ABSTRACT
Although supplemental vitamin D is used to promote bone health in the general population, data from randomized controlled trials (RCTs) have been inconsistent. We determined whether daily, vitamin D3 supplementation improves bone mineral density (BMD) and/or structure. VITamin D and OmegA‐3 TriaL (VITAL) is a double‐blind, placebo‐controlled RCT of supplemental vitamin D3 (2000 IU/d) and/or omega‐3 fatty acids (1 g/d) in 25,871 adults nationwide. This ancillary study included a subcohort of 771 participants (men ≥50 and women ≥55 years; not taking bone active medications) evaluated at baseline and at 2‐year follow‐up (89% retention). Total 25(OH)D levels were measured by liquid chromatography tandem mass spectrometry (Quest Diagnostics, San Juan Capistrano, CA, USA). Free 25(OH)D (FVD) levels were measured using the ELISA assay by Future Diagnostics Solutions BV (Wijchen, Netherlands). Primary endpoints were 2‐year changes in areal (a) BMD at the spine, hip, and whole body determined by dual‐energy X‐ray absorptiometry (DXA). Secondary endpoints were 2‐year changes in volumetric (v) BMD and cortical thickness at the radius and tibia assessed by peripheral quantitative computed tomography. Supplemental vitamin D3 versus placebo had no effect on 2‐year changes in aBMD at the spine (0.33% versus 0.17%; p = 0.55), femoral neck (−0.27% versus −0.68%; p = 0.16), total hip (−0.76% versus −0.95%; p = 0.23), or whole body (−0.22% versus −0.15%; p = 0.60), or on measures of bone structure. Effects did not vary by sex, race/ethnicity, body mass index, or 25(OH)D levels. Among participants with baseline FVD levels below the median (<14.2 pmol/L), there was a slight increase in spine aBMD (0.75% versus 0%; p = 0.043) and attenuation in loss of total hip aBMD (−0.42% versus −0.98%; p = 0.044) with vitamin D3. Whether baseline FVD levels help to identify those more likely to benefit from supplementation warrants further study. Supplemental vitamin D3 versus placebo for 2 years in general healthy adults not selected for vitamin D insufficiency did not improve BMD or structure. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Keywords: BONE QCT, DXA, GENERAL POPULATION STUDIES, OSTEOPOROSIS, PTH/VITD/FGF23
Introduction
Osteoporosis is a major health problem and primary prevention strategies are needed. Vitamin D supplements are widely recommended and prescribed in the general population to promote bone health. In the past decade, vitamin D supplement use has increased fourfold.1 Mechanisms by which vitamin D may support skeletal health include improved mineralization of bone through increased intestinal calcium absorption, prevention of secondary hyperparathyroidism, and direct effects on osteoblast formation.2, 3, 4, 5, 6 Observational studies have indicated that high 25‐hydroxyvitamin D [25(OH)D] levels are positively associated with areal bone mineral density (aBMD).7, 8, 9, 10 Data from large meta‐analyses and systematic reviews that support use of supplemental vitamin D alone (without calcium) to benefit bone are lacking.11, 12, 13, 14, 15 The few randomized controlled trials (RCTs) of vitamin D alone versus placebo have not shown significant changes in aBMD at the spine but showed small benefits at the femoral neck and/or a level of total 25(OH)D below which supplemental vitamin D increased spine and hip aBMD.16, 17, 18 Although RCTs provide the highest‐quality data, most previous RCTs of vitamin D versus placebo on aBMD were limited by design, including bolus dosing,17, 19, 20 short duration,21, 22 small sample sizes,21, 22 participants selected for vitamin D insufficiency,20 and/or inability to separate effects of supplemental vitamin D from calcium.23, 24, 25
Bone strength depends on bone density and quality. Components of bone quality include cortical and trabecular structure, which can be assessed using peripheral quantitative computed tomography (pQCT). Some but not other studies suggest an association of 25(OH)D levels with improved bone structure, though there are no large, long‐term RCTs of supplemental vitamin D versus placebo on bone structure.26, 27 A recent study from Canada raised concerns that high doses (4000 IU/d or 10,000 IU/d) versus a low dose of vitamin D (400 IU/d) resulted in loss of volumetric bone density at the radius and tibia.28
Recent estimates of vitamin D status among US middle‐age to older adults show that approximately 20% have 25(OH)D levels <50 nmol/L.29 Higher proportions of vitamin D insufficiency or deficiency have been reported among black adults (reduced cutaneous vitamin D synthesis),30 obese individuals (vitamin D sequestration in fat tissue),31 and older adults.32
Although serum 25(OH)D levels have been considered the clinical biomarker for vitamin D status, vitamin D circulates primarily bound to vitamin D binding protein. It is the free 25(OH) vitamin D (FVD) that may exert biological effects on bone.33, 34, 35, 36, 37 At present, there is no consensus on the optimal circulating total 25(OH)D or FVD level for bone, and it is unclear whether FVD may better predict effects of supplemental vitamin D on BMD and structure. The ancillary study “VITamin D and OmegA‐3 TriaL (VITAL): Effects on Bone Structure and Architecture” addresses these knowledge gaps, evaluating whether vitamin D3 supplementation (2000 IU/d), compared with placebo in the generally healthy population not selected for vitamin D insufficiency, produces small increases or reduces bone loss in spine, hip, and whole body aBMD or improves volumetric (v)BMD and bone strength measures at the radius and tibia. We also examined whether intervention effects were modified by baseline levels of total 25(OH)D and FVD.
Materials and Methods
Trial design and oversight
VITAL is a randomized, placebo‐controlled trial with a two‐by‐two factorial design investigating effects of vitamin D3 (cholecalciferol 2000 IU/d) and/or omega‐3 fatty acids (1 g/d) supplements in the primary prevention of cancer and cardiovascular disease. Calendar packs with trial capsules were mailed to the participants. This study included men ≥50 years and women ≥55 years from 50 US states and had a median follow‐up of 5.3 years. To ensure compliance, participants completed a 3‐month placebo run‐in phase and personal use of vitamin D3 was limited to 800 IU/d (US Recommended Dietary Allowance for older adults).38 More comprehensive protocol details have been reported.39, 40
The VITAL study was a hybrid design with the overall cohort of 25,871 participants and a subcohort of 1054 participants who lived within driving distance of the Harvard Clinical and Translational Science Center (CTSC) in Boston. Participants were eligible for this ancillary study if they were not on bisphosphonates within the past 2 years or other bone active agents ([Link]Supplemental Materials and Methods) within the past year. Of the CTSC participants, 771 completed assessments for bone and body composition at baseline, exceeding the enrollment goal of 600.41, 42 Participants received annual questionnaires evaluating risk factors for bone loss and fragility fractures, falls, medication/supplement use, and physical activity. Fasting blood samples were collected at baseline and year 2, matched by season, and levels of calcium, albumin, total 25(OH)D, and plasma phospholipid omega‐3 fatty acids were assayed by Quest Diagnostics (San Juan Capistrano, CA, USA). Total 25(OH)D, including both 25(OH)D2 and 25(OH)D3, and plasma phospholipid omega‐3 fatty acids levels were measured by liquid chromatography tandem mass spectrometry. Total 25(OH)D was calibrated to Centers for Disease Control and Prevention (CDC) standards. FVD levels, including both 25(OH)D2 and 25(OH)D3, were measured using the new ELISA assay by Future Diagnostics Solutions B.V. (Wijchen, Netherlands). See [Link]Supplemental Materials and Methods for serum measurement methods. The study was approved by the Institutional Review Board of Partners HealthCare–Brigham and Women's Hospital (BWH).
Ancillary study endpoints
In 771 participants at baseline and 687 at 2‐year follow‐up (89% retention), aBMD was assessed by dual‐energy X‐ray absorptiometry (DXA; Discovery W, APEX Software Version 4.2, Hologic, Bedford, MA, USA). If participants were found to have osteoporosis on DXA scans, they were sent letters indicating they had osteoporosis and recommending follow‐up with their health care providers. Participants who started treatment with bone active agents were not eligible to complete the 2‐year DXA scan and were excluded from 2‐year analyses. Primary endpoints were 2‐year changes in aBMD at the lumbar spine (L1 to L4), nondominant hip (total, femoral neck), and whole body. Least significant change at BWH is 0.024 g/cm2 at the spine, 0.021 g/cm2 at the femoral neck, 0.017 g/cm2 at the total hip, and 0.008 g/cm2 for males and 0.010 g/cm2 for females at the whole body. Guidelines from Hologic and the International Society for Clinical Densitometry were followed for all DXA scans. Detailed descriptions of the DXA protocol and reproducibility have been published.41 Inclusion and exclusion criteria for DXA scans are described in the [Link]Supplemental Materials and Methods.
In 677 participants at baseline and in 600 at 2‐year follow‐up (89%), pQCT scans were performed on the nondominant radius and tibia. Secondary endpoints included 2‐year changes in total, trabecular, and cortical vBMD, cortical thickness, and bone strength measures as assessed by pQCT (XCT 3000; Stratec Medizintechnik GmbH, Birkenfeld, Germany). At our site, precision (%CV) ranges from 0.02% to 2.87% at the radius and tibia.43 Details of pQCT measures are in the [Link]Supplemental Materials and Methods.
Statistical analysis
The intention‐to‐treat principle was used to analyze treatment effects between vitamin D3 and placebo groups. This ancillary study was designed to have 80% power to detect differences of 1.03%, 1.22%, and 0.42% in spine, femoral neck, and whole body aBMD, respectively, with a planned sample size of 600 and 10% loss to follow‐up.24 By exceeding this planned enrollment to 771 participants, detectable differences were reduced to 0.91%, 1.08%, and 0.37%, respectively. To assess whether balance was achieved by randomization among this subcohort, baseline characteristics were compared by treatment assignment. Continuous variables were first examined for normality. Means (standard deviation) or median (25th, 75th percentiles) are reported as appropriate. We used t tests and analysis of variance (or the Wilcoxon rank sum and Kruskal‐Wallis tests) to compare continuous variables across randomized groups. Chi‐square tests were used to compare proportions, using trend tests for ordinal data.
The primary analysis compared the effects of vitamin D3 versus placebo on changes in bone health measures, adjusted for the omega‐3 fatty acids intervention, age, sex, and race/ethnicity. Tests of significance for treatment effects were based on time by treatment interactions in repeated measures analyses. Thus, those with no follow‐up data were considered missing at random given their observed baseline data. Differences in treatment effects according to sex, race/ethnicity, body mass index (BMI), fat mass index (FMI), and baseline and achieved total 25(OH)D and FVD levels were specified a priori. Adherence‐based and other analyses were performed as secondary analyses. All analyses were generated using SAS (SAS Institute, Cary, NC, USA). Results were considered statistically significant when p < 0.05. There was no control for multiple hypothesis testing, and no formal adjustment was made to the p values. Thus, results regarding secondary, subgroup, and exploratory endpoints should be interpreted with caution.
Results
Ancillary study participants
The parent trial randomized 25,871 participants into four treatment groups (vitamin D3, omega‐3 fatty acids, both agents, or both placebos) between November 2011 and March 2014. A subcohort of 771 participants in the Boston area had detailed in‐person assessments at baseline. Testing at 2‐year follow‐up was completed by 687 participants (89% retention; Fig. 1). Follow‐up scans were not conducted for 19 participants who started taking bone active medications between baseline and 2‐year follow‐up. Other reasons for study discontinuation include lost to follow‐up, withdrawal of consent, subjects moved, did not want to drive back to Boston, were too busy, or did not want to return for a follow‐up visit.
Figure 1.
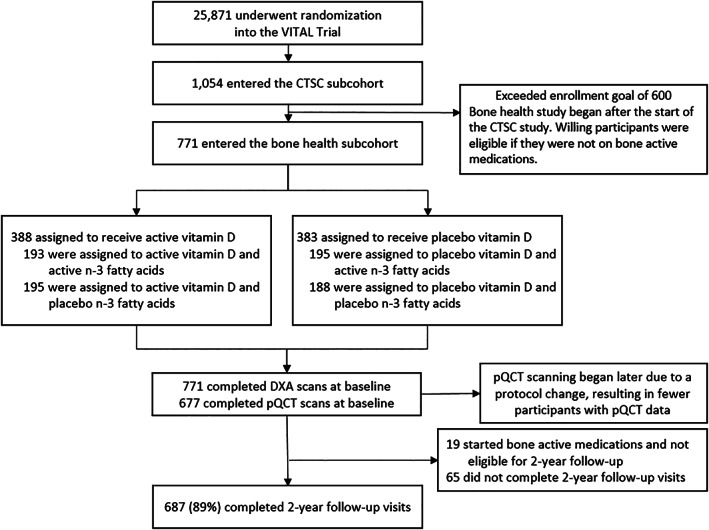
Randomization and follow‐up of participants.
Among participants answering the compliance question by questionnaire, 94.3% in the vitamin D group and 93.2% in the placebo group reported adherence to study pills at year 1. At 2 years, 93.0% of the vitamin D group and 92.1% of the placebo group reported study pill adherence (Supplemental Table S5).
Table 1 shows baseline characteristics of the bone health subcohort; most characteristics were balanced between the two groups. Of the 771 participants, 46.7% were women and 53.3% were men. The mean age was 63.8 years. At baseline, 42.3% were taking supplemental vitamin D (≤800 IU/d) and 17.1% were taking supplemental calcium (≤1200 mg/d). At baseline, 7.9% of participants had a history of fracture. A total of 80 participants had osteoporosis defined as a T‐score ≤ −2.5 at the spine or nondominant hip (n = 75) and/or reporting a fragility fracture at the hip, spine, forearm, or shoulder at baseline (n = 16). There were 402 participants who had osteopenia defined as a T‐score between −1 and − 2.5 at the spine or nondominant hip. The vitamin D3 group had a slightly lower total 25(OH)D level at baseline (67.4 versus 71.1 nmol/L, p = 0.025).
Table 1.
Characteristics of the Bone Health Subcohort at Baseline According to Randomized Assignment to Vitamin D3 Versus Placebo Groups
| Characteristic | Total (N = 771) | Vitamin D3 group (n = 388) | Placebo group (n = 383) | p Value |
|---|---|---|---|---|
| Female sex, n (%), N = 771 | 360 (46.7%) | 179 (46.1%) | 181 (47.3%) | 0.76 |
| Age (years), mean (SD), N = 771 | 63.8 (6.1) | 63.7 (6.0) | 63.9 (6.3) | 0.53 |
| Race or ethnic group,a n (%) | 755 (97.9%) | 0.28 | ||
| Non‐Hispanic white | 630 (83.4%) | 317 (82.8%) | 313 (84.1%) | |
| Black | 67 (8.9%) | 35 (9.1%) | 32 (8.6%) | |
| Nonblack Hispanic | 26 (3.4%) | 11 (2.9%) | 15 (4.0%) | |
| Asian | 15 (2.0%) | 9 (2.4%) | 6 (1.6%) | |
| Native American or Alaskan native | 5 (0.7%) | 2 (0.5%) | 3 (0.8%) | |
| Other or unknown | 12 (1.6%) | 9 (2.4%) | 3 (0.8%) | |
| Body mass index (kg/m2), mean (SD), N = 771 | 27.2 (4.8) | 27.2 (4.7) | 27.3 (4.8) | 0.91 |
| Fat mass index(kg/m2), mean (SD), N = 767 | 10.27 (3.89) | 10.26 (4.03) | 10.28 (3.74) | 0.94 |
| Leisure time physical activity (hr/wk), median (interquartile range) MET, N = 767 | 21.47 (7.86–37.11) | 21.61 (7.86–37.80) | 20.99 (7.97–36.00) | 0.62 |
| Diabetes history, n (%), N = 770 | 84 (10.9%) | 44 (11.4%) | 40 (10.4%) | 0.68 |
| Current smoking, n (%), N = 766 | 48 (6.3%) | 26 (6.8%) | 22 (5.8%) | 0.33 |
| Any fracture history,b n (%), N = 771 | 61 (7.9%) | 32 (8.3%) | 29 (7.6%) | 0.73 |
| Parental history of hip fracture, n (%), N = 733 | 102 (13.9%) | 54 (14.8%) | 48 (13.0%) | 0.49 |
| Baseline calcium supplement use,c n (%), N = 771 | 132 (17.1%) | 69 (17.8%) | 63 (16.5%) | 0.62 |
| Baseline vitamin D supplement use,c n (%), N = 771 | 326 (42.3%) | 157 (40.5%) | 169 (44.1%) | 0.30 |
| Baseline total 25(OH)D (nmol/L),d mean (SD), N = 770 | 69.1 (22.7) | 67.4 (22.2) | 71.1 (23.2) | 0.025 |
| Baseline free 25(OH)D (pmol/L), mean (SD), N = 770 | 14.6 (4.7) | 14.4 (4.5) | 14.8 (4.8) | 0.21 |
Race and ethnic groups self‐reported by participants.
Of those who reported fractures, 16 had a history of a fragility fracture (hip, spine, shoulder, and/or forearm fracture).
Calcium supplement intake ≤1200 mg/d; vitamin D intake ≤800 IU/d.
To convert values of 25(OH)D to ng/mL, multiply by 0.4.
Compared with the overall VITAL cohort of 25,871 participants,39 the bone health subcohort was slightly younger (mean age 63.8 versus 67.1 years) and healthier with fewer participants with obesity, hypertension, or diabetes. Although the overall cohort included an oversampling of black participants (20.2%), only 8.9% of the bone health subcohort was black given regional demographics of New England.41
At baseline, the mean serum total 25(OH)D level was 69.1 nmol/L, and 18.0% of participants had total 25(OH)D levels <50 nmol/L (n = 770). In the vitamin D3 group, mean total 25(OH)D levels increased by 46.2% to 98.6 nmol/L (n = 359). The total 25(OH)D level in the placebo group was similar at baseline and 2‐year follow‐up (71.1 nmol/L and 70.6 nmol/L, respectively; n = 354). Mean FVD level was 14.6 pmol/L at baseline (n = 770). FVD increased by 55.5% to 22.3 pmol/L at year 2 in the vitamin D3 group (n = 359). Calcium levels did not change in either group between baseline and year 2 (p = 0.27).
There were no increased incidences of hypercalcemia, kidney stones, or other adverse effects in the vitamin D3 versus placebo groups.39
Primary outcome: aBMD measures
Daily supplemental vitamin D3 did not increase aBMD or reduce bone loss at the spine, femoral neck, total hip, or whole body compared with placebo (Fig. 2; Supplemental Table S1). Overall, 2‐year changes in aBMD at all sites were minimal at <1%.
Figure 2.
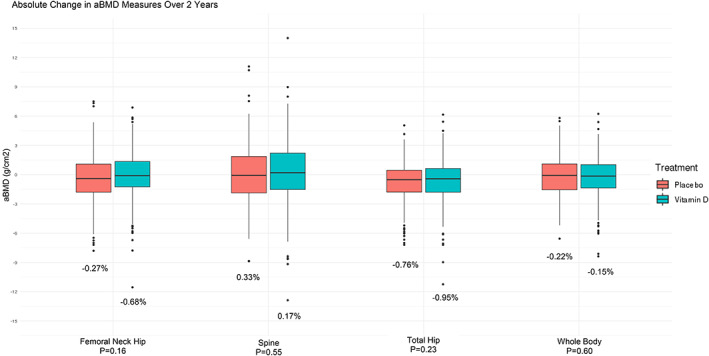
Mean absolute changes in areal bone mineral density (aBMD) from baseline to 2 years in the vitamin D3 and placebo groups. Percentages represent the percent change in aBMD over 2 years. All analyses adjusted for age, sex, and race.
Secondary outcomes: pQCT measures
There were no effects of daily supplemental vitamin D3 on pQCT outcomes at the radius or tibia. Changes in bone structure (total, cortical, and trabecular vBMD, cortical thickness) and bone strength indices (polar stress strength index, bone strength index) were similar for the vitamin D3 and placebo groups (Table 3).
Table 3.
Two‐Year Changes in pQCT Measurements
| Vitamin D3 group | Placebo group | ||||
|---|---|---|---|---|---|
| pQCT measurements | n | Mean (SD) | n | Mean (SD) | p Value |
| Radius | |||||
| Total vBMD (mg/cm3) | |||||
| Baseline | 326 | 369.678 (69.484) | 321 | 373.903 (72.498) | |
| Year 2 | 282 | 376.236 (74.480) | 278 | 382.252 (71.676) | |
| % Change | 277 | 2.16% | 269 | 1.23% | 0.31 |
| Trabecular vBMD (mg/cm3) | |||||
| Baseline | 326 | 196.447 (43.156) | 321 | 202.796 (46.074) | |
| Year 2 | 282 | 199.563 (44.647) | 278 | 205.112 (46.019) | |
| % Change | 277 | 0.59% | 269 | −0.21% | 0.099 |
| Bone strength index (mg*mm) | |||||
| Baseline | 326 | 46.429 (19.223) | 321 | 46.160 (18.378) | |
| Year 2 | 282 | 47.066 (19.627) | 278 | 47.722 (18.932) | |
| % Change | 277 | 1.48% | 269 | 0.76% | 0.44 |
| Cortical vBMD (mg/cm3) | |||||
| Baseline | 311 | 1197.75 (31.572) | 308 | 1196.98 (30.816) | |
| Year 2 | 258 | 1199.41 (33.686) | 257 | 1201.55 (30.098) | |
| % Change | 246 | 0.16% | 239 | 0.22% | 0.49 |
| Cortical thickness (mm) | |||||
| Baseline | 311 | 3.271 (0.584) | 308 | 3.221 (0.595) | |
| Year 2 | 258 | 3.248 (0.605) | 257 | 3.200 (0.603) | |
| % Change | 246 | −1.59% | 239 | −1.64% | 0.997 |
| Polar stress strength index (mm3) | |||||
| Baseline | 311 | 291.559 (98.278) | 308 | 277.702 (91.069) | |
| Year 2 | 258 | 297.382 (103.180) | 257 | 280.804 (94.408) | |
| % Change | 246 | 0.23% | 239 | 0.18% | 0.94 |
| Tibia | |||||
| Total vBMD (mg/cm3) | |||||
| Baseline | 331 | 295.291 (48.045) | 330 | 299.232 (49.285) | |
| Year 2 | 294 | 296.756 (49.312) | 284 | 303.213 (48.755) | |
| % Change | 291 | 0.05% | 283 | 0.26% | 0.30 |
| Trabecular vBMD (mg/cm3) | |||||
| Baseline | 331 | 246.626 (40.741) | 330 | 250.139 (42.283) | |
| Year 2 | 294 | 249.300 (41.539) | 284 | 254.067 (42.019) | |
| % Change | 291 | 0.47% | 283 | 0.42% | 0.76 |
| Bone strength index (mg*mm) | |||||
| Baseline | 331 | 105.338 (38.119) | 330 | 106.355 (38.763) | |
| Year 2 | 294 | 106.951 (39.314) | 284 | 109.583 (39.621) | |
| % Change | 291 | 0.26% | 283 | 0.48% | 0.56 |
| Cortical vBMD (mg/cm3) | |||||
| Baseline | 322 | 1167.39 (32.258) | 329 | 1162.02 (30.375) | |
| Year 2 | 294 | 1169.98 (32.739) | 285 | 1166.38 (30.624) | |
| % Change | 283 | 0.24% | 285 | 0.25% |
0.92 |
| Cortical thickness (mm) | |||||
| Baseline | 322 | 5.670 (0.881) | 329 | 5.648 (0.907) | |
| Year 2 | 294 | 5.645 (0.914) | 285 | 5.659 (0.905) | |
| % Change | 283 | −0.74% | 285 | −0.56% | 0.24 |
| Polar stress strength index (mm3) | |||||
| Baseline | 322 | 1922.60 (542.988) | 329 | 1867.05 (520.903) | |
| Year 2 | 294 | 1948.58 (557.398) | 285 | 1904.76 (529.863) | |
| % Change | 283 | 0.42% | 285 | 0.41% | 0.65 |
All analyses adjusted for age, sex, and race.
Subgroup analyses of aBMD on primary outcomes
Subgroup analyses are presented in Table 2 and the Supplemental Tables S2. The effect of vitamin D3 supplementation versus placebo on 2‐year changes in aBMD at all sites did not significantly differ by race/ethnicity, BMI, FMI, or baseline use of supplemental vitamin D (≤800 IU/d). In prespecified analyses, when stratified by sex, vitamin D3 supplementation versus placebo in women resulted in a trend for smaller decreases in aBMD at the spine (p = 0.062; p for interaction = 0.067). In exploratory analyses, we did not find any significant differences in response to vitamin D3 supplementation, compared with placebo, in those with osteopenia or osteoporosis versus those with normal aBMD. In participants taking calcium supplements (≤1200 IU/d) at baseline, there was attenuation of femoral neck aBMD loss with vitamin D3 supplementation versus placebo (p = 0.029); however, there was no significant interaction (p = 0.10; Supplemental Table S2).
Table 2.
Absolute 2‐Year Change in aBMD According to Subgroup, Comparing the Vitamin D3 Group With the Placebo Group
| Subgroup | Spine aBMD | |||||
|---|---|---|---|---|---|---|
| Vitamin D3 group | Placebo group | p Value | p Value for interaction | |||
| n | Absolute change (SD) g/cm2 | n | Absolute change (SD) g/cm2 | |||
| Sex | 0.067 | |||||
| Female | 149 | −0.001 (0.036) | 133 | −0.008 (0.033) | 0.062 | |
| Male | 177 | 0.007 (0.036) | 174 | 0.010 (0.035) | 0.46 | |
| Low bone density | 0.040 | |||||
| Normal | 110 | 0.002 (0.038) | 114 | 0.009 (0.039) | 0.17 | |
| Osteopenia/osteoporosis | 209 | 0.003 (0.035) | 192 | −0.003 (0.032) | 0.067 | |
| Race | 0.83 | |||||
| Non‐Hispanic white | 267 | 0.003 (0.036) | 248 | 0.002 (0.036) | 0.79 | |
| Black | 28 | 0.004 (0.044) | 28 | 0.005 (0.035) | 0.89 | |
| Body mass index (median) | 0.13 | |||||
| <Median (26.45 kg/m2) | 165 | 0.000 (0.038) | 152 | −0.006 (0.032) | 0.12 | |
| ≥Median (26.45 kg/m2) | 161 | 0.006 (0.034) | 155 | 0.009 (0.037) | 0.52 | |
| Fat mass index | 0.90 | |||||
| <Median (9.42 kg/m2) | 175 | 0.002 (0.036) | 149 | −0.001 (0.033) | 0.50 | |
| ≥Median (9.42 kg/m2) | 149 | 0.006 (0.038) | 158 | 0.004 (0.037) | 0.74 | |
| Vitamin D supplement use at baseline ≤800 IU/d | 0.88 | |||||
| Yes | 134 | 0.003 (0.031) | 138 | 0.000 (0.034) | 0.56 | |
| No | 192 | 0.004 (0.040) | 169 | 0.003 (0.037) | 0.76 | |
| Calcium supplement use at baseline ≤1200 mg/d | 0.40 | |||||
| Yes | 58 | 0.001 (0.033) | 51 | −0.007 (0.033) | 0.28 | |
| No | 268 | 0.004 (0.037) | 256 | 0.003 (0.035) | 0.83 | |
| Baseline total 25(OH)D Level | 0.09 | |||||
| <75 nmol/L | 209 | 0.006 (0.037) | 165 | 0.000 (0.037) | 0.10 | |
| ≥75 nmol/L | 116 | −0.001 (0.036) | 142 | 0.004 (0.034) | 0.24 | |
| Baseline total 25(OH)D Level | 0.061 | |||||
| <Median (70 nmol/L) | 179 | 0.006 (0.038) | 138 | −0.001 (0.035) | 0.066 | |
| ≥Median (70 nmol/L) | 146 | 0.001 (0.035) | 169 | 0.004 (0.035) | 0.30 | |
| Baseline total 25(OH)D Level | 0.42 | |||||
| <50 nmol/L | 60 | 0.002 (0.040) | 57 | 0.005 (0.036) | 0.76 | |
| ≥50 nmol/L | 265 | 0.004 (0.036) | 250 | 0.001 (0.035) | 0.39 | |
| Baseline total 25(OH)D Level | 0.92 | |||||
| <37 nmol/L | 29 | 0.005 (0.040) | 20 | 0.005 (0.045) | 0.77 | |
| ≥37 nmol/L | 296 | 0.003 (0.036) | 287 | 0.002 (0.035) | 0.59 | |
| Baseline total 25(OH)D Level | 0.14 | |||||
| <30 nmol/L | 12 | −0.014 (0.052) | 10 | 0.017 (0.051) | 0.17 | |
| ≥30 nmol/L | 313 | 0.004 (0.036) | 297 | 0.001 (0.035) | 0.30 | |
| Baseline free 25(OH)D | 0.026 | |||||
| <Median 14.2 pmol/L | 171 | 0.008 (0.040) | 149 | −0.000 (0.034) | 0.043 | |
| ≥Median 14.2 pmol/L | 154 | −0.001 (0.032) | 158 | 0.003 (0.036) | 0.170 | |
| Subgroup | Total hip aBMD | |||||
|---|---|---|---|---|---|---|
| Vitamin D3 group | Placebo group | LS mean, (95% CI), p‐value | P Value for interaction | |||
| n | Absolute change (SD) g/cm2 | n | Absolute change (SD) g/cm2 | |||
| Sex | 0.48 | |||||
| Female | 152 | −0.012 (0.024) | 150 | −0.016 (0.022) | 0.20 | |
| Male | 188 | −0.003 (0.023) | 189 | −0.004 (0.020) | 0.684 | |
| Low bone density | 0.32 | |||||
| Normal | 112 | −0.003 (0.025) | 118 | −0.007 (0.021) | 0.12 | |
| Osteopenia/osteoporosis | 214 | −0.009 (0.023) | 206 | −0.010 (0.022) | 0.73 | |
| Race | 0.81 | |||||
| Non‐Hispanic white | 282 | −0.008 (0.024) | 277 | −0.009 (0.021) | 0.44 | |
| Black | 27 | −0.003 (0.022) | 28 | −0.006 (0.018) | 0.56 | |
| Body mass index (median) | 0.31 | |||||
| <Median (26.45 kg/m2) | 173 | −0.007 (0.020) | 166 | −0.007 (0.018) | 0.96 | |
| ≥Median (26.45 kg/m2) | 167 | −0.007 (0.027) | 173 | −0.010 (0.024) | 0.13 | |
| Fat mass index | 0.52 | |||||
| <Median (9.42 kg/m2) | 183 | −0.005 (0.021) | 161 | −0.006 (0.019) | 0.84 | |
| ≥Median (9.42 kg/m2) | 154 | −0.009 (0.027) | 178 | −0.012 (0.023) | 0.19 | |
| Vitamin D supplement use at baseline ≤800 IU/d | 0.79 | |||||
| Yes | 141 | −0.008 (0.024) | 148 | −0.009 (0.020) | 0.67 | |
| No | 199 | −0.007 (0.024) | 191 | −0.009 (0.022) | 0.24 | |
| Calcium supplement use at baseline ≤1200 mg/d | 0.73 | |||||
| Yes | 59 | −0.009 (0.027) | 54 | −0.012 (0.019) | 0.46 | |
| No | 281 | −0.007 (0.023) | 285 | −0.008 (0.022) | 0.32 | |
| Baseline total 25(OH)D level | 0.09 | |||||
| <75 nmol/L | 216 | −0.005 (0.026) | 183 | −0.009 (0.023) | 0.09 | |
| ≥75 nmol/L | 123 | −0.010 (0.020) | 156 | −0.008 (0.020) | 0.59 | |
| Baseline total 25(OH)D level | 0.064 | |||||
| <Median (70 nmol/L) | 185 | −0.005 (0.025) | 148 | −0.010 (0.022) | 0.065 | |
| ≥Median (70 nmol/L) | 154 | −0.010 (0.022) | 191 | −0.008 (0.021) | 0.68 | |
| Baseline total 25(OH)D level | 0.18 | |||||
| <50 nmol/L | 62 | −0.003 (0.029) | 60 | −0.011 (0.022) | 0.12 | |
| ≥50 nmol/L | 277 | −0.008 (0.023) | 279 | −0.008 (0.021) | 0.61 | |
| Baseline total 25(OH)D level | 0.12 | |||||
| <37 nmol/L | 30 | 0.000 (0.028) | 20 | −0.013 (0.024) | 0.096 | |
| ≥37 nmol/L | 309 | −0.008 (0.024) | 319 | −0.009 (0.021) | 0.49 | |
| Baseline total 25(OH)D level | 0.36 | |||||
| <30 nmol/L | 13 | 0.002 (0.026) | 10 | −0.010 (0.028) | 0.29 | |
| ≥30 nmol/L | 326 | −0.007 (0.024) | 329 | −0.009 (0.021) | 0.33 | |
| Baseline free 25(OH)D | 0.047 | |||||
| <Median 14.2 pmol/L | 175 | −0.004 (0.024) | 159 | −0.009 (0.023) | 0.044 | |
| ≥Median 14.2 pmol/L | 164 | −0.010 (0.024) | 180 | −0.008 (0.020) | 0.62 | |
All analyses adjusted for age, sex, and race.
The vitamin D3 intervention had a slight benefit on spine and total hip aBMD among participants with baseline FVD levels below the median (14.2 pmol/L; prespecified) with significant interaction at both sites (p = 0.026 and 0.047, respectively). There were small increases in spine aBMD (0.75% versus 0.00%; p = 0.043) and smaller decreases in total hip aBMD (−0.42% versus −0.98%; p =0.044) with vitamin D3 compared with placebo in those with low FVD. In participants with baseline total 25(OH)D levels below the median (69.9 nmol/L; prespecified), there was a trend for greater attenuation of aBMD loss at the spine (p = 0.066) and total hip (p = 0.065) with vitamin D3 supplementation versus placebo. Using thresholds that were not prespecified (<75, <50, <37, or <30 nmol/L), there were no differences in changes in aBMD between the vitamin D3 and placebo groups (Table 2). Only 24 participants had 25(OH)D levels <30 nmol/L.
In exploratory analyses (Supplemental Tables S3 and S4), among participants in the vitamin D3 group, there was no beneficial effect on aBMD at any site between those who achieved 25(OH)D levels above versus below the median (97.3 nmol/L) or FVD levels above or below the median (21.3 pmol/L) at 2 years.
Discussion
Supplemental vitamin D3 (2000 IU/d for 2 years) without calcium, compared with placebo, did not significantly benefit bone density or structure in this large VITAL ancillary study. In contrast to our hypotheses, supplemental vitamin D3 did not increase aBMD or prevent bone loss at the spine, hip, or whole body. Vitamin D3 also did not improve or adversely affect total, trabecular, or cortical vBMD, cortical thickness, or bone strength at the radius or tibia compared with placebo. These effects were not modified by baseline BMI, FMI, age, race/ethnicity, or personal use of vitamin D supplements.
This VITAL ancillary study makes significant contributions to the literature as it is different from prior studies. This is the largest randomized, placebo‐controlled study that assessed effects of daily, supplemental vitamin D on bone density and structure in the general US population unselected for vitamin D insufficiency, and it is the first large RCT that measured FVD levels at baseline and 2 years of follow‐up.
RCTs of daily supplemental vitamin D on aBMD in the general population have shown either no benefits of vitamin D on aBMD or small improvements that have been interpreted as not clinically meaningful, consistent with our findings from our placebo‐controlled VITAL ancillary study. A meta‐analysis of RCTs found minimal differences (range 0.16% to 0.76%) in aBMD between vitamin D and placebo groups at the spine, total hip, and femoral neck with no differences at the whole body.15 Another meta‐analysis also did not support vitamin D supplementation for primary prevention of osteoporosis in healthy adults.14 In the New Zealand Vitamin D Assessment (ViDA) trial, bolus vitamin D supplementation of 100,000 IU/mo versus placebo for 2 years in community‐dwelling older adults (baseline 25(OH)D ~55 nmol/L) attenuated bone loss at the hip by 0.5%.17 In a 1‐year RCT among postmenopausal women in Scotland (baseline 25(OH)D 33.7 nmol/L), 1000 IU/d, but not 400 IU/d, of vitamin D prevented bone loss of ~0.6% at the hip but not at the spine.44 However, two additional studies found no effect of vitamin D supplements on aBMD.20, 45
A larger benefit may be found in those with high fracture risk. Although we found no benefit of vitamin D3 supplementation in participants with osteopenia or osteoporosis, most were mildly osteopenic. Jennings and colleagues showed that vitamin D3 supplementation (400 IU/d) had no effect on aBMD but, in exploratory analyses, attenuated femoral neck bone loss only in those with osteoporosis.46 In a UK study in elderly women after osteoporotic hip fractures, vitamin D3 supplementation versus placebo also improved aBMD at the femoral neck by 1.1% to 3.3% and at the total hip by 2.1% to 4.6%.47 In a RCT in the Netherlands of 348 elderly women at high fracture risk (mean age 80 years; baseline 25(OH)D ~26.0 nmol/L), 400 IU/d of vitamin D3 versus placebo improved femoral neck aBMD by 1.9% over 2 years.16, 48
There may be a total 25(OH)D threshold below which vitamin D supplementation benefits bone health, but this level is debated. While the Institute of Medicine recommends 25(OH)D levels ≥50 nmol/L for 97.5% of the population and suggests <30 nmol/L as deficient,49 the Endocrine Society and National Osteoporosis Foundation have recommended 25(OH)D levels >75 nmol/L and define 52 to 75 nmol/L as insufficient, particularly in those with osteoporosis.50, 51, 52, 53, 54 Total 25(OH)D levels <25 nmol/L are associated with osteomalacia, reduced bone mineralization, low aBMD, and secondary hyperparathyroidism.3, 4 The ViDA trial found that among participants with low baseline 25(OH)D ≤30 nmol/L (n = 25), the placebo group had significant spine and femoral neck aBMD loss (~2%), compared with stable aBMD in the vitamin D3 group; there was no difference in aBMD in participants with baseline 25(OH)D ≥30 nmol/L.17 Post hoc analyses of a UK trial in those with baseline total 25(OH)D levels ≤30 nmol/L, vitamin D supplements had a small treatment effect on spine and hip aBMD (0.6%).44 In contrast to the studies in New Zealand and the UK, in our VITAL ancillary study, we were unable to identify a vitamin D threshold for bone health using baseline total 25(OH)D levels of <30, <50, or <75 nmol/L. It is possible that our participants may have already reached the vitamin D level needed for bone health. The mean baseline 25(OH)D level of participants in this VITAL ancillary study was 69.1 nmol/L, 18.0% had 25(OH)D levels <50 nmol/L, and 3.1% <30 nmol/L (n = 25). This is consistent with recent US National Health and Nutrition Examination Survey data (2011–2014) showing that 2.9% of the population ≥60 years have 25(OH)D levels <30 nmol/L.29 Because a small percentage of older US adults have profound vitamin D deficiency,29 it would be neither ethical nor feasible to perform a supplemental vitamin D3 placebo‐controlled study in this population.
There is limited research investigating the relationship between FVD levels and effects of supplemental vitamin D on aBMD. In a vitamin D dose‐ranging study in 273 older women in the US with low baseline total 25(OH)D levels ≤50 nmol/L, aBMD changes were not associated with baseline total 25(OH)D or FVD levels; however, the study was not powered to detect changes in aBMD with each of the seven tested vitamin D doses.55 We found that baseline FVD levels, compared with total 25(OH)D levels, may better predict improvements in aBMD at the spine and total hip, though changes were small (0.56% to 0.75%). Whether baseline FVD levels help to identify those more likely to benefit from supplementation warrants further study. Given multiple comparisons, these results should be interpreted with caution.
This VITAL ancillary study evaluated the surrogate mechanisms through which supplemental vitamin D affects bone health and potential fracture risk. Other factors such as poor physical performance or balance, certain medical conditions (poor vision, cognitive impairment, neurological diseases, hypotension, diabetes, among others) or other intrinsic and environmental factors that contribute to falls can impact the risk of fractures.56, 57 In a parallel ancillary study, we are adjudicating incident fractures in the overall VITAL cohort (n = 25,871) for a median of 5.3 years to determine whether long‐term vitamin D3 supplementation reduces fracture risk in men and women nationwide.
This ancillary study to VITAL has many strengths, including being the largest RCT of supplemental vitamin D3 on aBMD at the spine and hip, and vBMD, structure, and strength measures at the radius and tibia. This study had high retention (89%) and adherence (~92%) and power to detect small effects on aBMD, the primary outcome. This study also evaluated effects of vitamin D3 supplementation versus placebo on bone health measures and analyzed outcomes according to FVD levels. Additionally, the vitamin D assays were calibrated to CDC standards. There were also limitations. The timeline for the bone density and structure outcomes was limited to 2 years of follow‐up. The results were not adjusted for multiple hypothesis testing, so the findings from the secondary and subgroup analyses should be interpreted as exploratory. These results do not generally apply to younger individuals or adults with osteoporosis or those with profound vitamin D deficiency, who otherwise warrant treatment.
In summary, this placebo‐controlled RCT found that daily vitamin D3 supplementation for 2 years did not improve bone density or structure in the general population of older adults in the US not selected for vitamin D insufficiency.
Disclosures
MSL, NRC, I‐ML, and VB report grants from the National Institutes of Health (NIH) during the conduct of the study. SM reports grants from NIH (DK112940, R01HL134811, and K24 HL136852) and non‐financial institutional support from Quest Diagnostics during the conduct of the study, as well as personal fees (modest) from Quest Diagnostics and an institutional research grant from Atherotech Diagnostics, outside the submitted work. JEB reports grants from NIH; non‐financial support from Pharmavite LLC of Northridge, CA, USA; non‐financial support from Pronova BioPharma of Norway and BASF, and non‐financial support from Quest Diagnostics (San Juan Capistrano, CA, USA) during the conduct of the study; other from Pharmavite, outside the submitted work. JEM reports grants from NIH, non‐financial support from Pharmavite LLC of Northridge, CA, USA; non‐financial support from Pronova BioPharma of Norway and BASF; and non‐financial support from Quest Diagnostics (San Juan Capistrano, CA, USA) during the conduct of the study. All other authors state that they have no conflicts of interest.
Supporting information
Supplemental Materials and Methods. Supplemental Fig. S1. Mean baseline and year 2 serum 25(OH)D levels (nmol/L) by demographic variables (sex, age, race/ethnicity) and body mass index (BMI) in the bone health subcohort (placebo group is not graphed: <2.5 nmol/L change at year 2).
Supplemental Table S1. aBMD From Baseline to Year 2
Supplemental Table S2. Absolute 2‐Year Change in aBMD According to Subgroup, Comparing the Vitamin D3 Group With the Placebo Group
Supplemental Table S3. Two‐Year Achieved Total 25(OH)D Levels in the Vitamin D3 Group
Supplemental Table S4. Two‐Year Achieved Free Vitamin D Levels in the Vitamin D3 Group
Supplemental Table S5. Participant‐Reported Adherence With the Vitamin D and Placebo Study Pills (Percent of Pills Taken) at Time Points Over 2 Years
Acknowledgments
We thank the trial participants, staff, and investigators. This project was supported by grants R01 AR059775, R01 AR070854, and R01 AR060574 (PI, MSL) from National Institute of Arthritis Musculoskeletal and Skin Diseases (NIAMS/NIH). This research was also supported by the VITAL parent grants and U01 CA138962 and R01 CA138962 (PIs, JEM and JEB) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH. This work was conducted with support from the Harvard Catalyst CTSC (NCRR and NCATS, NIH Award UL1TR001102) and the Office of Dietary Supplements.
We also acknowledge Cindy Yu for her precision and dedication in performing bone density and body composition scans, and Daniel Schiferl for his expertise on pQCT measurements and analysis. This study and the parent study are registered at clinicaltrials.gov (NCT01747447, NCT01169259). We also thank Quest Diagnostics, which performed the 25(OH)D, calcium, and omega‐3 laboratory measurements at no additional costs to the VITAL study. Pharmavite LLC of Northridge, CA, USA (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. We also thank the Centers for Disease Control and Prevention (Dr Hubert Vesper and Dr Julianne Cook Botelho) for their collaboration on the standardization and calibration of the 25(OH)D measurements throughout the study.
Authors’ roles: MSL had roles in conception and design, obtaining funding, data collection, analysis and interpretation, writing the article, critical revision of the article, and final approval of the article. SHC had roles in data collection, analysis and interpretation, writing the article, and final approval of the article. EMM had roles in data collection, writing the article, and final approval of the article. CMD had roles in data collection, writing the article, and final approval of the article. NRC had roles in conception and design, statistical expertise, analysis and interpretation, contributing to writing, critical revision of the article, and final approval of the article. SM had roles in provision of materials, critical revision of the article, final approval of the article. I‐ML had roles in conception and design, data collection, analysis and interpretation, and final approval of the article. GK and VB had roles in analysis and interpretation and statistical expertise and final approval of the article. JEB and JEM had roles in conception and design, obtaining funding, data collection, analysis and interpretation, critical revision of the article, final approval of the article.
References
- 1. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999‐2012. JAMA. 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demay MB. Physiological insights from the vitamin D receptor knockout mouse. Calcif Tissue Int. 2013;92(2):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El‐Desouki MI, Othman SM, Fouda MA. Bone mineral density and bone scintigraphy in adult Saudi female patients with osteomalacia. Saudi Med J. 2004;25(3):355–8. [PubMed] [Google Scholar]
- 4. Parfitt AM, Qiu S, Rao DS. The mineralization index—a new approach to the histomorphometric appraisal of osteomalacia. Bone. 2004;35(1):320–5. [DOI] [PubMed] [Google Scholar]
- 5. Geng S, Zhou S, Glowacki J. Effects of 25‐hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1alpha‐hydroxylase. J Bone Miner Res. 2011;26(5):1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. [DOI] [PubMed] [Google Scholar]
- 7. Cranney A, Horsley T, O'Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007. Aug;(158):1–235. [PMC free article] [PubMed] [Google Scholar]
- 8. Bischoff‐Ferrari HA, Dietrich T, Orav EJ, Dawson‐Hughes B. Positive association between 25‐hydroxy vitamin D levels and bone mineral density: a population‐based study of younger and older adults. Am J Med. 2004;116(9):634–9. [DOI] [PubMed] [Google Scholar]
- 9. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009. Aug;(183):1–420. [PMC free article] [PubMed] [Google Scholar]
- 10. Frost M, Abrahamsen B, Nielsen TL, Hagen C, Andersen M, Brixen K. Vitamin D status and PTH in young men: a cross‐sectional study on associations with bone mineral density, body composition and glucose metabolism. Clin Endocrinol. 2010;73(5):573–80. [DOI] [PubMed] [Google Scholar]
- 11. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta‐analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–38. [DOI] [PubMed] [Google Scholar]
- 12. Newberry SJ, Chung M, Shekelle PG, et al. Vitamin D and calcium: a systematic review of health outcomes (update). Evid Rep Technol Assess (Full Rep). Sep 2014;(217):1–929. [DOI] [PubMed] [Google Scholar]
- 13. Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta‐analysis. Lancet. 2014;383(9912):146–55. [DOI] [PubMed] [Google Scholar]
- 15. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta‐analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–58. [DOI] [PubMed] [Google Scholar]
- 16. Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double‐blind trial. J Clin Endocrinol Metab. 1995;80(4):1052–8. [DOI] [PubMed] [Google Scholar]
- 17. Reid IR, Horne AM, Mihov B, et al. Effect of monthly high‐dose vitamin D on bone density in community‐dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017;282(5):452–60. [DOI] [PubMed] [Google Scholar]
- 18. Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25‐Hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled. trial. J Bone Miner Res. 2018;33(8):1464–9. [DOI] [PubMed] [Google Scholar]
- 19. Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen KE, Johnson RE, Chambers KR, et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern Med. 2015;175(10):1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wamberg L, Pedersen SB, Richelsen B, Rejnmark L. The effect of high‐dose vitamin D supplementation on calciotropic hormones and bone mineral density in obese subjects with low levels of circulating 25‐hydroxyvitamin D: results from a randomized controlled study. Calcif Tissue Int. 2013;93(1):69–77. [DOI] [PubMed] [Google Scholar]
- 22. Bislev LS, Langagergaard Rodbro L, Rolighed L, Sikjaer T, Rejnmark L. Bone microstructure in response to vitamin D3 supplementation: a randomized placebo‐controlled trial. Calcif Tissue Int. 2019;104(2):160. [DOI] [PubMed] [Google Scholar]
- 23. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. [DOI] [PubMed] [Google Scholar]
- 24. Dawson‐Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–6. [DOI] [PubMed] [Google Scholar]
- 25. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–42. [DOI] [PubMed] [Google Scholar]
- 26. Callegari ET, Garland SM, Gorelik A, Wark JD. Determinants of bone mineral density in young Australian women; results from the Safe‐D study. Osteoporos Int. 2017;28(9):2619–31. [DOI] [PubMed] [Google Scholar]
- 27. Martin EN, Haney EM, Shannon J, et al. Femoral volumetric bone density, geometry, and strength in relation to 25‐hydroxy vitamin D in older men. J Bone Miner Res. 2015;30(3):562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high‐dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322(8):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. 2019;110(1):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alzaman NS, Dawson‐Hughes B, Nelson J, D'Alessio D, Pittas AG. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr. 2016;104(1):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 32. Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–43. [DOI] [PubMed] [Google Scholar]
- 33. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D‐binding protein modifies the vitamin D‐bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jemielita TO , Leonard MB, Baker J, et al. Association of 25‐hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: impact of vitamin D‐binding protein and its assays. Osteoporos Int. 2016;27(2):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bikle D, Bouillon R, Thadhani R, Schoenmakers I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J Steroid Biochem Mol Biol. 2017;173:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health . In Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US) National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 39. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA‐3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Donlon CM, LeBoff MS, Chou SH, et al. Baseline characteristics of participants in the VITamin D and OmegA‐3 TriaL (VITAL): effects on bone structure and architecture. Contemp Clin Trials. 2018;67:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL‐bone health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega‐3 fatty acid supplements on incident fractures and bone health outcomes in the Vitamin D and OmegA‐3 TriaL (VITAL). Contemp Clin Trials. 2015;41C:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rinaldi G, Wisniewski CA, Setty NG, Leboff MS. Peripheral quantitative computed tomography: optimization of reproducibility measures of bone density, geometry, and strength at the radius and tibia. J Clin Densitom. 2011;14(3):367–73. [DOI] [PubMed] [Google Scholar]
- 44. Macdonald HM, Wood AD, Aucott LS, et al. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1‐year double‐blind RCT in postmenopausal women. J Bone Miner Res. 2013;28(10):2202–13. [DOI] [PubMed] [Google Scholar]
- 45. Iuliano‐Burns S, Ayton J, Hillam S, et al. Skeletal and hormonal responses to vitamin D supplementation during sunlight deprivation in Antarctic expeditioners. Osteoporos Int. 2012;23(10):2461–7. [DOI] [PubMed] [Google Scholar]
- 46. Jennings A, Cashman KD, Gillings R, et al. A Mediterranean‐like dietary pattern with vitamin D3 (10 μg/d) supplements reduced the rate of bone loss in older Europeans with osteoporosis at baseline: results of a 1‐y randomized controlled trial. Am J Clin Nutr. 2018;108(3):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham Neck of Femur (NONOF) Study. Age Ageing. 2004;33(1):45–51. [DOI] [PubMed] [Google Scholar]
- 48. Lips P, Graafmans W, Ooms M, Bezemer P, Bouter L, supplementation VD. fracture incidence in elderly persons: a randomized, placebo‐controlled clinical trial. Ann Intern Med. 1996;124(4):400–6. [DOI] [PubMed] [Google Scholar]
- 49. Institute of Medicine . In Ross AC, Taylor CL, Yaktine AL, Del Valle HB e, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 50. Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for North America: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom Rev. 2011;14(2):79–84. [DOI] [PubMed] [Google Scholar]
- 51. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 52. Dawson‐Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–4. [DOI] [PubMed] [Google Scholar]
- 53. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54. [DOI] [PubMed] [Google Scholar]
- 55. Smith LM, Gallagher JC, Kaufmann M, Jones G. Effect of increasing doses of vitamin D on bone mineral density and serum N‐terminal telopeptide in elderly women: a randomized controlled trial. J Intern Med. 2018;284(6):685–93. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Take a stand on falls. 2017.
- 57. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Supplemental Fig. S1. Mean baseline and year 2 serum 25(OH)D levels (nmol/L) by demographic variables (sex, age, race/ethnicity) and body mass index (BMI) in the bone health subcohort (placebo group is not graphed: <2.5 nmol/L change at year 2).
Supplemental Table S1. aBMD From Baseline to Year 2
Supplemental Table S2. Absolute 2‐Year Change in aBMD According to Subgroup, Comparing the Vitamin D3 Group With the Placebo Group
Supplemental Table S3. Two‐Year Achieved Total 25(OH)D Levels in the Vitamin D3 Group
Supplemental Table S4. Two‐Year Achieved Free Vitamin D Levels in the Vitamin D3 Group
Supplemental Table S5. Participant‐Reported Adherence With the Vitamin D and Placebo Study Pills (Percent of Pills Taken) at Time Points Over 2 Years


