Abstract
Background
Recent American College of Cardiology/American Heart Association (ACC/AHA) Primary Prevention Guidelines recommended considering low-dose aspirin therapy only among adults 40–70 years of age who are at higher atherosclerotic cardiovascular disease (ASCVD) risk but not at high risk of bleeding. However, it remains unclear how these patients are best identified. The present study aimed to assess the value of coronary artery calcium (CAC) for guiding aspirin allocation for primary prevention using 2019 aspirin meta-analysis data on CVD relative risk reduction (RRR) and bleeding risk.
Methods
The study included 6,470 participants from the Multi-Ethnic Study of Atherosclerosis (MESA). ASCVD risk was estimated using the Pooled Cohort Equations (PCE) and 3 strata were defined: <5%, 5–20% and >20%. All participants underwent CAC scoring at baseline and CAC scores were stratified as =0, 1–99, ≥100 and ≥400. A 12% RRR in CVD events was used for 5-year number needed to treat (NNT5) calculations, and a 42% relative risk increase in major bleeding events was used for 5-year number needed to harm (NNH5) estimations.
Results
Only 5% of MESA participants would qualify for aspirin consideration for primary prevention according to ACC/AHA guidelines and using >20% estimated ASCVD risk to define “higher risk”. Benefit/harm calculations were restricted to aspirin-naïve participants <70 years not at high risk of bleeding (N=3,540). The overall NNT5 with aspirin to prevent one CVD event was 476 and the NNH5 was 355. The NNT5 was also greater than or similar to the NNH5 among estimated ASCVD risk strata. Conversely, CAC≥100 and CAC≥400 identified subgroups in which NNT5 was lower than NNH5. This was true both overall (for CAC≥100, NNT5=140 vs NNH5=518) as well as within ASCVD risk strata. Also, CAC=0 identified subgroups in which the NNT was much higher than the NNH5 (overall, NNT5=1,190 vs NNH5=567).
Conclusions
CAC may be superior to the PCE to inform allocation of aspirin in primary prevention. Implementation of current 2019 ACC/AHA guideline recommendations together with the use of CAC for further risk assessment may result in a more personalized, safer allocation of aspirin in primary prevention. Confirmation of these findings in experimental settings is needed.
Keywords: aspirin, bleeding, cardiovascular disease, coronary artery calcium, risk, safety
Introduction
The role of aspirin in the primary prevention of atherosclerotic cardiovascular disease (ASCVD) events is currently controversial (1, 2). Since 2018, three landmark randomized controlled trials and two large meta-analyses have suggested a limited benefit of low-dose aspirin for the primary prevention of ASCVD events, which on average is offset by an increased risk of bleeding in elderly individuals as well as in those at higher baseline hemorrhagic risk (3–7). Still, in the largest primary prevention meta-analysis available to date, a non-negligible 11% relative risk reduction (RRR) in ASCVD events was observed for aspirin (7).
Based on these updated data, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) Primary Prevention Guidelines altered their recommendation for aspirin use for primary prevention, from a previous class I to a much more tentative IIb recommendation “among select adults 40 to 70 years of age who are at higher ASCVD risk but not at increased bleeding risk” (8). It remains unclear, however, how should clinicians best identify patients likely to derive a net benefit from aspirin therapy in routine primary prevention.
Coronary artery calcium (CAC) is a robust marker of coronary atherosclerotic plaque burden (9, 10), and a powerful ACC/AHA guideline-endorsed prognostic tool for prediction of ASCVD events and personalized allocation of statin therapy (8, 11). It has been suggested that CAC may also have value guiding the allocation of other preventive pharmacotherapies, including aspirin, across estimated ASCVD risk groups (12–14). A 2014 analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) suggested that a CAC score ≥100 could help identify patients most likely to derive net benefit from chronic aspirin therapy, while in CAC=0 patients, aspirin would yield net harm (14). However, the study used 2009 meta-analysis data on aspirin safety and efficacy (15), and did not use observed bleeding data, instead relying on a fixed aspirin-related bleeding risk for all studied subgroups.
The aims of the present study were thus to describe the implications of the recent 2019 ACC/AHA Primary Prevention Guidelines (8) in terms of aspirin eligibility for primary ASCVD prevention purposes, and to assess the potential value of CAC for guiding allocation of aspirin therapy using the most recent, highest quality meta-analysis data available on aspirin-related ASCVD RRR and bleeding risk (7).
Methods
A detailed description of the research methods used is presented below. Requests to access the study dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Collaborative Health Studies Coordinating Center, University of Washington, at chsccweb@u.washington.edu. The analyses that support the findings of the present study are available from the corresponding author upon reasonable request.
Study design
MESA is an ongoing, NHLBI-funded, observational, community-based, prospective cohort study of 6,814 men and women of 4 self-identified racial/ethnic groups (non-Hispanic Whites, African Americans, Hispanics, Chinese Americans) living in the US. MESA participants were recruited between 2000 and 2002 in 6 field centers: Wake Forest University in Winston-Salem (NC), Columbia University in New York (NY), Johns Hopkins University in Baltimore (MD), University of Minnesota in Minneapolis (MN), Northwestern University in Chicago (IL), and University of California in Los Angeles (CA) (16). The study was approved by the institutional review committee of each site, and all participants provided written informed consent at study entry. The age range at baseline was 45 to 84 years, and participants had to be free of clinically overt cardiovascular conditions (including ASCVD) to be eligible for inclusion. Since then, participants have been followed for incident events for a median of more than 14 years. Further details on the MESA study design have been described elsewhere (10, 12, 14, 16).
Study population
All MESA participants were considered for inclusion in the present study. Of them, participants with missing information on CAC burden, aspirin use, variables used by the Pooled Cohort Equations (PCE; age, sex, race/ethnicity, systolic and diastolic blood pressure, medication use for hypertension, serum cholesterol levels, history of diabetes, and tobacco use), and longitudinal follow-up were excluded from the analyses (Figure 1).
Figure 1.

Flow of MESA participants included in the study.
*Participants included in the aspirin benefit/harm calculations
Abbreviations: MESA = Multi-Ethnic Study of Atherosclerosis; N = number
To assess the value of CAC to inform allocation of aspirin therapy under the 2019 ACC/AHA primary prevention guideline recommendations, additional exclusions were implemented for benefit/harm calculations: participants under chronic treatment with aspirin at baseline (defined as any aspirin dose taken 3 or more times per week), participants ages ≥70 years, and those with evidence of high bleeding risk features (8, 17, 18) at MESA Visit 1. The latter included a history of renal failure, severe liver disease, concurrent anticoagulation drug use, and uncontrolled hypertension, which was defined as either a systolic blood pressure >160mmHg, or a diastolic blood pressure >100mmHg at baseline (MESA Visit 1). This defined the “aspirin-naïve, <70 years, not high bleeding risk” subpopulation for the benefit/harm calculations. Information on history of upper gastrointestinal pain, gastrointestinal ulcers, bleeding disorders, thrombocytopenia, or chronic use of nonsteroidal anti-inflammatory drugs (8, 17, 18) was not available.
Baseline clinical evaluation in MESA
All MESA participants underwent a detailed baseline assessment as part of study Visit 1 (16). This included assessment of demographics, family history of cardiovascular disease (CVD), tobacco use, personal history of other cardiovascular risk factors and medical conditions, and medication use, which were evaluated using standardized questionnaires. Standardized measurements of body mass index, systolic and diastolic blood pressure were also performed, and blood samples were drawn from all participants to measure non-fasting levels of total and high-density lipoprotein cholesterol levels (16).
Ten-Year ASCVD risk estimation
For the purposes of the present study, 10-year ASCVD risk was estimated using the PCE for each participant using baseline demographics and relevant risk factor information. Three 10-year ASCVD risk strata were defined: <5%, 5 – 20%, >20%.
CAC measurement
All MESA participants underwent computed tomographic scanning for CAC assessment as part of Visit 1. Full details on the MESA computed tomographic scanning protocol have been reported elsewhere (10, 12, 14, 16). CAC was scored using the Agatston method (19). The following clinically relevant CAC groups were defined: CAC=0, CAC 1–99, and CAC≥100. In exploratory analyses, CAC≥400 was also evaluated.
Outcome definitions and event ascertainment
The outcomes of interest for the present study were CVD events (defined as a composite of fatal/non-fatal myocardial infarction [MI], fatal/non-fatal stroke, and other CVD death) and major bleeding events. The rates of coronary heart disease (CHD) events (defined as a composite of fatal/non-fatal MI and CHD deaths) were also calculated and reported. Major bleeding events were identified using International Classification of Diseases (ICD) codes (versions 9 and 10) for any bleeding in participants requiring a hospitalization during follow-up (Supplementary Table S1). In MESA, study personnel conduct active surveillance to systematically identify hospitalizations and deaths of study participants. When a hospitalization is identified, the full list of ICD codes is abstracted from the discharge record. Validation studies in other databases have shown that a strategy using ICD codes to identify bleeding events accurately rules out major bleeding cases (20).
Statistical analyses
The number and proportion of study participants in whom aspirin could be considered for primary prevention of ASCVD according to the 2019 ACC/AHA guideline recommendations (i.e., individuals age <70 years with no high bleeding risk features) were calculated using a >20% estimated 10-year ASCVD risk-based definition of “higher risk” (8).
The following calculations were conducted in the subpopulation of aspirin-naïve participants, <70 years of age and not at high risk of bleeding. First, their baseline characteristics were described, overall and by baseline CAC burden. Chi-squared tests were used to compare categorical variables across CAC strata, and ANOVA and Kruskal-Wallis tests were used to compare normally and non-normally distributed continuous variables, respectively. The interplay between estimated 10-year ASCVD risk categories and baseline CAC burden was described graphically. Crude 5-year and 10-year incidence rates of CVD, CHD and major bleeding events were also computed, overall as well as by estimated 10-year ASCVD risk and baseline CAC. Cox Proportional Hazards regression models were used to compare the risk of CVD, CHD and major bleeding events, respectively, for increasing CAC categories, overall and by estimated ASCVD risk strata. Regression analyses were adjusted for study site, the risk factors included in the PCE, and statin use, and used 10-year follow-up data to maximize statistical power and allow for adequate adjustment for potential confounders.
Using post-hoc estimates by Zheng et al. pooled from primary prevention randomized trials of low-dose aspirin versus placebo (excluding studies of participants with peripheral arterial disease [7]), a 12% expected 5-year RRR in CVD events with chronic low-dose aspirin therapy was applied to the observed 5-year incidence proportion of CVD events, overall as well as in strata defined by baseline CAC burden and by baseline estimated ASCVD risk. Using the reciprocal of the absolute risk reduction, the number needed to treat at 5 years (NNT5) was calculated for each group. Similarly, using the observed 5-year rates of major bleeding events in MESA overall and in each CAC and ASCVD risk strata, and assuming a 42% 5-year relative risk increase (RRI) in major bleeding events (7), the absolute risk increase was calculated, and the number needed to harm at 5 years (NNH5) was computed as its reciprocal. The NNT5 and NNH5 were then compared graphically to characterize the benefit/harm balance of aspirin therapy, overall as well as among baseline CAC strata and estimated ASCVD risk, respectively.
Subgroup analyses by sex were also conducted. To increase the number of observed bleeding events included in the analyses and to evaluate the robustness of the 5-year results, a sensitivity analysis was conducted using 10-year follow-up CVD and bleeding event data, and Altman-Anderson’s method was applied to scale back to 5 years for NNT5 and NNH5 calculations (21). A second sensitivity analysis was conducted using meta-analytic RRR and RRI estimates restricted to aspirin primary prevention trials published after 2000 (7). These had to be re-calculated replicating the methodology by Zheng et al. (7) but excluding two studies of participants with peripheral arterial disease (POPADAD [22] and AAA [23]), yielding a pooled RRR of 9% and a pooled RRI of 37%. In a third sensitivity analysis, interim aspirin users during the first 5 years of follow-up were excluded from the benefit/harm calculations.
In a post-hoc analysis, the benefit/harm calculations were replicated among MESA participants who were using aspirin at baseline, were <70 years of age and had no high bleeding risk features. For these calculations, CVD events without aspirin were estimated to be 12% higher than those observed with aspirin, and bleeding events without aspirin were estimated to be 42% lower than those observed with aspirin. Finally, in another post-hoc analysis the number and proportion of study participants in whom aspirin could be considered for primary prevention purposes were re-calculated using a ≥100 CAC-based definition of “higher risk” (8).
All statistical analyses were performed using Stata version 15. A p value <0.05 was used as threshold of statistical significance.
Results
Study Participants
Of the 6,814 MESA participants, 6,470 had data on baseline CAC scores, chronic aspirin use at baseline, the risk factors used by the PCE, and on incident events during follow-up (Figure 1). Of these, 1,287 were already using aspirin at baseline, 1,233 were ≥70 years of age, and 410 had at least one high bleeding risk feature. The remaining 3,540 participants defined the “aspirin-naïve, <70 year old, not high bleeding risk” subpopulation.
Participants qualifying for aspirin therapy consideration using a >20% ASCVD risk definition of “higher risk”
Overall, 316 of the 6,470 participants (4.9%) were younger than 70 years of age, had no evidence of high bleeding risk features, and had an estimated 10-year ASCVD risk >20%. The majority of these individuals were men (N=250, 79.1%), and their age was typically in the 60–69 years range (N=261, 82.6%). Although CAC≥100 was common (N=133, 42.1%), the prevalence of CAC=0 (N=100, 31.7%) and of CAC 1–99 (N=83, 26.3%) was also high. Figure S1 displays the proportion of participants qualifying for consideration of aspirin therapy (using a >20% ASCVD risk definition of “higher risk”) by key baseline characteristics.
Baseline characteristics of the aspirin-naïve, <70 year, not high bleeding risk subpopulation
Mean age of the participants included in the benefit/harm calculations (N=3,540) was 56.5 years, 55% were women, and the median estimated 10-year ASCVD risk using the PCE was 5.1% (Table 1). A total of 353 participants (10%) used statins at baseline. The higher the baseline CAC burden, the older the mean age, the lower the proportion of women, and the worse the cardiovascular risk profile of the participants.
Table 1.
Baseline characteristics of study participants not using aspirin at baseline, age <70 years and with no high bleeding risk features.
| All | CAC=0 | CAC 1–99 | CAC≥100 | |
|---|---|---|---|---|
| N | 3,540 (100.0) | 2,219 (62.7) | 871 (24.6) | 450 (12.7) |
| Age, years | 56.5 (7.2) | 54.9 (6.9) | 58.3 (6.9) | 61.2 (6.4) |
| Women | 1,952 (55.1) | 1,412 (63.6) | 393 (45.1) | 147 (32.7) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 1,215 (34.3) | 693 (31.2) | 318 (36.5) | 204 (45.3) |
| African American | 1,029 (29.1) | 692 (31.2) | 240 (27.6) | 97 (21.6) |
| Hispanic | 857 (24.2) | 566 (25.5) | 198 (22.7) | 93 (20.7) |
| Chinese | 439 (12.4) | 268 (12.1) | 115 (13.2) | 56 (12.4) |
| Body mass index, kg/m2 | 28.5 (5.7) | 28.4 (5.8) | 28.6 (5.4) | 28.8 (5.5) |
| Current smoker | 544 (15.4) | 310 (14.0) | 151 (17.3) | 83 (18.4) |
| Family history of ASCVD | 1,340 (39.9) | 763 (36.0) | 372 (45.1) | 220 (48.8) |
| Diabetes | 357 (10.1) | 173 (7.8) | 100 (11.5) | 84 (18.7) |
| Systolic blood pressure, mmHg | 120 (17) | 118 (17) | 123 (16) | 126 (17) |
| Diastolic blood pressure, mmHg | 72 (10) | 71 (10) | 73 (9) | 74 (9) |
| Medication use for hypertension | 961 (27.2) | 510 (23.0) | 274 (31.5) | 177 (39.3) |
| LDL cholesterol, mg/dL | 120 (32) | 118 (30) | 123 (34) | 122 (33) |
| HDL cholesterol, mg/dL | 51 (15) | 52 (15) | 49 (14) | 48 (15) |
| Statin use at baseline | 353 (10.0) | 163 (7.4) | 110 (12.6) | 80 (17.8) |
| Estimated 10-year ASCVD Risk* | 5.1 (2.4, 10.0) | 3.7 (1.6, 7.6) | 7.1 (4.0, 12.1) | 10.7 (6.2, 17.1) |
Using the Pooled Cohort Equations
Results presented as number (%), mean (standard deviation), or median (interquartile range). All p values for comparisons across CAC categories were <0.001, except for BMI (p=0.03)
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; HDL = high density lipoprotein; LDL = low density lipoprotein; N = number
Figure 2 displays the interplay between baseline estimated 10-year ASCVD risk and CAC burden in these individuals. The higher the CAC burden, the more frequent intermediate and high 10-year ASCVD risk estimations, and viceversa. Notably, CAC=0 was highly prevalent across all estimated ASCVD risk strata, including a 32% prevalence in the >20% estimated 10-year risk group. In individuals at 5 to 20% estimated risk, 49% had CAC=0, and 18% CAC≥100.
Figure 2.
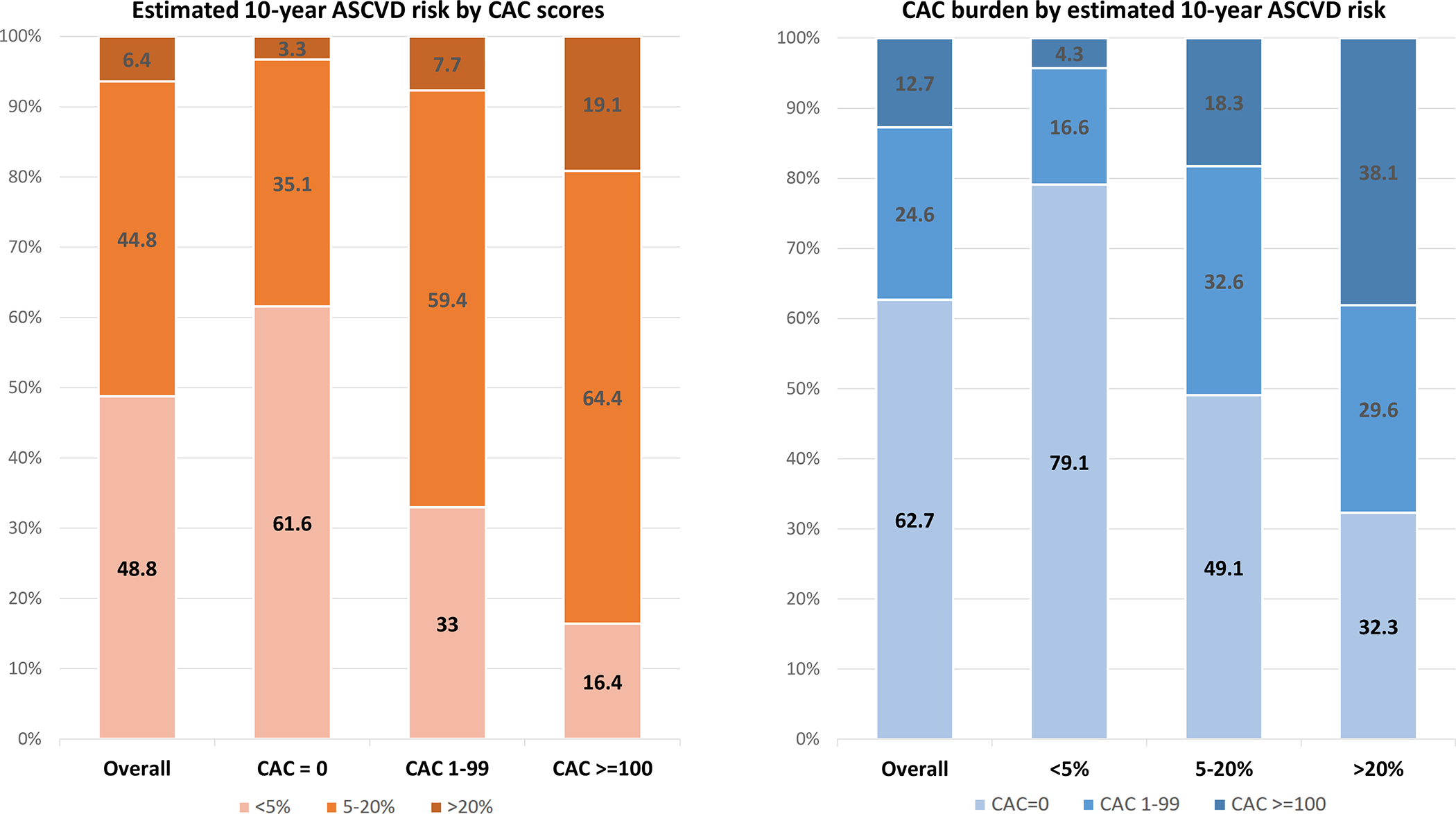
Interplay between 10-year estimated ASCVD risk and baseline CAC in the aspirin-naïve, <70 years, not high bleeding risk subpopulation.
Results presented in %. The 10-year ASCVD risk was estimated using the Pooled Cohort Equations.
Abbreviations: ASCVD = atherosclerotic cardiovascular disease events; CAC = coronary artery calcium
Incident events during follow-up
Overall crude rates per 1,000 person-years of CVD, CHD, and major bleeding events were 3.52 (95% confidence interval [CI] 2.73, 4.53), 2.34 (95% CI 1.72, 3.19) and 1.34 (95% CI 0.89, 2.02), respectively (Table 2). For CVD and CHD events, the higher the baseline CAC burden the higher the event rates, with a high of 12.4 per 1,000 person-years CVD events in the CAC≥100 group. The same was true for CVD events by CAC within estimated ASCVD risk categories. Similar trends were apparent using 10-year follow-up data, although event rates were generally higher (Table S2).
Table 2.
Crude 5-year incidence rates of CVD, CHD and major bleeding events.
| All | CAC=0 | CAC 1–99 | CAC≥100 | ||
|---|---|---|---|---|---|
| All | CVD | 3.52 (2.73, 4.53) | 1.39 (0.84, 2.30) | 4.58 (2.92, 7.18) | 12.35 (8.41, 18.14) |
| CHD | 2.34 (1.72, 3.19) | 0.65 (0.31, 1.36) | 2.88 (1.64, 5.08) | 9.92 (6.47, 15.22) | |
| Major bleeding | 1.34 (0.89, 2.02) | 0.83 (0.43, 1.60) | 2.89 (1.64, 5.09) | 0.93 (0.23, 3.70) | |
| ASCVD Risk <5% | CVD | 1.07 (0.56, 2.05) | 0.60 (0.23, 1.60) | 2.16 (0.70, 6.69) | 5.58 (1.40, 22.33) |
| CHD | 0.71 (0.32, 1.59) | 0.45 (0.15, 1.39) | 0.72 (0.10, 5.08) | 5.58 (1.40, 22.33) | |
| Major bleeding | 0.59 (0.25, 1.43) | 0.30 (0.08, 1.20) | 0.72 (0.10, 5.08) | 5.61 (1.40, 22.41) | |
| ASCVD Risk 5–20% | CVD | 5.80 (4.32, 7.80) | 2.64 (1.42, 4.91) | 6.13 (3.69, 10.16) | 14.04 (8.95, 22.01) |
| CHD | 3.94 (2.76, 5.64) | 1.05 (0.40, 2.81) | 4.08 (2.19, 7.57) | 11.76 (7.20, 19.19) | |
| Major bleeding | 2.09 (1.28, 3.42) | 1.85 (0.88, 3.88) | 3.67 (1.91, 7.05) | –* | |
| ASCVD Risk >20% | CVD | 6.69 (3.19, 14.03) | 2.90 (0.41, 20.61) | 3.23 (0.46, 22.96) | 12.72 (5.29, 30.56) |
| CHD | 3.80 (1.43, 10.13) | –* | 3.23 (0.46, 22.96) | 7.55 (2.43, 23.40) | |
| Major bleeding | 1.90 (0.48, 7.59) | –* | 6.58 (1.64, 26.29) | –* |
There were no 5-year events in this group.
Results presented as incidence rates per 1,000 person-years. The 95% confidence intervals were calculated using the quadratic approximation to the Poisson log likelihood for the log-rate parameter. Follow-up was censored at 5 years. The 10-year ASCVD risk was estimated using the Pooled Cohort Equations.
Abbreviations: ASCVD = atherosclerotic cardiovascular disease events; CAC = coronary artery calcium; CHD = coronary heart disease; CVD = cardiovascular disease
In multivariable regression analyses, CAC≥100 was independently associated with an increased risk of CVD events as compared to CAC=0 (hazard ratio [HR] 3.9, 95% CI 2.5 to 6.1) and with CHD events (HR 4.9, 95% CI 2.8 to 8.5; Table S2). On the other hand, no association was observed between CAC burden and bleeding events regardless of whether 5- or 10-year follow-up data were used. Although an association between estimated ASCVD risk and bleeding events was not evident using 5-year follow-up data, using 10-year follow-up data demonstrated such an association (Table S2).
NNT5 and NNH5 analyses
Overall, the estimated NNT5 with low-dose aspirin was 476 (Table 3). The higher the baseline CAC burden, the lower the NNT5, with a low of NNT5=100 among individuals with CAC≥400. The overall NNH5 with aspirin was 355, and there was no clear association between CAC burden and the NNH5 (Table 4). While the NNT5 exceeded the NNH5 in the overall study population, CAC≥100 and particularly CAC≥400 identified individuals likely to derive a net benefit from aspirin therapy (NNT5 lower than NNH5, Figure 3A). Also, CAC=0 identified individuals in whom aspirin would likely yield net harm (NNT5 greater than NNH5). The NNH5 could not be estimated for participants with CAC≥400 due to lack of observed bleeding events in this subgroup. The same trends were present in analyses stratified by sex (Figure 3B).
Table 3.
Number needed to treat with aspirin during 5 years to prevent one CVD event.
| Observed events at 5 years | Aspirin (assuming 12% RRR) | ||||
|---|---|---|---|---|---|
| Number | Incidence (%) | Incidence (%) | ARR (%) | NNT | |
| Overall | |||||
| All | 60 | 1.75 | 1.54 | 0.21 | 476 |
| CAC=0 | 15 | 0.70 | 0.62 | 0.08 | 1,190 |
| CAC 1–99 | 19 | 2.28 | 2.01 | 0.27 | 365 |
| CAC≥100 | 26 | 5.94 | 5.23 | 0.71 | 140 |
| CAC≥400 | 12 | 8.33 | 7.33 | 1.00 | 100 |
| ASCVD Risk <5% | |||||
| All | 9 | 0.54 | 0.48 | 0.06 | 1,543 |
| CAC=0 | 4 | 0.30 | 0.26 | 0.04 | 2,778 |
| CAC 1–99 | 3 | 1.08 | 0.95 | 0.13 | 772 |
| CAC≥100 | 2 | 2.78 | 2.45 | 0.33 | 300 |
| CAC≥400 | 1 | 8.33 | 7.33 | 1.00 | 100 |
| ASCVD Risk 5–20% | |||||
| All | 44 | 2.85 | 2.51 | 0.34 | 292 |
| CAC=0 | 10 | 1.31 | 1.15 | 0.16 | 636 |
| CAC 1–99 | 15 | 3.04 | 2.68 | 0.36 | 274 |
| CAC≥100 | 19 | 6.70 | 5.90 | 0.80 | 124 |
| CAC≥400 | 8 | 8.60 | 7.57 | 1.03 | 97 |
| ASCVD Risk >20% | |||||
| All | 7 | 3.32 | 2.92 | 0.40 | 251 |
| CAC=0 | 1 | 1.54 | 1.36 | 0.18 | 541 |
| CAC 1–99 | 1 | 1.67 | 1.47 | 0.20 | 499 |
| CAC≥100 | 5 | 6.14 | 5.40 | 0.74 | 136 |
| CAC≥400 | 3 | 7.77 | 6.84 | 0.93 | 107 |
Results presented as number or %. Follow-up was censored at 5 years. The 10-year ASCVD risk was estimated using the Pooled Cohort Equations.
Abbreviations: ARR = Absolute Risk Reduction; ASCVD = atherosclerotic cardiovascular disease events; CAC = coronary artery calcium; CVD = cardiovascular disease; NNT = number needed to treat; RRR = relative risk reduction
Table 4.
Number needed to treat with aspirin during 5 years to cause one major bleeding event.
| Observed events at 5 years | Aspirin (assuming 42% RRI) | ||||
|---|---|---|---|---|---|
| Number | Incidence (%) | Incidence (%) | ARI (%) | NNH | |
| Overall | 23 | 0.67 | 0.95 | 0.28 | 355 |
| By CAC Score | |||||
| CAC=0 | 9 | 0.42 | 0.60 | 0.18 | 567 |
| CAC 1–99 | 12 | 1.43 | 2.03 | 0.60 | 167 |
| CAC≥100 | 2 | 0.46 | 0.65 | 0.19 | 518 |
| CAC≥400 | 0 | 0.00 | –* | –* | –* |
| By ASCVD Risk | |||||
| ASCVD Risk <5% | 5 | 0.30 | 0.43 | 0.13 | 794 |
| ASCVD Risk 5–20% | 16 | 1.04 | 1.48 | 0.44 | 229 |
| ASCVD Risk >20% | 2 | 0.93 | 1.32 | 0.39 | 256 |
Could not be computed.
Results presented as number or %. Follow-up was censored at 5 years. The 10-year ASCVD risk was estimated using the Pooled Cohort Equations.
Abbreviations: ARI = Absolute Risk Increase; ASCVD = atherosclerotic cardiovascular disease events; CAC = coronary artery calcium; NNH = number needed to harm; RRI = relative risk increase
Figure 3.


Number needed to treat with low-dose aspirin during 5 years to prevent one CVD event and number needed to cause a major bleeding event by baseline CAC score, overall (Panel A) and by sex (Panel B).
Results presented as number of persons. Follow-up was censored at 5 years. Red horizontal lines represent NNH thresholds. Participants with CAC ≥400 had zero bleeding events and the NNH could not be computed. The exploratory NNT for participants with CAC ≥400 was computed only overall.
Abbreviations: CAC = coronary artery calcium; CVD = cardiovascular disease; NNH = number needed to harm; NNT = number needed to treat
In analyses by estimated 10-year ASCVD risk, the NNT with aspirin was 1,543 among individuals at <5% risk, 292 among individuals at 5–20% risk and 251 among individuals at >20% risk (Table 3). The corresponding NNH5 are presented in Table 4. The NNT5 exceeded the NNH5 in low and intermediate estimated risk individuals, and were very similar in individuals at high estimated risk (Figure 4). In all three risk strata, CAC≥100 and CAC≥400 identified subgroups with lower NNT5, and CAC=0 identified individuals in whom aspirin would likely yield net harm.
Figure 4.
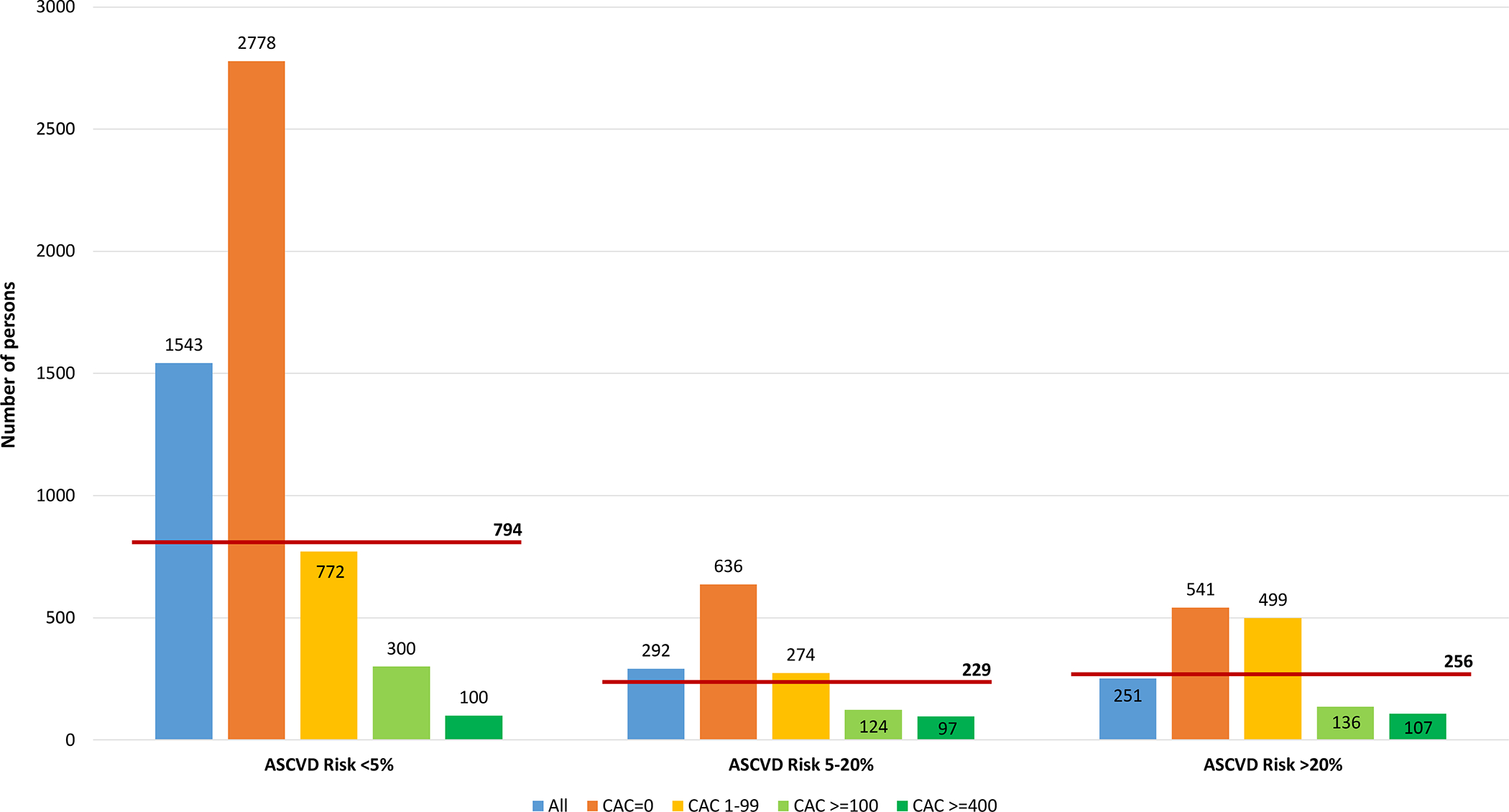
Number needed to treat with aspirin during 5 years to prevent one CVD event and number needed to cause a major bleeding event, by estimated ASCVD risk and CAC.
Results presented as number of persons. Follow-up was censored at 5 years. Ten-year ASCVD risk was estimated using the Pooled Cohort Equations. The red horizontal lines represent the NNH threshold for each ASCVD risk stratum.
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CVD = cardiovascular disease; NNH = number needed to harm
Sensitivity analyses
Similar results were observed in sensitivity analyses using 10-year follow-up data (Tables S3 and S4). However, the NNT5 and NNH5 were closer together in individuals with estimated ASCVD risk >20% and high CAC burden (Figure S2). The results of sensitivity analyses using alternative meta-analytic RRR (9%) and RRI (37%) estimates based on trials published after 2000 were also consistent with those from the main analyses, the NNT5 being consistently lower than the NNH5 for participants with CAC≥100 and particularly with CAC≥400, overall and across all estimated ASCVD risk strata (Tables S5 and S6, Figure S3).
Consistent results were also observed in sensitivity analyses excluding 1,011 interim aspirin users, which yielded a subcohort with lower incidence of ASCVD and bleeding events than that included in the main analyses (Tables S7 and S8). The NNH5 could not be estimated for participants with CAC≥100 due to lack of observed bleeding events in this subgroup.
Post-hoc analyses among baseline aspirin users
A total of 694 MESA participants were prevalent aspirin users at baseline, <70 years of age and had no high risk bleeding features. These individuals were older, more frequently male, and had a higher prevalence of diabetes, treated hypertension and statin use than those included in the main benefit/harm analyses (Table S9). Although there were only 7 bleeding events in this subpopulation, the results of the NNT/NNH analyses were qualitatively consistent with those form the main analyses (Tables S10 and S11).
Participants qualifying for aspirin therapy consideration using a CAC≥100 CAC definition of “higher risk”
Figure S4 displays the analyses assessing the proportion of participants who would qualify for consideration of aspirin therapy using a CAC-based definition of “higher risk”. Overall, 9.9% MESA participants would qualify, including 41.8% of those with a CAC score ≥100.
Discussion
In a contemporary, multi-ethnic population free of clinically overt CVD at baseline, <70 years of age and with no high bleeding risk features, bleeding risk was closely associated with estimated ASCVD risk but not with baseline CAC burden. In these individuals, ASCVD risk estimations and particularly an ASCVD risk threshold of >20% did not appear to identify individuals expected to derive a net benefit from chronic aspirin therapy. Conversely, CAC≥100 and ≥400 consistently identified subgroups of individuals in which the NNT5 was lower than NNH5 (i.e., likely to derive net benefit from aspirin), overall and across estimated ASCVD risk. However, the NNT5 with aspirin to prevent one CVD was relatively high (≥97) even among individuals with high CAC burden. Also, CAC=0 consistently identified individuals in whom chronic aspirin therapy would likely yield net harm. These trends were robust in various sensitivity and post-hoc analyses, and in subgroup analyses by sex.
Recent randomized controlled trials such as ASPREE (3), ARRIVE (4) and ASCEND (5) raised concerns regarding the potential for bleeding harm and limited efficacy of aspirin in the primary prevention of ASCVD. In ASPREE, which included 19,114 primary prevention elderly participants (median age 74 years), compared to placebo daily treatment with low-dose aspirin did not prolong disability-free survival or reduce the incidence of a composite secondary cardiovascular endpoint comprising fatal CHD, nonfatal MI, fatal or nonfatal stroke, or hospitalization for heart failure over 4.7 years of follow-up. On the other hand, there was a significantly higher rate of major hemorrhagic events in the aspirin arm. In ARRIVE, among 12,546 primary prevention participants without diabetes aged ≥55 years (men) or ≥60 years (women) at moderate cardiovascular risk, low-dose aspirin did not significantly reduce the incidence of the primary study endpoint (a composite of cardiovascular death, MI, unstable angina, stroke, or transient ischemic attack) compared to placebo, but was associated with a 2.1-fold increased risk of gastrointestinal bleeding events—most of which were classified as mild. In ASCEND, among 15,480 patients with diabetes free of known ASCVD (mean age 63 years), low-dose aspirin was associated with a 12% reduction in a composite endpoint comprising MI, stroke, transient ischemic attack, or death from any vascular cause (excluding any confirmed intracranial hemorrhage) but was also associated with a 29% increase in first major bleeding events.
A subsequent meta-analysis pooling these and 10 prior trials comparing low-dose aspirin with placebo among individuals without a history of MI or stroke reported a pooled 11% RRR in a composite cardiovascular outcome combining cardiovascular mortality, nonfatal MI and nonfatal stroke (HR 0.89, 95% CI 0.84–0.94), as well as a 43% RRI in major bleeding events (HR 1.43, 95% CI 1.30–1.56). The pooled estimates were 12% and 42% respectively after excluding studies in which participants had peripheral artery disease at baseline (POPADAD [22] and AAA [23]); and were 9% and 39% respectively when only studies published after 2000 were considered (7). Based on this updated evidence, in 2019 the ACC/AHA Primary Prevention Guidelines recommended consideration of low-dose aspirin therapy only in “select adults 40 to 70 years of age who are at higher ASCVD risk but not at increased bleeding risk” (8). Although the wording used in the guidelines intentionally left room for personalized medicine, incorporation of patient preferences and shared decision-making, it also left clinicians with limited objective guidance regarding which “higher risk” patients might receive the greatest benefit and least harm with low-dose aspirin therapy.
In this context of uncertainty, the present study suggests that ASCVD risk estimations using the PCE and particularly a 10-year ASCVD threshold of >20% fail to identify individuals who would derive net benefit from aspirin therapy for primary prevention. Conversely, these results suggest that CAC may be a useful decision-making tool informing aspirin allocation among individuals who are willing to consider therapy to further reduce their ASCVD risk (1, 2, 24). CAC may allow maximizing the benefits of aspirin while minimizing harms, and future ACC/AHA guidelines could consider specifying CAC thresholds to define “higher risk” in aspirin allocation decision-making (8). However, experimental studies are needed to confirm these observations.
It must be noted that in the present study, which comprised a relatively low ASCVD risk population (mean age 56 years, 10% participants with diabetes, median estimated ASCVD risk 5%) the calculated NNT5 was relatively high even among individuals with CAC≥400 (NNT5 ≥97). This further reinforces the notion that the value of low-dose aspirin for primary ASCVD prevention may be currently modest in the general primary prevention population. Future studies comprising individuals with a higher prevalence of clinical features associated with increased ASCVD risk (e.g., diabetes) and very high CAC scores may help further identify optimal candidates for aspirin therapy in primary prevention, who would maximize the 9% to 12% RRR of CVD events reported in the recent meta-analysis by Zheng et al.
In participants with CAC=0, the NNTs were clearly outweighed by the NNHs, even among intermediate and high estimated ASCVD risk groups. CAC=0 was a frequent finding, and was associated with very low CVD event rates in all risk strata, similar to prior analyses in MESA as well as in other cohorts (25–29). The 2019 ACC/AHA Primary Prevention Guideline authors indicated that “those with CAC scores of zero will have event rates <7.5%, which can help guide shared decision-making about statins or potentially even aspirin”. However, this was based on 2014 MESA data (14), and no formal recommendation for the use of CAC=0 in aspirin decision-making was listed. In this context, the present results provide further, updated observational support to include a formal recommendation to use CAC=0 to avoid aspirin therapy in individuals otherwise thought to be higher risk. This is important, as high ASCVD risk estimations may often signal a concomitant high risk of bleeding, particularly in the long term.
Finally, the present results also provide rationale for future randomized trials of aspirin in which CAC could be used to identify primary prevention populations at very high ASCVD risk. Experimental studies suggest that proton-pump inhibitors reduce gastrointestinal bleeding events in patients treated with aspirin, although the benefit may be limited in the absence of gastrointestinal lesions (30, 31). Therefore, a strategy combining aspirin and proton-pump inhibitors could be specifically evaluated in future CAC-based trials, as this might reduce harm while not affect benefit.
Study Strengths
The present study has important strengths. First, it was informed by the most updated, highest quality meta-analysis data on the efficacy and safety of aspirin in primary prevention settings (7). Also, it expands prior analyses in MESA (14), using actual 5-year rates of major bleeding events, which were computed overall as well as by CAC burden and estimated ASCVD risk strata. This allowed for a more granular and informative evaluation of the benefit/harm balance than prior MESA analyses on this topic (14). In addition, as compared to prior analyses, the PCE were now used to estimate 10-year ASCVD risk, which is considered the standard in cardiovascular risk prediction as of 2019. Overall, the study allowed evaluating the implications of the recent, 2019 ACC/AHA Primary Prevention Guideline recommendations relevant to aspirin use in primary preventive routine clinical practice, and to fully characterize the role that CAC may have in this setting using the most updated evidence available.
Study Limitations
Some limitations are worth discussing. First, the populations pooled by Zheng et al. were heterogeneous (7), and there are differences between those and MESA. This may limit the transferability of the pooled RRR and RRI to the MESA population. In this context, although the calculated NNTs and NNHs were somewhat sensitive to the characteristics of the subset of MESA participants included in the benefit/harm calculations, the robust set of main, subgroup, sensitivity and post-hoc analyses conducted yielded qualitatively consistent findings.
Second, our ability to identify individuals with high bleeding risk was limited as certain features such as history of gastrointestinal ulcers were not assessed at MESA Visit 1. Consequently, the proportion of individuals in whom aspirin might be considered may have been slightly overestimated. Nonetheless, such conditions would be expected to be very infrequent in the overall healthy MESA population, therefore the extent of this potential bias should be small.
Third, MESA was not originally designed to capture bleeding events, and the ascertainment of hospitalized bleeding cases using ICD codes may have led to some false positive events, as reported by Delate and colleagues (20). Moreover, some minor bleeding events occurring in patients hospitalized for another reason may have been listed in the hospital discharge reports, which would have inflated the observed rates of bleeding events. Non-detection of out-of-hospital deaths due to bleeding is also a concern, although such events would be expected to be very infrequent in a population with very low hospitalized bleeding events and very low 5-year death rates. Altogether, this could have biased the bleeding rates (which would most likely be overestimated) and the NNHs (which would most likely be underestimated). Nevertheless, this is unlikely to invalidate the compelling results observed in participants with CAC=0, and would further strengthen the findings of a favorable NNT/NNH balance in participants with high CAC scores. Also, the very low rates of bleeding events observed even in this setting suggest that when aspirin is used in individuals younger than 70 years of age with no high bleeding risk features (as recommended in recent ACC/AHA guidelines), the risk of bleeding is low.
Fourth, subgroup analyses by baseline statin use were precluded by the sample size of the statin user subgroup in MESA and the number of events. Further studies including larger populations of statin users are needed to better understand the potential value of CAC guiding the allocation of aspirin therapy in the setting of concomitant statin use. Notably, Zheng and colleagues observed that restricting their meta-analysis to randomized trials published later than 2000, which included increasing populations of statin users, still showed a 9% RRR in CVD events (7). The results of the sensitivity analyses using this more conservative RRR were consistent with those from the main analyses.
Fifth, although in the benefit/harm analyses atherothrombotic and bleeding events were assumed to be equivalent, clinicians and particularly patients may have their own perceptions and priorities. Efforts should be made to fully incorporate these into clinician-patient discussions involving consideration of aspirin therapy for primary prevention purposes.
Finally, given the observational nature of the study, the present findings should be considered hypothesis-generating. Evaluation in a randomized trial setting of the potential value of CAC for guiding a safe, effective allocation of aspirin for primary ASCVD prevention is needed.
Conclusions
The present study suggests that ASCVD risk estimations using the PCE and particularly an estimated ASCVD risk threshold of >20% may fail to identify individuals who would derive a net benefit from chronic aspirin therapy. On the other hand, CAC may be a valuable tool for aspirin therapy allocation in the context of current ACC/AHA Primary Prevention Guidelines, potentially aiding clinicians in the implementation of those recommendations. Specifically, detection of CAC≥100 and particularly of CAC≥400 might be used to identify asymptomatic individuals (younger than 70 years and with no high bleeding risk features) in whom the benefit/harm balance of aspirin is likely to be favorable. However, the 5-year NNT with aspirin would be relatively high in populations at low overall baseline ASCVD risk such as that included in the present study. On the other hand, detection of CAC=0 may be used to avoid aspirin therapy for primary prevention among individuals with high estimated ASCVD risk. Although studies in populations at higher baseline risk, with higher prevalence of very high CAC scores and greater baseline statin use are needed, the present results suggest that future guidelines could consider including CAC-based recommendations aimed at facilitating personalized, safe aspirin therapy allocation in routine clinical practice. This is particularly true for the inclusion of formal recommendations to consider avoiding aspirin therapy when CAC=0. Confirmation of these findings in experimental studies is warranted.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
The value of the coronary artery calcium (CAC) score to inform the allocation of aspirin in primary prevention had not been evaluated after publication of updated 2019 meta-analysis data on the efficacy and safety of aspirin and of the 2019 ACC/AHA Primary Prevention Guidelines
The Pooled Cohort Equations and particularly an estimated cardiovascular risk threshold of >20% failed to identify optimal candidates for aspirin therapy
The CAC score was able to identify subgroups of individuals (overall and within estimated risk strata) in which aspirin would yield net benefit (CAC≥100) and in which aspirin would yield net harm (CAC=0)
What are the clinical implications?
In primary prevention individuals considered potential good candidates for low-dose aspirin therapy (age <70 years, no high bleeding risk, believed to be at higher risk), clinicians may want to quantify the CAC score to guide personalized aspirin allocation
Individuals with CAC≥100 and particularly CAC≥400 may be good candidates for aspirin therapy for primary prevention, although the net expected benefit will likely be modest
In the presence of CAC=0, the risk of bleeding is greater than the potential benefit and aspirin therapy for primary prevention should be avoided
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
SOURCES OF FUNDING
This research was supported by contracts HHSN268201500003I, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, UL1-TR-001420 and UL1-TR-001881 from the National Center for Advancing Translational Sciences (NCATS).
ABBREVIATIONS AND ACRONYMS
- ACC/AHA
American College of Cardiology / American Heart Association
- ASCVD
atherosclerotic cardiovascular disease events
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- MI
myocardial infarction
- MESA
Multi-Ethnic Study of Atherosclerosis
- NNH(5)
number needed to harm (at 5 years)
- NNT(5)
number needed to treat (at 5 years)
- PCE
Pooled Cohort Equations
- RRI
relative risk increase
- RRR
relative risk reduction
Footnotes
DISCLOSURES
Michael J. Blaha declares that he has served on an Advisory Board of Bayer. The rest of the authors declare that they have no conflicts of interest relevant to the content of this manuscript.
REFERENCES
- 1.Fernandes A, McEvoy JW, Halvorsen S. “Doctor, Should I Keep Taking an Aspirin a Day?” N Engl J Med. 2019;380:1967–1970. [DOI] [PubMed] [Google Scholar]
- 2.Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, Blumenthal RS, McEvoy JW. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393:2155–2167. [DOI] [PubMed] [Google Scholar]
- 3.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, Storey E, Shah RC, Lockery JE, Tonkin AM, et al. ; ASPREE Investigator Group. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, et al. ; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, doubleblind, placebo-controlled trial. Lancet. 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoud AN, Gad MM, Elgendy AY, Elgendy IY, Bavry AA. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J. 2019;40:607–617. [DOI] [PubMed] [Google Scholar]
- 7.Zheng SL, Roddick AJ. Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA. 2019;321:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 ACC/AHA/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman MG, Blaha MJ, Budoff MJ, Rivera JJ, Raggi P, Shaw LJ, Berman D, Callister T, Rumberger JA, Rana JS, et al. Potential implications of coronary artery calcium testing for guiding aspirin use among asymptomatic individuals with diabetes. Diabetes Care. 2012;35:624–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, Blankstein R, Narula J, Rumberger J, Shaw LJ. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–168. [DOI] [PubMed] [Google Scholar]
- 14.Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 17.Guirguis-Blake JM, Evans CV, Senger CA, O’Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804–813. [DOI] [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 20.Delate T, Jones AE, Clark NP, Witt DM. Assessment of the coding accuracy of warfarin-related bleeding events. Thromb Res. 2017;159:86–90. [DOI] [PubMed] [Google Scholar]
- 21.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–8. [DOI] [PubMed] [Google Scholar]
- 24.Marquis-Gravel G, Roe MT, Harrington RA, Muñoz D, Hernandez AF, Jones WS. Revisiting the Role of Aspirin for the Primary Prevention of Cardiovascular Disease. Circulation. 2019;140:1115–1124. [DOI] [PubMed] [Google Scholar]
- 25.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, Miedema MD, Sibley CT, Shaw LJ, Blumenthal RS, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen MB, Fuster V, Muntendam P, Mehran R, Baber U, Sartori S, Falk E. Negative Risk Markers for Cardiovascular Events in the Elderly. J Am Coll Cardiol. 2019;74:1–11. [DOI] [PubMed] [Google Scholar]
- 27.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. [DOI] [PubMed] [Google Scholar]
- 28.Valenti V, Hartaigh BÓ, Cho I, Schulman-Marcus J, Gransar H, Heo R, Truong QA, Shaw LJ, Knapper J, Kelkar AA, et al. Absence of Coronary Artery Calcium Identifies Asymptomatic Diabetic Individuals at Low Near-Term But Not Long-Term Risk of Mortality: A 15-Year Follow-Up Study of 9715 Patients. Circ Cardiovasc Imaging. 2016;9:e003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. [DOI] [PubMed] [Google Scholar]
- 30.Vaduganathan M, Bhatt DL, Cryer BL, et al. Proton-Pump Inhibitors Reduce Gastrointestinal Events Regardless of Aspirin Dose in Patients Requiring Dual Antiplatelet Therapy. J Am Coll Cardiol. 2016;67:1661–1671. [DOI] [PubMed] [Google Scholar]
- 31.Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2019;157:403–412.e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


