Abstract
Background
Human exposure to mercury (Hg) is widespread and both organic and inorganic Hg are routinely found in the human brain. Millions of people are exposed to methyl Hg (MeHg) due to the consumption of fish and to inorganic Hg from dental amalgams, small scale gold mining operations, use of Hg containing products, or their occupations. Neuropathology information associated with exposures to different species of Hg is primarily based on case reports of single individuals or collections of case studies involving a single species of Hg at toxic exposure levels such as occurred in Japan and Iraq.
Methods/Results
This study brings together information on the neuropathological findings and deposition of Hg in the central nervous system of people exposed to different species of Hg at varying concentrations. The low dose exposures were lifetime exposures while the high dose exposures were generally acute or short term by different exposure routes with survival lasting various lengths of time. Total and inorganic Hg deposits were identified in formalin-fixed, paraffin embedded tissues from both low and high exposure Hg cases. Low concentration exposures were studied in adult brains from Rochester, New York (n=4) and the Republic of Seychelles (n=17). Rochester specimens had mean total Hg concentrations of 16–18 ppb in the calcarine, rolandic, and cerebellar cortices. Inorganic Hg averaged between 5–6 ppb or 30 – 37% for the cerebral and cerebellar cortices of the Rochester subjects. Total Hg was approximately 10-fold higher in specimens from Seychelles, where consumption of ocean fish is high and consequently results in exposure to MeHg. The predominant Hg species was MeHg in both the Rochester and Seychelles brain specimens. Histologically, cerebral and cerebellar cortices from Rochester and Seychelles specimens were indistinguishable. High concentration exposures were studied in brains from four adults who were autopsied at variable time periods after exposure to organic Hg (methyl or dimethyl) or inorganic Hg (inhaled vapor or intravenous injection of metallic Hg). In contrast to the Seychellois adults, these individuals had acute or subacute exposures to lethal or significantly higher concentrations. The pattern of Hg deposition differed between subjects with high organic Hg exposure and high inorganic Hg exposure. In the organic Hg cases, glia (astrocytes and microglia) and endothelial cells accumulated more Hg than neurons and there were minimal Hg deposits in cerebellar granule and Purkinje cells, anterior horn motor neurons, and neocortical pyramidal neurons. In the inorganic Hg cases, Hg was seen predominantly in neurons, vascular walls, brainstem, and cerebellar and cerebral deep gray nuclei. The presence of inorganic Hg in neural and neural supporting cells in the four high exposure Hg cases was not closely correlated with cellular pathology; particularly in the inorganic Hg cases.
Conclusions
Different Hg species are associated with differing neuropathological patterns. No neuropathological abnormalities were present in the brains of either Rochester or Seychelles residents despite substantial differences in dietary MeHg exposure. Increasing concentrations of inorganic Hg were present in the brain of relatively low exposure subjects with increasing age.
Keywords: Mercury, methyl mercury, organic mercury, inorganic mercury, neuropathology, Seychelles
1. Introduction
Mercury (Hg) is a natural element in the earth’s surface and as such, exposures can be reduced, but not eliminated. Recent efforts to reduce Hg exposures are the result of a meeting of more than 140 nations and produced a document referred to as the Minamata Convention, in which the countries agreed to take actions to limit the supply, trade, use, and anthropogenic emissions of Hg. The meeting at which the Convention was developed was preceded by a report prepared by the United Nations Environment Programme, which found Hg emissions were increasing in a number of developing nations particularly in relationship to emissions from coal-burning power plants and the use of Hg to recover gold (Outridge et al., 2018).
Mercury exposure can affect a number of organs including the kidneys, but the principal current health concern is its effects on the central nervous system, which is central to the World Health Organization’s listing Hg as one of the top ten chemicals of major public health concern (WHO, 2017). The neuropathological consequences of exposure to Hg differ with each Hg species, and it is important to understand the species if the neuropathological consequences are to be understood. Mercury is generally categorized as either inorganic or organic. Both types can form multiple chemical species and compounds. Different Hg species vary in their absorption, distribution, metabolism, elimination, and ability to cross the blood-brain-barrier and also in their effects on the adult human nervous system (Hunter and Russell, 1954; WHO, 1990; Clarkson and Magos, 2006).
The Hg atom has three naturally occurring oxidation states (WHO, 1990). In the zero oxidation state (Hg0) mercury is a liquid metal (quicksilver) or a monatomic gas (mercury vapor). This state is often referred to as “elemental” or “metallic” Hg. The Hg atom can also exist with either one or two electrons missing. With one electron missing it is termed the mercurous ion (Hg2++) and with two electrons missing the mercuric ion (Hg++). Mercurous mercury is unstable and in the presence of many common ligands dissociates into one atom of Hg0 and one of Hg++. Both mercurous and mercuric species of Hg are referred to as inorganic and can form numerous chemical compounds. They are readily interconverted in the body and the environment, and most analytic methods do not distinguish between the different forms (Clarkson and Magos, 2006). Although consumption of aquatic species is often discussed as a source of organic Hg exposure, some tissues used as food sources provide significant exposures to inorganic mercury as well (Lemes et al., 2011)
Metallic or liquid Hg0 readily vaporizes, and when inhaled rapidly crosses the alveolar membrane and passes into the circulation. Hg vapor is the primary exposure encountered in the recovery of gold during artisanal mining and some small-scale gold recovery processes. Inhaled Hg vapor may cause pulmonary inflammation at sufficient concentrations and can readily pass through the blood brain barrier. However, when Hg0 enters the circulation it is oxidized by the catalase-hydrogen peroxide pathway in erythrocytes and other cells to the mercuric state (Hg++). The mercuric ion (Hg++) ion crosses the blood-brain-barrier to only a limited degree and is thought to be the proximate toxic agent of this Hg species (Clarkson and Magos, 2006).
Organic mercurial species are formed when Hg in the mercuric oxidation state is attached to one or more carbon atoms. The most common form is (mono) methyl Hg, but dimethyl, phenyl, and ethyl Hg are other forms. These organic forms are termed short chain alkyl or aryl derivatives and the methyl and dimethyl forms are potent neurotoxicants. Methyl Hg in mammalian tissues forms numerous compounds and interacts especially with proteins containing a sulfhydryl (-SH) group (Clarkson and Magos, 2006). Both methyl and dimethyl Hg are readily absorbed through the lungs, skin, and mucosal surfaces. Once in the blood, they are distributed throughout the human body in about three days (Kershaw et al., 1980). The organic forms readily cross the blood-brain-barrier utilizing the amino acid transport pathway (Bridges and Zalups, 2020). In the brain, organic Hg can be demethylated to inorganic Hg and the majority of the Hg identified in the brain of individuals who survive an acute intoxication with organic mercury is inorganic (Davis et al., 1994). The mechanism and time period for this conversion are not known. However, it is known that inorganic Hg can persist in the brain for years (Davis et al., 1994), there is a good correlation between total Hg burden and the extent of brain damage with organic Hg exposure (Davis et al., 1994), and that specific brain regions appear to display selective vulnerability to the noxious effect of different Hg species (Hunter and Russell, 1954).
The form of organic mercury ingested from the consumption of fish muscle has been shown to be an aliphatic thiolate, similar to the methylmercury-cysteine complex (Harris et al., 2003). George et al. (2008) reported that this form of Hg is not modified by digestion with simulated gastric fluid and is presumably taken up into the human body intact. In skeletal muscle from beluga (Delphinapterus leucas) and pygmy sperm whales (Kogia breviceps), the predominant form of mercury is also suggested to be a methylmercury-cysteine complex (Lemes et al., 2011; George et al., 2011). A second methyl Hg-thiol complex identified in beluga muscle is methyl mercuric glutathione (Lemes et al., 2011). Muktuk, which is a form of whale skin consumed by Arctic populations, is reported to have a similar MeHg to inorganic Hg ratio as beluga muscle (Wagemann et al., 1998). However, consumption of whale liver results in exposure to large amounts of an inorganic Hg-selenium complex, which has not been completely identified (Lemes et al., 2011). Although consumption of muscle from fish or whales, or muktuk from whales presents similar exposures to Hg species, consumption of liver results in a greater exposure to inorganic Hg in addition to organic Hg species.
For this study, we assessed total (THg) and inorganic mercury levels, the distribution of mercury in brain regions and cell types, and pathologic changes in the nervous system using human autopsy cases submitted to the Departments of Environmental Medicine and Pathology, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA and the Department of Pathology, Victoria Hospital, Republic of the Seychelles. The cases involved exposures to different species of mercury and varying exposure conditions.
2. Materials and methods
2.1. Chemical analyses and pathology for subjects with relatively low brain mercury
2.1.1. Seychelles cases
Autopsy examinations were conducted at Victoria Hospital, which is the central hospital for the Republic of the Seychelles. Brains from 17 adults were collected from routine cases presented at the hospital. None of the cases included in this study were individuals with an infectious disease, a neoplasm, or brain malformation. Autolyzed and unidentified cases were not included. No cases of confirmed MeHg poisoning due to fish consumption are known to have occurred in Seychelles or elsewhere in the world apart from the poisonings in Japan (Clarkson and Magos, 2006). Brains were fixed by immersion in 10% formalin, later bisected in the mid-saggital plane, and cut in the coronal plane in 1 cm blocks. Typically, whole brains were received by the Department of Environmental Medicine, University of Rochester for analysis of total and inorganic mercury. A 1 – 3 cm length of scalp hair most proximal to the skin was also collected for mercury analysis. Hair was not available for some individuals due to natural hair loss associated with age and gender. None of the specimens were from individuals included in the Seychelles Child Development Study (SCDS) (Myers et al., 2003); although, they were from the same population.
Brain cortical specimens (calcarine, rolandic, and cerebellar ) from four adult autopsy cases from Victoria Hospital and four reference cases from Rochester, NY (see description below) were prepared for light microscopic examination using standard methods for hematoxylin-eosin, Luxol fast blue-cresyl violet, Bodian silver protargol, glial fibrillary acid protein, immunohistochemical expression of CD68 in activated microglia, and two different autometallographic procedures for mercury (Churukian et al., 2000 and Sakai et al., 1975 as modified by Tokunaga et al., 1988). The latter method has a limit of detection for mercury of 0.2 – 0.3 ppm. The cortical samples examined (calcarine, rolandic, and cerebellar) were selected as the small neurons in these areas of the brain are reported to show the most severe damage in alkyl mercury poisoning (Hunter and Russell, 1954). The slides were coded and examined without knowledge of source (Seychelles or USA) by four pathologists (O’Donoghue, Eto, Takahashi, and Marumoto).
Chemical analyses using cold vapor atomic absorption for total and inorganic mercury were conducted on formalin fixed tissue from tissue blocks adjacent to the areas selected for histochemistry and histopathology. The method used was that of Davis et al. (1994) as previously discussed in Lapham et al. (1995) and Cernichiari et al. (1995).
Mercury concentrations in hair and brain were analyzed using simple linear regression to examine the strength of relationships between variables. Residuals were checked for the assumptions of linearity, constant variance, and normally distributed errors. No strong deviations from these assumptions were found in this data; therefore, standard linear regression and the Pearson’s correlation coefficient were used to analyze the data.
2.1.2. Rochester reference cases
For comparison with the Seychelles cases, brain specimens from the Autopsy Service, at the University of Rochester Medical Center were collected and handled in a manner that was the same as that used for the Seychelles samples. Samples of brain were analyzed for mercury and prepared for histologic analysis in the same manner as for the Seychelles cases. Hair samples were not available for the Rochester cases.
2.2. Chemical analyses and pathology for subjects with high brain mercury
Paraffin embedded brain sections from four subjects who died following mercury exposure were received for analysis of pathologic changes associated with the regional deposition of mercury. The brains had all been fixed in formalin prior to paraffin embedding. Details of exposure, clinical findings for Subjects 1, 2, and 4, and neuropathology findings for Subjects 1 and 2 have been previously described (Davis et al., 1994; Giombetti et al., 1988; Nierenberg et al., 1998; Siegler et al, 1999).
2.2.1. Subject one
A 48-year-old chemistry professor had a single accidental dermal exposure in the laboratory to dimethyl mercury. She had severe neurological damage following a latency period of 5 months and died 10 months after exposure (Nierenberg et al., 1998; Siegler et al, 1999).
2.2.2. Subject two
A 29-year-old woman was exposed to methyl mercury for three months through the consumption of contaminated pork at age 8 years. The pork was from animals fed grain treated with an organic mercurial fungicide (cyano methyl mercury guanidine). She had severe neurological damage and died 21 years post-exposure (Davis et al., 1994).
2.2.3. Subject three
A 38-year-old woman was exposed to mercury vapor, reportedly from extracting gold at home using a Hg vaporization process. It was not clear if she had received single or multiple exposures to mercury vapor. She was brought to a hospital emergency department by her husband after complaining of progressively increasing shortness of breath for two days. Following admission, she had increasing respiratory insufficiency and multisystem organ failure. She died ten days after hospital admission. Her urinary Hg level on the day after admission was 682 mg/l. At autopsy, there was diffuse alveolar damage, acute renal tubular necrosis with interstitial hemorrhages, and minimal hepatic fatty change with early necrosis of hepatocytes. Her brain was swollen, weighed 1550 grams and had uncal grooving. Brain samples were received from the Fresno County Coroner’s Office, Fresno, CA.
2.2.4. Subject four
A 20-year-old man self-injected metallic Hg intravenously on two occasions. He had reportedly injected 1 ml of liquid mercury 21 months and 3 ml 16 months prior to committing suicide by a self-inflicted gunshot to the head (Giombetti et al., 1988). Pre-mortem imaging studies (X-ray and CT scans) revealed multiple densities in the head, chest, and the injection sites (left arm and left ankle), consistent with Hg emboli. At autopsy, there were skull fractures and a brain laceration secondary to the gunshot wound. Multiple Hg droplets were present in the lung and liver parenchyma. On microscopy, there were granulomas surrounding Hg droplets present diffusely throughout the lung and in sections of the right ventricle.
2.4. Hg chemical analyses
Chemical analyses for total and inorganic mercury were carried out on formalin-fixed brain sections from different cortical regions as well as the brainstem using the hot alkaline digestion method as previously described (Davis et al., 1994).
3. Results
3.1. Chemical analyses and pathology for subjects with low brain mercury
3.1.2. Rochester Cases
Hg analyses for the Rochester cases are shown in Table 1. In the Rochester reference cases, total mercury in cortical tissue from different regions of the brain was in the range of non-detectable to ~24 ppb. The range of inorganic mercury in different regions of the brain was non-detectible to 10 ppb. None of the Rochester brain samples were free of mercury.
Table 1:
Mercury Concentration in Cortical Sections from Rochester Reference Cases
| Case No. and Gender | Age (yr) | Cortical Region | Total Hg (ppb) | Inorganic Hg (ppb) | Inorganic Hg (%) |
| R1F | 67 | Calcarine | 13 | 10 | 77 |
| Rolandic | 17 | ND | 18 | ||
| Cerebellum | 21 | ND | 14 | ||
| R2F | 56 | Calcarine | 16 | ND | 19 |
| Rolandic | ND | ND | ND | ||
| Cerebellum | 13 | 6 | 46 | ||
| R3M | 66 | Calcarine | 21 | 7 | 33 |
| Rolandic | 24 | 8 | 33 | ||
| Cerebellum | 22 | 9 | 41 | ||
| R4F | 57 | Calcarine | 16 | ND | 19 |
| Rolandic | 21 | 8 | 38 | ||
| Cerebellum | 17 | ND | 20 | ||
| Mean ±SD | 62 ±6 | Calcarine | 16 ±3 | 6 ±3 | 37 ±27 |
| n=4 | Rolandic | 16 ±9 | 6±3 | 22 ±17 | |
| Cerebellum | 18 ±4 | 5±3 | 30 ±16 | ||
| Brain | 17±6 | 6±3 | 30±19 | ||
ND – None detected. The detection limit is 3 ppb. In calculating mean values, except in the case of % inorganic mercury where total mercury was also ND, a value of 3 ppb was substituted for ND.
3.1.3. Seychelles Cases
The ages of the individuals from whom brain and hair samples were collected ranged from 37 – 76 years with 12 males and 5 females. The results of Hg analyses of the calcarine cortex from 17 adult Seychellois at the time of death are shown in Table 2 and Figure 1. The concentration of total and inorganic Hg varied widely in the brain. The Hg concentrations (ppm) in the hair (see Figure 2), which represent mercury exposures occurring during the last months of life, were typically 1 to 3 orders of magnitude more than the concentrations of Hg in the brain (ppb).
Table 2:
Mercury Levels in the Calcarine Cortex and Hair of Adult Seychellois
| Case No. and Gender | Age (yr) | Brain Tot Hg ppb | Brain Inorg Hg ppb | Brain Inorg Hg % | Hair Tot Hg Ppm |
|---|---|---|---|---|---|
| S1F | 55 | 109 | 21 | 19 | 5.6 |
| S2F | 76 | 120 | 81 | 68 | 6.1 |
| S3F | 59 | 129 | 31 | 24 | NA |
| S4F | 44 | 130 | 24 | 18 | 12.3 |
| S5F | 56 | 159 | 25 | 16 | 19.1 |
| Mean ±SD n |
58±12 5 |
129±19 5 |
36±25 5 |
29±22 5 |
11±6 4 |
| S6M | 57 | 46 | 22 | 48 | 3.9 |
| S7M | 47 | 50 | 9 | 18 | NA |
| S8M | 66 | 64 | 39 | 61 | NA |
| S9M | 54 | 95 | 22 | 23 | 15.0 |
| S10M | 56 | 169 | 35 | 21 | 14.2 |
| S11M | 65 | 194 | 36 | 18 | 20.2 |
| S12M | 65 | 208 | 53 | 25 | 33.8 |
| S13M | 45 | 237 | 24 | 10 | NA |
| S14M | 57 | 252 | 62 | 24 | 22.6 |
| S15M | 37 | 263 | 25 | 10 | NA |
| S16M | 73 | 282 | 79 | 28 | 21.8 |
| S17M | 60 | 324 | 62 | 19 | NA |
| Mean±SD n |
57±10 12 |
182±97 12 |
39±21 12 |
25±15 12 |
19±9 7 |
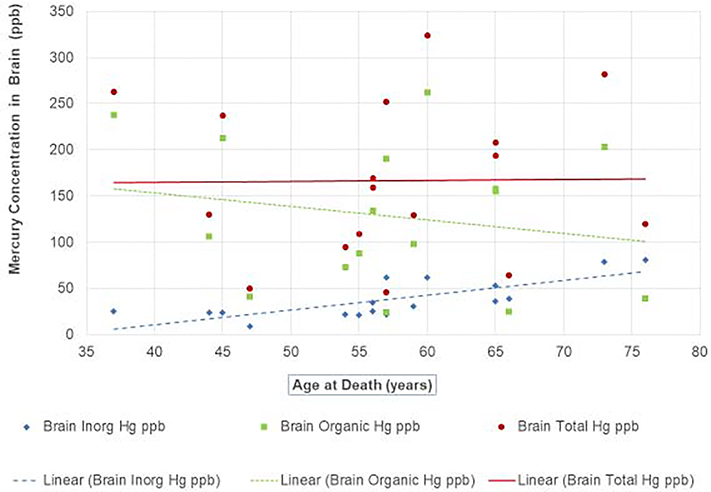
Figure 1. Mercury Concentrations in the Calcarine Cortex of Adult Seychellois at Time of Death.
Figure 1 shows the relationships between the concentrations of total, inorganic, and organic brain mercury (ppb) in adult Seychellois calcarine cortex and age at death with linear regression lines for each biomarker. Brain organic Hg (presumed to be MeHg) was calculated based on brain total Hg minus inorganic levels.
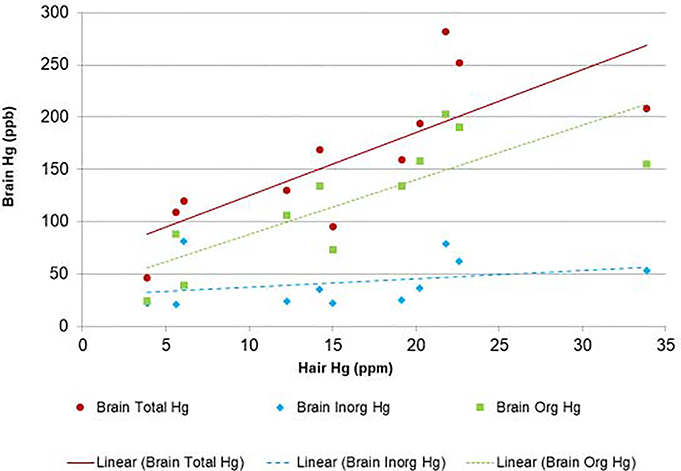
Figure 2. Hair and Calcarine Cortex Mercury Concentrations of Adult Seychellois at Time of Death.
The figure demonstrates the relationship of total, inorganic, and organic mercury (ppb) in adult Seychellois calcarine cortex and hair Hg (ppm) at death with linear regression lines for each biomarker.
While the regression lines in Figure 1 suggest that brain total Hg values were relatively constant at the various ages, the individual values were quite variable (R2 = 0.0002). The same was true for the brain organic Hg values (R2 = 0.0395). The regression slopes for brain total Hg (0.11) and organic Hg (−1.47) were not statistically significant (p = 0.96 and 0.44, respectively), perhaps due to the variable values and small sample size. However, the variability of the brain inorganic Hg concentrations was significantly less (R2 = 0.5703) and the slope (1.59) was statistically significant (p < 0.001), suggesting brain levels of inorganic Hg increase with age. For every increase of one year of life, there was on average an increase of 2 ppb of inorganic Hg in the brain for this Seychelles population (p<0.001). There was no apparent gender difference in the amount of inorganic Hg in the brain or the percentage of Hg that was inorganic. Our findings of increasing inorganic Hg levels in the brain with age are consistent with those suggested by Davis et al., (1994) in humans, and by Charleston et al. (1995), who found chronic dietary exposure of Macaca fascicularis to subclinical levels of MeHg increased brain inorganic Hg.
Total Hg (primarily organic Hg) levels in hair formed just prior to death are strongly correlated to brain levels of organic Hg (r=0.80), but not to levels of inorganic Hg in the brain (r=0.31). A similar finding of elevated organic Hg in the brain is seen experimentally in Macaca fascicularis killed immediately after chronic dietary MeHg exposure (Charleston et al., 1995). Hair Hg thus appears to be a robust marker of brain MeHg concentration from recent exposure.
On average total Hg concentrations in both the brain and hair were higher among the men; although both the highest and the lowest brain and hair Hg concentrations were seen among the men. Statistical analysis of brain and hair Hg levels showed that the correlation between total Hg measured in the hair and total Hg in the brain is strong (r=0.76, p=0.006). For every increase of one ppm of Hg in the hair, we expect on average an increase of 6.02 additional ppb of total Hg in the brain in this population.
Hg analyses results for the brain cortical samples examined microscopically from the Seychellois cases are shown in Tables 3. While among the Seychellois cases there was much variation in total Hg, inorganic Hg, and percent inorganic Hg between cases there was also much variation in each of these analyses by cortical region. There was no cortical region that obviously had a higher concentration of Hg among the three cortical regions examined. The Seychelles cases had Hg concentrations that were an order of magnitude higher than the Rochester cases. There was no preferential accumulation of Hg in one cortical region in comparison to the others.
Table 3:
Mercury Concentration in Cortical Sections from Seychelles Cases
| Case No. and Gender | Age (yr) | Cortical Region | Total Hg (ppb) | Inorganic Hg (ppb) | Inorganic Hg (%) | ||
|---|---|---|---|---|---|---|---|
| S2F | 76 | Calcarine | 120 | 81 | 68 | ||
| Rolandic | 167 | 117 | 70 | ||||
| Cerebellum | 53 | 23 | 42 | ||||
| S10M | 56 | Calcarine | 169 | 35 | 21 | ||
| Rolandic | 153 | 28 | 18 | ||||
| Cerebellum | 189 | 29 | 15 | ||||
| S11M | 65 | Calcarine | 194 | 36 | 18 | ||
| Rolandic | 209 | 40 | 19 | ||||
| Cerebellum | 255 | 43 | 17 | ||||
| S17M | 60 | Calcarine | 324 | 62 | 19 | ||
| Rolandic | 462 | 160 | 35 | ||||
| Cerebellum | 349 | 66 | 19 | ||||
| Mean ±SD | 64 ±9 | Calcarine | 202±87 | 54±22 | 32 ±24 | ||
| n=4 | Rolandic | 248±145 | 86±63 | 36 ±24 | |||
| Cerebellum | 211±124 | 40±19. | 23 ±13 | ||||
| Brain | 220±112 | 60±42 | 30±20 | ||||
Histologic analyses of the brain specimens from either Rochester or Seychelles cases did not identify any pathology considered to be associated with mercury exposure. The randomized Seychelles cases could not be distinguished microscopically from the Rochester referent cases. None of the cases from the Seychelles or Rochester exhibited microscopically evident mercury-positive granules. This result is somewhat surprising as some cell types, principally astrocytes and endothelial cells, have been reported to accumulate mercury at higher levels than neurons and other cell types (see case results below). Thus, although the overall tissue concentrations of Hg were significantly higher in the Seychelles cases versus the Rochester cases, astrocytes in the Seychelles did not accumulate sufficient mercury to be visible histochemically.
3.2. Chemical analyses and pathology for subjects with high brain mercury
The brains of Subjects 1 and 2 with clinically evident neurological deficits had severe gross and microscopic lesions typical of Hg toxicity as described previously (Davis et al., 1994; Siegler et al., 1999). These consisted of marked but uneven cerebral and cerebellar cortical neuronal loss. Subject 3 with Hg vapor inhalation had severe cerebral edema, probably due to Hg toxicity, potentiated by hypoxia-ischemia. The brain of Subject 4 did not show gross or microscopic signs typical of Hg toxicity, but imaging studies revealed the presence of intracranial mercury micro-emboli. These were not identified on brain sectioning. Clinical and toxicological information on these subjects is presented in Table 4.
Table 4:
Clinical and Toxicological Background Information for Subjects With High Brain Mercury Levels
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | |
|---|---|---|---|---|
| Age at Exposure (yrs) | 47 | 8 | 38 | 19 |
| Age at Death (yrs) | 48 | 29 | 38 | 20 |
| Time between exposure and death | 10 months | 21 years | 12 days | Injections 21 and 16 months pre-mortem |
| Gender | F | F | F | M |
| Exposure route | Dermal and possibly inhalation | Oral | Inhalation | Intravenous |
| Estimated dose | ∼1344 mg (∼0.44 ml) | NA | NA | 4 ml |
| Estimated exposure duration | Seconds to a few minutes | Multiple times over 3 months | Minutes to hours, probably once | Injections 21 and 16 months pre-mortem |
| Chelation treatment | Yes | Yes | No | Yes |
The levels of inorganic Hg (ppb) present in the brains of all subjects were elevated. The analytical results for total mercury concentration and the histochemical distribution of Hg deposition in various brain regions and cell types are shown in Table 5.
Table 5:
Mercury Exposure for Subjects With High Brain Mercury Levels
| Subject | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Mercury Exposure | Dimethyl Mercury | Dimethyl Mercury | Mercury Vapor | Mercury Liquid |
| Mercury Valence | Hg++ | Hg++ | Hg0 | Hg0 |
| Anatomical Site | (Total Hg, ppb) | |||
| Cerebral Cortex | 850 | |||
| Frontal Cortex | 2070 | 1790 | 44,600 | |
| Occipital Cortex | 1179 | 1974 | 69,200 | |
| Deep Gray Nuclei | 3,620 | 548 | 65,000 | NA |
| Cerebellar Cortex | 3,310 | 1,104 | 11,6700 | NA |
| Brainstem | 1,800 | 290 | 81,200 | NA |
| Average % Inorganic Hg in Brain for all sites | 60 – 83 (n = 5) | 82 – 100 (n = 5) | 100 (n = 5) | 100 (n = 1) |
3.3. Topography of Hg deposition
The intensity and topography of Hg deposition differed in the four subjects as might be expected by the diversity of Hg species, dose, exposure route, clinical presentation, and survival time. In all subjects, endothelial cells stored Hg intensely and oligodendroglia were generally spared.
3.3.1. Subject 1 [Dimethyl Hg; 10-month survival]
Cerebrum: the cortex showed more Hg staining than white matter and deep gray nuclei. Mercury staining was intense in endothelial cells and astrocytes. Oligodendrocytes were almost completely spared and neurons were variably loaded with granules, in part, corresponding to the intensity of the cortical damage. Minimal neuronal cytoplasmic Hg staining was seen in areas spared of damage, such as CA1 of the hippocampus. In necrotic areas of the cerebral cortex, scattered surviving neurons had substantial neuronal Hg staining. There was marked Hg staining of subpial astrocytes. Mercury was present in astrocyte processes, which in some cases surrounded neuronal cell bodies. In the white matter, there was abundant Hg staining of astrocytes and occasionally axons. Cerebellum: there was pancellular staining in areas of severe damage, but the Hg load of astrocytes and endothelial cells was higher than that of neurons. There was minimal Hg staining of granular neurons and weak Hg stippling of Purkinje cells. Brainstem and spinal cord: there was intense, pancellular Hg staining of neurons including cranial nerve nuclei and substantia nigra. There was abundant Hg staining in subpial astrocytes, mild to moderate staining of anterior horn nuclei, and none in the posterior horns.
3.3.2. Subject 2 [Methyl Hg; 21-year survival]
Cerebrum: there was abundant cytoplasmic Hg staining of variable intensity. In general, endothelial cells and astrocytes had the most intense Hg stain. The intensity of Hg stain correlated with the degree of cortical necrosis. In areas where the cortex was devastated, most remaining cells had intense Hg staining. In areas with intermediate degrees of damage, subpial and cortical astrocytes had a similar degree of intense Hg staining, but large pyramidal neurons had little to no staining. In areas of minimal damage, such as the hippocampus, the Hg stain was almost entirely limited to astrocytes (subpial and subependymal) and endothelium. In the deep gray nuclei, there was no appreciable neuronal loss. Nevertheless, there was intense neuronal Hg staining. Astrocytes of deep gray nuclei and white matter had Hg staining as well, but oligodendrocytes were almost universally spared. Cerebellum: the pattern and intensity of staining in cerebellar cortex was similar to cerebral cortex, corresponding in intensity with the tissue damage and showing Hg predominantly in astrocytes. Weak stippling of surviving granular neurons and Purkinje cells was present. Brainstem and spinal cord: there was mild Hg staining of neurons in the basis pontis, abundant staining of neurons in substantia nigra and cranial nerve nuclei, and mild staining in anterior and posterior horn neurons.
3.3.3. Subject 3 [Hg vapor]
The intensity of Hg staining was variable among the different brain regions. In general, the Hg staining was particularly intense in endothelial cells and in large pyramidal neurons, although fine stippling of all the tissue was seen in some regions. Cerebrum: neurons of deep gray nuclei stained more intensely than cortical neurons. The thalamus had some Hg staining, the globus pallidus and putamen less and the caudate nucleus had almost none. The mammillary bodies had intense Hg staining. Neurons of the cerebral cortex, specifically frontal and entorhinal cortices showed weak Hg deposition. Cortical areas with severe edema did not show enhanced Hg deposition. The CA1 region of the hippocampus was almost completely spared of Hg deposition with the exception of scattered single neurons. The ependymal layer showed no to minimal Hg staining, but there was moderate staining of subependymal astrocytes. Cerebellum: the cortex and white matter were almost entirely spared of Hg deposition, but the neurons of the dentate nuclei had intense Hg deposition. Brainstem and spinal cord: large neurons of the inferior olive and other scattered brainstem nuclei exhibited the highest intensity of Hg deposition. Neurons and capillaries of the basis pontis had intense Hg deposition as well, but the locus ceruleus was spared. The pattern of Hg deposition did not appear related to blood flow, neuronal density, or proximity to the brain surface. The degree of Hg deposition appeared to be symmetrical.
3.3.4. Subject 4 [Metallic Hg]
The most intense Hg staining was seen in vascular walls throughout the brain. Overall, the spinal cord and brainstem had relatively more Hg deposition than the cerebrum and the latter more than the cerebellum. Cerebrum: the deposition was of minimal to medium intensity and localized to subpial and cortical astrocytes with little neuronal Hg deposition. The only significant Hg deposition in large pyramidal neurons was localized to middle layers of the primary visual cortex. There were multifocal areas of axonal Hg deposition following axonal pathways in parietal and occipital white matter. Double staining for myelin (LFB) and immunostain for axons (NFP) demonstrated Hg deposition in axons rather than in myelin sheaths. We could not demonstrate the relationship, but we suspect areas of Hg deposition may have been related to Hg emboli in the brain with local diffusion of Hg. Hg deposition was minimal in the striatum. Cerebellum: besides vascular and endothelial Hg deposition, Hg deposition was minimal and localized predominantly to Bergmann astrocytes. Brainstem and spinal cord: neurons of basis pontis exhibited minimal Hg deposition, comparable to surrounding glial cells. In contrast, strong Hg deposition in inferior olivary neurons and most medullary cranial nerve nuclei was seen. Heavy Hg deposition was also present in anterior and posterior horns of cervical cord and dorsal root ganglia neurons. Hg deposition in nerve roots and cervical plexus was absent to minimal. There was focal circumferential Hg deposition of myelin sheaths in the spinal cord posteriorly. Stippling of intravascular as well as extravasated erythrocytes was prominent. This was investigated further by Hg staining of bone marrow and spleen. In the bone marrow, there was fine, prominent stippling of the bone spicules, abundant Hg deposition in macrophages and little to no Hg deposition of red blood cells or red cell precursors. On the other hand, in the spleen there was prominent Hg deposition in the red pulp with only focal Hg deposition in the white pulp within macrophages.
4. Discussion
4.1. Subjects with relatively low Hg exposure (Rochester / Seychelles)
We studied neuropathology material from the Seychelles population to determine if there were subtle changes that were apparent only at the cellular level. We found that the neuropathology in a population consuming large amounts of fish and having brain Hg concentration significantly higher than subjects from Rochester, New York, where fish consumption is significantly lower, were similar at the cellular level. The absence of clear neuropathological differences in these population supports our clinical studies in Seychelles that have not identified adverse associations between Hg exposure and developmental endpoints. Our pathology specimens were primarily from older subjects. In these subjects we found that the concentration of inorganic Hg in the brain slowly increased with age. The Hg concentrations in the Rochester brain samples we examined were similar to levels reported in other urban centers. Pamphlett and Waley (1996) reported mean normal values for Hg in the cerebrum of 113 Australians at autopsy as 0.08 μg/g. Weiner and Nylander (1993) analyzed total mercury levels in the brains of autopsy cases (mean age 52.6 years range 16 – 88 yrs) of mostly men who lived in Stockholm, Sweden. Total mercury in the occipital lobe averaged 10.6 ppb wet weight with a range of 2.4 – 28.7 ppb. Brain Hg concentrations increased with age and with the number of dental amalgam surfaces and decreased with alcohol abuse. Drasch et al. (1994) reported finding median total mercury levels of 14.7 ppb wet brain weight in adults (n = 39) age 16 – 45 years from Munich, Germany with 0 – 2 dental amalgam surfaces and 25.7 ppb in adults (n = 19) with greater than 10 amalgam surfaces.
Longitudinal epidemiologic studies in the Republic of the Seychelles have evaluated the neurodevelopment of children from families that consume ocean fish on average 12 times per week. These have revealed no consistent pattern of adverse associations (Myers et al., 2003). The cases studied in this report are adults from this same general population and are presumed to have had a similar diet. In the epidemiology studies, fish consumed by the Seychelles population contains MeHg at an average concentration of 0.3 ppm. This is similar to concentrations in ocean fish consumed in the developed countries such as the USA (Myers et al., 2003). Consequently, the Hg exposure of the Seychellois adults studied is greater than that of the general population in Europe and North America because total fish consumption in the Seychelles is greater than in European and American populations. In Seychellois, mercury exposure begins at conception and extends until death and the population’ principal source of methyl mercury exposure is fish consumption with smaller amounts of inorganic Hg exposure from dental amalgams.
Both total and inorganic Hg levels were higher in the Seychelles population in comparison to the Rochester population, but the relative amounts of inorganic Hg (30%) and by subtraction organic mercury (70%) were similar when the values for the calcarine, rolandic, and cerebellar inorganic mercury values were averaged. The highest total and inorganic brain Hg concentrations were recorded for Seychellois men; although there was overlap with levels reported for Seychellois women. Since Seychellois men and women consume fish with similar mercury concentrations, the difference in brain Hg levels may be related to differences in the size of food portions consumed. Pedersen et al. (1999) reported no gender differences for brain mercury levels among Greenlanders consuming a fish and sea mammal diet. Hg levels in hair tended to reflect Hg levels in brain, but the relationship was not uniform for Seychellois men possibly due to changes in fish consumption in the weeks or months prior to death. Among the three cortical brain areas analyzed, Hg concentration did not significantly differ by region; although, there was intra- and inter individual variation in Hg concentrations. We previously reported Hg levels in brains of Seychellois children at birth and prior to birth (Lapham et al., 1995). Interestingly, the total Hg concentration in the brain of Seychelles infants overlaps that in Seychellois adults. The total Hg in infant occipital cortex was 105 ± 51 ppb while that in adult female and male calcarine cortex was 129.4 ± 18.6 ppb and 182 +96.8 ppb respectively. This suggests that mercury concentrations for this population reach or approach a steady state in the brain during gestation or shortly thereafter.
Although analytically, Hg deposition was present in all cortical regions of the Seychelles adult samples and levels were at least 10 times greater than for the Rochester referent group, the morphology of the brains in these two groups was indistinguishable microscopically. There was no evidence in the brain samples of neuronal, myelin, or axonal changes, no morphological evidence of microglial or astrocyte activation, and no evidence of mercury deposition sufficient to microscopically induce silver deposition using histochemical procedures.
The results of the analysis of the Seychelles specimens are similar; although, not identical to the results reported for indigenous Greenlanders (Pedersen et al., 1999). Greenlanders like Seychellois have higher levels of oceanic fish consumption than is typical of Western diets. However, unlike the Seychellois, the diet of Greenlanders includes a significant amount of food based on marine mammals. Hg in the fish consumed by Greenlanders is significantly less than in marine mammals (fish 0.071 ppm vs. seal 0.355 ppm, toothed whale meat 0.583 ppm wet weight) (Jeppesen et al., 2012). Neither neurologic signs and symptoms nor neuropathological changes typical of mercury poisoning were reported in the 17 Greenlanders studied by Pedersen et al. (1999). The median concentration of total Hg in the brains of Greenlanders was 174 ppb of wet brain weight (range 29 – 4879) with the highest levels in the cerebellum (median 492 ppb; range 60 – 4879). Although the cerebellum had the highest total Hg level, it had the lowest fraction (median 6 %) as organic Hg while the sensory and motor cortices had organic fractions that were more than 5X higher. A significant difference between the two populations is a shift in the fraction of brain mercury in the organic form. While on average the organic mercury fraction in the Seychellois brains was 70 %, the median value in the Greenlander brains was 32 % with the average organic Hg fractions ranging from 1 – 60 %. This difference may be related to the significantly different diets these populations have since the whole blood Hg levels in Greenlanders correlates closely with seal consumption and not fish consumption (Jeppesen et al., 2012). As for the Seychellois, no one brain cortical area had the highest Hg concentration in all cases. There was no significant gender difference in brain Hg levels among the Greenlanders. Unlike the Seychellois, increasing total Hg concentration in the brain of Greenlanders was significantly correlated with increasing age over the period of 40 – 85 years. Like the Seychellois, the fraction of organic Hg in the brain decreased with age and the inorganic fraction increased with age. Histochemically, autometallography grains were observed infrequently in glial cells in 58 % of the Greenlanders and in neurons (pyramidal cells, Purkinje cells, and anterior horn cells) and a few endothelial cells in the two cases (17 % cases) with the highest brain Hg levels (mean total brain Hg of 1153 and 4782 ppb). These differences suggest that mercury derived from consumption of sea mammals may be biologically different than consumption of ocean fish.
The transformation of inorganic Hg to organic Hg is the basis for its bioaccumulation through the food chain and the transformation of organic Hg to insoluble inorganic species in the organism is the basis for deposition of inorganic Hg in the brain and a likely mechanism of detoxification. Hg forms complexes with Se and this is generally considered to significantly reduce its toxicity (Ralston and Raymond, 2018). Recently, Korbas et al. (2010) tentatively speciated the Hg present in human brain tissue and identified low levels of mercuric selenide and methylmercury cysteineate in brain tissue from a Seychelles autopsy case. The inorganic mercury present in the brain has generally been considered immobile (Clarkson and Magos, 2006). While this assessment appears to be largely correct based on the presence of large amounts of inorganic Hg in the brains of poisoned individuals, it may not be correct for populations consuming fish. There was no apparent age-related build-up of total Hg in the brain of either our Rochester reference population or the Seychellois cases.
The threshold brain Hg concentration when humans might develop evidence of neurotoxicity is uncertain. Macaca fasciclaris given 50 μg Hg/kg body weight as MeHg daily by mouth for 18 months did not reach brain organic Hg levels sufficient to develop adverse clinical signs, laboratory tests, neurobehavioral changes, or neuropathologic lesions (Vahter et al., 1995). The average level of organic Hg in their brain was 4.3 μg/g (4300 ppb) brain or about 27 times higher than that for Seychellois (total Hg minus inorganic Hg or 160 ppb) (Vahter et al., 1995). Total and inorganic Hg levels in the Macaca brains were similarly higher than the Seychelles brains with no reported adverse effects in the monkeys (Vahter et al., 1995). Morphometric analysis of the thalamus and calcarine cortex from these monkeys found no difference in the number of neurons, oligodendrocytes, endothelial cells, or pericytes (Charleston et al., 1994 and 1996). No significant change in astrocyte number was seen in these monkeys although other monkeys given methyl Hg for other time periods were reported to have reduced numbers of astrocytes in the thalamus, but not the calcarine cortex. No morphologic changes were identified in the astrocytes of these animals. Microglia were statistically significantly increased in the thalamus of these monkey and in monkeys allowed to recover from exposure for six months. Cells referred to as reactive glia were also reported to be elevated in the calcarine cortex (Charleston et al., 1996). Histochemical staining showed that most of the inorganic Hg in the brain was located in microglia or reactive glia. Although the significance, if any, of the difference is not clear, the fact that large amounts of inorganic Hg were localized in microglia/reactive glia in both the thalamus and calcarine cortex suggests that they are not only involved in converting methyl Hg into inorganic Hg but may also provide a physical process for removal of inorganic Hg from the brain. The report by Charleston et al. (1995) of a small number of phagocytic cells (gitter cells) in the calcarine cortex of these monkeys further suggests a clearance role for microglia, which can act as resident phagocytic cells in the brain. In this light, the microglial response would be an adaptive, rather than a pathological change in the monkey brain. Unlike the monkeys referred to above, monkeys receiving long-term exposure to 70 μg Hg/kg body weight/day are reported to develop adverse clinical signs and neuropathological effects (Stinson et al, 1989; Vahter et al., 1995) suggesting a threshold for clinical effects between 50 – 70 μg Hg/ kg body weight/day in this Macaca model of Hg exposure. The results of the Macaca experimental model showing no significant pathological change at exposure levels resulting in higher brain organic Hg levels than those we studied in Seychellois are supportive of our findings.
According to Berglund et al. (1970), a daily intake of 2 μg methyl mercury/day is estimated to result in a brain mercury concentration of 10 μg/kg (ppb) wet brain weight. This suggests that based on the data in Table 2 the Seychelles cases ingested 9.2 – 64.8 μg MeHg/ day (an estimated 0.13 – 0.93 μg MeHg/kg bodyweight for a 70 kg person). At an average mercury concentration of 0.3 μg/g of fish, this would amount to 31 – 216 g of fish/day for Seychellois men and women. This estimate is in line with recent reports that 98 % of pregnant Seychellois women consume on average 76 g of fish/day (Bonham et al., 2008).
4.2. Poisoning cases
In the two subjects we studied with organic Hg poisoning, the morphology of the brains and the results of the histochemical staining for mercury were significantly different than either the Rochester or Seychelles cases. The neuropathologic findings in these subjects with organic Hg exposure were similar to previously reported cases (Hunter and Russell, 1954; Davis et al., 1994; Eto, 1997; Nierenberg et al., 1998; Siegler et al, 1999). Organic Hg appears to be converted to inorganic Hg in the brain and can persist for years. We confirmed that abundant inorganic Hg deposits were present in the brains of both subjects despite exposure primarily to organic Hg many years ago followed by survival for 10 months (Subject 1) and 21 years (Subject 2), and chelation therapy. The finding of Hg deposits in neurons, astrocytes, perivascular cells (Eto, 1997), Bergmann’s glial cells, microglia or macrophages, and epithelial cells of the choroid plexus even after long survival has been reported (Takeuchi et al., 1989). Histochemically demonstrable Hg deposits were mainly observed in lysosomes on examination by electron microscopy (Eto, 1997). Takeuchi et al. (1989) reported that the accumulation of Hg in neurons in Minamata subjects who survived 26 years were located in discrete areas involving mainly the thalamus, calcarine cortex, and deep layers of the postcentral gyrus. In the cerebellum, Hg deposits were found in Bergmann’s glia and macrophages, which were more abundant in areas of tissue destruction. The location of pathologic lesions and inorganic Hg in the cerebral and cerebellar cortices are typical for intoxication with an organic mercurial in the Minamata cases, even though the exposures were from eating fish and shellfish that were contaminated with both organic and inorganic mercury, and other potential toxicants (Clarkson and Magos, 2006).
For Subject 3 in our case series, Hg exposure and the patient’s clinical course occurred over a relatively short period of time. However, under experimental conditions in laboratory animals, Hg deposition can be seen in the CNS of animals exposed to high vapor concentrations of Hg in as little as 30 min with females showing deposition of Hg at half the exposure time of males suggesting a greater risk to females from exposure to Hg vapor (Pamphlett and Coote, 1998). Hg vapor intoxications in the literature typically have been reported to occur over a longer period of time or have longer clinical courses. For example, Hg deposits have been described in the brain of patients up to 17 years following exposure to elemental Hg vapor using histochemical methods (Hargreaves et al., 1988; Opitz et al., 1996).
In the case reported by Opitz et al. (1996), a man was acutely exposed to mercury vapor while recycling dental amalgam. Although he excreted 1850 mg Hg/L in his urine, he did not present signs typical of mercurialism. At the time of his death 17 years post-exposure, neurons stained intensely for Hg in histochemical preparations. Hg deposition was most prominent in the basal ganglia and motor neurons of the ventral spinal cord. Neurons in the occipital l cortex stained more intensely than those of the frontal cortex. In the cerebellum, light Hg deposition was present in Purkinje cells, granule cells, and Bergmann glia. The brain stem nuclei were darkly stained with Hg deposits. Hg was observed by electron microscopy to be in lysosomes with lipofuscin. The Hg deposits were not associated with cellular or tissue damage.
In a case described by Hargreaves et al. (1988), a man developed typical signs of mercurialism while working for 18 months filling thermometers. At autopsy 16 years later, Hg deposition was present in many nerve cells in all regions of the brain that were examined. Hg deposition was present in the neurons of the substantia nigra. In the cerebral cortex, some large pyramidal cells contained heavy Hg deposits. Light deposits of Hg were seen in the Purkinje cells and granule cells of the cerebellum with heavier deposition of Hg in large neurons of the brainstem, ventral motor horn cells, and dorsal root ganglia. Small deposits of Hg were present in astrocytes and endothelial cells. Hg deposits were found only in lysosomes on electron microscopic examination.
Our Subject 3 differed from the reported cases in that the exposure level was higher and the survival time much shorter. There was only mild to moderate Hg staining in the subject’s brain despite very high levels of inorganic Hg measured in the urine. However, similar to the cases with longer duration cases, the pattern of Hg deposition was greatest in the deep cortical nuclei and brain stem with lesser amounts in cortical areas. Intoxication with inorganic Hg compounds in our cases did not produce gross or significant microscopic CNS pathology despite the clinical severity of the poisonings.
The literature also includes a case similar to Subject 4 in which inorganic Hg exposure was by injection intravenously. Pamphlett and Waley (1996) described neuropathologic findings 5 months following a suicidal attempt by intravenous injection with metallic Hg from two industrial thermometers. The exposure resulted in a Hg level of 0.1 μg Hg/g (100 ppb) in the cerebrum. The authors described Hg deposition in capillary walls and scattered glial cells throughout the brain with smaller deposits in scattered choroid plexus cells. Heavy Hg deposition was seen in the large neurons of primary motor cortex, but no other cortical neurons. Moderate amounts of Hg were seen in the mesencephalic trigeminal nucleus, locus ceruleus, lateral tegmental neurons with smaller amounts in some, but not all other brainstem nuclei. In the cerebellum, Hg granules were seen exclusively in dentate neurons.
Pamphlett and Jew (2018) described a second case in which a 24-year old man died 5 months following a suicidal attempt by intravenous injection of metallic Hg from thermometers. The man remained asymptomatic following the Hg exposure and at autopsy no neuropathology was described. Histologically, there was no evidence of neuronal or oligodendrocyte cell loss, astrocytic hypertrophy, or destructive tissue damage leading to microglial activation. Using a combination of autometallography and GFAP immunohistochemistry, Pamphlett and Jew were able to detect inorganic Hg in five major types of human astrocytes (grey matter subpial, interlaminar, protoplasmic and varicose astrocytes, and white matter fibrous astrocytes) They also detected inorganic Hg in grey but not white matter oligodendrocytes, corticomotoneurons but only in cortical layer 5 of the primary motor cortex, some locus ceruleus neurons, and the walls of some cortical venules. None of these cell types displayed pathologic changes associated with deposition of intracellular inorganic Hg. Neither an estimate of the dose of Hg received nor brain mercury levels were reported, but observation of collections of Hg in the heart, lungs and pelvic veins is consistent with heavy exposure to inorganic Hg as a metallic liquid continuously over the 5 month post-injection period.
In our Subject 4 who was autopsied 21 months following his first injection with metallic Hg, deposits of Hg were most conspicuous in vessel walls, neurons of brainstem and ventral and dorsal spinal neurons with very little present in glial elements and cortical areas of the cerebrum and cerebellum.
The distribution and pathologic effects of exposure to mercurous mercury is similar to that of our cases with elemental Hg, except that there may be slight cellular changes in the cerebellar cortex. Davis et al (1974) reported two cases of intoxication following long-term use of mercurous chloride laxatives. Both cases showed only mild loss of cerebellar granular neurons and slight global cerebellar atrophy. Hg granules were present in neurons of the inferior olive and dentate nucleus, endothelium, basement membrane of the choroid plexus, and in smaller amounts in Purkinje cells, and neurons in the anterior horn and substantia nigra. A pattern that is more similar to inorganic Hg deposition than methyl Hg deposition. Analytically, the Hg level in the inferior olivary nucleus of the brainstem was 32 times higher than in the occipital cortex of one of the patients.
The general picture of cellular distribution of inorganic mercury after exposure to either methyl Hg or elemental Hg is consistent with what is known about the mechanisms and cellular sites of the conversion process. The carbon - mercury bond of methyl mercury can be cleaved in phagocytic cells by the free radical cascade to produce mercuric mercury (Suda et al., 1992). Hargreaves et al (1985) demonstrated that inorganic Hg first appears in astrocytes after a single dose of methyl Hg in experimental animals. Likewise, we found in the high brain organic Hg cases that astrocytes, cells with phagocytic activity, are the principal site of deposition of inorganic mercury. On the other hand, we found inorganic Hg to be more or less equally distributed between all types of cells, both neuronal and non-neuronal, after exposure to elemental mercury. This is consistent with the ubiquitous distribution of catalase in mammalian cells as it functions as the major pathway for removal of hydrogen peroxide, a universal product of oxygen metabolism.
It is generally accepted that mercuric Hg produced in brain tissues by oxidation of elemental Hg is the proximate toxic agent in CNS poisoning after inhalation of Hg vapor. The vapor itself consists of uncharged atoms of elemental mercury that do not react with organic ligands in cells except as a substrate for catalase compound I (Wigfield and Tse, 1985), whereas its oxidation product mercuric Hg is chemically highly reactive and toxic to cells. The toxicological role of mercuric Hg produced by cleavage of the carbon - mercury bond in methyl Hg poisoning is not clear. Animal experiments indicate that the intact mercurial, CH3Hg, is responsible for brain damage at least in the acute form. A WHO expert committee has suggested that inorganic Hg remaining in brain tissues years after exposure to methyl Hg is in the form of an inert selenium complex. In autopsy cases with high brain mercury levels (Subjects 1 and 2), Korbas et al. (2010) reported elevated cortical selenium levels with significant proportions of nanoparticulate mercuric selenide plus some inorganic mercury and methylmercury bound to organic sulfur. This also may be the case after cessation of exposure to mercury vapor (Kosta, Byrne, and Zelenko, 1975). Thus, the inorganic Hg produced by oxidation of elemental Hg must undergo a transformation from its active state as the proximate toxic agent to a less active, if not inert, form. It is tempting to speculate that the reason that mercuric Hg is the proximate toxic agent after exposure to elemental Hg vapor is that it is produced in situ in neuronal cells. Its secondary role in toxicity, if any, after exposure to methyl Hg may be because it is produced mainly in non-neuronal cells.
Although there are reports of human poisonings due to other organic mercury compounds such as those that occurred in Iraq, autopsy reports from these situations are rare and confined to infancy (Choi et al., 1978). The clinical and pathologic effects appear to differ from that of methyl Hg and present limited evidence suggesting that the effects of these substances include similarities of both methyl Hg and inorganic Hg exposure. For example, Haq (1963) reported clinical effects in 34 Pakistani patients who had ingested seeds dressed with phenyl mercury acetate and ethyl mercury chloride that were similar to those for methyl Hg. While about a quarter of the cases had visual impairment, many of the cases exhibited signs and symptoms of inorganic Hg poisoning (erethism). The one case that was autopsied showed no abnormalities in the cerebrum, cerebellum or spinal cord but prominent lesions were present in the gastrointestinal tract and kidneys. Cinca et al. (1980) described four cases of poisoning due to ingestion of pork from a pig fed grain dressed with ethyl mercury chloride. At autopsy, the brains of two of the cases appeared normal macroscopically. However, microscopically neuronal loss and neuronophagia, and focal gliosis were present in the calcarine cortex, striate nuclei, and both the neo- and paleocerebellar cortex with extensive loss of ventral motor horn cells. Neuronal lesions were also found in the bulbar reticular formation in one of the two autopsied cases. The other two cases recovered significantly but both cases have residual concentric narrowing of the visual fields and one cases had mild intention tremor months after hospital discharge. A possible explanation for the effects seen with ethyl mercury chloride is that it is rapidly converted to inorganic Hg (Clarkson and Magos, 2006).
Although the results and conclusion of this study are constrained by the limited number of cases available for examination, they are of importance because they provide information about the target species for human risk assessment without the need for extrapolation from non-human species. The Seychelles data are consistent with the available data to date in that adults from populations consuming high dietary levels of non-piscivorous oceanic fish for their lifetime do not demonstrate obvious pathological lesions in areas of the brain known to be sensitive to organic mercury exposures. This is in contrast to populations such as those that surrounded Minamata Bay and consumed high levels of fish from near shore waters that were heavily polluted with industrial Hg waste. Hg exposure in each of our four subjects who had highly elevated brain Hg levels differed in severity, timing of exposure, interval between exposure and death, clinical course, and Hg species. Despite these variations, the distribution of Hg in the nervous system was primarily related to whether exposure was to an inorganic or MeHg species. The high brain Hg cases demonstrate a different pattern of cellular and brain region deposition of inorganic Hg with greater neuronal Hg deposition in cases of elemental Hg exposure and greater deposition in phylogenetically older regions of the brain (brain stem and nuclei of the deep grey matter) in contrast to heavier glial Hg deposition and greater cortical (cerebral and cerebellar) deposition in methyl Hg exposed cases. In their seminal case on the neuropathology of organic mercury, Hunter and Russell (1954) made a similar observation in that they stated that the sparing of the paleocerebellum was remarkable in contrast with the damage done to the neocerebellum by organic mercury. In our cases, heavy inorganic Hg deposits in neurons have been observed in cases of methyl and inorganic Hg exposure. However, histologic lesions are routinely associated with these heavy deposits in methyl Hg exposure cases and are not routinely associated with inorganic Hg exposure cases.
Although Hg in its various forms, whether organic or inorganic, has been shown to have adverse effects upon the nervous systems, the clinical and pathologic effects of these materials differ suggesting that the mechanism and the target cells differ depending on the molecular structure of the Hg compound to which people are exposed.
Much work still needs to be done to explain the mechanism(s) of action of mercury on the human brain. While the mercuric form of Hg is highly reactive and has been shown to interact with various cellular and tissue components, the exquisitely targeted effects of the short chain alkyl mercurials dimethyl- methyl- and ethylmercury suggest generalized protein or lipid interactions cannot explain the effects of Hg on developing and adult humans other Hg species do not completely share the effects of the alkyl mercury compounds. Thus, the neuropathology of Hg in humans suggests that alkyl mercurials may affect processes that are highly evolved in humans and less so in other species. The high specificity of organic Hg for localization and pathology in neocortical regions of the human brain suggests that there is an as yet unknown mechanism or mechanisms of action of short chain alkyl mercury compounds such as MeHg that affect more recent evolutionary regions or processes in the brain.
In conclusion, this work found no neuropathological abnormalities in the brains of adults from the Seychelle Islands even though brain mercury levels were substantially higher than for a continental American population, Seychellois adults and children at birth have brain Hg levels that are similar, increasing concentrations of inorganic Hg were present in the adult human brain with age, different Hg species are associated with differing patterns of Hg deposition and neuropathology.
Highlights.
Mean total mercury in the cerebrum of people with no known exposure was 16 ppb.
Mean total mercury in the cerebrum of Seychellois consuming fish was 129–182 ppb.
No pathology was observed in the brains of Seychellois who consumed fish regularly.
Total brain mercury approaches steady state during gestation or shortly thereafter.
Different Hg species are associated with differing neuropathological patterns.
Acknowledgements
We are grateful to Dr. Larry E. Davis (Neurology Service, New Mexico Veterans Affairs Health Care System), and Dr. David W. Nierenberg (Department of Medicine and Pharmacology/Toxicology, Dartmouth Medical School) for providing brain specimens. We are indebted to the late Dr. Ana Rubio (Medical Examiners Office, City of Baltimore, MD) for her neuropathological analysis of many of the specimens included in this study.
This research was funded by in part by NIEHS grants #ES-01247and ES-05497. A portion of this work was presented as a poster at the 5thth International Conference: “Mercury as a global pollutant”, Rio de Janeiro, Brazil.
Footnotes
Conflicts of Interest
The authors declare there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berglund F, berlin M, Birke G, Cederlöf R, von Euler U, Friberg L, Holmstedt B, Jonsson E, Lüning KG, Ramel C, Skerfving S, Swensson Å, Tejning S. Methylmercury in fish. A toxiclogic – epidemiologic risk evaluation. Nordisk Hygienisk Tidskrift: 1970; Suppl 3: 98–103. [Google Scholar]
- Bonham MP, Duffy EM, Robson PJ, Wallace JM, Myers GJ, Davidson PW, Clarkson TW, Shamlaye CF, Strain JJ, Livingstone MBE. Contribution of fish to intakes of micronutrients important for fetal development: a dietary survey of pregnant women in the Republic of Seychelles. Public Health Nutrition: 2008; 12:1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC and Zalups RK Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Hlth, Part B 2020; 13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernichiari E, Toribara TY, Liang L, Marsh DO, Berlin M, Myers GJ, Cox C, Choisy O, Davidson PW, Clarkson TW. The biological monitoring of mercury in the Seychelles study. Neurotoxicology 1995; 16: 613–28. [PubMed] [Google Scholar]
- Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure. Neurotoxicology 1996; 17: 127–138. [PubMed] [Google Scholar]
- Charleston JS, Bolender RP, Mottet NK, Body RL, Vahter ME, Burbacher TM. Increases in the number of reactive glia in the visual cortex of Macaca fascicularis following subclinical long-term methylmercury exposure. Toxicol App. Pharmacol 1994; 129: 196–206. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Mottet NK, Vahter ME, Burbacher TM. Autometallographic determination of inorganic mercury distribution in the cortex of the calcarine sulcus of the monkey Macaca fascicularis following long-term subclinical exposure to methylmercury and mercuric chloride. Toxicol Appl Pharmacol 1995;132: 325–33. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: A major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol 1978; 37: 719–733. doi.org/ 10.1097/00005072-197811000-00001 [DOI] [PubMed] [Google Scholar]
- Churukian CJ, Rubio A, Lapham LW. A simple colloidal silver method (autometallographic technique) for demonstrating inorganic mercury in brain sections. J Histotechnol 2000;23: 337–339. [Google Scholar]
- Cinca I, Dumitrescui I, Onaca P, Serbanescu A, Nestorescu B. Accidental ethyl mercury poisoning with nervous system, skeletal muscle, and myocardium injury. J Neurol Neurosurg Psychiat 1979; 43: 143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 2006; 36:609–62. [DOI] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW. Methylmercury poisoning: long term clinical, radiological, toxicological and pathological studies of an affected family. Ann Neurol 1994; 35:680–8. [DOI] [PubMed] [Google Scholar]
- Davis LE, Wands JR, Weiss SA, Price DL, Girling EF. Central nervous system intoxication from mercurous chloride laxatives. Arch Neurol 1974; 30:428–31. [DOI] [PubMed] [Google Scholar]
- Drasch G, Schupp I, Höfl H, Reinke R, Roider G. Mercury burden of human fetal and infant tissues. Eur J Pediatr 1994; 153: 607–610. [DOI] [PubMed] [Google Scholar]
- Eto K Pathology of Minamata disease. Toxicol Pathol 1997; 25: 614–23. [DOI] [PubMed] [Google Scholar]
- George GN, MacDonald TC, Korbas M, Singh SP, Myers GJ, Watson GE, O’Donoghue JL, Pickering IJ. The chemical forms of mercury and selenium in whale skeletal muscle. Metallomics: integrated biometal science 2011; 3: 1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George GN, Singh SP, Prince RC, Pickering IJ. Chemical forms of mercury and selenium in fish following digestion with simulated gastric fluid Chem Res Toxicol 2008; 21: 2106–10. [DOI] [PubMed] [Google Scholar]
- Giombetti RJ, Rosen DH, Kuczmierczyk AR, Marsh DO. Repeated suicide attempts by the intravenous injection of elemental mercury. Intl J Psychiat in Med 1988; 18: 153–67. [DOI] [PubMed] [Google Scholar]
- Haq IU. Argosan poisoning in man. Brit Med J 1963; 1 (5385): 1579–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HH, Pickering IJ, George GN. The chemical form of mercury in fish. Science 2003; 301: 1203. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Foster JR, Pelling D, Moorhouse SR, Gangolli SD, Rowland IR. Changes in the distribution of histochemically localized mercury in the CNS and in tissue levels of organic and inorganic mercury during the development of intoxication in methylmercury treated rats. Neuropathol Appl Neurobiol 1985; 11: 383–401. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Evans JG, Janota I, Magos L, Cavanagh JB. Persistent mercury in nerve cells 16 years after metallic mercury poisoning. Neuropathol Appl Neurobiol 1988; 14: 443–52. [DOI] [PubMed] [Google Scholar]
- Hunter D, Russell DS. Focal cerebral and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurosurg Psychiat 1954; 17: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen C, Jørgensen ME, Bjerregaard P. Assessment of consumption of marine food in Greenland by a food frequency questionnaire and biomarkers. Int J Circumpolar Health 2012; 71:18361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw TG, Clarkson TW, Dhahir PH. The relationship between blood levels and dose of methylmercury in man. Arch Environ Health 1980; 35: 28–36. [DOI] [PubMed] [Google Scholar]
- Korbas M, O’Donoghue JL, Watson GE. Pickering IJ, Singh SP, Myers GJ, Clarkson TW, George GN. The chemical nature of mercury in human brain following Poisoning or Environmental Exposure ACS Chem Neurosci. 2008; 21: 2106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosta LK, Byrne AR, Zelenko V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 1975; 254:238–9. [DOI] [PubMed] [Google Scholar]
- Lapham L, Cernichiari E, Cox C, Myers G J, Baggs RB, Brewer R, Shamlaye CF, Davidson PW, Clarkson TW. An analysis of autopsy brain tissue from infants prenatally exposed to methylmercury. Neurotoxicology 1995;16: 689–704. [PubMed] [Google Scholar]
- Lemes M, Wang F, Stern GA, Ostertag SK, Chan HM. Methylmercury and selenium speciation in different tissues of beluga whales (Delphinapterus leucas) from the western Canadian Arctic. Environ Toxicol Chem 2011; 30: 2732–8. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye E, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang L-S, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet 2003; 361: 1686–92, 2003. [DOI] [PubMed] [Google Scholar]
- Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, Toribara TY, Cernichiari E, Clarkson T. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N Eng J Med 1998; 338:1672–94. [DOI] [PubMed] [Google Scholar]
- Opitz H, Schweinsberg F, Grossmann T, Wendt-Gallitelli MF, Meyermann R. Demonstration of mercury in the human brain and other organs 17 years after metallic mercury exposure. Clin Neuropathol 1996; 15:139–44. [PubMed] [Google Scholar]
- Outridge PM, Mason RP, Wang F, Guerrero S, Heimburger L-E. Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018. Environ Sci Technol 2018; 11466–11477. doi: 10.1021/acs.est.8b01246 [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Coote P. Entry of low doses of mercury vapor into the nervous system. Neurotoxicology 1998; 19: 39–48. [PubMed] [Google Scholar]
- Pamphlett R, Waley P. Uptake of inorganic mercury by the human brain. Acta Neuropathol 1996; 92: 525–7. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Jew SK. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals 2018, 31: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MB, Hansen JC, Mulvad G, Pedersen HS, Gregersen M, Danscher G. Mercury accumulation in brains from populations exposed to high and low dietary levels of methyl mercury. Int J Circumpolar Health 1999; 58: 96–107. [PubMed] [Google Scholar]
- Ralston NVC, Raymond LJ. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochimica et Biophysica Acta - General Subjects, 2018; 1862: 2405–2416. doi: 10.1016/j.bbagen.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Sakai K, Okabe M, Eto K, Takeuchi T. Histochemical demonstration of mercury in human tissue cells of Minamata disease by use of autoradiographic procedure. Acta Histochem Cytochem 1975; 8: 257–64. [Google Scholar]
- Siegler RW, Nierenberg DW, Hickey W. Fatal poisoning from liquid methylmercury: a neuropathologic study. Human Pathol 1999; 30: 720–3. [DOI] [PubMed] [Google Scholar]
- Stinson CH, Shen DM, Burbacher TM, Mohamed MK, Mottet NK. Kinetics of methylmercury in blood and brain during chronic exposure in the monkey Macaca fascicularis. Pharmacol Toxicol 1989; 65: 223–230. [DOI] [PubMed] [Google Scholar]
- Suda I, Totoki S, Uchida T, Takahashi H. Degradation of methyl and ethyl mercury into inorganic mercury by various phagocytic cells. Arch Toxicol 1992; 66: 40–4. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Eto K, Tokunaga H. Mercury level and histochemical distribution in a human brain with Minamata disease following a long term clinical course of twenty-six years. Neurotoxicology 1989;10: 651–8. [PubMed] [Google Scholar]
- Tokunaga H, Furusawa R, Eto K. Identification of mercury granules from pigments in tissue using a photoemulsion method. Pathol Clin Med 1988; 6: 845–848. [Google Scholar]
- Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM. Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methylmercury exposure. Toxicol Appl Pharmacol 1995; 134: :273–284. [DOI] [PubMed] [Google Scholar]
- Wagemann R, Trebacz F, Boila G, Lockhart WI. Methylmercury and total mercury in tissues of arctic marine mammals. Sci Total Environ 1998; 218: 19–31. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Nylander M. The relationship between mercury concentration in human organs and different predictor variables. Sci Total Environ 1993; 138: 101–15. [DOI] [PubMed] [Google Scholar]
- Wigfield DC, Tse S. Kinetics and mechanism of the oxidation of mercury by peroxidases. Can J Chem 1985; 63:2940–4 [Google Scholar]
- World Health Organization (WHO). Methylmercury, in Environmental Health Criteria 101, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland: 1990. [Google Scholar]
- World Health Organization (WHO). Mercury and health fact sheet. Updated March 2017 Available at: http://www.who.int/mediacentre/factsheets/fs361/en/.