Abstract
Basement membranes are highly specialized extracellular matrices. More than providing scaffolds, basement membranes are recognized as dynamic and versatile structures that modulate cellular responses to regulate tissue development, function, and repair. Increasing evidence suggests that, in addition to providing structural support to adjacent cells, basement membranes serve as reservoirs and modulators of growth factors that direct and fine-tune cellular functions. Since the corneal stroma is avascular and has a relatively low keratocyte density, it’s likely that the corneal BM is different in composition from the BMs in other tissues. BMs are composed of a diverse assemblage of extracellular molecules, some of which are likely specific to the tissue where they function; but in general they are composed of four primary components—collagens, laminins, heparan sulfate proteoglycans, and nidogens—in addition to other components such as thrombospondin-1, matrilin-2, and matrilin-4 and fibronectin. Severe injuries to the cornea, including infection, surgery, and trauma, may trigger the development of myofibroblasts and fibrosis in the normally transparent connective tissue stroma. Ultrastructural studies have demonstrated that defective epithelial basement membrane (EBM) regeneration after injury to the cornea underlies the development of myofibroblasts from both bone marrow- and keratocyte-derived precursor cells. Defective EBM permits epithelium-derived and tear-derived transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), and possibly other modulators, to penetrate the stroma at sustained levels necessary to drive the development and persistence of vimentin+ alpha-smooth muscle actin+ desmin+ (V+A+D+) mature myofibroblasts. A recent discovery that has contributed to our understanding of haze development is that keratocytes and corneal fibroblasts produce critical EBM components, such as nidogen-1, nidogen-2 and perlecan, that are essential for complete regeneration of a normal EBM once laminin secreted by epithelial cells self-polymerizes into a nascent EBM. Mature myofibroblasts that become established in the anterior stroma are a barrier to keratocyte/corneal fibroblast contributions to the nascent EBM. These myofibroblasts, and the opacity they produce, often persist for months or years after the injury. Transparency is subsequently restored if the EBM is fully regenerated, myofibroblasts are deprived of TGF-β and undergo apoptosis, and keratocytes reoccupy the anterior stroma and reabsorb the disordered extracellular matrix.
Keywords: Cornea, epithelial basement membrane, histopathology, wound healing, corneal fibrosis, scarring, laminins, perlecan, nidogen-1, nidogen-2, collagen type IV
Basement membranes (BM) are highly specialized, thin, acellular extracellular matrices underlying cells that separate them from, as well as connect them to, their associated matrix.1 Basement membranes function not only in anchoring adjacent cells and providing scaffolding during embryonic development, but also in migration, differentiation, and maintenance of the differentiated phenotype of associated epithelial, endothelial, or parenchymal cells. In addition, BM control cellular functions by binding and modulating the activation, localization and concentrations of growth factors and cytokines that control the response to corneal injury (Yurchenco, 2011; Torricelli et al., 2013b). BMs also regulate cell polarity, cell adhesion, and migration via their effects on the cytoskeleton of attached cells (Yurchenco, 2011; Torricelli et al., 2013b).
1. Structure of the corneal epithelial basement membrane
The corneal epithelial BM is positioned between the basal epithelial cells and the stroma. It is first detected at 8 to 9 weeks of gestation in the human, and after the fourth month of development the corneal epithelium is separated from the stroma by a continuous BM. Evidence has been provided for a stromal cellular origin for some epithelial BM components in the cornea (Hassell et al, 1992; Kabosova et al., 2007; Santhanam et al., 2017). In adult humans, rabbits, mice, and many other species, the BM ultrastructure (Fig. 1) with transmission electron microscopy (TEM), using standard fixation methods, reveals adjacent layers termed the lamina lucida (layer between basal epithelial cell membrane and lamina densa) and the lamina densa (Torricelli et al., 2013a).
Fig 1.
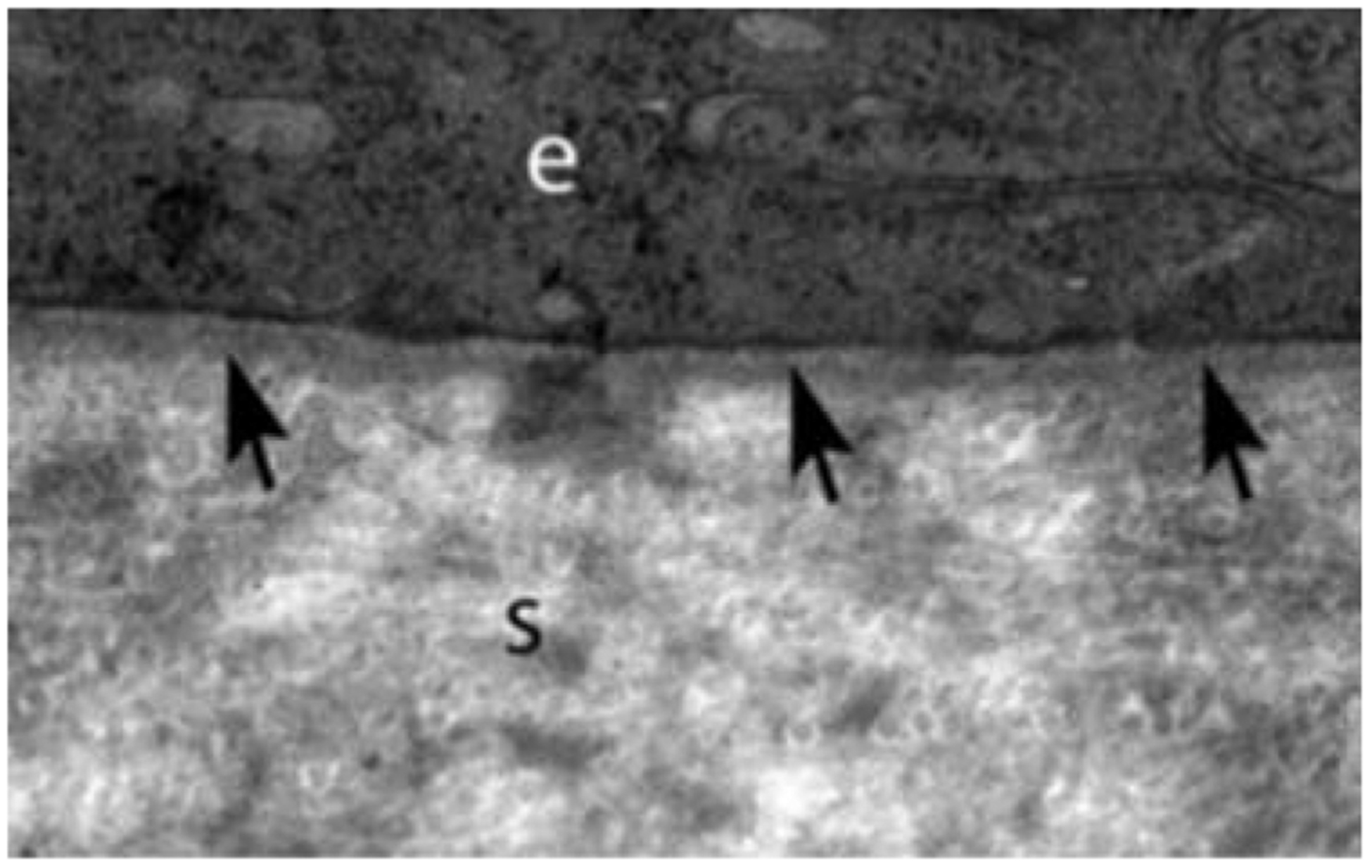
Transmission electron microscopy (TEM) images of central corneal epithelial basement membrane (EBM) in rabbits that includes the lamina densa (arrows) and lamina lucida (the less dense band between lamina densa and the basal epithelial cell) present between the epithelium (e) and the stroma (s). Mag. 23,000X.
The corneal stroma is normally avascular, and has a relatively low keratocyte density, and, thus, the corneal EBM is likely different in composition from the BMs in other tissues. Corneal epithelial BM undergoes considerable change during development and appears to have regional heterogeneity from central cornea to limbus to conjunctiva (Kabosova et al., 2007). Corneal epithelial BM is assembled from four primary components: collagens, laminins, heparan sulfate proteoglycans (HSPGs), and nidogens, although many other components such as fibronectin are also present—some of which may be tissue specific (Kruegel and Miosge, 2010).
2. Collagens
The presence of collagen type IV was at one time controversial, with some investigators failing in detect type IV collagen in the corneal BM. However, several immunohistochemical studies definitively localized type IV collagen beneath the human corneal epithelium. It appears the disparity between different studies arose as a result of the spatial variability (“horizontal” heterogeneity) in the BM composition between the central cornea, limbus, and conjunctiva (Ljubimov et al., 1995). It is also now recognized that collagen type IV has six α chains that can assemble into different heterotrimers, such as [α1(IV)2α2(IV)], [α3α4α5(IV)], [α3(IV)2(IV)], or [α5(IV)2α6(IV)]. This variability could also have contributed to early confusion about the presence of collagen IV in the corneal epithelial BM. One study (Ljubimov et al., 1995) showed in adult human corneas that central BM had type IV collagen α3 through α6 chains, whereas only limbal and conjunctival BM contained α1 and α2 chains. In addition, the study showed that limbal BM had collagen IV α5 and α6 chains. Limbal and conjunctival epithelial BM also had laminin α2 and α2 chains, whereas the central cornea BM did not. Laminin-332, perlecan, fibronectin, entactin/nidogen, and type VII collagen were detected in the entire ocular surface BM—central cornea, limbus, and conjunctiva. These authors suggested that the shifts in collagen IV chains and the appearance of additional laminins in the limbus may be related to the differentiation state of the corneal cells contributing to BM formation. Some studies have found other collagens in the corneal epithelial BM, including collagen type VII—as a primary structural element in anchoring fibrils, collagen type XV and collagen type XVIII—as active molecules in corneal wound healing and perhaps involved in the corneal avascularity, collagen type XVII—as adhesion molecules present in hemidesmosomes, and the long form of collagen type XII (Kabosova et al., 2007).
The collagen type IV self-polymerizing network associates with the parallel laminin network via specific associations with perlecan and nidogens 1/2 that crosslink these networks within the mature normal EBM (Boudko et al., 2018). Thus, nidogens have specific binding sites for both collagen type IV and laminins and, therefore, crosslink these two networks in the EBM, with the collagen type IV network serving as a scaffold that provides structural stability to the EBM. Perlecan also has specific domains through which it interacts with collagen type IV, nidogens and laminins (Kinsella and Wight, 2005). These multiple interactions of EBM components organize and stabilize the EBM.
Collagen type IV also interacts with integrins α1β1 and α2β1 via the central triple-helical domain of collagen type IV and thereby promotes the adhesion, activates migration, and stimulates proliferation of corneal epithelial cells (Boudko et al., 2018). Many of these responses are mediated by interaction of specific integrins, namely α1β1 and α2β1, with the central triple-helical domain of collagen IV proteins (Khoshinoodi et al., 2008; Leitinger and Hohenester, 2007).
3. Laminins
Laminins are the most abundant non-collagenous proteins in BM. Laminins are heterotrimeric glycoproteins that are composed of three chains, including one α, one β, and one γ chain. At present, five α, three β, and three γ peptides coded by different genes are known for mice and humans. The trimers were previously designated laminin-1 to −15 in order of their discovery, with no relationship to chain composition. According to the previous nomenclature, a trimer could be identified by either an Arabic numeral (e.g., 10) or its chains. An abbreviated nomenclature has been proposed (Aumailley et al., 2005). For example, with the new nomenclature 511 stands for α5β1γ1, and better identifies the peptide composition of individual laminins. Laminins have been shown to influence tissue development, and laminin gene defects have potential roles in diseases in many organs—including keratoconus, Fuchs’ dystrophy, and bullous keratopathy in the cornea. The expression of laminin chains is regulated both spatially and temporally, suggesting that different laminin isoforms have distinct roles. Laminins are vital for the assembly of BM and interact with collagen networks via nidogens and other extracellular matrix molecules. Several investigators have examined the range of laminins present in the corneal EBM. Central EBM was shown to have laminin alpha 2 (which may be a component of laminin 211) and beta 2 chains, and laminin 111 and 332 were detected in the entire EBM (Ljubimov et al., 1995). Byström et al (2007) found that normal cornea EBM contains laminin alpha 3, alpha 5, beta 1, beta 3, gamma 1 and gamma 2 chains, likely consistent with the presence of laminins 511/521, 332 and 111 being present in the EBM. Polisetti et al. (2017) found laminin chains alpha 2, alpha 3, alpha 5, beta 1, beta 2, beta 3, gamma 1, gamma 2 and gamma 3 in the human limbal EBM, but did not specify which laminin trimers with which these were associated.
In vivo and in vitro studies have suggested that laminins are principally responsible for initial organizing of BM assembly since they uniquely self-assemble into sheet-like structures on cell surfaces without the contribution of other components required for the assembly of a fully-functional BM, such as collagen type IV bound to nidogen-1 and nodogen-2, the HSPGs agrin and perlecan, and many other components (Yurchenco, 2011; Yurchenco et al., 1992).
4. Perlecan
Perlecan is the most prevalent HSPG in the EBM. It is a complex, multi-domain protein with several discrete binding partners, including collagen type IV, nidogens and laminins (Kinsella and Wight, 2005). The protein’s core consists of five domains that share homology with other molecules involved in nutrient metabolism, cell proliferation, and adhesion, including laminin, the low-density lipoprotein (LDL) receptor, epithelial growth factor (EGF), and the neural cell adhesion molecule (N-CAM) (Kruegel and Miosge, 2010; Wiradjaja et al., 2010). Perlecan, a typical proteoglycan, mediates the migration, proliferation, and differentiation of a variety of cells by modulating cell signaling events (Mongiat et al., 2000). Perlecan mediates these functions mainly by controlling the availability of fibroblast growth factors (FGF), bone morphogenic proteins (BMP), platelet-derived growth factor (PDGF), vascular endothelial growth factors (VEGF), transforming growth factor β−1 (TGFβ1), and insulin-like growth factors (IGF) to bind receptors on the cells they modulate (Iozzo, 2005), and likely also modulate TGFβ2 localization in the cornea. In vertebrates, perlecan functions in a diverse range of developmental and biological processes—from the development of cartilage to the regulation of wound healing. One study (Sher et al., 2006) found that perlecan regulates both the survival and terminal differentiation steps of keratinocytes and that it is critical for the formation of normal epidermis. Another study (Vittitow and Borras, 2004) reported that perlecan expression is upregulated after corneal stromal injury, as well as after an artificial increase in intraocular pressure. In that study, perlecan was identified in corneal epithelial BM, and the epithelium was shown to be thin and poorly differentiated in perlecan-deficient mice (Hspg2 / -TG) and accompanied by downregulation of Ki67, cytokeratin12, connexin43, Notch 1, and Pax6. These findings revealed that BM perlecan is likely critical for normal epithelial regeneration and terminal differentiation in the cornea.
Nidogens
Nidogen-1 and nidogen-2, also major BM components, are sulfated glycoproteins. Both nidogens have three globular domains separated by link-like and rod-like regions, and they have similar distribution within the corneal epithelial BM (Fox et al., 1991; Ho et al., 2008; Timpl et al., 1983). Due to their strong affinity to laminins and collagen IV, nidogens are considered to be link proteins in the EBM (Torricelli et al., 2015). Genetic deletion of either NID gene in mouse did not produce detectible alterations in tissue and BM architecture (Murshed et al., 2000; Schymeinsky et al., 2002). Redistribution and upregulation of the more restrictively expressed nidogen-2- in nidogen-1-deficient mice suggested compensatory functions of the two nidogens. Studies in mice lacking both nidogen isoforms showed that this is indeed the case, since the double knockouts had severe abnormalities in lungs, heart, and limbs that were directly related to BM defects (Bader et al., 2005; Bose et al., 2006). Surprisingly, however, ultrastructurally normal BM were seen in many other tissues—demonstrating that the other BM components may assemble and form BM structures without nidogens in some tissues. This also suggests there are tissue-specific requirements for nidogens. One study (Maguen et al., 2008) reported nidogen-2 accumulation around INTACS implanted in the corneal stroma, along with other known fibrotic extracellular matrix components.
A better appreciation of the structure, function, and regeneration of the BM is provided by understanding of the interactions between the overlying basal epithelial cells and the underlying anterior stroma in the regeneration process after severe fibrotic injuries (Fig. 2).
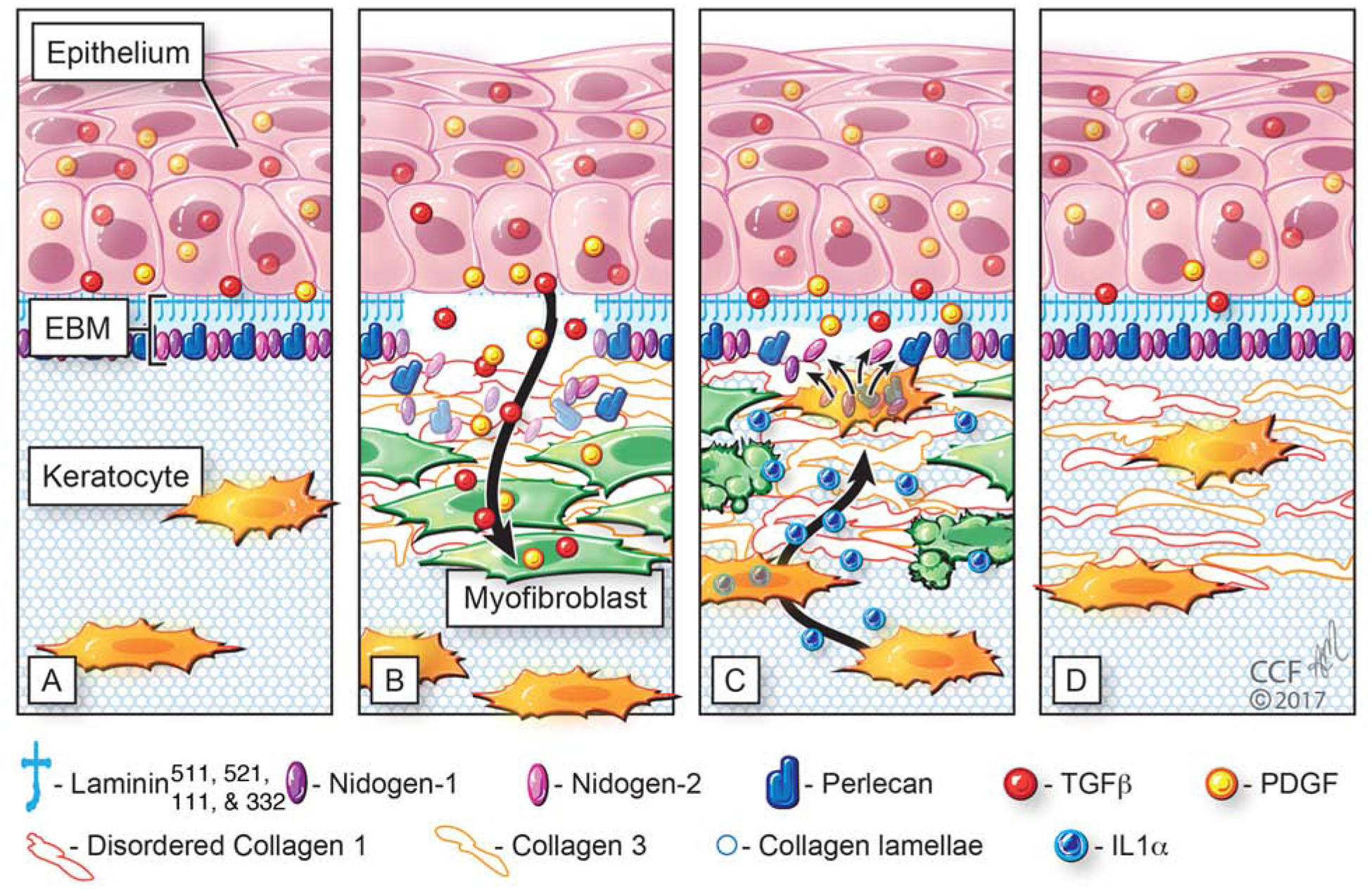
Fig. 2.
Schematic model of normal unwounded corneal epithelial basement membrane (EBM) and injured EBM with defective regeneration associated with anterior stromal myofibroblast development and fibrosis. Normal unwounded cornea (A) with intact epithelial basement membrane (EBM) comprised of laminins 511, 521, 111, 332, etc., in addition to perlecan, nidogen-1, nidogen-2, and other components not depicted, such as collagen type IV. The underlying stroma is populated with keratocytes within the highly-organized, transparent stromal collagen lamellae. Epithelial transforming growth factor beta (TGFβ and platelet-derived growth factor (PDGF) are blocked from penetration into the underlying stroma by binding components such as perlecan, nidogen and collagen type IV within the normal EBM. B) After severe epithelial-stromal injuries, such as infections, trauma or high-correction photorefractive keratectomy, the epithelium and EBM are disrupted and TGFβ and PDGF are activated and penetrate the underlying stroma at sufficient concentrations to drive the development of myofibroblasts from keratocyte-derived and bone marrow-derived (fibrocyte) precursors. Myofibroblasts are themselves opaque relative to keratocytes and secrete disordered collagen type 1, collagen type 3 and other matrix materials that disrupt the normal stromal lamellae to produce corneal opacity or scarring. C) Over months to years following the initial injury, keratocytes penetrate the anterior stromal myofibroblasts and facilitate EBM regeneration via the production of perlecan and nidogens in coordination with overlying epithelial cells producing other EBM components, such as laminins and collagen type IV. We hypothesize that once the nascent laminin layer containing laminins 111, 511, 521 and 332 is produced by the epithelium, more posterior EBM components must, at least in part, be derived from keratocytes and/or corneal fibroblasts to fully-regenerate the normal EBM. The resulting decrease in TGFβ and PDGF penetration from the epithelium into the stroma triggers myofibroblast apoptosis via unopposed paracrine IL-1α from adjacent keratocytes and/or autocrine IL-1α produced by the myofibroblasts themselves (autocrine suicide). This process begins in a random spotty distribution within the stromal opacity to produce clear areas of stroma called “lacunae” (Fig. 5A and 5B) that enlarge and coalesce over weeks to months to fully restore transparency. D) In some corneas, depending on the severity of the injury, myofibroblasts undergo apoptosis and keratocytes repopulate the anterior stroma and reabsorb disorganized collagens and other matrix materials secreted by the myofibroblasts, thereby restoring the normal morphology of the collagen lamellae and transparency. Revised with permission from Wilson et al., Matrix Biol 64, 17–26.
It remains somewhat unsettled which EBM components are made by epithelial cells and which are produced by keratocytes/corneal fibroblasts. Recent laser capture-reverse transcriptase-polymerase chain reaction studies found that laminin alpha 3, perlecan, nidogen-1 and nidogen-2 messenger RNAs are produced by keratocytes/corneal fibroblasts during the response to PRK injuries (Santhanam et al, 2017). These studies also showed that epithelium can produce these same components. Filenius and coworkers (2001) work suggested that laminin 332 was produced by corneal epithelial cells, but that laminin 511 was likely produced by keratocytes. Studies in human corneas at 30 minutes after epithelial scrape injury showed that perlecan and nidogen-2 proteins are produced in keratocytes (Torricelli et al. 2015), Also, recent studies in rabbits showed that perlecan and nidogen 1 and 2 proteins are produced in keratocytes and corneal fibroblasts (Saikia et al., 2018). Our most recent immunohistochemistry work in wounded rabbit corneas demonstrate that most, if not all, laminin isotypes and collagen type IV are produced primarily by the corneal epithelium, whereas perlecan, nidogen-1 and nidogen-2 are produced by keratocytes and corneal fibroblasts during regeneration of the EBM after injury (RC de Oliveira and SE Wilson, unpublished data 2019).
5. The epithelial basement membrane, myofibroblasts and corneal wound healing
Following severe injuries, infection or surgeries of the cornea in which the BM is damaged, large numbers of myofibroblasts are generated and persist in the corneal stroma (Wilson et al., 2017). These fibroblastic cells, and the disorganized extracellular matrix components they secrete, produce fibrosis that alters the structure and function of the corneal stroma and results in a loss of normal transparency (corneal scarring or haze). Studies using chimeric mice transplanted with bone marrow derived from green fluorescent protein (GFP)+ donors demonstrated conclusively that corneal myofibroblasts originate from both bone marrow-derived cells (likely fibrocytes) and resident stromal fibroblastic cells (keratocytes that transition to corneal fibroblasts following activation triggered by TGFβ and other cytokines upregulated and released into the stroma by corneal injury) (Singh et al., 2014a; Singh et al., 2014b; Torricelli and Wilson, 2014). The development of mature myofibroblasts from these precursor cells, and persistence in the stroma, is dependent on an adequate ongoing supply of TGF-β and PDGF. In the normal unwounded rabbit cornea, the epithelium produces TGFβ1 and PDGF, and after epithelial-stromal injury the epithelium produces TGFβ2 (G. Tye, R.C. de Oliveira, S.E. Wilson, unpublished data, 2019), but these growth factors cannot penetrate into the stroma at sufficient and sustained levels to drive myofibroblast development due to the barrier function of the normal epithelial BM. Small amounts of TGFβ1 and TGFβ2 are also produced transiently in stromal cells after injury. After minor injuries to the cornea that do not result in scarring—such as an abrasion—the epithelial BM is temporarily disrupted and epithelium-derived TGFβ1, TGFβ2 and PDGF penetrate the stroma and initiate the development of myofibroblasts from precursor cells. However, the epithelial BM is fully-regenerated within 8 to 10 day (Santhanam et al, 2017; Marino et al., 2017b), cutting off the supply of epithelium-derived TGFβ and PDGF. Therefore, the TGFβ- and PDGF-dependent immature myofibroblast precursors and myofibroblasts that have begun development undergo apoptosis before they produce sufficient disordered extracellular matrix to significantly reduce corneal transparency. With more severe injuries, such as bacterial infections or photorefractive keratectomy (PRK) surgery to correct high nearsightedness, normal regeneration of the EBM may be delayed (Torricelli et al., 2016). This defective epithelial BM allows ongoing penetration of high levels of TGFβ1, TGFβ2 and PDGF from the epithelium into the stroma to drive development and persistence of large numbers of myofibroblasts, resulting in fibrotic scarring of the cornea. Studies of this pathophysiology, including laser capture quantitative RT-PCR studies (Santhanam et al., 2017), demonstrated that defective epithelial BM regeneration may be associated with inadequate keratocyte production and/or localization of epithelial BM components, such as laminin 332 and nidogen-2, due to extensive apoptotic death of stromal keratocytes at the time of the injury. One explanation is that once the epithelium regenerates over the injured stroma, it lays down a nascent epithelial BM that in the cornea consists of self-polymerizing laminin 511/521, and then associating laminin 332, but full regeneration of mature functional epithelial BM requires keratocyte or corneal fibroblast BM component contributions to the more posterior epithelial BM. Our recent work found that laminin alpha 5, laminin beta 3, nidogen-1 and perlecan are all present at the site of the nascent regenerating EBM, and the process of EBM regeneration in −9D PRK corneas begins normally, but then goes awry as myofibroblast precursor cells develop in the anterior stroma (Saikia et al., 2018).
Specific components of the EBM bind profibrotic growth factors such as TGFβ1, TGFβ2, and PDGF isoforms, and regulate their penetration into the stroma in the unwounded cornea and inhibit ongoing penetration once the mature EBM regenerates. Thus, perlecan binds TGFβ1, TGFβ2, PDGF AA, and PDGF BB; nidogen 1 and 2 bind PDGF AA and PDGF BB; and collagen IV binds TGFβ1 and TGFβ2 (Yurchenco et al, 1986; Behrens et al., 2012; Paralkar et al., 1991; Shibuya et al., 2006; Iozzo et al., 2009: Gohring et al., 1998; Mongiat et al., 2001). Perlecan in EBM also produces a high negative charge due to its three heparan sulfate side chains and, therefore, produces a non-specific barrier to TGFβ penetration through the EBM and into the corneal stroma (Yurchenco et al, 1986; Behrens et al., 2012).
If large numbers of mature myofibroblasts develop and secrete disordered extracellular matrix, a physical tissue barrier is produced that blocks surviving keratocytes or corneal fibroblasts in the more peripheral and posterior corneal stroma from repopulating the anterior stroma (Fig 3). When this occurs, the working hypothesis is that normal keratocytes are blocked from proximity to the nascent EBM, where they could participate in regeneration of the EBM, and therefore, the defective EBM and myofibroblasts, and the disordered extracellular matrix they produce, persist for months to years.
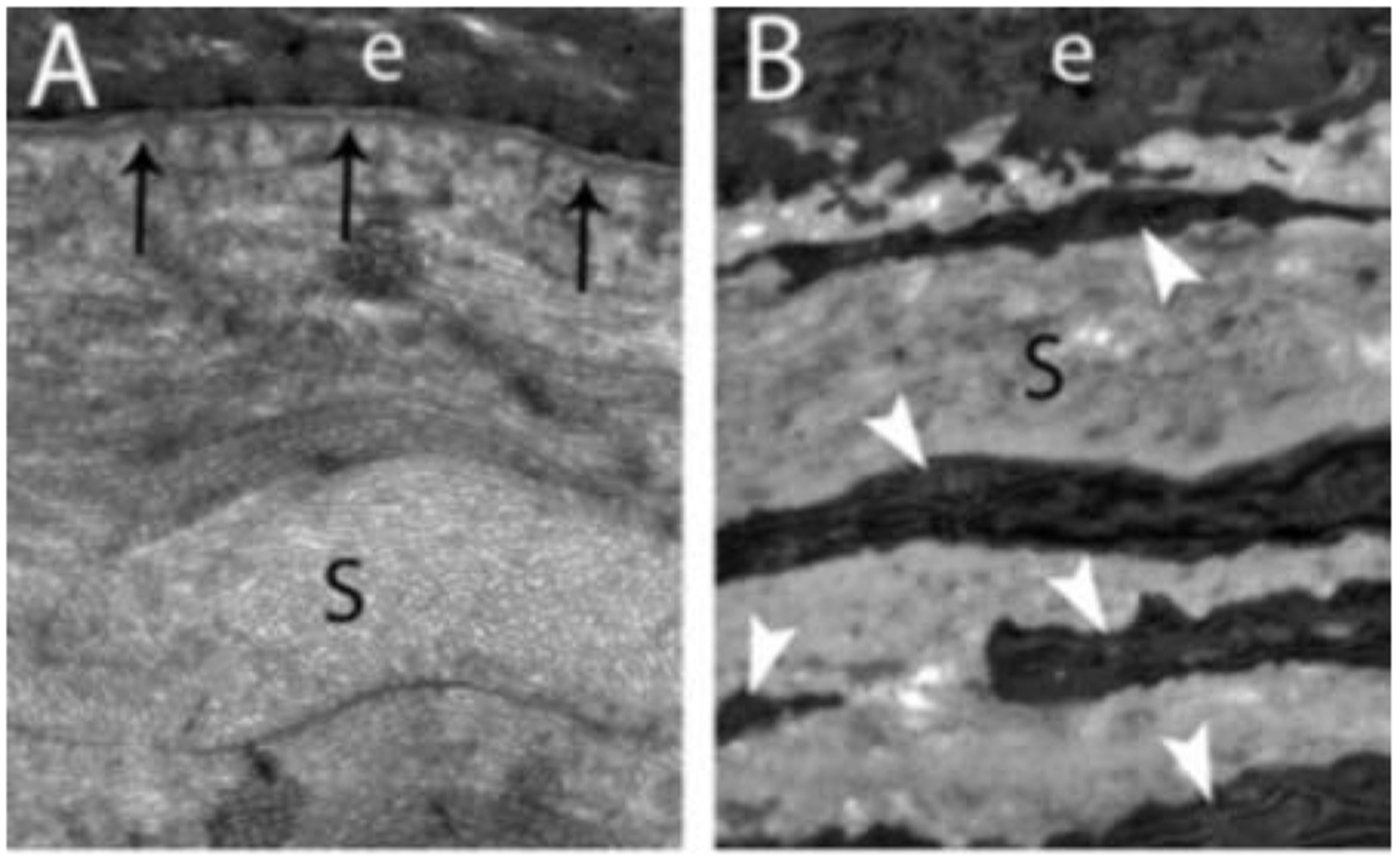
Fig. 3.
Transmission electron microscopy (TEM) images of rabbit corneas (e, epithelium; S, stroma). A. A clear cornea at two weeks after −4.5D PRK had normal regeneration of the EBM (arrows) with clear lamina densa and lamina lucida. B. A cornea with dense fibrotic haze at four weeks after −9.0D PRK showed a complete absence of regenerated EBM—although it is likely there is a nascent abnormal epithelial BM consisting of laminin and other components of EBM beneath the basal epithelial cells that doesn’t appear as the classic EBM on TEM. Also note the large numbers of myofibroblasts (cells indicated by arrowheads with prominent endoplasmic reticulum) in the anterior stroma surrounded by a disorganized extracellular matrix that would act as a barrier to keratocytes repopulating the anterior stroma.
Eventually, in many scarred corneas, after the source of injury is eliminated for a period of months to years, small areas of clearing called “lacunae” appear within the stromal fibrosis. In these clear areas, normal keratocytes have repopulated the stroma, mature epithelial BM has regenerated, and the underlying myofibroblasts—that are deprived of epithelium-derived TGFβ and PDGF—underwent apoptosis, whereas the EBM continues to be morphologically and functionally defective in adjacent scarred areas where underlying myofibroblasts persist (Fig. 4 and Fig. 5). Over time, there is a tendency for these lacunae to enlarge and coalesce, as more surrounding EBM regenerates and full transparency of the cornea can be restored (Medeiros et al., 2018).
Fig. 4. Defective regeneration of EBM at early time points after −9 diopter (D) photorefractive keratectomy (PRK) in rabbits.
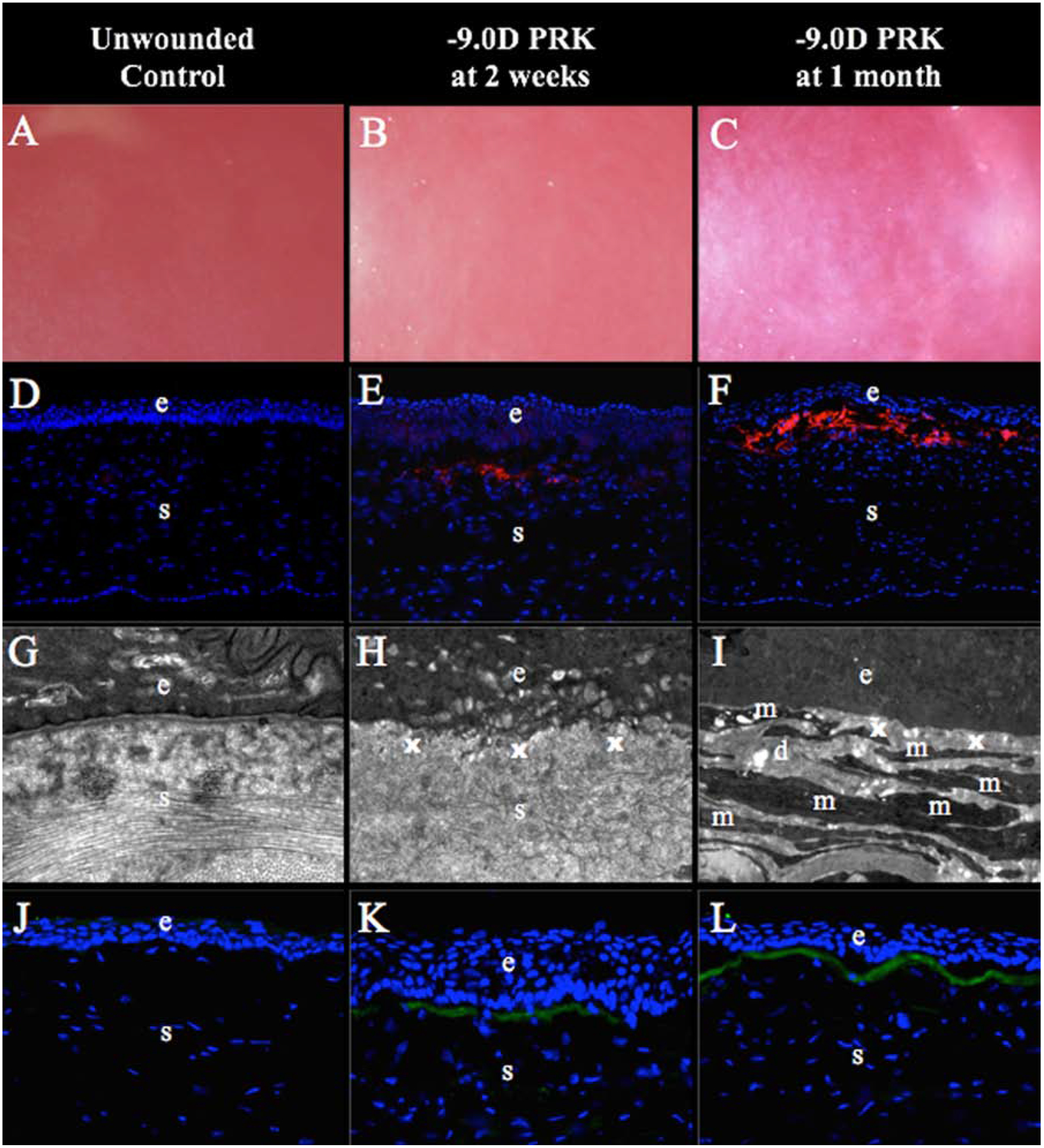
Unwounded corneas and corneas at two weeks or one month after −9 diopters (D) photorefractive keratectomy (PRK) are shown. (A) Control corneas were clear without fibrosis (mag 25X). (B) Mild fibrosis within the area of excimer laser ablation was noted in all corneas at two weeks after the PRK (mag 25X). (C) Dense subepithelial fibrosis was observed at one month after PRK (mag 25X). (D) Unwounded control corneas had no alpha-smooth muscle actin (α-SMA)+ cells (mag 200X). (E) At two weeks after −9D PRK, corneas had a few α-SMA+ cells (arrowheads) in the excimer laser ablated zone (mag 200X). (F) α-SMA+ myofibroblasts (arrowheads) were present at high density in the subepithelial excimer laser-ablated zone in all corneas at one month after −9D PRK (mag 200X). (G) Transmission electron microscopy (TEM) of control corneas shows normal epithelial basement membrane (EBM) lamina densa (arrows) and lamina lucida, and normal organized extracellular matrix in the stroma (mag 23,000X). (H) At two weeks after −9D PRK, there was no detectible lamina lucida and lamina densa where EBM is normally noted (x) within the excimer laser PRK zone and disorganized extracellular matrix was prominent in the subepithelial stroma (s) (mag 23,000X). (I) At one month after −9D PRK, no lamina lucida or lamina densa were detected within the PRK ablated zone where EBM would normally be detected (x) and it can be noted there were layers of cells (m) with large amounts of rough endoplasmic reticulum that correspond to the α-SMA+ cells (myofibroblasts) in Fig. 1F embedded in disorganized extracellular matrix (d) (mag 23,000X). (J) Control unwounded corneas had no detectible collagen type III (COL3) detected with immunohistochemistry (mag 400X). (K) At two weeks after −9D PRK, COL3 (arrows) was deposited in the subepithelial stroma (mag 400X). (L) At one month after −9D PRK, all corneas had large amounts of COL3 (arrows) in the subepithelial stroma (mag 400X). Blue is DAPI staining of cell nuclei; (e) epithelium; (s) stroma. Reprinted with permission from Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J. Ref Surg. 2017;33: 337–346.
Fig. 5. Regeneration of the EBM at later time points after −9D PRK in rabbits.
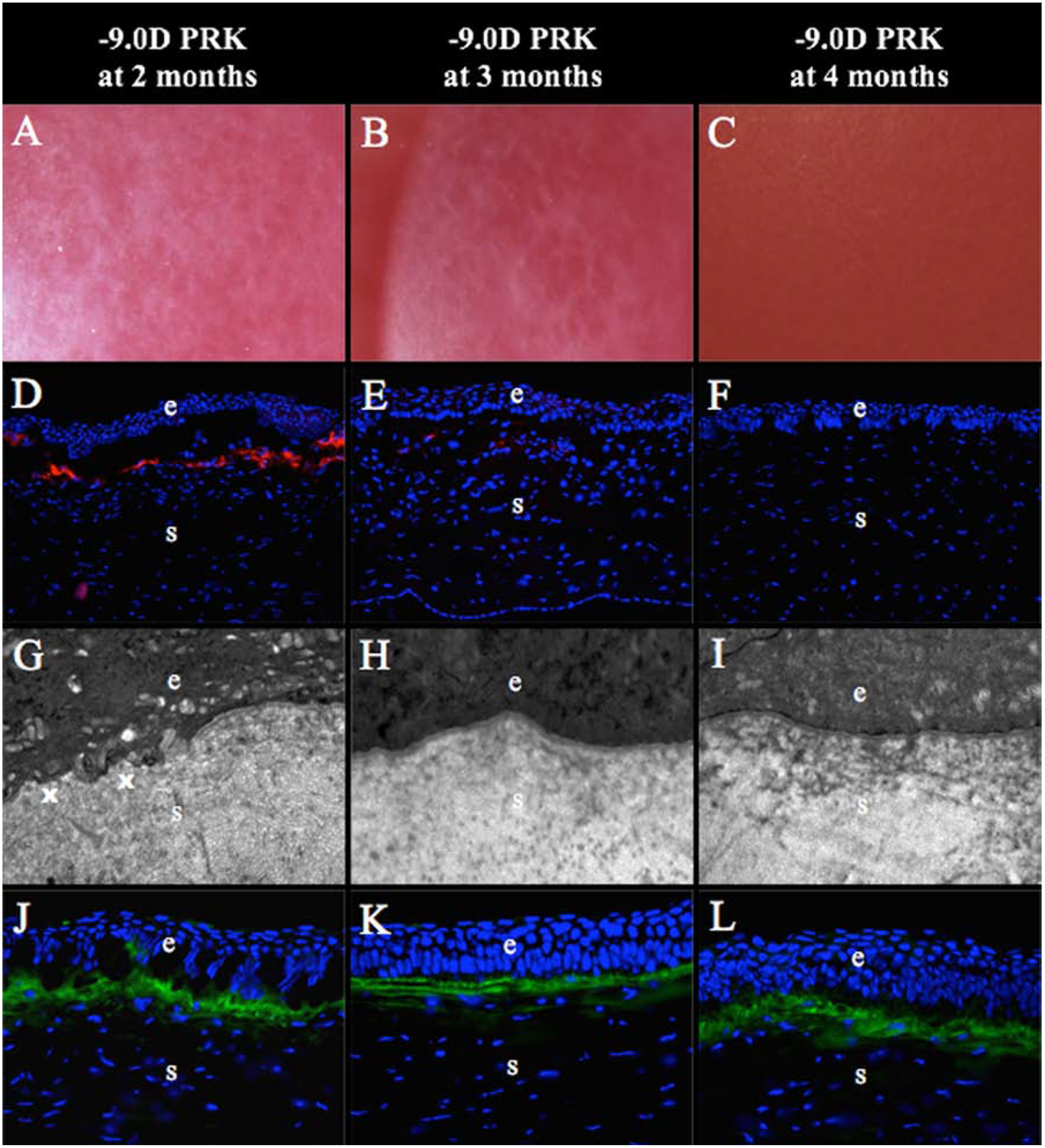
(A) At two months after −9D PRK, all the corneas had developed clear areas called “lacunae” (arrows) within the confluent corneal fibrosis in the PRK ablation zone (mag 25X). (B) At three months after −9D PRK, lacunae (arrows) had enlarged and some had coalescedin all corneas (mag 25X). (C) At four months after −9D PRK, corneal transparency was fully restored in all corneas (mag 25X). (D) At two months after −9D PRK, large numbers of α-SMA+ myofibroblasts (arrowheads) were present in the fibrotic subepithelial stroma, although less than corneas at one month after PRK (see Fig. 4F) (mag 200X). (*) is artifactual detachment of the epithelium from stroma that occurred during cryostat tissue sectioning—likely due to defective EBM. (E) At three months after −9D PRK, there were only a few remaining α-SMA+ myofibroblast (arrowheads) in the superficial stroma within the PRK ablated zone (mag 200X). (F) At four months after −9D PRK, there were no remaining α-SMA+ myofibroblasts in the superficial stroma (mag 200X). (G) At two months after −9D PRK, transmission electron microscopy (TEM) of the excimer laser ablated zone showed areas of fully-regenerated EBM with lamina lucida and lamina densa (arrows) adjacent to areas without normal EBM (x) (mag 23,000X). (H) At three months after −9D PRK, normal EBM (arrows) was regenerated in the PRK ablated zone of all corneas (mag 23,000X). (I) At four months after −9D PRK, normal EBM was present in the PRK ablated zone in all corneas (mag 23,000X). (J) At 2 months after −9D PRK, large amounts of collagen type III (COL3, arrows) were detected in superficial stroma of the PRK ablated zone (mag 400X). The level of COL3 deposited in the subepithelial stroma appeared unchanged at three months (K) and four months (L) after −9D PRK (mag 400X). Blue is DAPI staining of cell nuclei; (e) epithelium; (s) stroma. Reprinted with permission from Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J. Ref Surg. 2017;33:337–346.
After more extensive injuries to the cornea, such as severe microbial keratitis, both the epithelial BM and endothelial BM (Descemet’s membrane) can be damaged, leading to extraordinary myofibroblast generation and fibrosis of the full-thickness cornea. In this situation, the epithelial BM can eventually be repaired (Marino et al., 2017a), leading to apoptosis of anterior stromal myofibroblasts, while posterior stromal myofibroblasts survive due to persistent damage to Descemet’s BM that allows penetration of TGFβ from the aqueous humor within the anterior chamber of the eye into the posterior corneal stroma. In some species, in which the corneal endothelium can regenerate, Descemet’s BM may also eventually be repaired, leading to apoptosis of the posterior stromal myofibroblasts and restoration of full corneal transparency (Medeiros et al., 2018; Wilson et al., 2017). Alternatively, endothelial replacement surgeries such as DMEK or DSAEK, in which Descemet’s membrane is also transplanted, may facilitate apoptosis of posterior myofibroblasts and resolution of fibrosis.
The development of posterior scarring fibrosis after Descemetorhexis without graft or problematic Descemet’s membrane-endothelial replacement surgeries likely depends on the diameter of the Descemetorhexis and the species—with rabbits being more fibrinogenic than humans. However, posterior fibrosis has been reported after endothelial replacements in humans where there was poor adhesion of the graft or defects in the graft (Müller et al., 2016) and after Descemetorhexis without a graft (Iovieno et al., 2017).
Major components of the EBM are collagens, laminins, perlecan, and nidogens
Keratocytes and corneal fibroblasts contribute components during EBM regeneration
The EBM regulates the localization of TGFβ, PDGF, HGF and KGF
Defective regeneration of the EBM underlies stromal fibrosis
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, Department of Defense grant VR180066, and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
References
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD, 2005. A simplified laminin nomenclature. Matrix Biol 24, 326–332. [DOI] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R, 2005. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol 25, 6846–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, Hansen U, 2012. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem 287, 18700–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose K, Nischt R, Page A, Bader BL, Paulsson M, Smyth N, 2006. Loss of nidogen-1 and −2 results in syndactyly and changes in limb development. J Biol Chem 281, 39620–39629. [DOI] [PubMed] [Google Scholar]
- Boudko SP, Danylevych N, Hudson BG, Pedchenko VK, 2018. Basement membrane collagen IV: Isolation of functional domains. Methods Cell Biol 143, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byström B, Virtanen I, Rousselle P, Miyazaki K, Lindén C, Pedrosa Domellöf F, 2007. Histochem Cell Biol 127, 657–67. [DOI] [PubMed] [Google Scholar]
- Filenius S, Hormia M, Rissanen J, Burgeson RE, Yamada Y, Araki-Sasaki K, Nakamura M, Virtanen I, Tervo T, 2001. Laminin synthesis and the adhesion characteristics of immortalized human corneal epithelial cells to laminin isoforms. Exp Eye Res 72, 93–103. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, et al. , 1991. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J 10, 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohring W, Sasaki T, Heldin CH, Timpl R, 1998. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur J Biochem 255, 60–66. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Schrecengost PK, Rada JA, SundarRaj N, Sossi G, Thoft RA,1992. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest Ophthalmol Vis Sci 33, 547–57. [PubMed] [Google Scholar]
- Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N, 2008. Nidogens-Extracellular matrix linker molecules. Microsc Res Tech 71, 387–395. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, 2005. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6, 646–656. [DOI] [PubMed] [Google Scholar]
- Iovieno A, Neri A, Soldani AM, Adani C, Fontana L., 2017. Descemetorhexis without graft placement for the treatment of Fuchs endothelial dystrophy: Preliminary results and review of the literature. Cornea 36, 637–641. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Zoeller JJ, Nystrom A, 2009. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol. Cells 27, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV, 2007. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48, 4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella MG, Wight TN, 2005. Perlecan: An Extracellular Matrix Heparan Sulfate Proteoglycan that Regulates Key Events in Vascular Development and Disease in Chemistry and Biology of Heparin and Heparan Sulfate, eds. Garg HG, Linhardt RJ and Hales CA, Elsevier; London, UK, pp 607–635. [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG, 2008. Mammalian collagen IV. Microscopy Research and Technique 71, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel J, Miosge N, 2010. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci 67, 2879–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hohenester E, 2007. Mammalian collagen receptors. Matrix Biology. 26, 146–155. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC, 1995. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72, 461–473. [PubMed] [Google Scholar]
- Maguen E, Rabinowitz YS, Regev L, Saghizadeh M, Sasaki T, Ljubimov AV, 2008. Alterations of extracellular matrix components and proteinases in human corneal buttons with INTACS for post-laser in situ keratomileusis keratectasia and keratoconus. Cornea 27, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Bose K, Tam KP, Wilson SE, 2017a. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res 161, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Torricelli AAM, Wilson SE, 2017b. Regeneration of Defective Epithelial Basement Membrane and Restoration of Corneal Transparency After Photorefractive Keratectomy. J Refract Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Santhiago MR, Wilson SE, 2018. The Corneal Basement Membranes and Stromal Fibrosis. Invest Ophthalmol Vis Sci 59, 4044–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock JM, Iozzo RV, 2000. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J Biol Chem 275, 7095–7100. [DOI] [PubMed] [Google Scholar]
- Müller TM, Verdijk RM, Lavy I, Bruinsma M, Parker J, Binder PS, Melles GR, 2016. Histopathologic features of Descemet membrane endothelial keratoplasty graft remnants, folds, and detachments. Ophthalmology 123, 2489–2497. [DOI] [PubMed] [Google Scholar]
- Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R, 2000. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 20, 7007–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VM, Vukicevic S, Reddi AH, 1991. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol 143, 303–8. [DOI] [PubMed] [Google Scholar]
- Polisetti N, Sorokin L, Okumura N, Koizumi N, Kinoshita S, Kruse FE, 2017. Schlötzer-Schrehardt U.. Laminin-511 and −521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci Rep 7, 5152 10.1038/s41598-017-04916-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia P, Thangavadivel S, Medeiros CS, Lassance L, de Oliveira RC, Wilson SE, 2018. IL-1 and TGF-β modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest Ophthalmol Vis Sci. 59, 5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Marino GK, Torricelli AA, Wilson SE, 2017. EBM regeneration and changes in EBM component mRNA expression in stromal cells after corneal injury. Mol Vis 23, 39–51. [PMC free article] [PubMed] [Google Scholar]
- Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL, 2002. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol 22, 6820–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, Ron D, 2006. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem 281, 5178–5187. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Okamoto O, Fujiwara S, 2006. The bioactivity of transforming growth factor-beta1 can be regulated via binding to dermal collagens in mink lung epithelial cells. J Dermatol Sci 41, 187–195. [DOI] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE, 2014a. Transforming growth factor beta and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp Eye Res 120, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jaini R, Torricelli AA, Santhiago MR, Singh N, Ambati BK, Wilson SE, 2014b. TGFbeta and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp Eye Res 121, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G, 1983. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem 137, 455–465. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE, 2015. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res 134, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE, 2016. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res 142, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE, 2013a. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci 54, 4026–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE, 2013b. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Wilson SE, 2014. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res 129, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittitow J, Borras T, 2004. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol 201, 126–137. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Marino GK, Torricelli AAM, Medeiros CS, 2017. Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: A paradigm for fibrosis in other organs? Matrix Biol 64, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiradjaja F, DiTommaso T, Smyth I, 2010. Basement membranes in development and disease. Birth Defects Res C Embryo Today 90, 8–31. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Cheng YS, Colognato H, 1992. Laminin forms an independent network in basement membranes. J Cell Biol 117, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H, 1986. Models for the self-assembly of basement membrane. J Histochem Cytochem 34, 93–102. [DOI] [PubMed] [Google Scholar]


