To the Editor:
In endemic areas around the world, galactose-alpha-1,3-galactose (alpha-gal) sensitivity has emerged as an etiology of mammalian meat allergy that is causally associated with bites from sensitizing ticks.1 Alpha-gal syndrome (AGS) typically presents with delayed anaphylaxis after consumption of mammalian meat, and less commonly with similar reactions to mammalian milks and gelatin.1 Mammalian-derived products are common in medications, both as main and excipient ingredients, and are therefore a potential safety risk for patients with AGS. We have previously reported that a subset of alpha-gal allergic patients will react to the parenteral administration of alpha-gal contained in gelatin and gelatin containing vaccines.2–4 A recent report by Swiontek et al.5 identified a group of 17 AGS patients who demonstrated positive skin prick and ex vivo testing to porcine derived pancreatic enzyme replacement. Since these patients had no need for pancreatic enzyme replacement they were not orally challenged to confirm reactivity versus tolerance. Their report, therefore, highlights the further need to determine the safety of porcine pancreatic replacement in AGS patients.5, 6
To assess the safety of porcine pancreatic enzyme replacement in AGS patients, we evaluated two patients (Table 1) with a history of AGS and an indication for treatment with this class of medication. Skin prick testing to different commercially available porcine pancreatic enzymes was followed by oral ingestion challenge to determine tolerance. Finally, because of literature indicating increases in total IgE after splenectomy,7 we observed the effect of splenectomy on total IgE and alpha-gal specific IgE (sIgE) in our second AGS case who was undergoing a planned pancreatectomy/splenectomy.
Table 1:
Comparison of two cases of alpha-gal syndrome (AGS) with a treatment indication for porcine pancreatic enzyme replacement
| Case 1 | Case 2 | |
|---|---|---|
| Year of AGS symptom onset | 2011 | 2014 |
| Year of AGS diagnosis | 2016 | 2014 |
| Year of consultation for porcine pancreatic enzyme replacement | 2016 | 2018 |
| Timeline in order |
2011- onset of symptoms: episodic tachycardia, gastrointestinal distress, intermittent urticaria 2014- evidence of pancreatitis on CT scan 2014- failed treatment with pancrelipase, did not improve symptoms (see below) April 2016- diagnosis of AGS, alpha-gal sIgE = 6.18kU/L (evaluation prompted by systemic urticaria 4 hours after eating pepperoni pizza, other similar triggering foods listed) May 2016- cessation of mammalian meat consumption with improvement of tachycardia/urticaria and gastrointestinal symptoms with some residual intermittent diarrhea July 2016- ongoing concern for chronic pancreatitis due to residual diarrhea, allergy consulted to resume pancrelipase September 2016 - asymptomatic tolerance of pancrelipase challenge and treatment, alpha-gal sIgE = 1.24 kU/L December 2016/2017 - patient with continued tolerance of enzyme replacement. |
2014- onset of symptoms: overnight delayed anaphylaxis after steak consumption requiring ER treatment with epinephrine, leading to diagnosis of alpha-gal syndrome: alpha-gal sIgE =5.65 kU/L March 2018- follow up for alpha- gal syndrome after four years of meat and tick avoidance: alpha-gal sIgE=1.83 kU/L July 2018- patient reports another tick bite with lone star tick August 2018- diagnosis of pancreatic cancer Nov 2018- pre-clinic laboratory testing: alpha-gal sIgE >100 kU/L January 2019- pancrelipase skin testing performed Feb 2019- asymptomatic pancrelipase challenge performed in drug clinic: alpha-gal sIgE =93.90 kU/L April 2019- 1 day pre-operative for pancreatectomy/splenectomy: alpha-gal sIgE =37.4 kU/L, total IgE=2,088 kU/L April 2019- pancrelipase started on post-op day 3 May 2019- 6 weeks post-operative alpha-gal sIgE : 57.70 kU/L, total IgE=7,088 kU/L |
| Previous receipt of porcine pancreatic enzymes | 2014, discontinued because of nausea, vomiting and intermittent urticaria. Retrospectively, symptoms possibly consistent with undiagnosed AGS. | No |
| AGS food triggers | Beef, pork, dairy | Beef, pork, dairy |
| Amount of alpha-gal containing foods previously known to trigger symptoms | 2 slices pepperoni pizza- urticaria and gastrointestinal symptoms cup of beef broth- urticaria and gastrointestinal symptoms ham sandwich - urticaria and gastrointestinal symptoms yogurt- urticaria and gastrointestinal symptoms | Small steak - anaphylaxis 1 piece of pork bacon - anaphylaxis Butter - Flushing/urticaria |
| AGS food avoidances | Mammalian meats, dairy (at times) | Mammalian meats, dairy, gelatin |
| Pre-challenge serological testing | Alpha-gal sIgE = 1.24 kU/L Total IgE = 21.0 kU/L Porcine gelatin IgE <0.10 kU/L |
Alpha-gal sIgE > 100 kU/L Beef sIgE = 41 kU/L Lamb sIgE = 16.5 kU/L Pork sIgE = 16.5 kU/L Porcine gelatin sIgE = 0.9 kU/L |
| Skin testing | Negative to Creon™ 24K lipase unit capsule contents (erythema only) Positive to Viokase™ (10mm wheal and 15 mm flare) Positive to Zenpep™ (8mm wheal and 10 mm flare) Positive to Pertyze™ (6mm wheal and 8mm flare). |
Positive to Creon™ 3K lipase unit capsule contents (4mm wheal and 20mm flare) Positive to Zenpep™ (5 mm wheal and 25mm flare) Gelatin skin test negative |
| Oral tolerance of porcine pancreatic enzymes | Tolerance of oral Creon™ 6K lipase unit gelcap on outpatient challenge followed by immediate treatment initiation at 36K lipase unit gelcaps three times a day Asymptomatic tolerance during follow up. |
Tolerance of oral Creon™ 3K lipase units removed from gelcap on outpatient challenge and on hospital rechallenge with 24K lipase units followed by immediate treatment initiation and titration to 24K lipase unit capsules three times a day. Asymptomatic tolerance during follow up. |
| Unique features of patient presentation |
|
|
The first patient was a 41-year-old female seen in 2016 at the University of North Carolina, who developed recurrent heart racing, headache, and nightly gastrointestinal distress following two tick bites in May 2011. In June 2014, she was diagnosed with chronic pancreatitis when she presented with nausea, vomiting and chronic diarrhea and an elevated serum lipase level 3 times the upper limit of normal with evidence of pancreatic inflammation on CT scan. She was seen by gastroenterology and started on pancrelipase (Creon™) which is a combination of porcine-derived lipases, proteases and amylases. From the onset of treatment, she experienced nausea, vomiting, and intermittent urticaria; thus, therapy was discontinued after 4 months. In 2016, she was formally evaluated by an allergist after the recognition of delayed symptoms that occurred 4–6 hours after consumption of mammalian foods. A diagnosis of AGS was established by serologic testing (alpha-gal sIgE= 6.18kU/L). Mammalian meat withdrawal led to resolution of urticaria and improvement in her initial gastrointestinal symptoms, but she had lingering chronic diarrhea consistent with chronic pancreatitis. She was therefore referred for re-evaluation for reintroduction of porcine pancreatic enzyme replacement. At that time, she was avoiding all mammalian meats with alpha-gal sIgE = 1.24 kU/L, total IgE = 21.0 kU/L and porcine gelatin sIgE = <0.10 kU/L, reference all tests <0.10 kU/L. Skin prick testing was performed to FDA approved porcine derived pancreatic enzyme replacement products using a protocol similar to Swiontek et al.5 Testing was negative to pancrelipase (Creon™) 24K lipase unit capsule contents (no wheal and flare < 3mm) and positive to three other porcine derived formulations of pancrelipase: Viokase™ (10mm wheal and 15 mm flare), Zenpep™ (8mm wheal and 10 mm flare), and Pertyze™ (6mm wheal and 8mm flare). (Figure 1) Gelatin skin testing was not performed. Due to the lesser reactivity on skin prick, an oral challenge to Creon™ was performed. While on 5mg levocetirizine twice daily, the patient tolerated an oral ingestion challenge to a Creon™ 6K lipase unit gelcap inside the capsule, developing only itching without rash, and subsequently tolerated Creon™ 36K lipase unit gelcaps three times a day with meals during 6 months of follow up.
Figure 1:
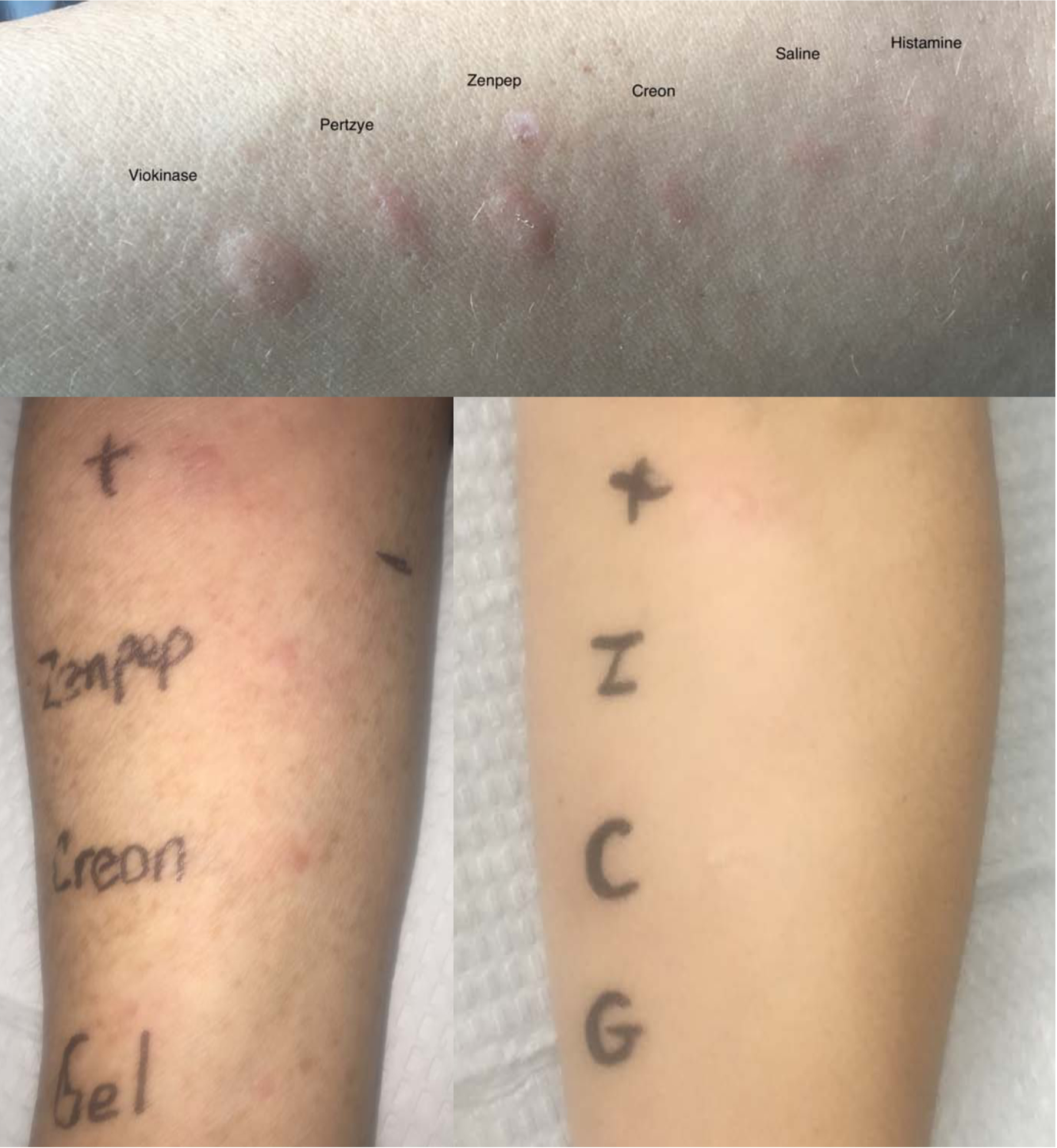
Skin testing to porcine pancreatic enzymes in Case 1 (top panel), Case 2 (bottom left panel) and a healthy control (bottom right panel): In Case 1, skin testing was negative to Creon™ (erythema only) and positive to Viokase™, Zenpep™, and Pertzye™. The image shown was taken at 30 minutes after test placement. In Case 2, skin testing was positive to Creon™ and Zenpep™ and negative to gelatin. The same reagents tested simultaneously in a healthy control produced no response. The images shown were taken at 15 minutes after placement.
The second patient was a 58-year-old female with known AGS since 2014, who was subsequently diagnosed with pancreatic cancer in 2018. She was seen in consultation at the Vanderbilt Asthma, Sinus and Allergy Program drug allergy clinic ahead of a planned pancreatectomy and splenectomy in April 2019 and the post-operative need for porcine pancreatic enzyme replacement. She was avoiding all mammalian meats, dairy, and gelatin, and had known symptoms of 2–3 hour delayed onset urticaria, angioedema, gastrointestinal distress, and respiratory compromise with beef or pork ingestion. Serologic evaluation at the time of initial consultation in 2018 was alpha-gal sIgE > 100 kU/L, beef sIgE = 41 kU/L, lamb sIgE = 16.5 kU/L, pork sIgE = 16.5 kU/L, and porcine gelatin sIgE = 0.9 kU/L, reference all tests <0.35 kU/L. Skin prick testing was positive to Creon™ 3K lipase unit capsule contents (4mm wheal and 20mm flare) and Zenpep™ (5mm wheal and 25mm flare) prepared as per the protocol described by Swiontek et al.5 with appropriate positive and negative controls. A gelatin skin prick test was negative.5, 10 (Figure 1) The same skin testing was negative in a healthy non-alpha-gal allergic control. The patient subsequently tolerated a 4 hour in-office oral ingestion challenge to the contents of a 3K lipase unit Creon™ capsule removed from its gelcap mixed with water. Alpha-gal sIgE at the time of challenge had decreased to 93.9kU/L. Two months later, after pancreatectomy with splenectomy, she was started on one 24K lipase unit capsule of Creon™ removed from its gelcap with meals upon resumption of enteral feeding. She underwent a stepwise increase to one intact capsule with meals on day 2, then to three intact capsules of 24K lipase unit Creon™ on day 3 by adding one additional capsule with every meal. By discharge she was tolerating three 24K lipase unit capsules three times a day with meals and one capsule with snacks which she continues to tolerate 8 months post-operatively. To examine IgE post-splenectomy,7 a 1month post-splenectomy total IgE was compared to a baseline drawn immediately after splenectomy, showing a 3.5fold increase in total IgE to 7088 kU/L from 2088 kU/L. Alpha-gal sIgE obtained at the same time points also showed an increase to 57.7 kU/L from 37.4 kU/L. During her surgery all porcine derived hemostatic agents (Gelfoam™, Surgifoam™) were avoided, but in the preoperative period she had tolerated parenteral porcine heparin flushes through an implanted central venous access port one month prior to initial consultation, with ongoing heparin use for central line maintenance following her surgery.8, 9 The patient’s Creon™ and inadvertent exposure to porcine derived heparin therapies were her only known exposure to mammalian products in the pre- and post-operative period and they were both tolerated.
We next evaluated if alpha-gal sIgE containing sera would interact with components of the porcine pancreatic enzymes. To do so, we performed an overnight incubation at 4°C of alpha-gal sIgE containing sera with the capsule contents of three porcine enzyme products (Creon 24K lipase, Zenpep 24K lipase, and Viokase 16K lipase) diluted 1:100 in saline. Forty microliters of undiluted serum from Case 1 along with two additional subjects with alpha-gal allergy were used, similar to previously published methods.1, 8 We then compared pre-incubation measurements of serum alpha-gal sIgE to post-incubation measurements. We performed the same assay in a healthy control without alpha-gal, examining total IgE as a proxy measure, to check for dilutional effects or non-specific IgE binding by the products.
Measured sIgE to alpha-gal from allergic patient sera decreased when incubated overnight in the presence of any of the three pancreatic porcine enzyme products selected, suggesting the presence of alpha-gal (Online Table EIA). In contrast, total IgE from a non-allergic subject did not decrease in the presence of the same products, suggesting that the observed decreases in alpha-gal specific IgE are not because of dilution or non-specific IgE binding to these products (Online Table EIB).
Our case study of these two alpha-gal allergic patients therefore confirms the presence of positive prick testing to porcine pancreatic enzyme replacement, and that in vitro binding of alpha-gal sIgE to these products can be detected in the laboratory.
We also demonstrate that the same two patients tolerated porcine pancreatic enzymes despite positive skin prick testing and in vitro sIgE binding, suggesting that oral provocation is still required to ascertain tolerance in these cases. Our report is currently limited by diagnoses of alpha-gal allergy based upon clinical history, blood testing, and the skin testing that we report here, whereas an oral challenge might have more definitively proven the diagnosis for Case 1. We also do not currentlyhave any information on how much alpha-gal is present in pancreatic enzymes. Future studies comparing the relative binding of alpha-gal sIgE to a suspect drug with alpha-gal sIgE binding to standardized concentrations of alpha-gal containing positive control substances (cetuximab, bovine thyroglobulin) may provide important information about the concentrations of alpha-gal in a drug. However, we postulate that there is sufficient alpha-gal to demonstrate a positive skin test in these patients but that the amount was below the threshold to elicit a challenge response. In keeping with this, the absolute reductions in alpha-gal sIgE binding post-absorption were modest in comparison to binding seen with thyroglobulin or gelatin-containing vaccines.3,4 This may reflect a limited absorption of alpha-gal in the setting of porcine pancreatic enzymes. It is possible that the slow post-operative introduction of enzymes in Case 2 may have served as a desensitization, but this patient was also challenged directly, twice, with no symptoms or pre-medication. In Case 2, splenectomy appeared to increase circulating total IgE and alpha-gal sIgE, but didn’t change the outcome of subsequent tolerance. In terms of safety and tolerability, the route of administration of medications (parenteral versus gastrointestinal) is likely to be important in alpha-gal allergy.2, 4 The amount of alpha-gal that is absorbed from oral medications containing mammalian ingredients is currently unknown and the safety of these products in patients with alpha-gal allergy requires further prospective research with defined provocation protocols.
Supplementary Material
Clinical Implications:
Patients with alpha-gal syndrome (AGS) can have positive skin testing to porcine pancreatic enzyme replacement. We report two patients with drug tolerance despite positive skin testing, identifying that at least in some circumstances these drugs do not elicit a reaction.
Acknowledgements:
The authors would like to acknowledge their appreciation of the patients described in this report and also Dr. Kenneth Babe, MD, Dr. Dana Cardin, MD, Dr. Marcus Tan, and Michelle Moore, PharmD.
Funding Sources:
Dr. Stone receives funding from NIH/NIGMS T32 HL87738.
Dr. Phillips receives funding related to this project from: National Institutes of Health (1P50GM115305-01, R21AI139021 and R34AI136815) and the National Health and Medical Research Foundation of Australia.
Dr. Commins receives funding related to this project from: NIH R56AI113095 & R01AI135049
Abbreviations:
- AGS
alpha-gal syndrome
- alpha-gal
galactose-alpha-1,3-galactose
- sIgE
specific IgE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IRB: This study was done under IRB approved protocols from Vanderbilt University and the University of North Carolina.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Levin M, Apostolovic D, Biedermann T, Commins S, Iweala O, Platts-Mills T, et al. Galactose alpha-1,3-galactose phenotypes: Lessons from various patient populations. Ann Allergy Asthma Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins R, James H, Platts-Mills T, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy and Clin Immunology 2012; 129:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone C Jr., Commins S, Choudhary S, Vethody C, Heavrin J, Wingerter J, et al. Anaphylaxis After Vaccination in a Pediatric Patient: Further Implicating Alpha-Gal Allergy. J Allergy Clin Immunol Pract 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone C Jr., Hemler J, Commins S, Schuyler A, Phillips E, Peebles R Jr., et al. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol 2017; 139:1710–3 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiontek K, Morisset M, Codreanu-Morel F, Fischer J, Mehlich J, Darsow U, et al. Drugs of porcine origin-A risk for patients with alpha-gal syndrome? J Allergy Clin Immunol Pract 2018. [DOI] [PubMed] [Google Scholar]
- 6.Dunkman W, Rycek W, Manning M. What Does a Red Meat Allergy Have to Do With Anesthesia? Perioperative Management of Alpha-Gal Syndrome. Anesth Analg 2018. [DOI] [PubMed] [Google Scholar]
- 7.Balsalobre B, Enguidanos M, Hernandez-Godoy J, Planelles D, Mir A. Changes in total serum IgE concentrations after splenectomy. J Investig Allergol Clin Immunol 1993; 3:268–70. [PubMed] [Google Scholar]
- 8.Kendall P, Marney SJ. Porcine Heparin Allergy in a Pork Allergic Patient: A Case Report. J Allergy Clin Immunol 2005; 115:S181. [Google Scholar]
- 9.Mawhirt S, Banta E. Successful intravenous heparin administration during coronary revascularization surgery in a patient with alpha-gal anaphylaxis history. Ann Allergy Asthma Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 10.Kelso J, Greenhawt M, Li J, Nicklas R, Bernstein D, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter update. J Allergy and Clin Immunology 2012; 130:25–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


