Abstract
The synthetic cathinone α-pyrrolidinovalerophenone (α-PVP) continues to be abused despite being banned by regulatory agencies. The abused formulation of α-PVP is a racemic mixture consisting of two enantiomers, S-α-PVP and R-α-PVP. In this study, we investigated the neurochemical, behavioral and cardiovascular effects of racemic α-PVP and its enantiomers in male rats. Racemic α-PVP blocked the uptake of both dopamine and norephinephrine ex vivo, but did not block the uptake of serotonin (5-HT), at their respective transporters. S-α-PVP was slightly more potent than racemic α-PVP, while R-α-PVP was 10–20 times less potent at blocking dopamine and norepinephrine uptake. In microdialysis studies, racemic and S-α-PVP increased extracellular dopamine levels in the nucleus accumbens, but not levels of 5-HT. Racemic and S-α-PVP also increased locomotor activity. When tested at the same doses, S-α-PVP produced larger effects than racemic α-PVP. R-α-PVP also increased extracellular dopamine levels and locomotor activity, but only at 30 times higher doses than S-α-PVP. Racemic and S-α-PVP were self-administered by rats at 0.03 mg/kg/injection, whereas R-α-PVP was self-administered at a 10 times higher dose. Dose-effect determinations following acquisition suggested that R-α-PVP was at least 30 times less potent than S-α-PVP. Finally, racemic and S-α-PVP increased blood pressure and heart rate at doses approximately 30 times less than was required for R-α-PVP to produce similar effects. These results show that the neurochemical, behavioral and cardiovascular effects of racemic α-PVP most likely reflect the actions of S isomer.
1. INTRODUCTION
Synthetic cathinones are new psychoactive substances that have been used as recreational drugs (i.e., for non-medical purposes) in the United States (US) and elsewhere during the past decade.1,2 These drugs are structurally-related to cathinone, a naturally-occurring compound in the khat plant Catha edulis, whose leaves are chewed for their psychomotor stimulant effects. 3,4-Methylenedioxypyrovalerone (MDPV) was one of the first synthetic cathinones to gain widespread use and was subsequently classified as a Schedule I controlled substance by the US Drug Enforcement Administration.3 Like other psychomotor stimulant drugs of abuse, MDPV increases extracellular concentrations of dopamine in brain reward pathways, promotes locomotor activity, and supports self-administration behavior in laboratory animals.4,5 The mechanism of action for MDPV resembles that of cocaine since the drug potently blocks the uptake of dopamine and norepinephrine through their respective transporters (DAT and NET). In contrast to cocaine, MDPV has weak activity at serotonin (5-HT) transporters (SERT).6–8
α-Pyrrolidinovalerophenone (α-PVP) is a structural analog of MDPV which lacks the 3,4-methylenedioxy ring substituent. α-PVP was part of the second generation of synthetic cathinones that saw widespread abuse and, like MDPV, was classified as a Schedule I controlled substance by the DEA.9 Despite this classification, α-PVP continues to be abused10, often with fatal consequences.11,12 Like MDPV, α-PVP is an uptake blocker at DAT and NET, with little effect at SERT13–16 and α-PVP does not act as a substrate at the transporters.17,18 The potent action of α-PVP at DAT leads to an increase in extracellular dopamine concentrations in the nucleus accumbens as measured by microdialysis.4,19,20 α-PVP increases locomotor activity in mice and rats21–25, an effect blocked by dopamine antagonists.19,21,23 α-PVP has effects that are predictive of its abuse in humans. It produces place preference in mice and rats21,22,26, it reduces intracranial self-stimulation thresholds27 and is self-administered by rats.20,24,28–33 α-PVP also generalizes to other psychomotor stimulants in drug discrimination procedures carried out in rodents and monkeys.22,34,35
MDPV has two enantiomers, R and S, based on absolute configuration. These two isomers show dramatic differences in potency. S-MDPV is 50–100 times more potent than R-MDPV in blocking uptake at DAT and NET.36 In mice trained to discriminate cocaine from saline, both isomers of MDPV fully substitute for cocaine, but the S-isomer is about 100 times more potent than the R-isomer.37 S-MDPV also produces clear increases in activity in mice, while the R-isomer does not. The S-isomer also produces robust increases in blood pressure and heart rate in rats, while the R-isomer fails to do so when administered over a similar dose range.38 In rats trained to self-administer racemic MDPV, the S-isomer is about 50 times more potent than the R-isomer in tests of self-administration on a progressive ratio schedule.30 Because of the structural similarity between MDPV and α-PVP, it might be predicted that similar differences in potency between the isomers of α-PVP would be observed. In studies in rats, Gannon et al.30 showed that S-α-PVP is about 30 times more potent than R-α-PVP in supporting self-administration using procedures identical to those used for MDPV self-administration, although both isomers induced similar maximal effects. Using a taste avoidance procedure, Nelson et al.39 found that racemic and S-α-PVP support taste avoidance learning, while across a similar dose range, R-α-PVP does not. Previous studies have uncovered potency differences between R and S enantiomers of α-PVP in behavioral assays, but no studies have examined neurochemical or adverse effects of the compounds. To this end, the purpose of this study was to carry out a comprehensive pharmacological characterization of α-PVP and its enantiomers in a variety of procedures examining neurochemical, behavioral and cardiovascular effects in male rats.
2. MATERIALS AND METHODS
2.1. Drugs and Reagents
Racemic α-PVP and its R and S enantiomers were synthesized as HCl salts. Chemical and structural analysis included proton nuclear magnetic resonance, gas chromatography/mass spectrometry, thin layer chromatography, and melting point determination.40 All data confirmed the expected structures. [3H]Transmitters (specific activity ranging from 30–50 Ci/mmol) were purchased from Perkin Elmer (Shelton, CT, USA). All other chemicals and reagents used for the in vitro assays, microdialysis methods, and high-pressure liquid chromatography with electrochemical detection (HPLC-ECD) were acquired from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
2.2. Animals
Male Sprague-Dawley rats weighing 300–400 g were housed under conditions of controlled temperature (22±2 1C) and humidity (45±5%), with food and water freely available. Rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the NIDA IRP. For rats used in uptake and microdialysis experiments, lights were on from 0700–1900 h and experiments were carried out between 0900–1500 h. For rats used in the telemetry and self-administration experiments, lights were on from 1900–0700 h (i.e., reverse light-dark phase) and experiments were carried out in the dark phase.
2.3. In Vitro Uptake Assays in Synaptosomes
Rats were euthanized by CO2 narcosis, and the brains were processed to yield synaptosomes as previously described.41,42 Synaptosomes were prepared from caudate tissue for DAT assays whereas synaptosomes were prepared from whole brain, minus caudate and cerebellum, for NET and SERT assays. For uptake inhibition assays, 5 nM [3H]dopamine, 10 nM [3H]norepinephrine, and 5 nM [3H]5-HT were used to assess transport activity at DAT, NET and SERT, respectively. The selectivity of uptake assays was optimized for a single transporter by including unlabeled blockers to prevent the uptake of [3H]transmitter by competing transporters. Uptake inhibition assays were initiated by adding 100 ml of tissue suspension to 900 ml Krebs-phosphate buffer (126 mM NaCl, 2.4 mM KCl, 0.83 mM CaCl2, 0.8 mM MgCl2, 0.5 mM KH2PO4, 0.5 mM Na2SO4, 11.1mM glucose, 0.05mM pargyline, 1mg/ml bovine serum albumin, and 1 mg/ml ascorbic acid, pH 7.4) containing test drug and [3H]transmitter. Drug concentrations ranged from 0.1 nM to 10 μM. Uptake inhibition assays were terminated by rapid vacuum filtration through Whatman GF/B filters, and retained radioactivity was quantified by liquid scintillation counting.
2.4. In Vivo Microdialysis in Rats
Microdialysis sampling was carried out as described.43 Twenty-seven rats were anesthetized with ketamine/xylazine (75/5 mg/kg, i.p.) and received a surgically implanted jugular catheter and an intracranial guide cannula aimed at the nucleus accumbens. Rats were allowed at least 1 week for recovery from surgery. On the evening before an experiment, a 2 × 0.5-mm dialysis probe (CMA/12, Harvard Apparatus, Holliston, MA, USA) was inserted into the guide cannula, and an extension tube was attached to the jugular catheter. Each rat was placed into its own enclosure and connected to a tethering system. Enclosures were equipped with a row of photobeams to detect movements (TruScan, Harvard Apparatus). Probes were perfused overnight with Ringers’ salt solution pumped at a flow rate of 0.6 ml/min. On the next morning, dialysate samples were collected at 20-min intervals. Samples were immediately assayed for dopamine and 5-HT by HPLC-ECD.44 Rats were randomly assigned to groups receiving either drug or saline (n = 6–7 / group) injections. Once three stable baseline samples were obtained, rats received two sequential intravenous (i.v.) injections of drug: one dose at time zero, followed by a threefold higher dose 60 min later (0.1 and 0.3 mg/kg for racemic and S-α-PVP and 1.0 and 3.0 mg/kg for R-α-PVP). Doses were chosen based on previous results with MDPV.6,45 Control rats received sequential i.v. injections of saline (1 ml/kg) according to the same schedule. Microdialysis samples were collected every 20 min throughout the post-injection period of 120 min. Ambulation and stereotypy were measured during microdialysis sampling via photobeam breaks. Ambulation was defined as forward movement in the horizontal plane, whereas stereotypy was defined as repetitive back-and-forth movement within a 2 sec window that did not exceed a 1.5 beam spaces. At the end of the experiments, rats were euthanized with CO2 and decapitated. The brain sections were examined to verify placement of microdialysis probe tips within the nucleus accumbens. Only those rats with correct placements were included in data analyses.
2.5. Self-Administration in Rats
Twenty-seven rats were anesthetized with ketamine/xylazine (80/9.6 mg/kg, i.p.) and intravenous (i.v.) jugular catheters were implanted as previously described.45 Concentration of drug for self-administration was adjusted for rat body weight to give the desired dose. Catheters were flushed before and after each training session with 0.1 ml of a saline solution containing 1.25 units/ml heparin and 0.08 mg/ml gentamicin. If a catheter failed during the study, a second catheter was implanted on the opposite side. After the second catheter failure, the rat was removed from the study.
Ten training chambers were used (ENV-008CT, Med Associates, St. Albans, VT). Each chamber was enclosed in a sound-attenuation cubicle equipped with a fan to provide ventilation and stable background noise. Each chamber had a grid floor and two nose-poke response holes (ENV-114BM, Med Associates), one on each side of a food trough (not used in this study). The nose-poke holes could be illuminated from inside the hole by a dim yellow light. A houselight was situated above the nose-poke holes. Experimental events were controlled by a MED-PC computer system (Med Associates). Following surgery and at least 7 days of recovery, the catheters were connected to the extension tubing, and the rats were placed in the training chambers every weekday. Sessions began with the illumination of the houselight and the two nosepoke holes. A nose-poke in the active hole was reinforced on a fixed-ratio 1 schedule with an infusion of drug, and the houselight and nose-poke hole lights were turned off and a 20-s timeout began. Nose-pokes in the inactive hole were recorded but had no scheduled consequence. During the timeout, responses were recorded, but not reinforced. Following the timeout, the houselight and nose-poke hole lights were turned on and active nose-pokes were again reinforced. Whether the right or left nose-poke was active was counterbalanced across subjects. Sessions lasted for 2 h. Initial acquisition doses were 0.03 mg/kg/inj R,S-α-PVP (n = 9), 0.03 mg/kg/inj S-α-PVP (n = 10), 0.03 mg/kg/inj R-α-PVP (n = 8), and 0.3 mg/kg/inj R-α-PVP (n = 8). Doses were chosen based on previous results with MDPV.45
At least 20 injections/session and a clear separation of active/inactive responses was taken of evidence of acquisition, however, this clear evidence of acquisition was not required to advance to dose-effect testing. Rats were required to have at least 10 days of acquisition training. If day 10 fell at the end of the week, additional acquisition sessions may have been given prior to dose-effect testing. Only the first 10 days of acquisition training are reported.
Following acquisition training, dose-effect testing began for all groups except those originally trained on 0.03 mg/kg/inj R-α-PVP, since this dose did not support acquisition and were not tested further. The dose of each drug was varied to three additional doses. Each dose was available for self-administration for 2 hours/day for 3 days, with results for days 2 and 3 averaged for analysis. Doses were presented in a non-systematic order. Following dose-effect testing, the rats were returned to the original training dose for at least 3 days. After responding stabilized, saline was substituted for each drug and training continued for 10 days to evaluate extinction.
2.6. Telemetric Assessment of Cardiovascular Parameters in Rats
Twelve rats were used in the telemetry experiments. Rats implanted with HD-S10 biotelemetry transmitters were purchased from Data Sciences International (DSI, St. Paul, MN). After surgical recovery at DSI, the rats were shipped to NIDA in Baltimore and underwent a 7-day quarantine. Following release from quarantine, food was restricted to maintain a constant or slowly increasing weight not to exceed 500 gms over the course of the experiment. This was done so the position of the pressure transducer within the aorta was not significantly changed over the course of the experiment.
The rats were subsequently adapted to the experimental chambers and injection procedure over a period of 3–4 weeks. Each weekday, the rats were transported to the procedure room where the food and water were removed from the home cage and the entire home cage was placed on top of a telemetry receiver (RPC-001, Data Sciences) inside a small acoustical chamber (BRS/LVE, Laurel, MD). Transmitters were turned on by placing a magnet near the abdomen of the rat. The chambers were then closed and experimental parameters were monitored for 3 hrs. Mean arterial blood pressure, heart rate and body temperature were sampled for 10 s every minute for 3 h. At the end of the session, the transmitters were turned off by again placing a magnet near the abdomen of the rats, water and food were returned to the home cage, and the rats were returned to the housing room. Once experimental parameters were stable during daily exposure to the chambers and injection (s.c.) procedures, seven rats were tested with R,S-α-PVP and S-α-PVP over a dose range of 0.3 – 3.0 mg/kg. Five additional rats were tested with R-α-PVP over a dose range of 3.0 – 30 mg/kg. Doses were chosen based on previous results with MDPV.38
On any one day, all rats were tested at the same drug and dose, however, drug and dose order were non-systematic (i.e., not simply increasing or decreasing doses of the same drug). The rats were tested with drug or vehicle no more frequently than twice per week, typically on Tuesday and Friday.
2.7. Data Analysis and Statistics
All statistical analyses were carried out using GraphPad Prism (v. 5.0 or newer; GraphPad Scientific, San Diego, CA, USA), except for the self-administration data that were analyzed using R version 3.4.4. For synaptosome assays, IC50 values for inhibition of uptake were calculated based on non-linear regression analysis. Neurochemical data from microdialysis experiments were normalized to percentage of preinjection baseline (based on three pre-injection samples) and evaluated using two-way analysis of variance (dose × time) followed by Bonferroni’s post hoc test. For self-administration studies, only responses during the reinforcement period were used for analysis. Timeout responses were not included but were generally very low following the first couple of days of training. Data from the self-administration experiments were analyzed using a linear mixed-effects model. For the acquisition and extinction analysis, day and response (active/inactive) were treated as within-subject factors. For the dose-effect study, dose was treated as a within-subject factor. Dose/h was calculated by multiplying the total number of infusions by the dose/infusion and dividing by the 2 h session time. The telemetry data were collapsed across the 3-h sampling period to yield mean heart rate (beats/min), blood pressure (mm Hg) and temperature (°C) at each drug dose. These parameters were used to construct dose–effect curves, which were analyzed by one-factor analysis of variance (dose) followed by Dunnet’s post hoc test to determine differences from control. To calculate ED50 values for blood pressure and heart rate, the average values for saline and the average maximal effect at the highest dose tested were calculated. The halfway point between these two values was used to determine the ED50 (109.5 mm Hg for blood pressure and 315.4 beats/min for heart rate). P < 0.05 was the minimum criterion for statistical significance.
3. RESULTS
3.1. [H3]Neurotransmitter uptake
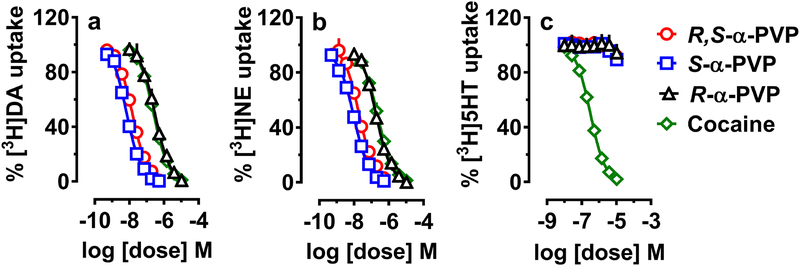
Fig. 1 depicts the effects of α-PVP and its enantiomers on inhibition of uptake at DAT, NET and SERT as compared to (−)-cocaine. Racemic α-PVP and its enantiomers blocked the uptake of [3H]dopamine and [3H]norepinephrine by their respective transporters, as did cocaine. Unlike cocaine, α-PVP and its enantiomers did not block the uptake of [3H]5-HT by SERT. Cocaine was non-selective, showing similar potency in blocking uptake at all three transporters. R-α-PVP was 20–40 times less potent than S-α-PVP at the NET and DAT respectively. S-α-PVP was about 2 times more potent than the racemate at DAT and NET (Table 1). The weaker R-isomer was nearly identical in potency to cocaine at DAT and NET suggesting potential in vivo activity similar to cocaine. Racemic α-PVP and S-α-PVP had similar potency at DAT and NET, with R-α-PVP showing a slight preference for NET over DAT. All the drugs tested appear to be fully efficacious as uptake blockers at DAT and NET.
Fig.. 1.
Effects of racemic α-PVP and its enantiomers in comparison to cocaine on uptake at DAT (a), NET (b) and SERT (c) in rat brain synaptosomes. Synaptosomes were incubated with different concentrations of α-PVP or its enantiomers or cocaine in the presence of 5 nM[3H]dopamine for DAT, 10 nM [3H]norepinephrine for NET or 5 nM [3H]5-HT for SERT. Data are percentage of [3H]neurotransmitter uptake expressed as mean ± SEM for n = 3 experiments performed in triplicate.
Table 1.
Effects of α-PVP and its enantiomers in comparison to cocaine on transporter-mediated inhibition of [3H]transmitter uptake in rat brain synaptosomesa
| R,S-α-PVP | S-α-PVP | R-α-PVP | Cocaine | |
|---|---|---|---|---|
| DAT | 15 ± 1.0 | 7 ± 0.5 | 307 ± 41.8 | 256 ± 12.5 |
| NET | 19 ± 2.4 | 10 ± 1.0 | 181 ± 15.3 | 247 ± 20.7 |
| SERT | >10,000 | >10,000 | >10,000 | 318 ± 7.2 |
Data are IC50 values (nM) expressed as mean ± SEM for n = 3 separate experiments performed in triplicate.
3.2. Microdialysis
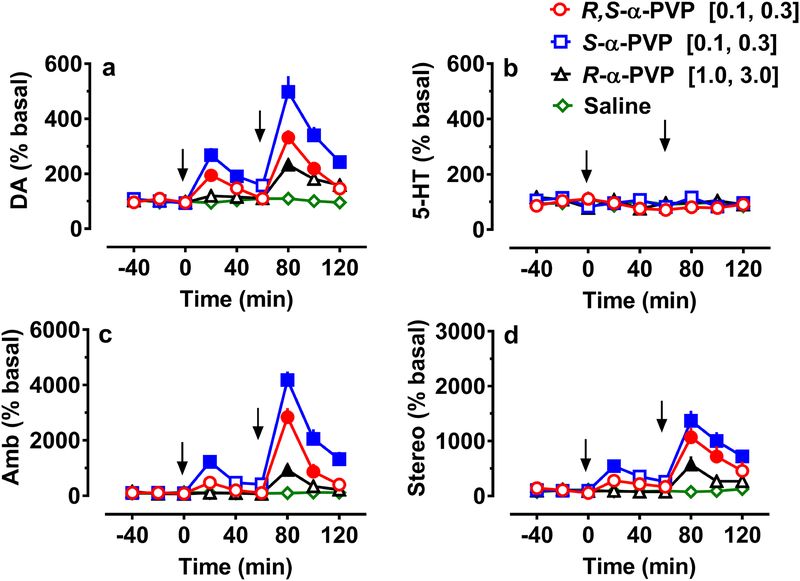
Fig. 2 illustrates the effects of α-PVP and its enantiomers on neurochemistry and locomotor behavior in rats undergoing in vivo microdialysis in the nucleus accumbens. α-PVP produced increases in extracellular levels of dopamine (Fig. 2a, F24,207 = 11.8, p < 0.001), but no changes in extracellular levels of 5-HT (Fig. 2b, F24,207 = 1.0, p > 0.4). The increases in dopamine were most pronounced with S-α-PVP, (n = 7) with racemic α-PVP (n = 7) producing smaller increases over saline (n = 7) at the same doses (0.1 and 0.3 mg/kg). It required a dose of 3 mg/kg R-α-PVP (n = 6) to produce a significant increase in dopamine, and that effect was less than half that seen with 0.3 mg/kg S-α-PVP.
Fig.. 2.
Effects of racemic α-PVP and its enantiomers in comparison to saline on extracellular dopamine (DA) (a) and 5-HT (b) in the nucleus accumbens, and on horizontal ambulation (Amb) (c) and stereotypy (stereo) (d) over the same time period in the same rats. Rats received an i.v. injection of drug at time zero followed by a 3-fold higher dose 60 min later (arrows). Vehicle controls received i.v. saline on the same time schedule. Data are expressed as a percentage of basal ± SEM for n = 6–7 rats/group. Solid symbols represent significant difference from saline at the specified time point (p < 0.05).
At the same time rats were undergoing the microdialysis testing, they were also monitored for locomotor activity. Overall activity (Fig. 2c, F24,207 = 20.6, p < 0.001) and stereotypy (Fig. 2d, F24,207 = 6.5, p < 0.001) were increased in parallel to the increases in dopamine in the nucleus accumbens. S-α-PVP increased overall activity and stereotypy at the lowest dose (0.1 mg/kg) tested with even larger increases seen at 0.3 mg/kg. Racemic α-PVP also increased activity and stereotypy at 0.3 mg/kg, but this increase was less than that seen with S-α-PVP. Finally, only at a higher dose of 3 mg/kg of R-α-PVP were significant increases in activity and stereotypy observed. These results suggest that in vivo, S-α-PVP is at least 30 times more potent than R-α-PVP on these behavioral measures.
3.3. Self-administration
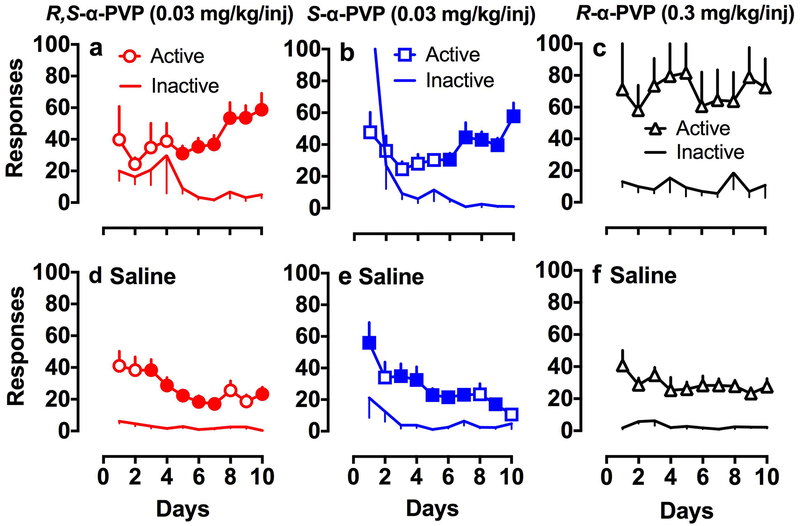
Fig. 3 panels a and b show 10 days of acquisition for rats trained with 0.03 mg/kg/injection of racemic and S-α-PVP. Rats trained with 0.03 mg/kg/injection R-α-PVP did not show evidence of acquisition (data not shown, F9,68 = 1.99). Rats trained at a 10-fold higher dose of R-α-PVP (0.3 mg/kg/injection) did show evidence of acquisition (Fig. 3c). All rats completed 10 days of acquisition with the exception of 2 rats trained with S-α-PVP. For those 2 rats, one completed 8 days of training and another completed 9 days prior to catheter loss. In general, rats responded in both the active and inactive nose-poke hole over the first few days of training of self-administration for racemic (Fig. 3a) and S-α-PVP (Fig. 3b), with a clear separation between active and inactive holes by the 6th day of training (racemic α-PVP F9.80 = 2.2, p < 0.05, S-α-PVP F9,87 = 2.3, p < 0.05). The high number of inactive responses on day 1 for S-α-PVP was due to a high number of responses (891) for one rat. All 10 rats trained with S-α-PVP showed clear evidence of self-administration and 8 of the 9 rats trained on the racemic showed clear evidence of acquisition. For the higher dose of R-α-PVP, there was no clear evidence of a change in active/inactive responding over days, but there was a significant overall effect of response (F1,70 = 9.8, p < 0.01). Six of the 8 rats trained on 0.3 mg/kg injection R-α-PVP showed clear evidence of acquisition. In general, responding was more variable for R-α-PVP in comparison to the other two drugs.
Fig.. 3.
Acquisition of nose-poke responding for 0.03 mg/kg/inj racemic (a, n = 9) and S-α-PVP (b, n = 10) and 0.3 mg/kg/inj R-α-PVP (c, n = 8). Lines with symbols are active responses. Lines without symbols are inactive responses. Solid symbols are for days during which active responses were significantly different (p < 0.05) from inactive responses on that day. For R-α-PVP the main effect of response was significant, but not the response x day interaction. The bottom panels show extinction for those rats who maintained catheters through acquisition and dose-effect (Fig. 4) testing (d racemic n = 7, e S-α-PVP n = 8, f R-α-PVP n = 6). Symbol designations are the same as for acquisition. Each point is the mean ± SEM.
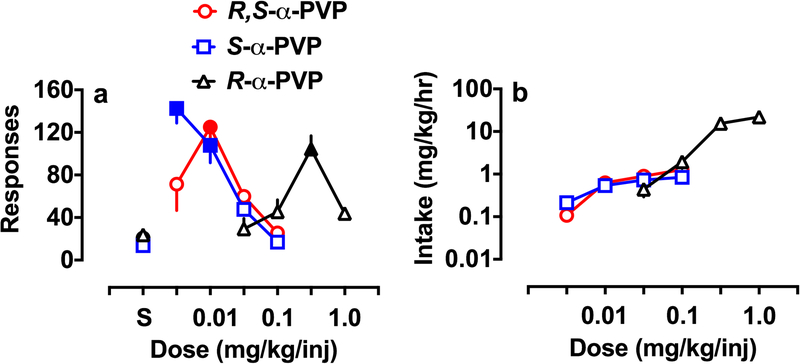
Following acquisition, the rats were tested with a range of doses of the drug they were trained with, and the results are shown in Fig. 4 (responses Fig. 4a, intake Fig. 4b). Each dose was tested for 3 days, with the last 2 days averaged. Due to catheter loss, not all rats received all doses. For racemic α-PVP, dose-effect data are from 7–9 rats, for S-α-PVP data are from 8 rats and for 3.0 mg/kg R-α-PVP data are from 6 rats. Results for saline are following 10 days of extinction with all rats completing dose-effect testing also completing extinction. Racemic α-PVP showed a typical inverted U-shaped dose-effect function with a peak of responding at 0.01 mg/kg/injection (F4,36 = 10.1, p < 0.001) that was significantly above saline control levels. Intake increased across all four doses of racemic α-PVP tested, with intake of the 3 higher doses being significantly above that of 0.003 mg/kg/injection (F3,30 = 33.8, p < 0.001). Responses for S-α-PVP were highest at the lowest dose tested (0.003 mg/kg/injection) and decreased in a dose-dependent manner at higher doses. Both of the lower doses tested were significantly above extinction levels (F4,35 = 33.6, p < 0.001) and both doses showed more responding than the highest dose tested. The 0.003 and 0.01 mg/kg/injection doses did not differ significantly. Again, intake increased in a dose-dependent manner across dose of S-α-PVP. Intake for the 0.003 mg/kg/injection dose was significantly lower than for the other 3 doses. Intake for 0.01 mg/kg/injection was lower than 0.1 mg/kg/injection. The dose-effect function for R-α-PVP was also an inverted U-shape but was shifted to the right when compared to the other two drugs, indicating R-α-PVP was around 30-fold less potent than racemic α-PVP. Responses were increased over saline extinction levels at 0.3 mg/kg/injection R-α-PVP (F4,25 = 13.2, p < 0.001). Responding at 0.3 mg/kg/injection was also higher than the other 3 doses tested. Like with the other two drugs tested, intake increased in a dose-dependent manner (F3,20 = 46.9, p < 0.001). Intake at the 0.3 and 1.0 mg/kg/injection doses were not significantly different. All other dose comparison showed a significant difference.
Fig.. 4.
Dose-effect functions for active responses (a) and mg/kg/hr intake (b) for racemic α-PVP and the enantiomers. Each rat was tested for 3 days at each dose with the final 2 days averaged for presentation and analysis. The point above S is the average for the last 2 days of extinction. Solid symbols for the response function are significantly different from the respective saline (p < 0.05). See text for description of statistics for intake. Each point is the mean ± SEM of 6–8 rats/group.
Following dose-effect testing, saline was substituted for drug and the rats were trained for 10 additional days (Fig. 3, lower panels). For both racemic (Fig. 3d) and S-α-PVP (Fig. 3e), responding in the active nose-poke hole decreased over the 10 days of extinction (racemic F9,108 = 3.7, p < 0.001, S-α-PVP F9,126 = 5.0, p < 0.001) although active hole responding was still significantly higher than inactive hole responding on some days toward the end of extinction. Responding for R-α-PVP (Fig. 3f) did not decrease significantly over days of extinction (F9,90 = 0.8), although responding in the first 3 days of extinction was slightly higher than the last 3 days.
3.4. Telemetry
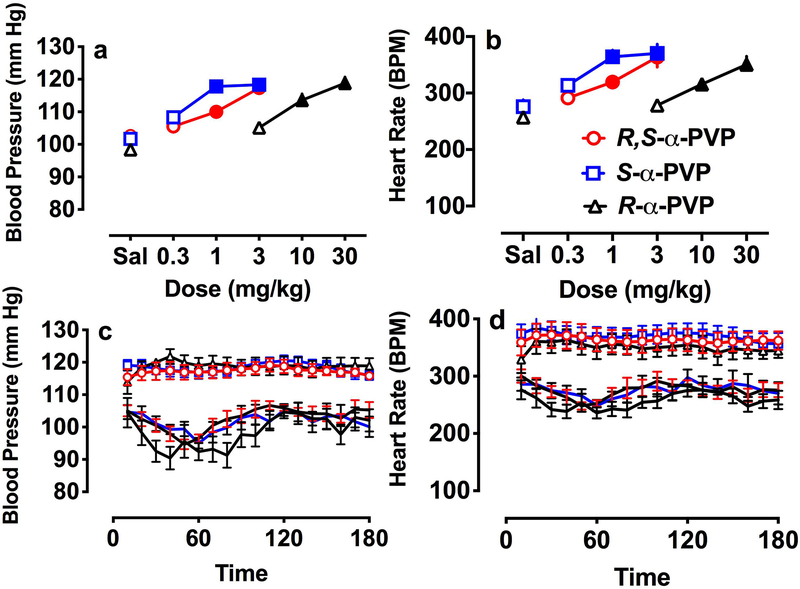
Fig. 5 shows the effects of the drugs on blood pressure and heart rate. Racemic α-PVP and its two enantiomers increased blood pressure (Fig. 5a). Both racemic α-PVP (F3.23 = 14.6, p < 0/001) and S-α-PVP (F3,24 = 32.1, p < 0.001) significantly increased blood pressure (Fig. 5a) over saline control at 1 and 3 mg/kg, although S-α-PVP appeared to be more potent. R-α-PVP, over a higher dose range, also significantly increased blood pressure (F3.16 = 18.3, p < 0.001). Significant increases over saline were seen at both 10 and 30 mg/kg, suggesting that R-α-PVP was approximately 10 times less potent than racemic α-PVP. An almost identical pattern of results was seen with heart rate (Fig. 5b). Dose-dependent increases in heart rate were seen with all three drugs. Racemic α-PVP (F3.23 = 11.2, p < 0/001) and S-α-PVP (F3,24 = 13.3, p < 0.001) significantly increased heart rate over saline at 1 and 3 mg/kg. Again, S-α-PVP appeared to be more potent than racemic α-PVP. At the higher dose range, R-α-PVP increased heart rate (F3,24 = 12.1, p < 0.001) at the 10 and 30 mg/kg dose over saline control. Calculated ED50 values supported these conclusions. For blood pressure S-α-PVP was approximately 2 times more potent than racemic (ED50 = 0.29 mg/kg, 95% CI 0.1 – 0.49 for S-α-PVP; 0.69 mg/kg, 95% CI 0.39 – 1.06 for racemic) while R-α-PVP was much less potent (ED50 = 5.85 mg/kg, 95% CI 2.83 – 9.10). Similar patterns were seen for heart rate (ED50 = 0.25 mg/kg, 95% CI 0.02 – 0.51 for S-α-PVP; 0.72 mg/kg, 95% CI 0.38 – 1.16 for racemic; ED50 = 9.85 mg/kg, 95% CI 5.63 – 17.5 for R-α-PVP). Time course data for saline and the highest dose of each drug are shown in the bottom panels of Fig. 5. The time course for the 3 saline determinations were clearly overlapping and the maximal effect of all three drugs was similar. In general, the effect of the highest doses of the α-PVP drugs persisted across the 3 hr session.
Fig.. 5.
Effects of racemic α-PVP and its enantiomers on dose-effect functions for blood pressure (a) and heart rate (b). Rats were implanted with telemetry transmitters that sampled blood pressure and heart rate every 1 min for 3 hr. Data are expressed as mean ± SEM or sum (for activity counts) for n = 5–7 rats/group for the entire 3 hr sampling period. Solid symbols represent significant difference from respective saline (p < 0.05). The bottom panels present time-course for blood pressure (c) and heart rate (d) with the 1-min samples averaged over 10-min periods for the entire 3-hr session. Data are presented for saline sessions and the highest dose of each drug tested.
Only small changes in body temperature (data not shown) were observed, with increases of less than 1 °C. Significant changes in temperature were only seen with S-α-PVP (F3,24 = 5.1, p < 0.01), with an increase seen at 0.3 mg/kg, and at the higher dose range for R-α-PVP (F3,24 = 4.5, p < 0.05), with an increase in body temperature seen at the 10 mg/kg dose.
4. DISCUSSION
The major purpose of this study was to characterize the neurochemical, behavioral and cardiovascular effects of the abused synthetic cathinone compound α-PVP and determine the contribution of its two enantiomers to those effects. Since α-PVP is structurally related to MDPV, we hypothesized that the two drugs would display comparable pharmacological effects. This notion was supported across all assays for racemic α-PVP. α-PVP blocked uptake at DAT and NET with low nM potency, similar to the effects of MDPV.6 The presence of the 3,4-methylenedioxy ring substituent of MDPV confers somewhat greater potency at SERT when compared to α-PVP.15,17,18,31 Nevertheless, both MDPV and α-PVP are highly selective for DAT and NET over SERT. While not tested here, previous studies have shown that α-PVP does not serve as a substrate for DAT, NET or SERT.17,18 In vivo, α-PVP increases dopamine in the nucleus accumbens as measured by microdialysis at doses similar to MDPV.4,6 As expected from its weak effects on SERT uptake, α-PVP did not increase levels of 5-HT in the nucleus accumbens. Like MDPV, α-PVP increased distance travelled and stereotypy in the rats undergoing microdialysis sampling in the nucleus accumbens.
MDPV produces clear increases in blood pressure and heart rate, which may be responsible for some of the mortality seen with MDPV misuse in humans. In rats implanted with telemetry transmitters, α-PVP produced increases in blood pressure and heart rate similar to other psychomotor stimulants, including MDPV.38 MDPV and α-PVP were tested over an identical dose range suggesting a similar potency. The effects of α-PVP on temperature were small and failed to reach significance, though racemic α-PVP tended to increase body temperature. Our results agree with the findings of Nelson et al.26 who found no change in body temperature after α-PVP administration (0.3 – 3.0 mg/kg) in Sprague-Dawley rats bearing s.c. implanted temperature probes. By contrast, Aarde et al.24 found decreases in body temperature after α-PVP injection in Wistar rats bearing surgically-implanted telemetry transponders similar to those used here. In addition to using a different strain of rats, Aarde et al.24 also tested α-PVP over a higher (1–10 mg/kg) but overlapping dose range.
The presence of the 3,4-methylenedioxy moiety does not seem to alter pharmacokinetics of pyrrolidine-containing cathinones since α-PVP and MDPV have a similar time-course of action after i.v. administration.4 Additionally, the time course of cardiovascular effects produced by α-PVP shown here is similar to that of MDPV shown previously, which suggests these two agents display similar pharmacokinetics after s.c. administration. By contrast, the presence of the 3,4-methylenedioxy group markedly affects metabolism of pyrrolidine-containing cathinones.46 More specifically, the major pathway for MDPV metabolism involves demethylenation of the phenyl ring to form hydroxylated metabolites, whereas the major pathway for metabolism of α-PVP involves formation of a lactam on the pyrrolidine ring. Future studies are warranted to compare the metabolism of α-PVP and MDPV in rodent models
Finally, confirming what others have shown for racemic α-PVP20,24,28–33, rats self-administered α-PVP at doses similar to racemic MDPV.45 Self-administration was acquired rapidly with α-PVP, and the drug produced a typical inverted U-shaped dose-effect function comparable to other psychomotor stimulants.
Like MDPV, α-PVP has two enantiomers, R and S based on absolute configuration, and it was expected that these enantiomers would show potency differences to the comparable enantiomers of MDPV. For MDPV, the S-enantiomer is 50–100 times more potent than the R-enantiomer. In general, both enantiomers of MDPV produce similar maximal effects, with the primary difference being potency. Here we show that S-α-PVP is about 30 times more potent than R-α-PVP in blocking uptake at DAT, and these findings agree with the earlier findings of Meltzer and colleagues.13 S-α-PVP was also about 30 times more potent than cocaine in blocking uptake at DAT. R-α-PVP was slightly more potent at NET than DAT, with S-α-PVP showing similar potency at DAT and NET. Like the racemate, neither of the enantiomers displayed substantial inhibition of SERT, at doses up to 10 μM. Given that the racemate is half as potent as S-α-PVP at catecholamine uptake sites, and given that the racemate contains equal amounts of each enantiomer, it is likely that S-α-PVP constitutes the primary effect of the racemate.
The robust stereoselective effects α-PVP and other pyrrolidine-containing cathinones on inhibition of dopamine uptake differs from the relative lack of stereoselectivity for stimulation of dopamine release induced by simple ring-substituted cathinones, which act as transporter substrates. As a specific example, R and S isomers of 4-methyl-N-methylcathinone (mephedrone) have nearly equal potency to induce release at DAT but differ in their potency to induce release at SERT.47 Likewise, Mayer et al.48 demonstrated that R and S stereoisomers of mephedrone metabolites, including 4-hydroxytolyl-mephedrone and nor-mephedrone, display equivalent potency at DAT but differential potency at SERT. Thus, it appears that stereoselectivity at DAT is more prominent for transporter inhibitors than for substrates, at least for the cathinone-related compounds examined thus far.
The potent uptake inhibition at DAT would be expected to produce increases in extracellular dopamine in the nucleus accumbens, and also increases in locomotor activity, with S-α-PVP being approximately 30 times more potent than R-α-PVP. The results of the microdialysis experiments confirmed this prediction with an i.v. dose of 0.1 mg/kg S-α-PVP producing significant increases in dopamine and locomotor activity, while it required an i.v. dose of 3.0 mg/kg R-α-PVP to produce comparable effects. The results of the uptake assays would also predict that neither enantiomer would produce changes in 5-HT in the nucleus accumbens and in fact that is what was observed.
In comparison to the other measures described here, the enantiomers of α-PVP have been tested in a self-administration model in rats. Gannon et al.30 reported that S-α-PVP was about 40 times more potent than R-α-PVP when tested on a progressive ratio (PR) schedule in rats originally trained with racemic α-PVP. The results of the current study support that finding. In dose-effect testing following training on the specific enantiomer being tested, the i.v. dose of R-α-PVP supporting peak responding was 0.3 mg/kg/inj, while the peak i.v. dose of S-α-PVP supporting the most responding was ≤ 0.003 mg/kg/inj. Acquisition of responding for S-α-PVP followed a course that was very similar to the racemic mixture. By contrast, for R-α-PVP, rats appeared to respond at a high rate from the beginning of training, with much larger variability in responding on the active lever. This situation may reflect the fact that 2 of the 8 rats trained with R-α-PVP showed only minimal evidence (at least 10 active lever responses and a clear preference for the active vs inactive response) of self-administration. In contrast, all of the rats trained with S-α-PVP showed clear evidence of self-administration by the end of training. Given that none of the rats trained on a lower dose (0.03 mg/kg/inj) of R-α-PVP showed evidence of self-administration and the 0.3 mg/kg/inj dose was the peak of the dose-effect function, it appears that an appropriate dose range of R-α-PVP was tested. Whether the differences in the acquisition curves between the enantiomers of α-PVP represents any fundamental difference between the drugs in not clear.
The dose-intake function for many psychomotor stimulants flatten out at higher doses as animals appear to regulate their intake to maintain a constant blood level of drug).49 While maybe not as clear for α-PVP, it appears that some degree of regulation was evident at doses above 0.01 mg/kg/inj for the racemic and S-α-PVP as the dose-effect functions appear to flatten out at these doses. For R-α-PVP the function appears to plateau above 0.3 mg/kg/inj. Thus, with both response rate and drug intake, α-PVP appears to function like a typical psychomotor stimulant, although it is clearly more potent than typical psychomotor stimulants such as cocaine.
The R-enantiomer of α-PVP showed slightly increased potency on the uptake assays at NET in comparison to DAT, suggesting that R-α-PVP might show increased potency on cardiovascular function as opposed to the behavioral effects of the drug thought to be mediated by dopamine. Despite this, it appeared that the same potency relationship held for the cardiovascular effects as seen with microdialysis and locomotor activity. It took a s.c. dose of 30 mg/kg R-α-PVP to produce effects comparable to a s.c. dose of 1 mg/kg S-α-PVP on blood pressure and heart rate. The calculated ED50 values suggested a 20 – 40-fold difference in potency. It is possible that the small changes in potency at NET are not readily observable in the in vivo models used in this study, or more likely, the changes observed on the uptake assays do not fully reflect the full nature of the role of norepinephrine in the cardiovascular effects of α-PVP. With the small changes observed in body temperature, it is impossible to observe any clear potency relationship between the enantiomers.
In conclusion, α-PVP produces pharmacological effects that are very similar to those produced by MDPV, blocking uptake at DAT and NET with minimal activity at SERT. These effects produce a spectrum of in vivo effects that are typical of psychomotor stimulants. α-PVP increases extracellular dopamine concentrations in the nucleus accumbens, increases locomotor activity, increases blood pressure and heart rate, and is self-administered by rats. The enantiomers of α-PVP also mimic the effects of the MDPV enantiomers, with S-α-PVP being about 30-fold more potent than R-α-PVP. Given that potency difference, it is likely that S-α-PVP is the main compound responsible for pharmacological effects of the racemate.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health (NIH) (Z01DA000523, Z01DA000532).
References
- 1.Baumann MH, Walters HM, Niello M, Sitte HH (2018) Neuropharmacology of Synthetic Cathinones. Handb Exp Pharmacol 252:113–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glennon RA, Dukat M (2017) Structure-Activity Relationships of Synthetic Cathinones. Curr Top Behav Neurosci 32:19–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drug Enforcement Administration DoJ (2011) Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist 76:65371–65375. [PubMed] [Google Scholar]
- 4.Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA (2017) Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs. Curr Top Behav Neurosci 32:93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glennon RA, Young R (2016) Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolindinovalerophenone (α-PVP). Bran Res Bull 126:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drug Enforcement Administration DoJ (2014) Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final order. Fed Regist 79:12938–12943. [PubMed] [Google Scholar]
- 10.Beck O, Backberg M, Signell P, Helander A (2018) Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin Toxicol (Phila) 56:256–263. [DOI] [PubMed] [Google Scholar]
- 11.Beck O, Franzen L, Backberg M, Signell P, Helander A (2016) Toxicity evaluation of alpha-pyrrolidinovalerophenone (alpha-PVP): results from intoxication cases within the STRIDA project. Clin Toxicol (Phila) 54:568–575. [DOI] [PubMed] [Google Scholar]
- 12.Potocka-Banas B, Janus T, Majdanik S, Banas T, Dembinska T, Borowiak K (2017) Fatal Intoxication with alpha-PVP, a Synthetic Cathinone Derivative. J Forensic Sci 62:553–556. [DOI] [PubMed] [Google Scholar]
- 13.Meltzer PC, Butler D, Deschamps JR, Madras BK (2006) 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem 49:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA (2015b) Structural Modification of the Designer Stimulant alpha-Pyrrolidinovalerophenone (alpha-PVP) Influences Potency at Dopamine Transporters. ACS Chem Neurosci 6:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA (2017) Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr Top Behav Neruosi 32:93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky A (2017) Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J Pharmacol Exp Ther 360:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickli A, Hoener MC, Liechti ME (2015) Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol 25:365–376. [DOI] [PubMed] [Google Scholar]
- 19.Kaizaki A, Tanaka S, Numazawa S (2014) New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci 39:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (2018) Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL (2016) Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology 55:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatch MB, Dolan SB, Forster MJ (2015) Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther 354:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojcieszak J, Andrzejczak D, Wojtas A, Golembiowska K, Zawilska JB (2018) Effects of the new generation alpha-pyrrolidinophenones on spontaneous locomotor activities in mice, and on extracellular dopamine and serotonin levels in the mouse striatum. Forensic Toxicol 36:334–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA (2015) In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 232:3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannotti G, Canazza I, Caffino L, Bilel S, Ossato A, Fumagalli F, Marti M (2017) The Cathinones MDPV and alpha-PVP Elicit Different Behavioral and Molecular Effects Following Acute Exposure. Neurotox Res 32:594–602. [DOI] [PubMed] [Google Scholar]
- 26.Nelson KH, Hempel BJ, Clasen MM, Rice KC, Riley AL (2017) Conditioned taste avoidance, conditioned place preference and hyperthermia induced by the second generation ‘bath salt’ alpha-pyrrolidinopentiophenone (alpha-PVP). Pharmacol Biochem Behav 156:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watterson LR, Burrows BT, Hernandez RD, Moore KN, Grabenauer M, Marusich JA, Olive MF (2014) Effects of alpha-pyrrolidinopentiophenone and 4-methyl-N-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD (2017) Active vaccination attenuates the psychostimulant effects of alpha-PVP and MDPV in rats. Neuropharmacology 116:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology (Berl) 234:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gannon BM, Rice KC, Collins GT (2017) Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and alpha-pyrrolidinopentiophenone and their enantiomers. Behav Pharmacol 28:578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, Collins GT (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA (2018a) Binge-like acquisition of alpha-pyrrolidinopentiophenone (alpha-PVP) self-administration in female rats. Psychopharmacology (Berl) 235:2447–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA (2018b) Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology 134:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL (2015) Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML (2017) Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol 22:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA (2015a) Stereoselective Actions of Methylenedioxypyrovalerone (MDPV) To Inhibit Dopamine and Norepinephrine Transporters and Facilitate Intracranial Self-Stimulation in Rats. ACS Chem Neurosci 6:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J Pharmacol Exp Ther 356:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindler CW, Thorndike EB, Suzuki M, Rice KC, Baumann MH (2016b) Pharmacological mechanisms underlying the cardiovascular effects of the “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV). Br J Pharmacol 173:3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson KH, Lopez-Arnau R, Hempel BJ, To P, Manke HN, Crissman ME, Clasen MM, Rice KC, Riley AL (2019) Stereoselective effects of the second-generation synthetic cathinone alpha-pyrrolidinopentiophenone (alpha-PVP): assessments of conditioned taste avoidance in rats. Psychopharmacology (Berl) 236:1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, Deschamps JR, Jacobson AE, Rice KC (2015) Chiral resolution and absolute configuration of the enantiomers of the psychoactive “designer drug” 3,4-methylenedioxypyrovalerone. Chirality 27:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41. [DOI] [PubMed] [Google Scholar]
- 42.Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA (2003) In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther 307:138–145. [DOI] [PubMed] [Google Scholar]
- 43.Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monamine transporters in brain tissure. Neuropsyhopharmaocology 37:1192–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB (2011) In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther 337:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH (2016a) Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negreira N, Erratico C, Kosjek T, van Nuijs AL, Heath E, Neels H, Covaci A (2015) In vitro phase I and phase II metabolism of α-pyrrolidinovalerophenone (α-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal Bioanal Chem 407:5803–5816. [DOI] [PubMed] [Google Scholar]
- 47.Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Velvadapu V, Smith GR, Peet MM, Reitz AB, Negus SS, Rawls SM (2015) Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br J Pharmacol 172:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer FP, Cintulova D, Pittrich DA, Wimmer L, Luethi D, Holy M, Jaentsch K, Tischberger S, Gmeiner G, Hoener MC, Liechti ME, Mihovilovic MD, Sitte HH (2019) Stereochemistry of phase-1 metabolites of mephedrone determines their effectiveness as releasers at the serotonin transporter. Neuropharmacology 148:199–209. [DOI] [PubMed] [Google Scholar]
- 49.Tsibulsky VL, Norman AB (1999) Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res 839:85–93. [DOI] [PubMed] [Google Scholar]