A major area of interest regarding human P450s is the relationship to disease. One major issue is single nucleotide variants in the population that link to lack of function and lead to maladies. Classic examples are seen in endocrinology due to defects in steroid metabolism [5]. Another area of interest is nearly the opposite, in a sense, where drugs are used to inhibit a P450 in certain medical conditions, e.g. blocking P450 19A1 (steroid aromatase) to lower estrogen levels in breast cancer [6, 7].
What has been more difficult is to define the contributions of human P450s in cases where the roles may be less obvious, e.g. chemical carcinogenesis [8]. Although studies with experimental animals were quite definitive in associating cancer with P450 expression [9] and these findings have been extended in very elegant matter with transgenic animals [10, 11], application to problems in human medicine has been less straightforward. It is in this latter category that P450 2E1 fits.
2. Background on P450 and ethanol oxidation
Historically most of the interest in ethanol oxidation involved alcohol and aldehyde dehydrogenases [12-14]. Catalase can also oxidize ethanol [15].
In 1965 Orme-Johnson and Ziegler [16] demonstrated the NADPH-dependent oxidation of ethanol and methanol in rat, rabbit, and hog microsomes. Interestingly, the system was not inhibited by carbon monoxide and no activity was seen with longer-chain alcohols. Similar activity was also reported by Lieber and DeCarli [17], who also found that the activity was enhanced by feeding ethanol to rats. The work in the initial report was expanded in 1970 [18] and the activity was found to be inhibited by carbon monoxide.
A role for P450 in oxidation was difficult to accept, in light of the general nature of the known larger and more hydrophobic substrates. However, Mezey et al. [19] reported that a partially purified rat liver P450 preparation could oxidize ethanol to acetaldehyde. In 1978 Miwa et al. [20] could demonstrate ethanol oxidation by highly purified rat and rabbit liver P450 preparations, demonstrating a lack of both alcohol dehydrogenase and catalase in their systems.
Coon’s laboratory isolated a P450 enzyme from ethanol-treated rabbits (3a, now known as 2E1) that oxidized ethanol [21]. P450 2E1 (then termed P450j) was also purified and characterized from rat and human liver [22]. In 1986 Gonzalez’s group [23] cloned the rat and human cDNAs for P450 2E1 and later characterized the gene [24].
Subsequent work by Gonzalez and others revealed the complexity of mechanisms involved in the regulation of P450 2E1 [25]. The in vivo activity is difficult to study because ethanol is both an inducer and an inhibitor of the enzyme. P450 2E1 is quite different in its regulatory mechanisms from many other major P450 enzymes involved in the oxidation of xenobiotics, which are dominated largely by nuclear receptor activation mechanisms. Transcriptional regulation is involved, at least in part [26]. There is also a role of ethanol in stabilization of the protein and a role of the proteasome. The difficulties in studying regulation are exemplified by the induction with placental lactogen and through the phosphoinositol 3-kinase pathway [27]: CYP2E1-humanized mice did not exhibit enhanced P450 2E1 expression during pregnancy due to interspecies differences in placental lactogen physiology.
A significant development in the field was the generation of Cyp2e1−/− knockout mice by the Gonzalez laboratory, which have proven to very useful in evaluation of in vivo roles of the enzyme, at least in mice. These have proven to be valuable in the assessment of the role of mouse P450 2e1 and human P450 2E1 in the metabolism of drugs and potentially toxic chemicals [11, 28-31]. Further, “humanized” P450 2E1 mice (devoid of mouse P450 2e1, but expressing human P450 2E1) have been very useful [32, 33]. A biomarker of P450 2e1 (2-piperidone) has been identified using these animals (although it is not known if this is useful in humans) [34]. There are still some caveats in interpretation of results with these animals though, e.g. P450 2E1-humanized mice showed acute proximal renal tubule injury (as did wild-type control mice) but did not show hepatic lipid accumulation with high doses of perchloroethylene [33].
3. Metabolism of drugs by P450 2E1
Probably because of its preference for small molecules, P450 2E1 does not contribute to the metabolism of many drugs. One group of drugs where P450 2E1 is a major factor is anesthetics (Figure 1). P450 2E1 is a major factor in the disposition of halothane, isoflurane, sevoflurane, enflurane, and desflurane [35-39].
Figure 1.
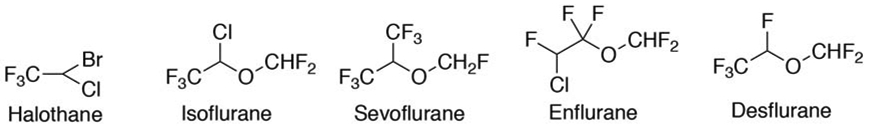
Anesthetic substrates for P450 2E1.
Another low molecular weight drug that P450 2E1 is involved in the metabolism of is acetaminophen. In mice, P450 2e1 is a major factor in toxicity, as shown with transgenic animals [29, 30]. Of interest is a report in which very obese humans were shown to have elevated levels of acetaminophen-derived cysteine conjugates, ascribed to P450 2E1 oxidation, as a result of elevated P450 2E1 levels [40].
Another issue with anesthetics is with halothane, a substrate for P450 2E1 (Figure 1). A large fraction of patients with halothane-induced hepatitis have autoantibodies that recognize P450 2E1 [41, 42]. These antibodies are also found in anesthesiologists, presumably due to exposure (to halothane?), but even in the absence of injury [43]. The role of the antibodies in the etiology remains unclear, as has been the case with other drugs whose use is associated with autoantibodies [44].
4. P450 2E1 and carcinogen metabolism
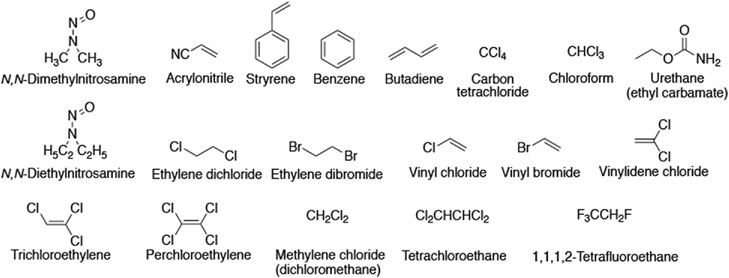
Early work by Wrighton et al. [22] and in Yang’s laboratory [45] showed the involvement of what is now known as P450 2E1 in the metabolic activation of N,N-dimethylnitrosamine and other carcinogenic nitrosamines, the oxidation of which had been difficult to identify catalysts for [46]. The toxicology literature had numerous reports of the effects of ethanol and disulfiram (Antabuse®) on the metabolism and carcinogenicity of various chemicals (e.g., ethylene dibromide [47]), which had been difficult to explain. P450 2E1 was demonstrated to be involved in the metabolism of a variety of low molecular weight chemicals, many of which are cancer suspects, including not only the smaller alkyl nitrosamines but also vinyl monomers, alkyl halides, and others (Figure 2) [48-50].
Figure 2.
An interesting case is urethane, or ethyl carbamate. Work in the Millers’ laboratory had suggested that vinyl carbamate might be formed and then converted to an epoxide, which could modify DNA [51], but it was difficult to detect vinyl carbamate. Subsequently our laboratory was able to demonstrate that P450 2E1 could slowly desaturate ethyl carbamate and then the resulting vinyl carbamate was rapidly oxidized by the same enzyme to the epoxide, which reacts with DNA to form etheno adducts (Figure 3) [52]. In mice the expression of P450 2e1 is involved in urethane-induced lung tumors [53].
Figure 3.
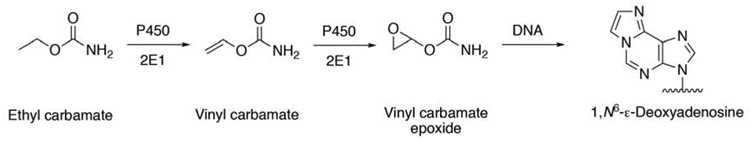
Activation of ethyl carbamate (urethane) by P450 2E1 [51-53].
Ethanol treatment has also been shown to increase the levels of etheno adducts ~ 2-fold in mice treated with high chronic doses of ethanol, presumably due to P450 2e1 induction [54-56].
The significance of P450 2E1 in carcinogen metabolism has been demonstrated with knockout mouse models. For instance, Cyp2e1−/− mice were not susceptible to benzene toxicity or genotoxicity [28]. It is also possible to interpret the effects of P450 2E1 inhibitors. For instance, disulfiram increased the carcinogenicity of ethylene dibromide by blocking the detoxication and thus making more of the compound available for bioactivation by a glutathione conjugation pathway [57]. In a similar way, knocking out Cyp2e1 in mice diverted the pathway for trichloroethylene from oxidation to conjugation [33]. Dietary ethanol enhanced the levels for O6-methyldeoxyguanosine adducts in rats treated with the carcinogen N,N-dimethylnitrosamine [54], presumably due to P450 2E1 induction.
5. Reactive oxygen species (ROS)
The literature is replete with discussion of ROS production due to P450 2E1. The vast majority of this has been developed in vitro with liver microsomes [58, 59] and cultured cells [60]. Many of the commonly used ROS assays are not validated, e.g. in vivo malondialdehye assays [61] and dichlorofluorescein fluorescence [62]. In the ROS field, the most appropriate “gold standard” for ROS is F2-isoprostane production, which can be measured both in vitro and in vivo [63, 64].
We questioned whether P450 2E1 (2e1 in mice) was really so highly uncoupled and could produce large scale levels of ROS. Treatment of animals with ethanol is complicated in that it can be both an inducer and inhibitor (vide supra). Accordingly, we treated rats with the P450 2E1 inducer isoniazid and did not see an increase in F2-isoprostane levels [65]. Subsequent work showed that Cyp2e1−/− mice had very similar levels of liver, brain, and urinary isoprostanes as the wild-type animals [66]. P450 2E1 does not appear to increase global levels of ROS, and any increases due to ethanol treatment are not related to P450 2E1 induction. Although purified P450 2E1 is not well-coupled to NADPH consumption, neither are several other P450s that we and others have examined [67-69]. Others have suggested that ROS production may be coupled to the production of CH3CHO· radicals [70, 71].
Although P450 2E1 does not appear to be involved in large changes in global ROS, the results do not rule out the possibility of localized ROS production. In this regard, mutation of the N-terminal sequence of rat P450 2E1 or protein kinase A-mediated phosphorylation of Ser-129 enhanced mitochondrial translocation of the protein due to enhanced affinity for binding to the HSP70 chaperone protein [72]. Hepatic mitochondria isolated from ethanol-treated rats showed enhanced isoprostane levels after eight weeks, but microsomes did not [73]. The relevance of the enhanced mitochondrial ROS production may be seen in the distribution of mitochondrial versus microsomal localization of P450 2E1 in human liver samples, i.e. in some individuals a large fraction of P450 2E1 was localized in mitochondria [74]. The ROS may be the result of poor (mitochondrial) P450 2E1 coupling with the alternate electron transfer accessory protein adrenodoxin.
6. Structures of P450 2E1
Some X-ray crystal structures of P450 2E1 have been published by Scott and associates [75-77]. These include both a small ligand and a larger one, a fatty acid.
The existence of multiple conformations of the enzyme raises questions about the origin of these. Two general models can explain the results. In a conformational selection model an equilibrium exists between different conformations, in the absence of ligands, and one of these binds the ligand [78]. Alternatively, in a true induced fit model there is a single conformation of the unbound enzyme, and the initial binding of the ligand to the enzyme induces a conformation change that changes the enzyme into a more efficient catalytic state (Figure 4) [79, 80]. Several human P450s have now been shown to operate primarily through conformational selection modes [81, 82], but kinetic studies with P450 2E1 and its substrate hexyl isonicotinate could not distinguish between the conformational selection and induced fit models for P450 2E1 [82].
Figure 4.
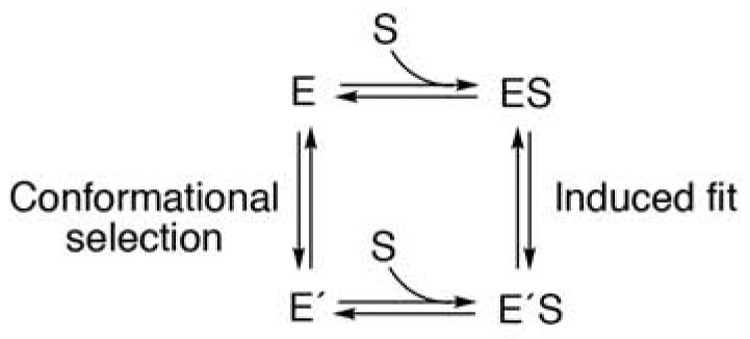
Thermodynamic box for complex substrate binding possibilities. The free energy for the conversion of E to E’S is identical for the conformational selection and induced fit routes [81].
7. Catalytic mechanism
The catalytic mechanism involves the usual steps of substrate binding, reduction of ferric iron to ferrous, O2 binding, introduction of a second electron into the iron-oxygen complex, transformation of the iron-oxygen complex to Compound I (FeO3+), abstraction of a hydrogen atom, oxygen rebound, and product release (Figure 5) [83, 84].
Figure 5.
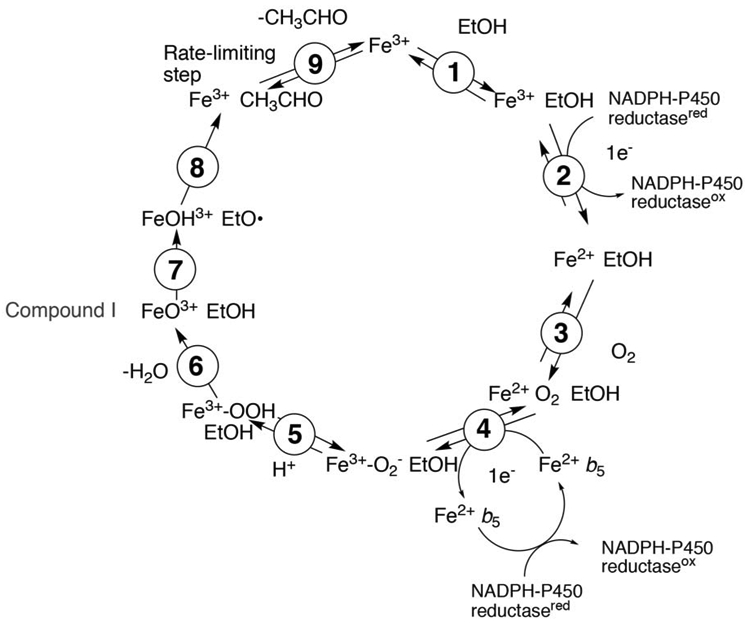
P450 catalytic cycle with P450 2E1 features.
There are two anomalies regarding catalytic mechanisms of P450 2E1. The first is a role for cytochrome b5 (b5), which seems to be the case in most reactions [85-89]. In contrast to several other P450s, apo-b5 (devoid of heme) is ineffective in stimulating (chlorzoxazone 6-hydroxylation catalyzed by) P450 2E1 [89] and the conclusion is that electron transfer to the Fe2+O2 complex is done by b5.
Another anomaly is the kinetic deuterium isotope effect on C-H bond-breaking. We were lead to pursue this research area because of microsomal results on the oxidation of N,N-dimethylnitrosamine showing a kinetic deuterium isotope effect on Km and not Vmax [90], plus the newer knowledge that P450 2E1 was a major P450 enzyme involved in that reaction [45]. We initiated studies with human P450 2E1 and the oxidation of ethanol, finding the same pattern of a strong kinetic isotope effect on Km but not kcat [87]. The basis of this effect was attributed to the “burst kinetics,” i.e. a rate-limiting step following product formation, which results in expression of the kinetic deuterium isotope effect in the Km [87]. Pre-steady-state kinetic measurements showed the isotope effect on C-H bond-breaking [87]. Accordingly, consideration of the relevant expression shows that Km is not an independent parameter [91].
The kinetics of P450 2E1, with a rate-limiting step, after product formation, produce some kinetics that can be considered unusual. With a simplified system
where S is the substrate and P the product,
If k3 > k5 then these reduce to
and
As discussed elsewhere [87, 93], this analysis has a number of implications for kinetic deuterium isotope effects.
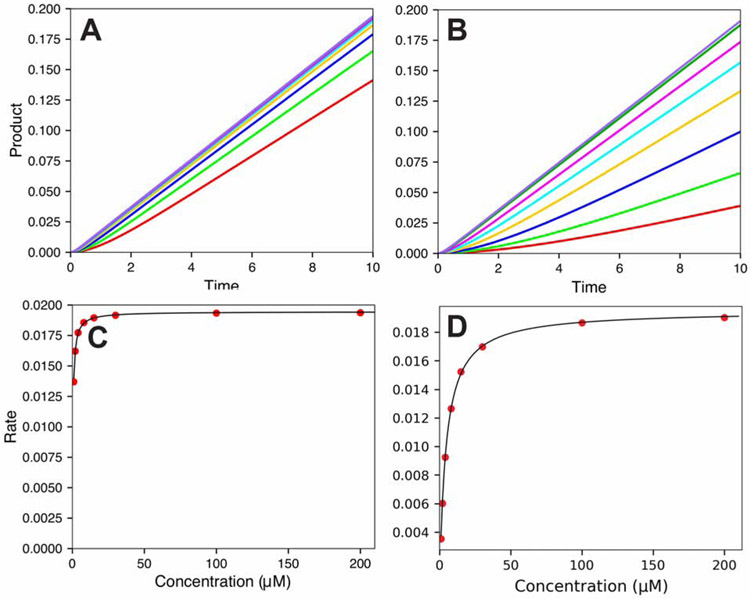
The kinetics is also relevant to inhibition. For instance, consider a model reaction (Figure 6), simulated in KinTek software [94]. A system was set up with the following rate constants
and
where E is P450 2E1, S is the substrate, P is the product, and I is an inhibitor so that the designed Kd for S is 10 μM and the Ki (Kd for I) is 5 μM. The fits (Figure 6) give kcat = 0.02 s−1. As shown in Figure 6 the actual Kd values for S and I are 10 μM and 0.5 μM, respectively, as set up in the model. The Km value for S is 0.41 μM (Figure 6C) and the value of Ki calculated using a classic competitive inhibition model is 0.056 μM. Thus, due to the kinetic nature of the system, the Km and Ki values are much lower than the actual affinities (i.e., dissociation constants) for S and I.
Figure 6.
8. Single nucleotide variants (SNVs)
The number of reported SNVs for the CYP2E1 gene is relatively small, with only 19 reported in www.pharmvar.org/gene/CYP2E1 (Table 1). (Note: the term SNV is used instead of single nucleotide polymorphism, which by definition is a variant found at a frequency of ≥1% in a population.) Of these, there are only four with distinct amino acid sequence changes (CYP2E1.1 and R76H, V389I, and V179I). Of the SNVs, the specific catalytic activity (chlorzoxazone 6-hydroxylation) was similar to CYP2E1.1 but the level of expression in COS-7 cells was 40% that for CYP2E1.1, which is not a large change [95].
Table 1.
CYP2E1 alleles (www.pharmvar.org/gene/CYP2E1) (accessed 11 December 2019)
| Allele | Protein | Nucleotide changes, Gene |
Effect | Enzyme activity | |
|---|---|---|---|---|---|
| CYP2E1*1A | CYP2E1.1 | None | In vivo | In vitro | |
| CYP2E1*1B | CYP2E1.1 | 9896C>G | Normal | Normal | |
| CYP2E1*1C | CYP2E1.1 | 6 repeats in the 5’ flanking region | |||
| CYP2E1*1Cx2 | CYP2E1.1 | ||||
| CYP2E1*1D | CYP2E1.1 | 8 repeats in the 5’ flanking region | Increased activity after alcohol exposure and in obese subjects | ||
| CYP2E1*2 | CYP2E1.2 | 1132G>A | R76H | Reduced | |
| CYP2E1*3 | CYP2E1.3 | 10023G>A | V389I | Normal | |
| CYP2E1*4 | CYP2E1.4 | 4768G>A | V179I | Normal | |
| CYP2E1*5A | CYP2E1.1 | −1293G>C; −1053C>T (c1>c2); 7632T>A | |||
| CYP2E1*5B | CYP2E1.1 | −1293G>C; −1053C>T (c1>c2) | |||
| CYP2E1*6 | CYP2E1.1 | 7632T>A | |||
| CYP2E1*7A | CYP2E1.1 | −333T>A | |||
| CYP2E1*7B | CYP2E1.1 | −71G>T; −eeeT>A | |||
| CYP2E1*7C | CYP2E1.1 | −333T>A; −352A>G | |||
| Additional SNVs, where the haplotype has not yet been determined | |||||
| 6431C>A | |||||
| 9630T>G | |||||
| 1031C>T; 1199G>A; 1316C>T; 4451C>G; 4486G>T; 4529C>T; 4696G>A; 4845T>C; 4904T>C; 5625G>A; 6317C>T; 9745C>T; 9987C>G; 11024C>G; 11276T>C; 11356A>C | |||||
| 11112A>T | H457L | ||||
Whether the non-coding region changes have effects on expression levels or not is unknown. In a review in 2015, Daly [8] considered a *5 (rs2031920) variant (“RasI”) and its relationship to lung cancer, although the results seem to be equivocal [96-98]. A decreased risk was seen with the SNV in a meta analysis of 15 studies but only in Asians [98], and no mechanism is proposed. Hakenewereth et al. [99] identified two minor alleles (rs38138675, rs8192772) that had odds ratios of 1.6-2.0 for decreased head and neck cancer survival, but these are also non-coding region differences. These were not among the eight CYP2E1 loci identified in a more recent genome-wide association study (for oral/pharyngeal cancer) by the same author [100].
A PubMed search for CYP2E1 polymorphisms and diseases yielded 493 hits. Included among the study topics were alcoholism, systolic disfunction, coronary artery lesions, ischemic stroke, liver function, pre-term birth, semen quality, hepatitis, gout, oral leukoplakia, lupus, pancreatitis, Parkinson’s disease, non-alcoholic fatty liver disease, hypertension, oral fibrosis, leprosy, tuberculosis, schizophrenia, endometriosis, chronic obstructive pulmonary disease, oral cleft, cirrhosis, gastritis, amyotrophic lateral sclerosis, and various cancers including bladder, lung, gastric, Hodgkin’s and non-Hodgkin’s lymphoma, cervical, leukemia, head and neck, stomach, prostate, ovarian, breast, liver, and pancreatic. As pointed out by Daly [8], there may prove to be some associations with alcoholic liver disease and nasopharyngeal and lung cancer, but overall the associations are still not very striking. It is possible that stronger associations may be seen in future studies.
9. Conclusions and Future Directions
More than fifty years after the first evidence that a microsomal oxidation system might be involved in ethanol metabolism, what have we learned? We definitely know that some P450s can oxidize ethanol to acetaldehyde, the major one being P450 2E1. P450 2E1 appears to be one of the more invariant P450s across species [101], but it does not have a critical role in physiology as judged by the mouse Cyp2e1−/− phenotype [29]. P450 2E1 can contribute to in vivo ethanol metabolism, although this role is shared with alcohol dehydrogenase and possibly other systems. We know that the structure of P450 2E1 is such that it can explain the preference of this P450 in oxidizing small molecules [48, 75], but the enzyme can also change conformations and expand its active site to accommodate larger molecules [76, 82]. P450 2E1 can oxidize acetaldehyde to acetic acid [88, 102, 103], and the kinetics of this process are also unusual [88]. In our experience, the appearance of a rate-limiting step after product formation [87, 88] is still unusual among P450s and is not explained by product affinity in the case of acetaldehyde [104].
Although localized production of ROS can be attributed to P450 2E1, its contribution to systemic ROS cannot, at least in rodents [66]. P450 2E1 clearly contributes to the metabolism of many cancer suspects, including vinyl monomers, halogenated hydrocarbons, and dialkylnitrosamines [45, 48, 105]. Epidemiological studies have not revealed strong associations between SNVs and cancer or any other diseases to date, but it is possible that analysis of subsets of the population exposed to known pro-toxicants (e.g., vinyl chloride) might be more revealing if enough individuals could be identified. One issue is that the number of known SNVs is small and knowledge of their effects on enzyme function is limited. The regulation of expression—and inhibition—are complex in animal models [25]. The concentration of human P450 2E1 does vary considerably (≥ 10-fold) [106-108]. It is possible that a phenotypic analysis of P450 2E1 (function) (e.g., chlorzoxazone phenotyping) [109] might be more revealing in the analysis of disease states with P450 2E1.
Highlights:
P450 2E1 contributes to ethanol metabolism.
P450 2E1 also has a role in the oxidation of some drugs and chemical carcinogens.
P450 2E1 does not cause global oxidative stress but it can be local.
The kinetic mechanism is unusual and leads to some apparent anomalies.
Single nucleotide variations exist but have not been implicated in diseases much.
Acknowledgments
Grant support: This work was supported by the National Institutes of Health grant R01 GM118122.
Abbreviations used:
- P450 (or CYP)
cytochrome P450
- ROS
reactive oxygen species
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rendic S, Guengerich FP, Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals, Chem. Res. Toxicol, 28 (2015) 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ortiz de Montellano PR, Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015. [Google Scholar]
- [3].McLean KJ, Leys D, Munro AW, Microbial cytochromes P450, in: Ortiz de Montellano PR (Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015, pp. 261–407. [Google Scholar]
- [4].Guengerich FP, Cytochrome P450 catalysis in natural product biosynthesis, in: Bollinger M, Booker S, Bandarian V (Eds.), Comprehensive Natural Products, III: Chemistry and Biology Elsevier, New York, 2020, in press. [Google Scholar]
- [5].Auchus RJ, Miller WL, P450 enzymes in steroid processing, in: Ortiz de Montellano PR (Ed.), Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015, pp. 851–879. [Google Scholar]
- [6].Brodie AMH, Aromatase inhibition and its pharmacologic implications, Biochem. Pharmacol, 34 (1985) 3213–3219. [DOI] [PubMed] [Google Scholar]
- [7].Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A, History of aromatase: saga of an important biological mediator and therapeutic target, Endocrin. Rev, 30 (2009) 343–375. [DOI] [PubMed] [Google Scholar]
- [8].Daly AK, Polymorphic variants of cytochrome P450: Relevance to cancer and other diseases, Adv. Pharmacol, 74 (2015) 85–111. [DOI] [PubMed] [Google Scholar]
- [9].Guengerich FP, Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy, Cancer Res, 48 (1988) 2946–2954. [PubMed] [Google Scholar]
- [10].Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, Luch A, Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation, Chem. Res. Toxicol, 15 (2002) 1127–1135. [DOI] [PubMed] [Google Scholar]
- [11].Kang JS, Wanibuchi H, Morimura K, Gonzalez FJ, Fukushima S, Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo, Cancer Res, 67 (2007) 11141–11146. [DOI] [PubMed] [Google Scholar]
- [12].Bosron WF, Li TK, Vallee BL, Heterogeneity and new molecular forms of human liver alcohol dehydrogenase, Biochem. Biophys. Res. Commun, 91 (1979) 1549–1555. [DOI] [PubMed] [Google Scholar]
- [13].Edenberg HJ, Bosron WF, Alcohol dehydrogenases, in: Guengerich FP (Ed.), Vol. 3, Biotransformation, McQueen CA (Series Ed.), Comprehensive Toxicology, Elsevier, Oxford, U.K., 2010, pp. 111–130. [Google Scholar]
- [14].Vasilou V, Petersen DR, Aldehyde dehydrogenases, in: Guengerich FP (Ed.), Vol. 3, Biotransformation, McQueen CA (Series Ed.), Comprehensive Toxicology, Elsevier, Oxford, U.K., 2010, pp. 131–147. [Google Scholar]
- [15].Bradford BU, Seed CB, Handler JA, Forman DT, Thurman RG, Evidence that catalase is a major pathway of ethanol oxidation in vivo: dose-response studies in deer mice using methanol as a selective substrate, Arch. Biochem. Biophys, 303 (1993) 172–176. [DOI] [PubMed] [Google Scholar]
- [16].Orme-Johnson WH, Ziegler DM, Alcohol mixed function oxidase activity of mammalian liver micoromes, Biochem. Biophys. Res. Commun, 21 (1965) 78–82. [DOI] [PubMed] [Google Scholar]
- [17].Lieber CS, DeCarli LM, Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding, Science, 162 (1968) 917–918. [DOI] [PubMed] [Google Scholar]
- [18].Lieber CS, DeCarli LM, Hepatic microsomal ethanol oxidizing system: In vitro chracteristics and adaptive properties in vivo, J. Biol. Chem, 245 (1970) 2505–2512. [PubMed] [Google Scholar]
- [19].Mezey E, Potter JJ, Reed WD, Ethanol oxidation by a component of liver microsomes rich in cytochrome P-450, J. Biol. Chem, 248 (1973) 1183–1187. [PubMed] [Google Scholar]
- [20].Miwa GT, Levin W, Thomas PE, Lu AYH, The direct oxidation of ethanol by a catalase- and alcohol dehydrogenase-free reconstituted system containing cytochrome P-450, Arch. Biochem. Biophys, 187 (1978) 464–475. [DOI] [PubMed] [Google Scholar]
- [21].Koop DR, Morgan ET, Tarr GE, Coon MJ, Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits, J. Biol. Chem, 257 (1982) 8472–8480. [PubMed] [Google Scholar]
- [22].Wrighton SA, Thomas PE, Ryan DE, Levin W, Purification and characterization of ethanol-inducible human hepatic cytochrome P-450HLj, Arch. Biochem. Biophys, 258 (1987) 292–297. [DOI] [PubMed] [Google Scholar]
- [23].Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ, Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s: transcriptional and post-transcriptional regulation of the rat enzyme, J. Biol. Chem, 261 (1986) 16689–16697. [PubMed] [Google Scholar]
- [24].Umeno M, McBride OW, Yang C-S, Gelboin HV, Gonzalez FJ, Human ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression, Biochemistry, 27 (1988) 9006–9013. [DOI] [PubMed] [Google Scholar]
- [25].Gonzalez FJ, The 2006 Bernard B. Brodie Award Lecture. Cyp2e1, Drug Metab. Dispos, 35 (2007) 1–8. [DOI] [PubMed] [Google Scholar]
- [26].Takahashi T, Lasker JM, Rosman AS, Lieber CS, Induction of cytochrome P-4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA, Hepatology, 17 (1993) 236–245. [PubMed] [Google Scholar]
- [27].Lee JK, Chung HJ, Fischer L, Fischer J, Gonzalez FJ, Jeong H, Human placental lactogen induces CYP2E1 expression via PI 3-kinase pathway in female human hepatocytes, Drug Metab. Dispos, 42 (2014) 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Valentine JL, Lee SS, Seaton MJ, Asgharian B, Farris G, Corton JC, Gonzalez FJ, Medinsky MA, Reduction of benzene metabolism and toxicity in mice that lack CYP2E1 expression, Toxicol. Appl. Pharmacol, 141 (1996) 205–213. [DOI] [PubMed] [Google Scholar]
- [29].Lee SST, Buters JTM, Pineau T, Fernandez-Salguero P, Gonzalez FJ, Role of CYP2E1 in the hepatotoxicity of acetaminophen, J. Biol. Chem, 271 (1996) 12063–12067. [DOI] [PubMed] [Google Scholar]
- [30].Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ, The Cyp2e1-humanized transgenic mouse: role of Cyp2e1 in acetaminophen hepatotoxicity, Drug Metab. Dispos, 33 (2005) 449–457. [DOI] [PubMed] [Google Scholar]
- [31].Kang JS, Wanibuchi H, Morimura K, Wongpoomchai R, Chusiri Y, Gonzalez FJ, Fukushima S, Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity, Toxicol. Appl. Pharmacol, 228 (2008) 295–300. [DOI] [PubMed] [Google Scholar]
- [32].Yamasaki C, Kataoka M, Kato Y, Kakuni M, Usuda S, Ohzone Y, Matsuda S, Adachi Y, Ninomiya S, Itamoto T, Asahara T, Yoshizato K, Tateno C, In vitro evaluation of cytochrome P450 and glucuronidation activities in hepatocytes isolated from liver-humanized mice, Drug Metab. Pharmacokinet, 25 (2010) 539–550. [DOI] [PubMed] [Google Scholar]
- [33].Luo YS, Furuya S, Soldatov VY, Kosyk O, Yoo HS, Fukushima H, Lewis L, Iwata Y, Rusyn I, Metabolism and toxicity of trichloroethylene and tetrachloroethylene in cytochrome P450 2E1 knockout and humanized transgenic mice, Toxicol. Sci, 164 (2018) 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng J, Chen C, Kristopher KW, Manna SK, Scerba M, Friedman FK, Luecke H, Idle JR, Gonzalez FJ, Identification of 2-piperidone as a biomarker of CYP2E1 activity through metabolomic phenotyping, Toxicol. Sci, 135 (2013) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kharasch ED, Thummel KE, Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane, Anesthesiology, 79 (1993) 795–807. [DOI] [PubMed] [Google Scholar]
- [36].Kharasch ED, Thummel KE, Mautz D, Bosse S, Clinical enflurane metabolism by cytochrome P450 2E1, Clin. Pharmacol. Therapeut, 55 (1994) 434–440. [DOI] [PubMed] [Google Scholar]
- [37].Kharasch ED, Hankins DC, Cox K, Clinical isoflurane metabolism by cytochrome P450 2E1, Anesthesiology, 90 (1999) 766–771. [DOI] [PubMed] [Google Scholar]
- [38].Wandel C, Neff S, Keppler G, Bohrer H, Stockinger K, Wilkinson GR, Wood M, Martin E, The relationship between cytochrome P4502E1 activity and plasma fluoride levels after sevoflurane anesthesia in humans, Anesthesia Analges, 85 (1997) 924–930. [DOI] [PubMed] [Google Scholar]
- [39].Restrepo JG, Garcia-Martin E, Martinez C, Agundez JA, Polymorphic drug metabolism in anaesthesia, Curr. Drug Metab, 10 (2009) 236–246. [DOI] [PubMed] [Google Scholar]
- [40].van Rongen A, Valitalo PAJ, Peeters MYM, Boerma D, Huisman FW, van Ramshorst B, van Dongen EPA, van den Anker JN, Knibbe CAJ, Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen, Clin. Pharmacokinet, 55 (2016) 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bourdi M, Chen W, Peter RM, Martin JL, Buters JTM, Nelson SD, Pohl LR, Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis, Chem. Res. Toxicol, 9 (1996) 1159–1166. [DOI] [PubMed] [Google Scholar]
- [42].Eliasson E, Kenna JG, Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis, Mol. Pharmacol, 50 (1996) 573–582. [PubMed] [Google Scholar]
- [43].Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, Pohl LR, Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists, Anesthesia Analges, 94 (2002) 243–249. [DOI] [PubMed] [Google Scholar]
- [44].Boitier E, Beaune P, Xenobiotic-metabolizing enzymes as autoantigens in human autoimmune disorders. An update, Clin. Rev. Allergy. Immunol, 18 (2000) 215–239. [DOI] [PubMed] [Google Scholar]
- [45].Yang CS, Tu YY, Koop DR, Coon MJ, Metabolism of nitrosamines by purified rabbit liver cytochrome P-450 isozymes, Cancer Res, 45 (1985) 1140–1145. [PubMed] [Google Scholar]
- [46].Lai DY, Arcos JC, Dialkylnitrosamine bioactivation and carcinogenesis, Life Sci, 27 (1980) 2149–2165. [DOI] [PubMed] [Google Scholar]
- [47].Wong LCK, Winston JM, Hong CB, Plotnick H, Carcinogenicity and toxicity of 1,2-dibromoethane in the rat, Toxicol. Appl. Pharmacol, 63 (1982) 155–165. [DOI] [PubMed] [Google Scholar]
- [48].Guengerich FP, Kim DH, Iwasaki M, Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects, Chem. Res. Toxicol, 4 (1991) 168–179. [DOI] [PubMed] [Google Scholar]
- [49].Guengerich FP, Avadhani NG, Roles of cytochrome P450 enzymes in the metabolism of ethanol and carcinogens, in: Vasilou V, Zakhari S, Mishra L, Seitz HK (Eds.), Adv. Exp. Med. Biol., Alcohol and Cancer Proc, 3rd Int. Conf. Alcohol and Cancer Crete, Greece, 2018, pp. 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Raucy JL, Kraner JC, Lasker JM, Bioactivation of halogenated hydrocarbons by cytochrome P4502E1, Crit. Rev. Toxicol, 23 (1993) 1–20. [DOI] [PubMed] [Google Scholar]
- [51].Dahl GA, Miller JA, Miller EC, Vinyl carbamate as a promutagen and a more carcinogenic analog of ethyl carbamate, Cancer Res, 38 (1978) 3793–3804. [PubMed] [Google Scholar]
- [52].Guengerich FP, Kim DH, Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine, Chem. Res. Toxicol, 4 (1991) 413–421. [DOI] [PubMed] [Google Scholar]
- [53].Ghanayem BI, Inhibition of urethane-induced carcinogenicity in Cyp2e1−/− in comparison to Cyp2e1+/+ mice, Toxicol. Sci, 95 (2007) 331–339. [DOI] [PubMed] [Google Scholar]
- [54].Navasumrit P, Ward TH, O'Connor PJ, Nair J, Frank N, Bartsch H, Ethanol enhances the formation of endogenously and exogenously derived adducts in rat hepatic DNA, Mutat. Res, 479 (2001) 81–94. [DOI] [PubMed] [Google Scholar]
- [55].Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, Bartsch H, Seitz HK, Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease, Hepatology, 50 (2009) 453–461. [DOI] [PubMed] [Google Scholar]
- [56].Peccerella T, Arslic-Schmitt T, Mueller S, Linhart KB, Seth D, Bartsch H, Seitz HK, Chronic ethanol consumption and generation of etheno-DNA adducts in cancer-prone tissues, Adv. Exp. Med. Biol, 1032 (2018) 81–92. [DOI] [PubMed] [Google Scholar]
- [57].Kim D-H, Guengerich FP, Formation of the DNA adduct S-[2-(N7-guanyl)ethyl]glutathione from ethylene dibromide: effects of modulation of glutathione and glutathione S-transferase levels and the lack of a role for sulfation, Carcinogenesis, 11 (1990) 419–424. [DOI] [PubMed] [Google Scholar]
- [58].Ekström G, Ingelman-Sundberg M, Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1), Biochem. Pharmacol, 38 (1989) 1313–1319. [DOI] [PubMed] [Google Scholar]
- [59].Rashba-Step J, Cederbaum AI, Generation of reactive oxygen intermediates by human liver microsomes in the presence of NADPH or NADH, Mol. Pharmacol, 45 (1994) 150–157. [PubMed] [Google Scholar]
- [60].Cederbaum AI, Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells, J. Gastroenterol. Hepatol, 21 Suppl 3 (2006) S22–S25. [DOI] [PubMed] [Google Scholar]
- [61].Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI, Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice, Free Rad. Biol. Med, 49 (2010) 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJH 2nd, Ischiropoulos, Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations, Free Rad. Biol. Med, 52 (2012) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC, Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning?, Free Rad. Biol. Med, 38 (2005) 698–710. [DOI] [PubMed] [Google Scholar]
- [64].Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, Fitzgerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ 2nd, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP, Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning, Free Rad. Biol. Med, 38 (2005) 711–718. [DOI] [PubMed] [Google Scholar]
- [65].Dostalek M, Brooks JD, Hardy KD, Milne GL, Moore MM, Sharma S, Morrow JD, Guengerich FP, In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers, Mol. Pharmacol, 72 (2007) 1419–1424. [DOI] [PubMed] [Google Scholar]
- [66].Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, Martin MV, Guengerich FP, Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems, J. Biol. Chem, 283 (2008) 17147–17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shinkyo R, Guengerich FP, Cytochrome P450 7A1 cholesterol 7α-hydroxylation: individual reaction steps in the catalytic cycle and rate-limiting ferric iron reduction, J. Biol. Chem, 286 (2011) 4632–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yun CH, Kim KH, Calcutt MW, Guengerich FP, Kinetic analysis of oxidation of coumarins by human cytochrome P450 2A6, J. Biol. Chem, 280 (2005) 12279–12291. [DOI] [PubMed] [Google Scholar]
- [69].Peng HM, Im SC, Pearl NM, Turcu AF, Rege J, Waskell L, Auchus RJ, Cytochrome b5 activates the 17,20-lyase activity of human cytochrome P450 17A1 by increasing the coupling of NADPH consumption to androgen production, Biochemistry, 55 (2016) 4356–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kono H, Bradford BU, Yin M, Sulik K, Koop D, Peters J, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, Mason R, Thurman RG, CYP2E1 is not involved in early alcohol-induced liver injury, Am. J. Physiol. Gastrointest. Liver Physiol 277 (1999) G1259–G1267. [DOI] [PubMed] [Google Scholar]
- [71].Yin M, Gabele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG, Alcohol-induced free radicals in mice: direct toxicants or signaling molecules?, Hepatology, 34 (2001) 935–942. [DOI] [PubMed] [Google Scholar]
- [72].Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NB, Gordon DM, Pain D, Avadhani NG, Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation, J. Biol. Chem, 277 (2002) 40583–40593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bansal S, Liu CP, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG, Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress, J. Biol. Chem, 285 (2010) 24609–24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bansal S, Anandatheerthavarada HK, Prabu GK, Milne GL, Martin MV, Guengerich FP, Avadhani NG, Human cytochrome P450 2E1 mutations that alter mitochondrial targeting efficiency and susceptibility to ethanol-induced toxicity in cellular models, J. Biol. Chem, 288 (2013) 12627–12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Porubsky PR, Meneely KM, Scott EE, Structures of human cytochrome P-450 2E1. Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates, J. Biol. Chem, 283 (2008) 33698–33707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Porubsky PR, Battaile KP, Scott EE, Human cytochrome P450 2E1 structures with fatty acid analogs reveal a previously unobserved binding mode, J. Biol. Chem, 285 (2010) 22282–22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].DeVore NM, Meneely KM, Bart AG, Stephens ES, Battaile KP, Scott EE, Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine, FEBS J, 279 (2012) 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Vogt AD, Di Cera E, Conformational selection or induced fit? A critical appraisal of the kinetic mechanism, Biochemistry, 51 (2012) 5894–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Koshland DE Jr., Nemethy G, Filmer D, Comparison of experimental binding data and theoretical models in proteins containing subunits, Biochemistry, 5 (1966) 365–385. [DOI] [PubMed] [Google Scholar]
- [80].Johnson KA, Role of induced fit in enzyme specificity: a molecular forward/reverse switch, J. Biol. Chem, 283 (2008) 26297–26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Guengerich FP, Wilkey CJ, Glass SM, Reddish MJ, Conformational selection dominates binding of steroids to human cytochrome P450 17A1, J. Biol. Chem, 294 (2019) 10028–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Guengerich FP, Wilkey CJ, Phan TTN, Human cytochrome P450 enzymes bind drugs and other substrates mainly through conformational-selection modes, J. Biol. Chem, 294 (2019) 10928–10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ortiz de Montellano PR, Substrate oxidation, in: Ortiz de Montellano PR (Ed.), Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015, pp. 111–176. [Google Scholar]
- [84].Guengerich FP, Perspective: Mechanisms of cytochrome P450-catalyzed oxidations, ACS Catal., 8 (2018) 10964–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gillam EM, Guo Z, Guengerich FP, Expression of modified human cytochrome P450 2E1 in Escherichia coli, purification, and spectral and catalytic properties, Arch. Biochem. Biophys, 312 (1994) 59–66. [DOI] [PubMed] [Google Scholar]
- [86].Yamazaki H, Nakano M, Gillam EM, Bell LC, Guengerich FP, Shimada T, Requirements for cytochrome b5 in the oxidation of 7-ethoxycoumarin, chlorzoxazone, aniline, and N-nitrosodimethylamine by recombinant cytochrome P450 2E1 and by human liver microsomes, Biochem. Pharmacol, 52 (1996) 301–309. [DOI] [PubMed] [Google Scholar]
- [87].Bell LC, Guengerich FP, Oxidation kinetics of ethanol by human cytochrome P450 2E1. Rate-limiting product release accounts for effects of isotopic hydrogen substitution and cytochrome b5 on steady-state kinetics, J. Biol. Chem, 272 (1997) 29643–29651. [DOI] [PubMed] [Google Scholar]
- [88].Bell-Parikh LC, Guengerich FP, Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde, J. Biol. Chem, 274 (1999) 23833–23840. [DOI] [PubMed] [Google Scholar]
- [89].Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, Yokoi T, Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli, Prot. Express. Purif, 24 (2002) 329–337. [DOI] [PubMed] [Google Scholar]
- [90].Wade D, Yang CS, Metral CJ, Roman JM, Hrabie JA, Riggs CW, Anjo T, Keefer LK, Mico BA, Deuterium isotope effect on denitrosation and demethylation of N-nitrosodimethylamine by rat liver microsomes, Cancer Res, 47 (1987) 3373–3377. [PubMed] [Google Scholar]
- [91].Guengerich FP, Bell LC, Okazaki O, Interpretations of cytochrome P450 mechanisms from kinetic studies, Biochimie, 77 (1995) 573–580. [DOI] [PubMed] [Google Scholar]
- [92].Kuby SA, A Study of Enzymes, Vol. I, Enzyme Catalysis, Kinetics, and Substrate Binding, CRC Press, Boca Raton, FL, 1991. [Google Scholar]
- [93].Guengerich FP, Kinetic deuterium isotope effects in cytochrome P450 enzyme reactions, Methods Enzymol, 596 (2017) 217–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Johnson KA, Simpson ZB, Blom T, Global Kinetic Explorer: A new computer program for dynamic simulation and fitting of kinetic data, Anal. Biochem, 387 (2009) 20–29. [DOI] [PubMed] [Google Scholar]
- [95].Hu Y, Oscarson M, Johansson I, Yue QY, Dahl ML, Tabone M, Arinco S, Albano E, Ingelman-Sundberg M, Genetic polymorphism of human CYP2E1: characterization of two variant alleles, Mol. Pharmacol, 51 (1997) 370–376. [PubMed] [Google Scholar]
- [96].Hildesheim A, Anderson LM, Chen C, Cheng Y, Brinton LA, Daly AK, Reed CD, Chen I, Caporaso NE, Hsu M, Chen J, Idle JR, Hoover RN, CYP2E1 genetic polymorphisms and risk of nasopharyngeal carcinoma in Taiwan, J. Natl. Cancer Inst, 89 (1997) 1207–1212. [DOI] [PubMed] [Google Scholar]
- [97].Hayashi S, Watanabe J, Kawajiri K, Genetic polymorphisms in the 5'-flanking region change transcriptional regulation of the human P450IIE1 gene, J. Biochem, 110 (1991) 559–565. [DOI] [PubMed] [Google Scholar]
- [98].Ye XH, Song L, Peng L, Bu Z, Yan SX, Feng J, Zhu XL, Liao XB, Yu XL, Yan D, Association between the CYP2E1 polymorphisms and lung cancer risk: a meta-analysis, Mol. Genet. Genom, 290 (2015) 545–558. [DOI] [PubMed] [Google Scholar]
- [99].Hakenewerth AM, Millikan RC, Rusyn I, Herring AH, Weissler MC, Funkhouser WK, North KE, Barnholtz-Sloan JS, Olshan AF, Effects of polymorphisms in alcohol metabolism and oxidative stress genes on survival from head and neck cancer, Cancer Epidemiol, 37 (2013) 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lesseur C, Diergaarde B, Olshan AF, Wunsch-Filho V, Ness AR, Liu G, Lacko M, Eluf-Neto J, Franceschi S, Lagiou P, Macfarlane GJ, Richiardi L, Boccia S, Polesel J, Kjaerheim K, Zaridze D, Johansson M, Menezes AM, Curado MP, Robinson M, Ahrens W, Canova C, Znaor A, Castellsague X, Conway DI, Holcatova I, Mates D, Vilensky M, Healy CM, Szeszenia-Dabrowska N, Fabianova E, Lissowska J, Grandis JR, Weissler MC, Tajara EH, Nunes FD, de Carvalho MB, Thomas S, Hung RJ, Peters WH, Herrero R, Cadoni G, Bueno-de-Mesquita HB, Steffen A, Agudo A, Shangina O, Xiao X, Gaborieau V, Chabrier A, Anantharaman D, Boffetta P, Amos CI, McKay JD, Brennan P, Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer, Nat. Genet, 48 (2016) 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Guengerich FP, Comparisons of catalytic selectivity of cytochrome P450 subfamily enzymes from different species, Chem.-Biol Interact, 106 (1997) 161–182. [DOI] [PubMed] [Google Scholar]
- [102].Terelius Y, Norsten-Höög C, Cronholm T, Ingelman-Sundberg M, Acetaldehyde as a substrate for ethanol-inducible cytochrome P450 (CYP2E1), Biochem. Biophys. Res. Commun, 179 (1991) 689–694. [DOI] [PubMed] [Google Scholar]
- [103].Kunitoh S, Imaoka S, Hiroi T, Yabusaki Y, Monna T, Funae Y, Acetaldehyde as well as ethanol is metabolized by human CYP2E1, J. Pharmacol. Expt. Therapeut, 280 (1997) 527–532. [PubMed] [Google Scholar]
- [104].Chowdhury G, Calcutt MW, Nagy LD, Guengerich FP, Oxidation of methyl and ethyl nitrosamines by cytochrome P450 2E1 and 2B1, Biochemistry, 51 (2012) 9995–10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Constan AA, Sprankle CS, Peters JM, Kedderis GL, Everitt JI, Wong BA, Gonzalez FL, Butterworth BE, Metabolism of chloroform by cytochrome P450 2E1 is required for induction of toxicity in the liver, kidney, and nose of male mice, Toxicol. Appl. Pharmacol, 160 (1999) 120–126. [DOI] [PubMed] [Google Scholar]
- [106].Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP, Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians, J. Pharmacol. Expt. Therapeut, 270 (1994) 414–423. [PubMed] [Google Scholar]
- [107].Guengerich FP, Human cytochrome P450 enzymes, in: Ortiz de Montellano PR (Ed.), Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015, pp. 523–785. [Google Scholar]
- [108].Yang X, Zhang B, Molony C, Chudin E, Hao K, Zhu J, Gaedigk A, Suver C, Zhong H, Leeder JS, Guengerich FP, Strom SC, Schuetz E, Rushmore TH, Ulrich RG, Slatter JG, Schadt EE, Kasarskis A, Lum PY, Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver, Genome Res, 20 (2010) 1020–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kim RB, Yamazaki H, Chiba K, O'Shea D, Mimura M, Guengerich FP, Ishizaki T, Shimada T, Wilkinson GR, In vivo and in vitro characterization of CYP2E1 activity in Japanese and Caucasians, J. Pharmacol. Expt. Therapeut, 279 (1996) 4–11. [PubMed] [Google Scholar]