Abstract
As conversion from calcineurin inhibitor (CNI) to sirolimus (SRL), an mTOR-inhibitor (mTOR-I), has been shown to enhance immunoregulatory profiles in liver transplant recipients (LTR), mTOR-I therapy might allow for increased success with immunosuppression withdrawal. Our aim was to determine if operational tolerance could be observed in LTR withdrawn from SRL and if blood/graft tolerance biomarkers were predictive of successful withdrawal. We performed a prospective trial of SRL monotherapy withdrawal in non-immune, non-viremic LTR > 3 years post-LT. Sirolimus was weaned over ~6 months and biopsies performed 12 months post-weaning or at concern for acute rejection (AR). Twenty-one LTR were consented; 6 were excluded due to subclinical AR on baseline biopsy or other reasons; 15 underwent weaning (age 61.3±8.8 yrs; LT to SRL weaning 6.7±3 yrs). Eight (53%) achieved operational tolerance (TOL). Of the 7 non-TOL, 6 had mild AR on biopsy near the end of weaning or at study end; 1 was removed due to liver cancer recurrence. At baseline preweaning, there were statistically higher blood tolerogenic dendritic cells, regulatory B cells, and cell phenotypes correlating with chronic antigen presentation in the TOL vs. non-TOL groups. At baseline preweaning, a previously identified biopsy gene signature accurately predicted TOL vs. non-TOL in 12/14 LTR. At study end, biopsy staining revealed statistically significant increases in antigen presenting cell:leukocyte pairings, Foxp3+CD4+ T cells, T-bet+CD8+ T cells, and lobular dendritic cells in the non-TOL group.
Conclusion:
This study is the first to evaluate IS withdrawal directly from mTOR-I therapy in LTR and achieved >50% operational tolerance. Pre-weaning blood/graft gene expression and PBMC profiling may be useful as predictors of successful mTOR-I therapy withdrawal. NCT02062944
Keywords: tolerance, molecular target of rapamycin inhibitors, tolerogenic dendritic cells, T cell exhaustion, genomics
INTRODUCTION
Lifetime immunosuppression (IS) with standard agents, calcineurin inhibitors (CNI) cyclosporine and tacrolimus (TAC), is currently required to prevent rejection in liver transplant recipients (LTR). However, this occurs at the significant expense of long-term CNI toxicity, i.e. chronic kidney disease (CKD), hypertension, diabetes, infections and malignancy (1, 2). One such class of agents, mechanistic target of rapamycin inhibitor (mTOR-I) therapies (SRL; sirolimus; EVL; everolimus), has a different mechanism of action. Some studies have shown that CNI to SRL conversion can stabilize renal function with a low risk of rejection (3, 4). Yet even with these possible benefits, patients on SRL are still subject to lifetime IS therapy, highlighting the need to investigate strategies that promote full IS withdrawal without rejection, also known as operational tolerance.
With this in mind, an additional advantage of mTOR-I therapy lies in its potential to promote an immunoregulatory state that could facilitate safe IS withdrawal. As the most immune-privileged solid organ transplanted, the liver houses numerous hematopoietic cells, a large mass of less immunogenic cells (hepatocytes, stellate cells, endothelial cells), and secretes immunoregulatory proteins (HLA-G) (5). The abundance of resident immunocytes, Kupffer cells, and antigen presenting cells (APC) protect against graft rejection. Donor-specific immunoregulatory effects, dilution or inhibition of alloantibody, and mixed hematopoietic microchimerism are also putative components of liver transplant tolerance (6–8). The percentage of LT recipients able to undergo complete IS withdrawal is the highest of all organ transplant recipients, although still only successful in the minority and mainly performed in CNI-treated patients (9). While speculative, this low percentage could be due to known CNI mechanisms inhibiting immunoregulation.
A key difference between mTOR-I and CNI is their effect on regulatory T cells (Tregs; CD4+CD25highFoxp3+) and tolerogenic dendritic cells important in the suppression of immune responses. As an inhibitor of interleukin-2 (IL-2) signaling, SRL blocks proliferation of alloreactive T cells but facilitates the generation of Tregs, tolerogenic DC, and a regulatory cytokine environment in vitro (10, 11). In contrast, CNIs block T cell receptor signal transduction and IL-2 transcription, both inhibiting Treg generation (12–14). We have recently demonstrated systemic Treg/tolerogenic DC ratio increases and enhanced tolerogenic proteogenomic markers in LT recipients converted from CNI to SRL (15), as well as augmented allo-specific Treg function by mTOR-I in vitro (16). Other reports demonstrate a high percentage of Tregs, tolerogenic DC, γδ Tcells and specific gene signatures in tolerant LT recipients weaned mainly from CNI therapy (17–20).
With this rationale, we conducted a pilot clinical trial of SRL monotherapy withdrawal in select LTR with serial peripheral blood and graft biomarker assessments. We hypothesized that the clinical use of SRL promotes beneficial immunoregulatory pathways that may lead to a higher success of IS withdrawal than CNI weaning. We also hypothesized that PBMC profiles and gene expression patterns associated with immunoregulation, potentially enhanced by SRL conversion, would correlate with operational tolerance, supporting their use in future studies to predict weaning success.
METHODS
Subjects and Assessments
The study was a prospective single-arm trial of SRL monotherapy withdrawal in stable non-immune, non-viremic LT recipients (ClinicalTrials.gov: NCT02062944). The study was approved by the Northwestern University Institutional Review Board. To identify our cohort, we screened our LT database for all living recipients transplanted at Northwestern (1995-2012) who met the following inclusion criteria: 1) adult LT ≥18 years of age; 2) ≥3 months of SRL monotherapy with trough levels between 3-8 ng/ml; 3) ≥3 years post-LT (primary living or deceased donor). We selected ≥3 years from LT given the known low rates of withdrawal success prior to this time point (21) and to more directly compare with reported CNI withdrawal success (42%) after this time point (22). Patients were excluded based on any of the following: 1) acute cellular rejection within 12 months prior to enrollment; 2) abnormal liver function tests within 12 months prior to enrollment: direct bilirubin ≥1 mg/dL; ALT, AST, GGT or alkaline phosphatase ≥2x ULN; 3) viral [viremic hepatitis B virus (HBV) or hepatitis C virus (HCV)] or immune causes of liver disease (autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis); 4) re-transplantation or combined liver-other organ; 5) human immunodeficiency virus (HIV) infection; 6) glomerular filtration rate (GFR) <30 ml/min by MDRD-4; 5) Inability to provide informed consent or comply with the protocol. If the prescreening criteria were met, subjects were consented in-person.
Figure 1 shows the study timeline, visits and assessments. Baseline physical examination and laboratory tests were performed and if acceptable per criteria above, a baseline liver biopsy was performed within one month. Patients were excluded from IS withdrawal if any of the following were present on local biopsy read: a) ≥ grade 2 inflammation or stage 2 fibrosis; b) acute or chronic rejection; c) de-novo autoimmune hepatitis; d) inflammation of >50% of portal tracts; e) other pathology not-specified but deemed high risk per the pathologist.
Figure 1: Study design.
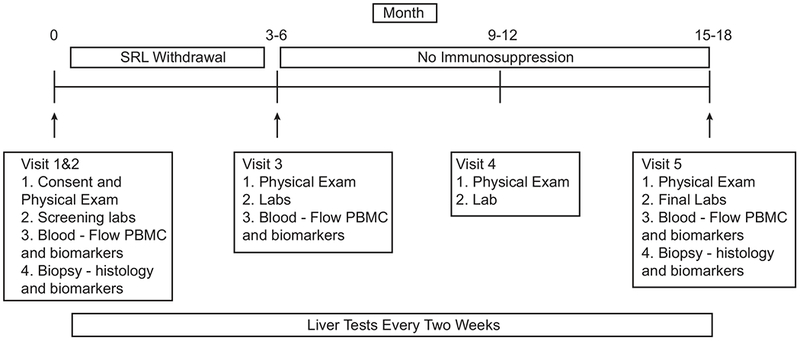
Timing of visits, SRL presence/absence, and clinical and mechanistic tests are displayed over the study period.
For enrollment participants, SRL was minimized slowly over approximately 3-6 months, dictated by starting dose. Specifically, SRL was reduced every month by 50% of total dose until 0.5 mg daily for one month. Each month, SRL was then reduced to 0.5 mg every other day, then 0.5 mg twice weekly, and finally once weekly dosing. Repeat laboratory and biomarker blood tests were performed after one month of weekly SRL dosing. If liver tests were normal, SRL was discontinued completely. Subjects were seen six months later and all baseline assessments were repeated for the final study visit 12 months post-full withdrawal. Liver tests were performed every 2 weeks at the patient’s local laboratory throughout the trial. At any concern for rejection, defined by abnormal liver tests in the criteria above, liver biopsy and blood/tissue biomarkers assays were performed. If rejection was diagnosed, the patient was withdrawn from further weaning and the trial, making this the end of study visit. Rejection was managed by reinstitution of full dose IS therapy with or without corticosteroid treatments, depending on severity.
The primary outcome was the proportion of operationally tolerant patients off SRL therapy with normal liver biochemistry and graft histology at 12 months. Secondary outcomes included the incidence, severity and reversibility of rejection, resolution of SRL-associated and other non-specific IS effects (hyperlipidemia, hyperglycemia, etc.), and the assessment of tolerance biomarkers described below.
Histological Assessments
Following study completion, all biopsy slides (baseline, for-cause, end of study) were sent for blinded external confirmation of histology by a central pathologist and scored for inflammation, fibrosis, features of rejection, and any other notable pathological features, based on established criteria (23). Central reports were compared to local reports generated at the time of the biopsies during the active clinical trial, for concordance and assessment of the primary endpoint (operational tolerance on histology 12 months after full SRL withdrawal).
Blood and Graft Biomarker Assessments
Blood and graft samples were collected, frozen, and stored in Northwestern’s Comprehensive Transplant Center biorepository, with the exception of fresh PBMC for initial immunophenotyping (below). Stored samples were batched and sent to various laboratories via collaborative agreements between NU and Scripps (blood gene expression) and between NU and the Immune Tolerance Network (graft gene expression; frozen PBMC immunophenotyping; DSA assessment; graft histology and immunohistochemistry).
PBMC Immunophenotyping
Flow cytometric analyses were performed in fresh PBMC at Northwestern during the trial and later in frozen PBMC in collaboration with the Immune Tolerance Network. The fresh and frozen samples were analyzed with different panels.
Fresh PBMC were isolated from heparinized 10 ml samples on Ficoll-Hypaque gradients. Surface markers were detected with monoclonal antibodies for the T cell subsets CD3, CD4, CD8, CD25, CD45RA, CTLA4 and CD127 (Beckman Coulter) followed by fixation/permeabilization/incubation with human Foxp3 antibodies (eBiosciences) and final flow cytometric analysis. RBC-lysed peripheral blood were also labeled with monoclonal antibodies for T cell subsets (as above), B cells (CD19), monocytes (CD14) and NK cells (CD56) to quantify the absolute cell numbers per μL. Dendritic cell (DC) assessments included markers for monocytoid vs. plasmacytoid (CD11c; CD123) ratio, antigen processing (CD83; CD205), and regulatory T cell induction (ILT3; ILT4). Tolerogenic DCs were identified as HLA-DR+CD11c+ILT3+ILT4+ (24).
Frozen PBMC were stained as detailed in the Supplemental Methods. Samples were acquired on an X20 Fortessa (BD, Franklin Lakes, NJ) with FACSDiva software (BD). Data were analyzed using FlowJo version 9.9.6 (Ashland, OR). Additional antibody information can be found in Supplemental Table 1. For statistical analysis of all PBMC, we performed group (TOL vs. non-TOL) comparisons of pre- vs. post withdrawal measurements using appropriate analyses (paired t-test or Wilcoxon Signed Rank test). Two-sided F-test statistics are applied; alpha level of 0.05.
HLA Typing and Serum Assessment for Donor Specific Antibodies
Donor and recipient DNA were extracted from buffy layer and typed at intermediate resolution using sequence-specific oligonucleotide primed PCR (PCR-SSO), for both class I and class II loci. Serum was collected and stored for anti-HLA antibody screening (FlowPRA Screening™) and specificity (LabScreen® Single Antigen™). Samples were acquired on a FACSCanto II (Becton-Dickinson, San Jose CA) and LABScreen 200 instrument (One Lambda, Canoga Park, CA), respectively. HLA pattern analysis and LabScreen® bead mean fluorescence intensities of (MFI) >1000 were used to determine DSA+ samples.
Blood Gene Expression Profiling
Blood was collected in PaxGene tubes for gene expression profiling with the Affymetrix Human Genome U133 Plus PM GeneChip. Total RNA was extracted from the PAXgene tubes using the PAXgene Blood microRNA (miRNA) reagents on a QIAcube instrument (Qiagen). Total RNA yields and concentrations were determined using a Nanodrop 8000 (Thermo Fisher Scientific). Blood samples underwent a globin RNA reduction step using the Ambion GLOBINclear Human kit (Thermo Fisher Scientific). In vitro transcription and probe labeling was done using the Affymetrix 3’ IVT (in vitro transcript) PLUS labeling system with starting input of 200ng of globin-reduced RNA. Array hybridization washing, staining and scanning was done using standard manufacturer’s protocols (Affymetrix, Santa Clara, CA).
Liver Graft Gene Expression Profiling
Biopsy samples were prepared as detailed in the Supplemental Methods. Gene expression was profiled with Affymetrix Human Genome U133PM. Analysis for differential expression on a probe basis was done by LIMMA, including multiple testing corrections using the False Discovery Rate (FDR) method. Details for analysis are provided in the Supplemental Methods. To predict the outcome of drug withdrawal from baseline liver biopsies, we quantified the relative expression of 5 genes (SOCS1, TFRC, PEBP1, MIF, CDHR2) employing real time PCR (Applied Biosystems 7900HT platform using commercially available primer/probe combinations) and a previously described logistic regression gene classifier (19).
Immunohistochemical Biopsy Staining
Immunohistochemical biopsy staining compared pre- and post-withdrawal cell populations within the graft to correlate with blood PBMC markers in TOL vs. non-TOL recipients. Batched slide sets were stained as described in the Supplemental Methods and previously (25, 26). Fully automated tissue-tethered cytometry was then performed using internally developed image analysis software (NearCYTE; http://nearcyte.org) that co-localizes multiple analytes via a defined nuclear marker and parametric segmentation. Multiplex staining panels included: 1) CD4/CD8/T-bet/Foxp3 (Tregs, Th1regs, Th1 cells, and CD4:CD8 ratios), and 2) IRF4/IRF8/HLA-DPB1/ILT4/CD11c (Th2 cells and differentiating DC). Additional information can be found in Supplemental Table 2.
Statistical Analysis
Immunosuppression withdrawal outcome and clinical differences and differences between baseline and last study visits for TOL and non-TOL participants were compared using Wilcoxon rank sum tests, as appropriate. For statistical analysis of all PBMC, we performed exploratory group (TOL vs. non-TOL) comparisons of pre- vs. post withdrawal measurements using appropriate analyses (paired t-test or Wilcoxon Signed Rank test). Two-sided F-test statistics are applied; alpha level of 0.05. All analyses were performed using SAS software (version 9.4, SAS Institute, Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
Figure 2 is a flow chart of the screening procedures that led to the final group meeting weaning criteria. As shown, 30 met inclusion criteria, of which 21 consented to participate. Three were medically excluded after the initial evaluation and three more were excluded due to mild rejection on biopsy by local read. Of the latter three, two were read as having non-alcoholic steatohepatitis and only one with rejection on central pathology review. However, in the remaining 15 patients who underwent SRL weaning, there was 100% concordance between local and central pathology reads for all pre-weaning, rejection, and end of study biopsies.
Figure 2: Diagram of study enrollment.
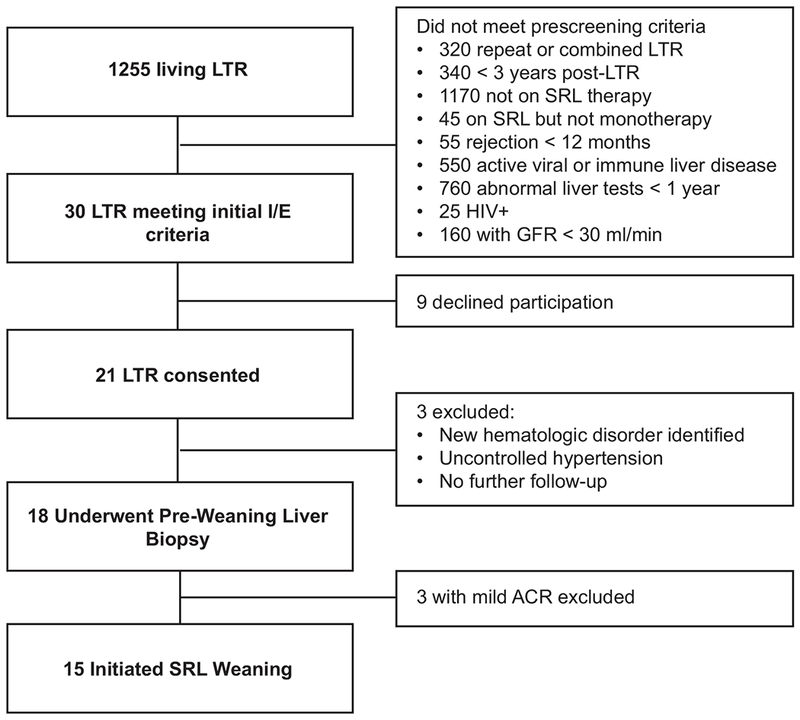
The number of participants eligible for the study based on the inclusion/exclusion criteria is indicated.
Table 1 shows the baseline characteristics between the 8 patients who were successfully withdrawn from IS (TOL) and the 7 who were not (non-TOL). There was no difference between the median age [63.7 (range 47.3, 76.3) vs. 62.7 (range 44, 67.6) years], time on SRL monotherapy [4.2 (range 0.62, 5.5) vs. 4.1 (range 2.5, 5.4) years], or time from LT to weaning [8.1 (range 4.5, 12.0) vs. 6.9 (range 3.0, 10.9) years] between the groups, respectively. Other baseline parameters (sex, hypertension, diabetes, liver tests, pre-enrollment histology, eGFR, lipid profile, urine prot/creat ratio, HbA1C, blood pressure, hematological parameters) were also not different (Table 1; Supplemental Table 3).
TABLE 1:
Baseline Clinical Characteristics
| Age (years) | Sex | Transplant to enrollment (years) | Years on SRL monotherapy | Reason for initial SRL conversion¥ | SRL daily dose (trough - ng/ml) | Cause of Liver Disease | Liver Tests (TB/ALT/AlkPhos) | eGFR¥ | Hypertension | Diabetes | Pre-Enrollment Biopsy Features | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOLERANT GROUP | Inflammation | Ishak Fibrosis | ||||||||||
| 76.3 | F | 7.5 | 4.5 | eGFR 30-60 | 0.5 mg (4.0) | NASH | 0.7/14/72 | 36 | y | n | - | 0/6 |
| 64.4 | M | 4.5 | 3.6 | eGFR 30-60 | 2 mg (4.9) | Alcohol | 0.3/29/97 | 35 | n | y | Minimal portal | 0/6 |
| 56.7 | M | 7.6 | 4.9 | eGFR 30-60 | 1.5 mg (4.7) | NASH | 0.3/16/68 | 46 | n | n | - | 1/6 |
| 64.3 | M | 8.8 | 5.5 | eGFR 30-60 | 1 mg (4.4) | Cryptogenic | 0.4/31/63 | 67 | y | n | NASH Activity Index 3/8 | 1/6 |
| 47.3 | M | 12.1 | 2.4 | eGFR 30-60 | 1 mg (5.0) | Cryptogenic | 0.6/25/92 | 65 | n | n | Rejection Activity Index 2/9 | 1/6 |
| 75.2 | F | 6.9 | 0.6 | eGFR 30-60 | 1 mg (2.2) | Cryptogenic | 0.4/44/108 | 46 | y | n | NASH Activity Index 5/8 | 1/6 |
| 58.8 | M | 10.3 | 4.8 | eGFR 30-60 | 1 mg (3.6) | Alcohol | 0.6/17/89 | 50 | y | y | Minimal portal | 0/6 |
| 64.1 | M | 10.3 | 4.4 | eGFR 30-60 | 1 mg (3.9) | Alcohol | 0.4/25/37 | 46 | y | n | Minimal portal | 0/6 |
| NON-TOLERANT GROUP | ||||||||||||
| 67.1* | M | 5.1 | 3.3 | Hepatoma | 2 mg (4.8) | HCV | 0.5/40/75 | 47 | y | y | - | 1/6 |
| 64.0 | F | 10.9 | 4.9 | eGFR 30-60 | 1 mg (4.3) | Alcohol | 0.4/36/53 | 74 | n | n | NASH Activity Index 5/8 | 0/6 |
| 53.3 | M | 8.8 | 5.4 | eGFR 30-60 | 1 mg (5.6) | Alpha-1 Antitrypsin | 0.6/43/112 | 72 | y | n | Rejection Activity Index 2/9 | 1/6 |
| 67.6 | M | 3.0 | 2.7 | eGFR 30-60 | 1 mg (5.3) | Alcohol | 0.4/23/85 | 45 | n | n | NASH Activity Index 3/8 | 0/6 |
| 62.8 | M | 9.4 | 4.7 | eGFR 30-60 | 1 mg (4.6) | HCV | 0.4/33/73 | 53 | n | n | - | 1/6 |
| 54.4 | F | 6.9 | 4.1 | eGFR 30-60 | 0.5 mg (2.9) | Cryptogenic | 0.7/15/109 | 46 | y | n | Minimal portal | 1/6 |
| 44.0 | M | 3.0 | 2.5 | HEHE | 2 mg (4.5) | HEHE | 1.1/15/56 | 76 | n | n | Minimal portal | 0/6 |
Removed due to hepatoma recurrence;
all eGFR values in mL/min/1.73 m2 per MDRD-4.
Patient Clinical Course
The 8 TOL patients were successfully withdrawn from SRL at a median of 18 weeks (range 12, 24) from enrollment. One patient had elevation of routine liver tests (TB 0.4 mg/dL, ALT 93 U/L, AlkPhos 111 U/L) 6 months after weaning and underwent for-cause liver biopsy. Biopsy showed mild non-alcoholic steatohepatitis without rejection or fibrosis. Liver test abnormalities resolved in conjunction with weight loss. All 8 patients had end-of-study biopsies which were negative for T-cell mediated rejection (TCMR) and showed no change or worsening in inflammation/fibrosis (all minimal). Three patients had steatosis and/or steatohepatitis with no progression in fibrosis.
The 7 non-TOL patients failed withdrawal from SRL at different time points (Supplemental Table 4). Three subjects had abnormal liver tests at week 21, 26, and 35 near the end of weaning when on once weekly SRL; all three biopsies demonstrated mild TCMR. Three subjects had normal liver tests 12 months post-SRL withdrawal but the end study protocol biopsy revealed mild TCMR. One patient did not have rejection during withdrawal but was withdrawn from the trial due to an unexpected adrenal metastasis of hepatocellular carcinoma. This patient resumed SRL, was treated with resection of the adrenal metastasis followed by sorafenib therapy, and had normal liver tests at 12 months of follow up.
There was no statistical difference between the following clinical variables on paired testing (pre- vs. post-withdrawal) between the TOL and non-TOL groups: 1) blood pressure; 2) body weight; 3) hematological parameters; 4) renal function parameters; 5) glucose control; 6) lipid profile (Supplemental Table 3).
Peripheral Blood Mononuclear Cell Immunophenotyping Analysis
In freshly isolated PBMC at baseline (pre-weaning), TOL vs. non-TOL participants had a higher percentage of HLA-DR+CD11c+ILT3+ILT4+ DC (p<0.01, Figure 3A). There were no differences in the percentages of PBMC that were Breg (Figure 3B) or Tregs (Figure 3C), as well as CD3+, CD4+, CD8+, CD14+, CD19+, or CD56+ at baseline (data not shown). At the end of study, TOL subjects had increased percentages of HLA-DR+CD11c+ILT3+ILT4+ DC compared to baseline and to non-TOL participants (p<0.05). However, by the end of study, non-TOL participants had a significantly higher percentage of Breg compared to TOL participants (p<0.01, Figure 3B).
Figure 3: Real-time flow cytometry detects baseline (pre-weaning) differences in DC and B cell populations between TOL and non-TOL participants.

PBMC were processed and stained in real-time for surface and intracellular markers to detect cells with regulatory phenotypes. Panel A: Tolerogenic DC (HLA-DR+CD11c+ILT4+ILT3+); Panel B: Bregs (CD19+IgM+IgD+IL-10+); Panel C: Tregs (CD4+Foxp3+, panel C). Patient numbers: TOL (n = 8 at baseline and n = 7 at study end for Panel A; n=8 at baseline and at study end for Panels B and C) and non-TOL (n=7 at baseline; n=6 at study end). Symbols represent medians; bars represent IQR. Medians are presented as the percentage of the parent population (DC scatter, CD19+ B cells, or CD4+ T cells, as appropriate). *p<0.05; **p<0.01; ***p<0.001.
In frozen PBMC samples, at baseline, TOL participants had increased cell populations suggestive of previous activation and differentiation (CD45RA+, CCR7−) (27) when compared to non-TOL participants (Figure 4). These differences were concentrated in CD8+ vs. CD4+ lymphocytes (Supplemental Table 5). Naïve CD8+ T cells made up a greater proportion of T cells in non-TOL participants both at baseline and at the end of study visit (TOL vs. non-TOL, p<0.01 and 0.05, respectively, Figure 4A). Conversely, CD8+ TEMRA (T effector memory subsets; Figure 4B) and Eomes+ (Figure 4C) CD8+ T cells made up a greater proportion of CD8+ T cells in TOL participants at baseline (p<0.05). CD8+ TEMRA, Eomes+, and Eomes+T-bet+ (Figure 4D) T cells made up a greater proportion of CD8+ T cells in TOL participants at the end of study (p<0.01, <0.001, and <0.05, respectively). Markers for additional cell populations tested in the frozen PBMC flow are detailed in Supplemental Table 1.
Figure 4: Batched flow cytometry detects baseline (pre-weaning) differences in antigen non-specific memory cell populations between TOL and non-TOL participants.
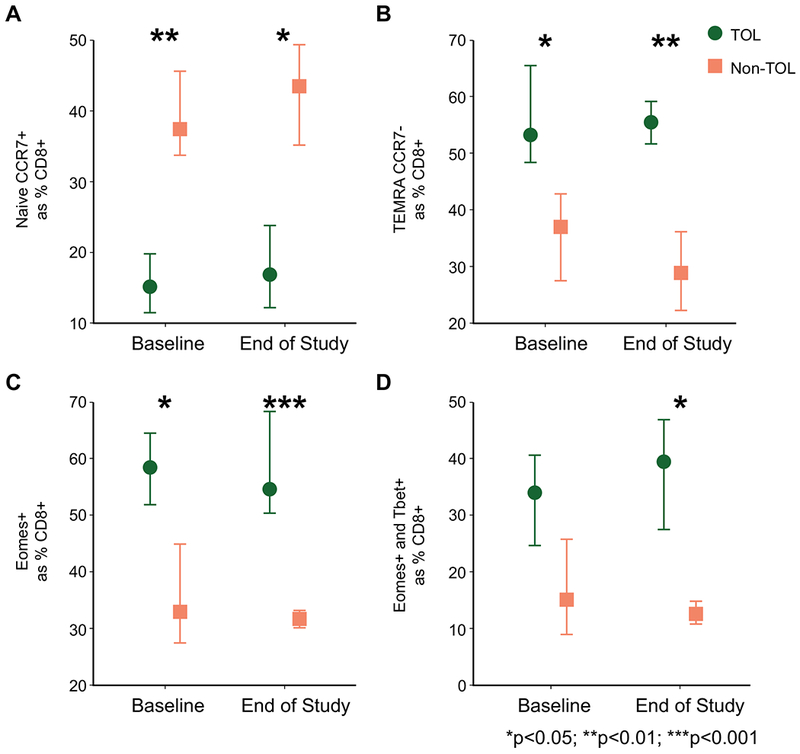
PBMC were processed in real-time and frozen until staining for surface and intracellular markers to detect naïve and memory cell populations. Naïve CD8+ T cells (Panel A), CD8+ TEMRA T cells (Panel B), CD8+Eomes+ T cells (Panel C), and CD8+Eomes+T-bet+ T cells (Panel D) are depicted for TOL and non-TOL participants (n=8 and n=6 at baseline and end of study, respectively). Symbols represent medians; bars represent IQR. Medians are presented as the percentage of the parent population, CD8+ T cells. *p<0.05; **p<0.01; ***p<0.001.
Donor Specific Antibody Assessments
We performed DSA testing on serum samples collected from TOL and non-TOL participants at screening, before their last dose of IS, and following completion or termination of IS withdrawal. Three of 6 non-TOL participants, and 4 of 8 TOL participants were DSA negative throughout the trial. One TOL and one non-TOL participant developed DSA following IS withdrawal. No participants lost DSA positivity during the study. Both the maximum MFI value of DSA (Figure 5A) and the summation of all DSA MFI values (Figure 5B) are summarized.
Figure 5: DSA presence, absence, or development did not correlate with IS withdrawal outcome.
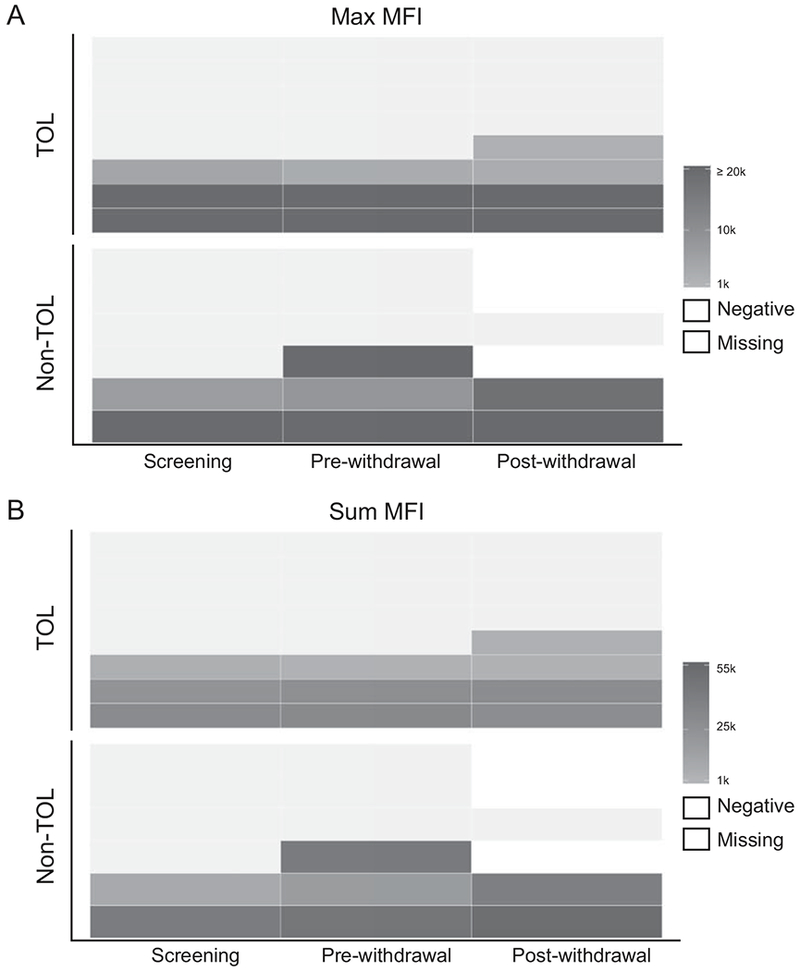
Serum samples were tested for DSA from TOL (n=8) and non-TOL (n=6) participants at screening, TOL (n=8) and non-TOL (n=5) before their last dose of SRL, and TOL (n=8) and non-TOL (n=3) following completion or termination of SRL withdrawal. The maximum MFI detected (Panel A) and sum of all DSA MFI detected (Panel B) are depicted. The upper limit of the linear range of detection for this assay is an MFI of 20,000; changes in DSA concentration above this MFI may not be detectable due to bead saturation. MFI values below 1,000 are considered negative and imputed for representation only.
Gene Expression Profiling in Biopsy and Peripheral Blood
We hypothesized that baseline pre-weaning gene expression could also predict tolerance. Therefore, in the current mTOR-I cohort, we tested a previously identified real-time PCR based biopsy signature relating to iron metabolism (see methods above) that predicted tolerance in a prior CNI withdrawal study (19). Using pre-weaning biopsies, this same signature accurately predicted TOL in 12/14 (85.7%), with 88% sensitivity, 83% specificity, 88% positive predictive value and 83% negative predictive value.
To test for new markers of a tolerant state, we examined transcriptional differences between blood and biopsy. There were 258 blood probesets and 210 tissue probesets distinguishing TOL vs. non-TOL at baseline (p<0.005, Supplemental Tables 6 and 7, respectively). However, only four genes were common to both blood and biopsy at p<0.005, and three were differentially expressed as down-regulated in blood and up-regulated in biopsy: abhydrolase domain containing 4 , pyrroline-5-carboxylate reductase 2, and signal regulatory protein alpha. Only one gene (NOP9 nucleolar protein) was downregulated in both blood and biopsy.
Immunohistochemistry Biopsy Staining Differences at Baseline and End of Study
Biopsies obtained from subjects both at baseline and at their last study visit (end of study protocol biopsy or at rejection) were analyzed by fluorescent immunohistochemistry for presence and phenotype of both T cells and antigen presenting cells (Figure 6). Unsupervised, automated analysis detected the load (number of positive events per mm2 of biopsy) of CD4+ T cells was increased in TOL vs. non-TOL participants at baseline (median [IQR]; 178.06 [168.03-204.76] vs. 85.23 [68.70-157.90], p<0.05). The intragraft Foxp3+CD4+ load remained stable before and after IS withdrawal for TOL participants. For non-TOL participants, the load of CD3+ T cells increased between baseline and last study visit (158.11 [89.40-219.87] vs. 435.61 [323.72-652.46], p<0.01). Compared to cells identified in the baseline biopsy, the last biopsy from non-TOL participants also contained increased Foxp3+CD4+ T cells (0.68 [0.66-0.77] vs. 5.48 [0.86-7.32], p<0.05) and T-bet+CD8+ T cells (29.48 [16.09-35.63] vs. 65.40 [39.28-68.90], p<0.05). Interestingly, the number of APC:lymphocyte pairings per mm2 of tissue (CD45+ cells located within 5 μm of an MHCII+ cell) in the tissue increased in non-TOL participants (18.52 [15.24-22.60] vs. 36.31 [32.40-43.50], p<0.05). While there was no difference in the load of APC:lymphocyte pairs between TOL and non-TOL at baseline, non-TOL participants had significantly more of these pairings at the last study visit (15.77 [3.54-18.28] vs. 36.31 [32.40-43.50], p<0.05). These findings are suggestive of increased inflammation in non-TOL biopsies. In addition, lobular MHCII+ILT4+CD11c+ DC were increased at the last biopsy in non-TOL vs. baseline (66.34 [40.30-83.16] vs. 14.27 [10.21-16.56], p<0.01), and vs. TOL participants (4.69 [3.84-38.09], p<0.01).
Figure 6: Immunohistochemistry detects differences between biopsies at baseline and end of study.
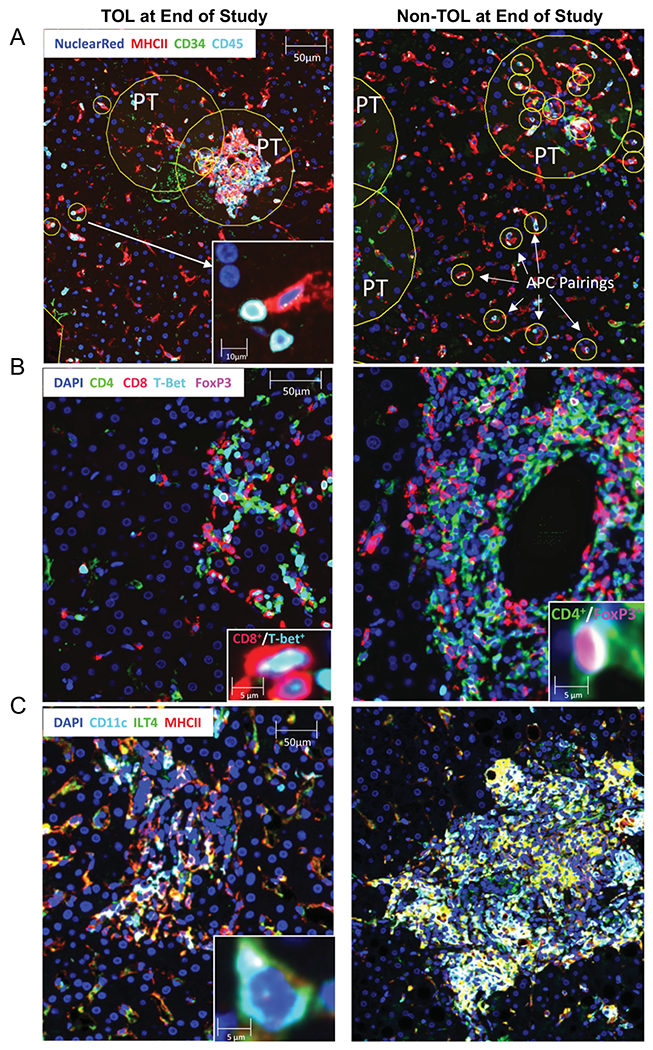
Biopsies were obtained from TOL and non-TOL participants at baseline and end of study. P values and n values for each stain/group combination can be found in Supplemental Table 8. Panels show representative images from end of study biopsies for TOL (left column) and non-TOL (right column) participants stained for APC:lymphocyte pairings (Panel A: in yellow circles; PT = portal tract), Foxp3+ and T-bet+ T cells (Panel B), and MHCII+CD11c+ILT4+ DC (Panel C). Main image bar = 50 μm, inset image bar = 10 μm or 5μm as indicated.
DISCUSSION
Prior studies have reported variable success of IS withdrawal in highly selected LTR, likely due to differences in patient populations, clinical approaches, and definitions of success. Fortunately, rejection occurring within monitored withdrawal trials does not appear to negatively affect liver grafts, as most are diagnosed early and responsive to IS augmentation. While the clinical impact of IS withdrawal has not been established, most trials have been performed late after LT in patients exposed to years of IS therapy (9). A recent large withdrawal study performed 1-2 years after LT demonstrated a trend toward reduced IS complications in those withdrawn vs. maintained on therapy (28). Yet as most patients fail full IS withdrawal, particularly early after LT, alternative approaches are needed to improve the current success rates and potential for clinical benefit. One major question is whether the low IS withdrawal success rates could be related to weaning from chronic CNI therapy, which has both anti-rejection and anti-tolerogenic properties. As laboratory and clinical data support pro-tolerogenic properties of mTOR-I, particularly demonstrated by our previous conversion study, the use of these agents as an interim step to facilitate higher weaning success is of significant interest (15).
This current study is the first to withdraw LTR from mTOR-I instead of CNI therapy. It was designed to generate preliminary data on the success rate of this approach and identify blood and graft tolerance markers that may be specific to mTOR-I therapy. While more than half of our recipients were successfully withdrawn from mTOR-I therapy, this rate is comparable to CNI-withdrawal studies of similar patients who are older and further from LT (22, 29). Whether we can achieve mTOR-I withdrawal earlier after LT in younger patients, where the benefit may be higher, needs to be tested in larger trials.
Importantly, we have detected PBMC populations that may be predictive of tolerance and perhaps specific to mTOR-I treatment. High percentages of tolerogenic DC (HLA-DR+CD11c+ILT3+ILT4+ DC) were seen prior to and after successful SRL withdrawal, compared to those who failed. In culture, IL-10 treated tolerogenic DC have been reported to induce anergy in both CD4+ and CD8+ T cells (30, 31). mTOR-I can induce tolerogenic DC that inhibit T cell proliferation in culture (32), and we and others have shown that conversion from CNI to mTOR-I therapy increases the percentage of tolerogenic DC and Tregs in LTR (33). Given tolerogenic DC increased significantly from baseline, this may indicate their importance in maintaining tolerance - dampening antigen presentation and protecting the graft from rejection.
Interestingly, in our tolerant recipients, we also observed increases in cell populations (CD8+ TEMRA and CD8+Eomes+T-bet+ T cells) that have surface and intracellular phenotypes of hyporesponsiveness and prior antigen exposure, and this could be related to interactions with tolerogenic DCs. These populations accumulate with age, display surface markers of terminal differentiation, and are hyporesponsive to proliferative challenges (34–36). T cell hyporesponsiveness has been reported to correlate with clinical tolerance (37). As tolerogenic DC and hyporesponsive PBMC phenotypes were increased before and after successful SRL withdrawal, this suggests that terminal differentiation resulting in T cell hyporesponsiveness may be one path to tolerance. In addition, increased exhaustion at baseline may suggest greater susceptibility to activation-induced cell death promoted by antigen exposure in the presence of mTOR inhibition. Alternatively, increased naive cells at baseline in the non-tolerant groups may overcome antigen-driven exhaustion and lead to rejection with ISwithdrawal. Whether these mechanisms are entirely unique to mTOR-I therapy needs further investigation but remain plausible.
We also were interested if these immune cells were present in the liver graft itself and correlated with tolerance, which might further assist in pre-weaning prediction. On staining, intragraft CD3+ T cells, CD4+Foxp3+ T cells, and CD8+T-bet+ increased only in the non-TOL group over time. While it is not known whether the Foxp3+ cells are effector cells or Tregs responding to rejection, it is common to see them at sites of inflammation. These T cell populations correlated with an increased number of APC:leukocyte pairings in non-TOL, which is perhaps a more direct sign of immune activation. Intragraft MHCII+ILT4+CD11c+ DC were higher in non-TOL at study end and may home to the graft to dampen inflammatory responses.
We have also confirmed data from prior studies that blood and graft gene expression profiles may help predict success with IS withdrawal. Similar to other reports, the gene expression profile appears different between blood and biopsy compartments (15, 38). The only common gene downregulated in both blood and biopsy at baseline in the TOL group was Nop9, a nucleolar protein essential for 18S rRNA maturation (39). The significance of this finding to tolerance and our study is not clear and needs further investigation. Interestingly, a biopsy gene signature profile shown to predict CNI withdrawal in a prior study was also highly predictive in our cohort, supporting the robustness of this signature that now appears independent of IS therapy used (19). Currently, a European trial is underway to test the ability of this biopsy profile to guide patient selection for withdrawal.
Finally, we did not find a correlation between DSA and the ability to achieve tolerance. While emerging data have linked DSA with adverse outcomes in LTR (40, 41), the data on DSA as a biomarker for liver tolerance are inconsistent. A recent study showed that de novo DSA development was a risk factor for acute rejection during early IS minimization post-LT; however, a high percentage who achieved withdrawal without rejection had de novo DSA (42). The available data, including this current study, support that the presence of DSA does not appear to prohibit the development of tolerance or correlate with histologic injury in LTR (22, 29, 43–45). We also performed C4d staining on all of our biopsies (data not shown) to identify any features of antibody-mediated rejection. While C4d staining was not detected, it was performed on stored formalin fixed, paraffin-embedded tissue which may have been suboptimal or inaccurate compared to fresh or stored frozen samples. Certainly, longer-term follow-up in tolerant recipients with DSA and proper C4d staining are needed to confirm graft stability and whether these recipients are truly tolerant.
This report has limitations that need to be addressed in subsequent studies. We did not test for donor-specific hyporesponsiveness in functional assays, which is the most robust definition of tolerance compared to clinical definitions used in most studies (46). These functional assays are unfortunately rarely used in tolerance studies as they are expensive, not always validated, and require donor sample collections that may not have been obtained at transplantation. As an exploratory trial, the study cohort was small and only a small percentage of our LT population. While most transplant tolerance studies, including ours, have used one year off IS therapy with normal graft function and histology to define the primary endpoint of operational tolerance (9), we recognize that long term follow-up clinical, laboratory, and biopsy assessments, such as recently reported in pediatric LT (26), are needed to confirm true maintenance of tolerance. Without knowledge of donor-specific tolerance, these operationally tolerant recipients could still develop rejection related to immune-stimulating events, such as infections, vaccinations, pregnancy, transfusions, etc. and need to be followed closely. To address this, our plan is to perform long term biopsies and clinical assessments in this current cohort and in all future withdrawal studies.
Finally, no CNI-treated control group was enrolled to directly compare the clinical success of withdrawal. We can only state that in this pilot study mTOR-I withdrawal had similar success to CNI withdrawal when considering older LTR (22). In addition, while we do not have direct blood and graft biomarker comparisons between CNI and mTOR-I treated patients, prior data from in vitro studies, direct monotherapy comparisons and CNI to mTOR-I conversion studies have shown differences with respect to tolerogenic DC, Tregs, and other systemic immunoregulatory markers (12–16, 47, 48).
With this pilot study and recent approval of another similar mTOR-I, everolimus, larger studies using mTOR-I as a pathway toward tolerance with direct comparisons to CNI withdrawal, more focused biomarker assessments and longer follow-up can now be planned more readily. Knowledge of specific clinical immunological characteristics and using an individualized IS approach with biomarker assessments may allow transplant clinicians to more accurately select appropriate candidates for these tolerance interventions.
Supplementary Material
ACKNOWLEDMENTS:
From the Northwestern Comprehensive Transplant Center, we would like to thank Patrice Al-Saden and the research coordinators for clinical trial coordination and taking care of the enrolled patients, and Drs. Jessica Voss, Xuemei Huang and Jie He of the Immune Monitoring Core for flow data acquisition and analysis.
Financial support: Internal funds from the Northwestern University Division of Gastroenterology and Hepatology supported the clinical trial portion of the study, as well as the fresh PBMC and blood gene expression analysis. The Immune Tolerance Network, supported by the National Institute of Allergy and Infectious Diseases, funded additional mechanistic studies (graft gene expression; frozen PBMC immunophenotyping; DSA assessment; graft histology and immunohistochemistry) under award number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations:
- APC
antigen presenting cell
- AR
acute rejection
- Breg
regulatory B cells
- CNI
calcineurin-inhibitor
- DC
dendritic cells
- eGFR
estimated glomerular filtration rate
- HEHE
Hepatic Epithelioid Hemangioendothelioma
- HCV
Hepatitis C Virus
- HTN
hypertension
- ILT
immunoglobulin like transcript
- IS
immunosuppression
- LTR
liver transplant recipients
- NASH
non-alcoholic steatohepatitis
- Non-TOL
non-tolerant
- PBMC
peripheral blood mononuclear cells
- SRL
sirolimus
- TEMRA
T effector memory RA+
- TOL
operationally tolerant liver transplant recipient
- Treg
regulatory T cells
Contributor Information
Josh Levitsky, Northwestern University Feinberg School of Medicine, Chicago, IL.
Bryna E. Burrell, Immune Tolerance Network, Bethesda, MD.
Sai Kanaparthi, Immune Tolerance Network, Bethesda, MD.
Laurence A. Turka, Immune Tolerance Network, Bethesda, MD; Massachusetts General Hospital, Boston, MA.
Sunil Kurian, Scripps Clinic Bio-Repository and Transplantation Research, La Jolla, California, United States.
Alberto Sanchez-Fueyo, King’s College London, London, UK.
Juan J. Lozano, Biomedical Research Center in Hepatic and Digestive Diseases, Carlos III Health Institute, Barcelona, Spain.
Anthony Demetris, University of Pittsburgh, Pittsburgh, PA.
Andrew Lesniak, University of Pittsburgh, Pittsburgh, PA.
Allan D. Kirk, Department of Surgery, Duke University, Durham, NC.
Linda Stempora, Department of Surgery, Duke University, Durham, NC.
Guang-Yu Yang, Northwestern University Feinberg School of Medicine, Chicago, IL.
James M. Mathew, Northwestern University Feinberg School of Medicine, Chicago, IL.
REFERENCES
- 1.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 2.Levitsky J, O’Leary JG, Asrani S, Sharma P, Fung J, Wiseman A, Niemann CU. Protecting the Kidney in Liver Transplant Recipients: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016;16:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morard I, Dumortier J, Spahr L, Hadengue A, Majno P, Morel P, Mentha G, et al. Conversion to sirolimus-based immunosuppression in maintenance liver transplantation patients. Liver Transpl 2007;13:658–664. [DOI] [PubMed] [Google Scholar]
- 4.Fairbanks KD, Eustace JA, Fine D, Thuluvath PJ. Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus. Liver Transpl 2003;9:1079–1085. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE. The “privileged” liver and hepatic tolerogenicity. Liver Transpl 2001;7:918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamada N, Wight DG. Antigen-specific immunosuppression induced by liver transplantation in the rat. Transplantation 1984;38:217–221. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, et al. Systemic chimerism in human female recipients of male livers. Lancet 1992;340:876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson AW, Lu L, Wan Y, Qian S, Larsen CP, Starzl TE. Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation 1995;60:1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitsky J, Feng S. Tolerance in clinical liver transplantation. Hum Immunol 2018;79:283–287. [DOI] [PubMed] [Google Scholar]
- 10.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005;105:4743–4748. [DOI] [PubMed] [Google Scholar]
- 11.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007;178:7018–7031. [DOI] [PubMed] [Google Scholar]
- 12.Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, Balk AH, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 2005;80:110–117. [DOI] [PubMed] [Google Scholar]
- 13.Szabo G, Gavala C, Mandrekar P. Tacrolimus and cyclosporine A inhibit allostimulatory capacity and cytokine production of human myeloid dendritic cells. J Investig Med 2001;49:442–449. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant 2007;7:1722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitsky J, Mathew JM, Abecassis M, Tambur A, Leventhal J, Chandrasekaran D, Herrera N, et al. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology 2013;57:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitsky J, Miller J, Huang X, Gallon L, Leventhal JR, Mathew JM. Immunoregulatory Effects of Everolimus on In Vitro Alloimmune Responses. PLoS One 2016;11:e0156535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, Thomson AW. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant 2003;3:689–696. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benitez C, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest 2008;118:2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohne F, Martinez-Llordella M, Lozano JJ, Miquel R, Benitez C, Londono MC, Manzia TM, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 2012;122:368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonaccorsi-Riani E, Pennycuick A, Londono MC, Lozano JJ, Benitez C, Sawitzki B, Martinez-Picola M, et al. Molecular Characterization of Acute Cellular Rejection Occurring During Intentional Immunosuppression Withdrawal in Liver Transplantation. Am J Transplant 2016;16:484–496. [DOI] [PubMed] [Google Scholar]
- 21.Shaked A, DesMarais MR, Kopetskie H, Feng S, Punch JD, Levitsky J, Reyes J, et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am J Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benitez C, Londono MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martinez-Llordella M, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013;58:1824–1835. [DOI] [PubMed] [Google Scholar]
- 23.Banff Working Group on Liver Allograft P. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl 2012;18:1154–1170. [DOI] [PubMed] [Google Scholar]
- 24.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol 2003;11:245–258. [DOI] [PubMed] [Google Scholar]
- 25.Feng S, Bucuvalas JC, Demetris AJ, Burrell BE, Spain KM, Kanaparthi S, Magee JC, et al. Evidence of Chronic Allograft Injury in Liver Biopsies From Long-term Pediatric Recipients of Liver Transplants. Gastroenterology 2018;155:1838–1851 e1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S, Demetris AJ, Spain KM, Kanaparthi S, Burrell BE, Ekong UD, Alonso EM, et al. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology 2017;65:647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745–763. [DOI] [PubMed] [Google Scholar]
- 28.Shaked A, DesMarais MR, Kopetskie H, Feng S, Punch JD, Levitsky J, Reyes J, et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am J Transplant 2019;19:1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 2012;307:283–293. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 2002;99:2468–2476. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol 2010;184:1765–1775. [DOI] [PubMed] [Google Scholar]
- 32.Fedoric B, Krishnan R. Rapamycin downregulates the inhibitory receptors ILT2, ILT3, ILT4 on human dendritic cells and yet induces T cell hyporesponsiveness independent of FoxP3 induction. Immunol Lett 2008;120:49–56. [DOI] [PubMed] [Google Scholar]
- 33.Stallone G, Pontrelli P, Infante B, Gigante M, Netti GS, Ranieri E, Grandaliano G, et al. Rapamycin induces ILT3(high)ILT4(high) dendritic cells promoting a new immunoregulatory pathway. Kidney Int 2014;85:888–897. [DOI] [PubMed] [Google Scholar]
- 34.Knox JJ, Cosma GL, Betts MR, McLane LM. Characterization of T-bet and eomes in peripheral human immune cells. Front Immunol 2014;5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, et al. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukoc Biol 2013;93:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, Loh L, et al. Age-Related Decline in Primary CD8(+) T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8(+) T Cells. Cell Rep 2018;23:3512–3524. [DOI] [PubMed] [Google Scholar]
- 37.Bohne F, Londono MC, Benitez C, Miquel R, Martinez-Llordella M, Russo C, Ortiz C, et al. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci Transl Med 2014;6:242ra281. [DOI] [PubMed] [Google Scholar]
- 38.Kurian SM, Heilman R, Mondala TS, Nakorchevsky A, Hewel JA, Campbell D, Robison EH, et al. Biomarkers for early and late stage chronic allograft nephropathy by proteogenomic profiling of peripheral blood. PLoS One 2009;4:e6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson E, Rappsilber J, Tollervey D. Nop9 is an RNA binding protein present in pre-40S ribosomes and required for 18S rRNA synthesis in yeast. RNA 2007;13:2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitsky J, Kaneku H, Jie C, Walsh RC, Abecassis M, Tambur AR. Donor-Specific HLA Antibodies in Living Versus Deceased Donor Liver Transplant Recipients. Am J Transplant 2016;16:2437–2444. [DOI] [PubMed] [Google Scholar]
- 41.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant 2013;13:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Prevalence and Impact of De Novo Donor-Specific Antibodies During a Multicenter Immunosuppression Withdrawal Trial in Adult Liver Transplant Recipients. Hepatology 2019;69:1273–1286. [DOI] [PubMed] [Google Scholar]
- 43.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, Watanabe M, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016;64:632–643. [DOI] [PubMed] [Google Scholar]
- 44.Girnita A, Mazariegos GV, Castellaneta A, Reyes J, Bentlejewski C, Thomson AW, Zeevi A. Liver transplant recipients weaned off immunosuppression lack circulating donor-specific antibodies. Hum Immunol 2010;71:274–276. [DOI] [PubMed] [Google Scholar]
- 45.Waki K, Sugawara Y, Mizuta K, Taniguchi M, Ozawa M, Hirata M, Nozawa M, et al. Predicting operational tolerance in pediatric living-donor liver transplantation by absence of HLA antibodies. Transplantation 2013;95:177–183. [DOI] [PubMed] [Google Scholar]
- 46.Mathew JM, Ansari MJ, Gallon L, Leventhal JR. Cellular and functional biomarkers of clinical transplant tolerance. Hum Immunol 2018;79:322–333. [DOI] [PubMed] [Google Scholar]
- 47.Levitsky J, Gallon L, Miller J,Tambur A,, Leventhal JFC, Huang X, Sarraj B, Wang E,, J. M The Differential Effects of Tacrolimus and Sirolimus in the Treg MLR. Am J Transplant 2010;10:483. [Google Scholar]
- 48.Levitsky J, Miller J, Wang E, Rosen A, Flaa C, Abecassis M, Mathew J, et al. Immunoregulatory profiles in liver transplant recipients on different immunosuppressive agents. Human Immunology 2009;70:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


