Abstract
Objective:
Resistance to chemo-radiation therapy is a substantial obstacle that compromises treatment of advanced cervical cancer. The objective of this study was to investigate if a proteomic panel associated with radioresistance could predict survival of patients with locally advanced cervical cancer.
Methods:
A total of 181 frozen tissue samples were prospectively obtained from patients with locally advanced cervical cancer before chemoradiation. Expression levels of 22 total and phosphorylated proteins were evaluated using well-based reverse phase protein arrays. Selected proteins were validated with western blotting analysis and immunohistochemistry. Performances of models were internally and externally validated.
Results:
Unsupervised clustering stratified patients into three major groups with different overall survival (OS, P = 0.001) and progression-free survival (PFS, P = 0.003) based on detection of BCL2, HER2, CD133, CAIX, and ERCC1. Reverse-phase protein array results significantly correlated with western blotting results (R2 = 0.856). The C-index of model was higher than clinical model in the prediction of OS (C-index: 0.86 and 0.62, respectively) and PFS (C-index: 0.82 and 0.64, respectively). The Kaplan-Meier survival curve showed a dose-dependent prognostic significance of risk score for PFS and OS. Multivariable Cox proportional hazard model confirmed that the risk score was an independent predictor of PFS (HR: 1.6; 95% CI: 1.4–1.9; P < 0.001) and OS (HR: 2.1; 95% CI: 1.7–2.5; P < 0.001).
Conclusion:
A proteomic panel of BCL2, HER2, CD133, CAIX, and ERCC1 independently predicted survival in locally advanced cervical cancer patients. This prediction model can help identify chemoradiation responsive tumors and improve prediction for clinical outcome of cervical cancer patients.
Keywords: Chemoradiotherapy, cluster analysis, prognosis, proportional hazards models, protein array analysis, uterine cervical neoplasms
1. Introduction
Although concurrent chemoradiotherapy improves progression-free survival (PFS) and overall survival (OS) in patients with locally advanced cervical cancer [1], recurrence is frequent. Better tool for risk stratification is an unmet clinical needs [2]. It is valuable to identify markers that can predict recurrence after chemoradiation therapy to facilitate the selection of alternative therapeutic strategies and further improve clinical outcomes.
There are several predictors of chemoradiation resistance, including tumor size [3], regional metastases [3], and histology [4]. Additional predictors such as tumor hypoxia [5], interstitial tumor pressure, and standard uptake value (SUV) of positron emission tomography (PET) image [6] have also been suggested. However, none of these predictors fulfills clinical need. Interestingly, molecular studies have led to the discovery of biomarkers for predicting response to chemoradiation therapy. The most well-established molecular biomarkers include genes in hypoxia-regulating pathways [7], excision repair cross-complementing1 (ERCC1) [8], and EGFR pathways [9]. However, most studies were relatively small. In addition, no predictive marker or set of markers has been validated yet in an independent population.
Although transcriptional profiling divides molecular subtypes with different clinical features in several cancers, it does not capture all levels of biological complexity. Additional information may reside in the proteome [10]. Proteins are direct effectors of cellular function. Levels and function of proteins depend on translation and post-translational modifications [11] that can influence protein stability and activity [12]. Reverse phase protein array (RPPA) can accomplish proteomic profiling of clinical samples and quantitative detection of signaling proteins present in low abundance with high sensitivity and precision [13].
Here, we used well-based RPPAs [14] to assess expression levels of 22 selected markers in 181 frozen tissue lysates obtained from patients with locally advanced cervical cancer. Results were used to construct predictive models that could effectively discriminate between chemoradiation sensitive and resistance patient groups. The aim of the present study was to assess if these predictive models of multiple panel proteins with/without clinical indicators could be used as reliable biomarkers for the prediction of chemoradiation resistance in a cohort of patients treated with chemoradiation.
2. Patients and methods
2.1. Patient samples and tissue processing
Tumors were prospectively collected from patients diagnosed with locally advanced cervical cancer treated uniformly with concurrent chemoradiation at Samsung Medical Center (Seoul, South Korea) between 2002 and 2009. All procedures were conducted in accordance with the Declaration of Helsinki. Tumor tissues were sampled before starting concurrent chemoradiation. They were snap-frozen in liquid nitrogen within 20-30 minutes after collection. Tumor content was verified by histopathology. When tumor content was < 70%, tumors were excluded. Tissue samples and medical records were obtained with informed consent of all patients and approval by the Institutional Review Board (IRB) of Samsung Medical Center (IRB# SMC 2009-09-002). Samples were divided to training set and test set for the development and validation of the model, respectively. Study scheme is shown in Supplementary Fig. S1 Frozen tumor samples were homogenized using a Precellys Evolution Tissue Homogenizer (Bertin Instruments, France) and tissue protein extraction buffer supplemented with complete mini protease inhibitor cocktail (Roche Science, Manheim, Germany) and PhosSTOP phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA).
2.2. Well-based reverse phase protein arrays (RPPAs)
Proteomic expressional profiling was performed by analyzing expression/activation of selected markers (Supplementary Table S1). Final condition of well-based RPPA assay was decided after extensive antigen-antibody titration assays, including the availability of high quality antibody. Well-based RPPA assays were performed as described previously [14]. Electrochemiluminescence signals were detected using MSD-T read buffer on an MSD Sector Imager 2400 (Meso Scale Diagnostics, Gaithersburg, MD, USA).
2.3. Treatment and primary outcome
Pretreatment workup included clinical examination, chest radiography, abdomino-pelvic magnetic resonance imaging, cystoscopy, and proctoscopy for cases with clinical suspicion of invasion of the bladder and/or rectum. External beam radiotherapy (EBRT) and high-dose rate (HDR) intracavitary brachytherapy were done according to schedule [15,16]. The whole pelvis total dose was 50.40 gray (Gy) with 1.8 Gy daily fractions administered 5 times a week. HDR brachytherapy was started 4-5 weeks after initiation of EBRT. The dose of HDR brachytherapy was 24 Gy at point A, with 4 Gy per fraction twice a week for 3 weeks. After treatment, patients were followed up every 3 months during the first 2 years and every 6 months thereafter.
Overall survival (OS) was primary outcome. It was considered a surrogate of chemoradioresistance. OS was defined as the time interval from treatment to death or the last follow-up. Progression-free survival (PFS) was measured from the date of diagnosis to the date of progression or the last follow-up. Patients who died before progression were censored at the date of death. Early complete response was defined as complete disappearance of tumor at 1 month after treatment. It was measured by MRI/CT. The median duration of follow up was 53.7 months (range, 1.5 – 137.5 months).
2.4. Western blotting
Tissue lysates were subjected to SDS-polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane using an iBlot™ dry blotting system (Invitrogen, Carlsbad, CA, USA). Detailed western blotting conditions are described in Supplementary Table S2. Immunoreactive bands were detected with horseradish peroxidase-labeled anti-rabbit or anti-mouse IgG antibodies and enhanced with a SuperSignal Chemiluminescence Kit (Thermo Scientific). Signals were detected with Kodak Biomax MR X-ray films (Kodak, Rochester, NY, USA). The intensity of western blot signal was quantified using an ImageQuant version 5.2 (Molecular Dynamics, Sunnyvale, CA, USA).
2.5. Immunohistochemistry
To validate protein expressions measured by RPPAs, immunohistochemical expressions of selected proteins were measured and compared with those of RPPAs. Selected protein markers were used in survival predicting model. Immunohistochemical analysis was performed for 21 representative FFPE samples from the same cohort. Nineteen of these cases (90.5%) were squamous cell carcinoma. Five significant proteins (BCL2, HER2, CD133, CAIX, and ERCC1) were tested. Detailed immunohistochemistry conditions are described in Supplementary Table S3. The overall protein expression was expressed as mean value of histoscore, which is a result of multiplying the intensity score (0-3) and percentage of stained cells, with a maximum of 300.
2.6. Development of prediction model
An unsupervised hierarchical clustering was performed for normalized data from a 22-protein panel to distinguish groups based on the expression pattern of each proteins. Expression values of these proteins in 181 samples (where rows indicate the identity of the proteins, and columns indicate the identity of the patients) were clustered using hierarchical clustering with Euclidean distance and ward linkage by heatmap.2 function in the gplots R package. Clinical characteristics was then compared between clusters. Cox regressions with stepwise and backward selection (significance level for stay = 0.1) were used identify proteins and construct a multivariate protein-marker model using training data set. We then calculated a risk score (RS) for each patient. RS was the sum of estimated coefficients from the selected protein multivariate model multiplied by their expression in the training set [17]. We also used this formula to compute RS for the test set. Detailed information of validation of models is described in Supplementary information (Supplementary methods).
2.7. Statistical analysis
All statistical analyses were performed using R software version 3.1.2. Survival distributions were estimated using the Kaplan–Meier method. The relationship between survival and each parameter was analyzed with log-rank test. A Cox proportional hazards model was created to identify independent predictors of survivals. P-values of 0.050 or less were considered statistically significant.
To evaluate the prognostic significance of the identified model, data from The Cancer Genome Atlas (TCGA) Research Network were also analyzed (http://cancergenome.nih.gov/). The pan-cancer normalized form of the cervical cancer RNA-seq data, which had been obtained using Illumina HiSeq (Illumina, SanDiego, CA, USA), were downloaded (version: 2015-02-24). We used RNA expression data to make up for the lack of some protein data, and stages were limited to IB2, IIA2, IIB, III, and IVA. The characteristics are shown in Supplementary Table S4.
3. Results
3.1. Patient characteristics
The median age of 181 patients was 56 years (range, 28–85 years). Fifty-six (30.9 %) patients were at stage III or higher while others were at stage IIB. One hundred and sixty-one (89.0%) patients had squamous cell carcinoma and only 20 patients (11.0%) had adenocarcinoma (Supplementary Table S5). With a median follow-up of 53.7 months (range, 1.5–137.5 months), there were 76 (42.0%) recurrences. Five-year progression-free survival was 53.1% (95% CI: 45.7–61.7). Overall survival was 66.4% (95% CI: 59.5–74.2). Patients were randomly allocated to either train set or test set (Supplementary Table S5).
3.2. Unsupervised hierarchical clustering of 22 proteins
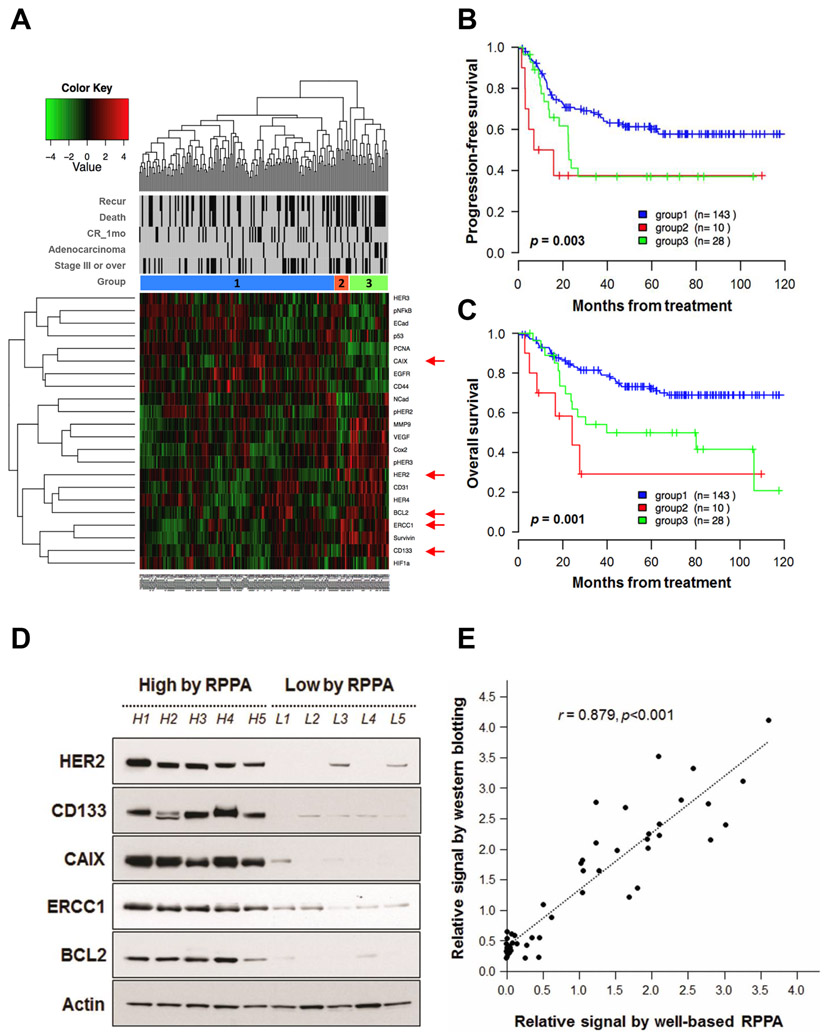
In recent years, great knowledge has been gained about effects of hypoxia, DNA repair, EGFR pathway, apoptosis, angiogenesis, cancer stem cell, proliferation, cell adhesion, cyclooxygenases, and hypoxia on radiation, making it possible to predict radiation response of cervical cancer (Supplementary Table S1) [18,19]. Of them, 22 most representative proteins of those pathways were selected in this study. Unsupervised clustering of proteomic profiles these 22 proteins is shown in Fig. 1A. These 22 proteins stratified locally advanced cervical cancers into three major groups with different PFS (P = 0.003, Fig. 1B) and OS (P = 0.001, Fig. 1C). Group 1 was characterized by high PCNA. Group 2 was characterized by high Her3, PCNA, HER2, BCL2, ERCC1, Survivin, and HIF1a while Group 3 was characterized by high p53, N-cadherin, pHER2, VEGF, Cox2, and pHER3 (Supplementary Fig. S2). Adenocarcinoma was more frequent in group 2 (20.0%) or group 3 (32.1%) than that in group 1 (6.3%) (Supplementary Table S6). The prognostic significance of the clustering was evaluated after removing adenocarcinoma patients, and though decreased, the clinical significance persisted in OS (P = 0.05) (data not shown).
Fig. 1.
Unsupervised hierarchical clustering. (A) Hierarchical clustering analysis of 22-protein expressional data by well-based RPPA divided patients into three groups. Adenocarcinoma was more frequent in groups 2 and 3. Proteins indicated by red arrow head are tested with western blotting analysis. Progression-free survival (B) and overall survival (C) according to groups. We subsequently validated well-based RPPA results using western blotting analysis and locally advanced cervical cancer samples. (D) Representative western blot images in 10 locally advanced cervical cancer specimens. Protein expression levels of HER2, CD133, CAIX, ERCC1, and BCL2 are confirmed by western blot analysis. All of the samples were squamous cell carcinomas cases. (E) Comparison of relative signal by western blotting and expressional values by well-based RPPA. Protein expressional levels are normalized against β-actin expression. There was a significant correlation between expression signal from western blotting and well-based RPPA score (r = 0.879, P < 0.001). r, correlation coefficients.
To validate these well-based RPPA findings, we analyzed whether expressional signals from well-based RPPA were correlated with data from conventional western blots in 10 samples, including 5 samples with low value (< 0.02) by well-based RPPA and 5 samples with high value (> 1.00) by well-based RPPA. As shown in Figs. 1D and E, the signal in well-based RPPA was significantly correlated with that in western blotting analysis (r = 0.879, P < 0.001). The expression pattern was subsequently confirmed by immunohistochemistry in 21 samples (r = 0.448, P < 0.001, Supplementary Fig. S3). BCL2 (r = 0.448, P = 0.047), CD133 (r = 0.560, P = 0.010), and ERCC1 (r = 0.501, P = 0.024) values by well-based RPPA showed positive correlations with immunohistochemical assessment (Supplementary Fig. S3B).
3.3. Prediction model for overall survival
Results of univariate analysis for survival are presented in Supplementary Tables S7 & S8. Multivariate analysis implemented with Cox proportional hazard model showed that age, FIGO stage (III/IV vs IIB), ERCC1, CD133, HER2, BCL2, and CAIX were predictors for overall survival (Supplementary Table S9). We then defined RS for each patient as estimated coefficients from variables selected multiplied by their expression: RS = (0.04*Age + 0.85*stage) + (1.26*ERCC1 + 1.12*HER2 + 3.54*BCL2) − (4.61*CD133 + 1.80*CAIX).
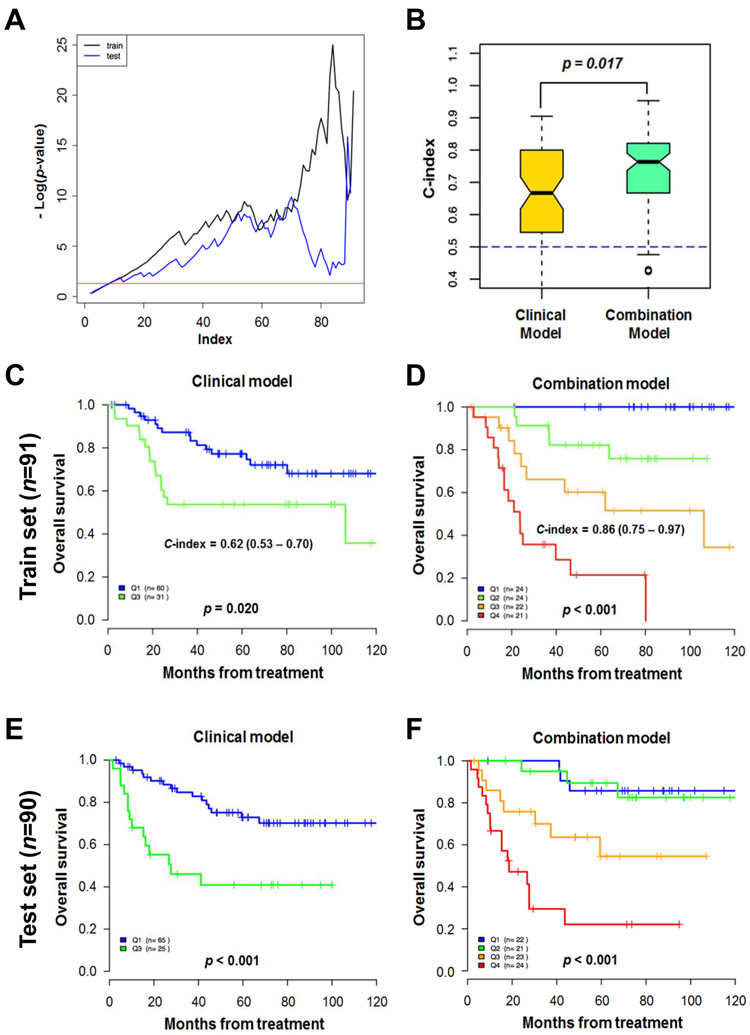
Statistical significance [−Log (p value) of log rank test] was plotted with each cut-off point of RS, showing significance (area over the red line) for most cut-off points in train set and test set (Fig. 2A). Random survival forest model showed that combined clinical and proteomic model (combined model) predicted overall survival better than clinical variables-only model (clinical model) (P = 0.017, Fig. 2B).
Fig. 2.
Model predicting overall survival. (A) Minus log p-value of log rank test for each cut-off point of risk score of a combined model. Area above the red line means statistical significance. (B) C-index of a random survival forest model. Combined clinical and protein-variable model showed better performance than the model only based on clinical variables in predicting death (P = 0.017). (C-F) Kaplan-Meier survival curve according to risk score of each model in each set. C-index of combined model was higher than that of clinical model (C-index: 0.62 and 0.86, respectively). C-index, concordance-index.
The performance of RS was compared between clinical model and combined model. C-index of combined model was higher than that of clinical model [C-index: 0.86 (95% CI: 0.7–1.0) and 0.62 (95% CI, 0.5–0.7), respectively] (Figs. 2C and D). Kaplan-Meier survival curve was plotted according to RS in training and validation cohorts, showing dose-dependent prognostic significance of RS (Figs. 2C-F).
The final multivariable Cox proportional hazard model confirmed that RS was an independent predictor of PFS (HR: 1.6; 95% CI: 1.4–1.9; P < 0.001) and OS (HR: 2.1; 95% CI: 1.7–2.5; P < 0.001) after adjusting for other known prognostic factors including age, FIGO stage, tumor size, SCC Ag level, and early complete response (Table 1).
Table 1.
Multivariate analysis of overall survival.
| Factors | OS | |
|---|---|---|
| HR (95% CI) | P-value* | |
| Age | 1.01 (0.98 – 1.04) | 0.485 |
| Stage (III/IV vs II) | 1.67 (0.83 – 3.39) | 0.154 |
| Tumor size | 0.83 (0.68 – 1.03) | 0.091 |
| SCC-Antigen | 1.00 (0.99 – 1.00) | 0.725 |
| Early CR | 0.28 (0.11 – 0.74) | 0.010 |
| RS | 2.07 (1.71 – 2.50) | <0.001 |
Abbreviations: HR, hazard ratio; CR, complete response; RS, risk score; CI, confidential interval; PFS, progression-free survival; OS overall survival Significant P-values in bold.
Cox proportional hazard analysis
For the external validation, the model was tested in the TCGA using RNA expression dataset. Model using 7 factors (2 clinical and 5 molecular) was developed, which showed trend of better prediction of overall survival than clinical model (Supplemental Fig. S4). Lack of clear reproduction of clinical significance might be due to the use of RNA expression dataset and limited sample size.
3.4. Prediction model for progression-free survival
For progression free survival age, FIGO stage (III/IV vs. IIB), tumor size, SCC antigen (log scale), Survivin, CD133, CD31, CD44, HER2, CAIX, VEGF, and pHER were selected in multivariate analysis (Supplementary Fig. S5A). We then defined RS for each patient as estimated coefficients from variables selected multiplied by their expression: RS = (0.05*Age + 1.32*stage − 0.31*tumor size + 0.91*SCC-Ag) + (1.17*Survivin + 1.18*CD31 + 1.32*CD44 + 1.03*HER2 + 2.66*VEGF) − (3.47*CD133 + 1.66*CAIX + 2.82*pHER3).
Minus Log (p value) of log rank test showed significance (area over the red line) for most cut-off points in the train set and some cut-off points in the test set (Supplementary Fig. S5B). The C-index of the combined model was higher than that of the clinical model [C-index: 0.82 (95% CI: 0.7–0.9) and 0.64 (95% CI: 0.6–0.7), respectively] (Supplementary Figs. S5C and D). The Kaplan-Meier survival curve was plotted according to RS in training and validation cohorts, showing dose-dependent prognostic significance of the RS (Supplementary Figs. S5C-F).
4. Discussion
In this study, we described a prediction model based on multiple protein markers that could effectively classify cervical cancer patients into long-term and short-term survivors in training and validation cohorts. We assessed expressional levels of 22 proteins by a well-based RPPA and identified a panel of five markers that showed powerful prognostic significance in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy treatment. Their prognostic significance was validated with an independent test set. The model could also be applied to PFS outcomes representative of radiosensitivity. To the best of our knowledge, the present study is first assessment of a model based on multiple proteins that can help predict chemoradiotherapy response in patients with cervical cancer.
In the current study, our prediction model for chemoradiotherapy response was established by proteomic profiling. According to this prediction model, BCL2 and CD133 showed the most relative significances, followed by CAIX, CRCC1, and HER2. BCL2 is a well-known anti-apoptotic regulator. Overexpression of BCL2 is associated with resistance to radiation in several types of cancer cells including cervical cancer [20,21]. HER2, one of the EGFR family, has been reported to be frequently overexpressed in carcinoma of the uterine cervix [22]. Patients with overexpression of EGFR have higher probability of pelvic relapses and decreased disease-free survival treated with chemoradiation. The poor prognosis of these tumors may be due to increased radioresistance [23]. Interestingly, overexpression of HER2 can modulate the expression of Bcl-2 and Bcl-XL in human breast cancer cell lines [24]. Milella et al. [25] have demonstrated that disruption of anti-apoptotic cross-talk between Bcl2 and HER2 can potentiate apoptosis efficiently in breast cancer cells that are resistant to anti-cancer therapy targeting Bcl2. These studies suggest that our proteomic profiling of multiple factors is more reliable and effective in predicting results of anticancer therapy.
CD133 is probably one of the most studied markers of cancer stem cells. High expression of CD133 expression has been correlated with poor prognostic features and chemoresistance [26,27]. However, patients with cervical cancer expressing high level of CD133 demonstrated better response to CCRT in the current study. The discrepancy between CD133 expression and CCRT susceptibility in the current study suggests that other molecules might be related to cancer stem cells for predicting anti-cancer therapy response. Similarly, carbonic anhydrase-9 (CAIX) is a transmembrane protein overexpressed in a wide variety of tumor types. It is induced by hypoxia. It is also known to be predictor for radiation-resistant hypoxic cells [28]. Elevated CAIX expression has been correlated with high-risk tumor features such as higher stage and greater depth of invasion [7]. The correlation between CAIX overexpression and prognosis has been shown to be diverse, depending on tumor types and its expression site. Interestingly, overexpression of CAIX is related to better survival in several carcinomas [29,30].
Excision repair cross-complementing 1 (ERCC1) involved in nucleotide excision repair is associated with resistance to chemoradiotherapy in various types of cancer [8]. Consistent with findings in other cancer types, in a small study of patients with cervical cancer, high ERCC1 levels are associated with inferior disease-free survival among patients treated with chemoradiation therapy with cisplatin [31].
Our study has limitations. First, our study was based on evaluation of markers previously suggested to have prognostic value in cervical cancer without seeking to identify new makers. Second, this study was performed with a single institution cohort and a relatively small sample size. It was not a prospective validation study. Therefore, further prospective large-scale multi-institutional validation studies are necessary to confirm these findings.
In conclusion, we observed that three patient subtypes derived from unsupervised clustering analysis on RPPA-based proteomic profiles had significantly different PFS and OS. Notably, the proposed five-marker panel was demonstrated to be a robust classification model for predicting chemoradiotherapy responsiveness in patients with locally advanced cervical cancer. It could serve as a predictive tool for clinical outcome. However, the RPPA-based model warrants further testing to evaluate its prognostic potential and clinical applicability in locally advanced cervical cancer.
Supplementary Material
Highlights.
Age, FIGO stage, ERCC1, CD133, HER2, BCL2, and CAIX were associated with OS.
Age, FIGO stage, tumor size, SCC antigen, Survivin, CD133, CD31, CD44, HER2, CAIX, VEGF, and pHER were related with PFS.
Proteomic panel of BCL2, HER2, CD133, CAIX, and ERCC1 independently predicted survival in locally advanced cervical cancer.
Prediction model can help identify chemoradiation sensitive tumors and predict clinical outcome of cervical cancer patients.
Funding
This research was supported by a grant (2017R1D1A1B05035844) and an Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research.
Footnotes
Disclosures
The authors have no conflicts of interest relevant to this study to disclose.
Data Availability Statements: All data generated or analyzed during this study are available on request from the corresponding authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. , Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis, Lancet 358 (2001) 781–786. [DOI] [PubMed] [Google Scholar]
- [2].Brabec V, Kasparkova J, Modifications of DNA by platinum complexes. Relation to resistance of tumors to platinum antitumor drugs, Drug Resist Updat 8 (2005) 131–146. [DOI] [PubMed] [Google Scholar]
- [3].Eifel PJ, Jhingran A, Levenback CF, Tucker S, Predictive value of a proposed subclassification of stages I and II cervical cancer based on clinical tumor diameter, Int J Gynecol Cancer 19 (2009) 2–7. [DOI] [PubMed] [Google Scholar]
- [4].Monk BJ, Tian C, Rose PG, Lanciano R, Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials, Gynecol Oncol 105 (2007) 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, et al. , Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer, J Clin Oncol 20 (2002) 680–687. [DOI] [PubMed] [Google Scholar]
- [6].Xue F, Lin LL, Dehdashti F, Miller TR, Siegel BA, Grigsby PW, F-18 fluorodeoxyglucose uptake in primary cervical cancer as an indicator of prognosis after radiation therapy, Gynecol Oncol 101 (2006) 147–151. [DOI] [PubMed] [Google Scholar]
- [7].Woelber L, Kress K, Kersten JF, Choschzick M, Kilic E, Herwig U, et al. , Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer, BMC Cancer 11 (2011) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin M, Yan J, Martinez-Balibrea E, Graziano F, Lenz HJ, Kim HJ, et al. , ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis, Clin Cancer Res 17 (2011) 1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. , Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy, Int J Radiat Oncol Biol Phys 56 (2003) 922–928. [DOI] [PubMed] [Google Scholar]
- [10].Lee YY, Kim TJ, Kim JY, Choi CH, Do IG, Song SY, et al. , Genetic profiling to predict recurrence of early cervical cancer, Gynecol Oncol 131 (2013) 650–654. [DOI] [PubMed] [Google Scholar]
- [11].Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, et al. , A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells, Nat Cell Biol 6 (2004) 308–318. [DOI] [PubMed] [Google Scholar]
- [12].Amerik AY, Hochstrasser M, Mechanism and function of deubiquitinating enzymes, Biochim Biophys Acta 1695 (2004) 189–207. [DOI] [PubMed] [Google Scholar]
- [13].Liotta LA, Espina V, Mehta AI, Calvert V, Rosenblatt K, Geho D, et al. , Protein microarrays: meeting analytical challenges for clinical applications, Cancer Cell 3 (2003) 317–325. [DOI] [PubMed] [Google Scholar]
- [14].Perry C, Conway CM, Ha JW, Braunschweig T, Morris J, Ylaya K, et al. , HER-2 assessment in formalin-fixed paraffin-embedded breast cancer tissue by well-based reverse phase protein array, Clin Proteomics 11 (2014) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim MK, Kim TJ, Sung CO, Choi CH, Lee JW, Bae DS, et al. , Clinical significance of HIF-2alpha immunostaining area in radioresistant cervical cancer, J Gynecol Oncol 22 (2011) 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choi CH, Lee YY, Kim MK, Kim TJ, Lee JW, Nam HR, et al. , A matched-case comparison to explore the role of consolidation chemotherapy after concurrent chemoradiation in cervical cancer, Int J Radiat Oncol Biol Phys 81 (2011) 1252–1257. [DOI] [PubMed] [Google Scholar]
- [17].Verweij PJ, Van Houwelingen HC, Penalized likelihood in Cox regression, Stat Med 13 (1994) 2427–2436. [DOI] [PubMed] [Google Scholar]
- [18].Qin C, Chen X, Bai Q, Davis MR, Fang Y, Factors associated with radio sensitivity of cervical cancer, Anticancer Res 34 (2014) 4649–4656. [PubMed] [Google Scholar]
- [19].Klopp AH, Eifel PJ, Biological predictors of cervical cancer response to radiation therapy, Semin Radiat Oncol 22 (2012) 143–150. [DOI] [PubMed] [Google Scholar]
- [20].Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y, Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis, Neoplasia 4 (2002) 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saegusa M, Takano Y, Hashimura M, Shoji Y, Okayasu I, The possible role of bcl-2 expression in the progression of tumors of the uterine cervix, Cancer 76 (1995) 2297–2303. [DOI] [PubMed] [Google Scholar]
- [22].Cerciello F, Riesterer O, Sherweif M, Odermatt B, Ciernik IF, Is EGFR a moving target during radiotherapy of carcinoma of the uterine cervix?, Gynecol Oncol 106 (2007) 394–399. [DOI] [PubMed] [Google Scholar]
- [23].Perez-Regadera J, Sanchez-Munoz A, De-la-Cruz J, Ballestin C, Lora D, Garcia-Martin R, et al. , Impact of epidermal growth factor receptor expression on disease-free survival and rate of pelvic relapse in patients with advanced cancer of the cervix treated with chemoradiotherapy, Am J Clin Oncol 34 (2011) 395–400. [DOI] [PubMed] [Google Scholar]
- [24].Kumar R, Mandal M, Lipton A, Harvey H, Thompson CB, Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells, Clin Cancer Res 2 (1996) 1215–1219. [PubMed] [Google Scholar]
- [25].Milella M, Trisciuoglio D, Bruno T, Ciuffreda L, Mottolese M, Cianciulli A, et al. , Trastuzumab down-regulates Bcl-2 expression and potentiates apoptosis induction by Bcl-2/Bcl-XL bispecific antisense oligonucleotides in HER-2 gene--amplified breast cancer cells, Clin Cancer Res 10 (2004) 7747–7756. [DOI] [PubMed] [Google Scholar]
- [26].Artells R, Moreno I, Diaz T, Martinez F, Gel B, Navarro A, et al. , Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients, Eur J Cancer 46 (2010) 642–649. [DOI] [PubMed] [Google Scholar]
- [27].Li Z, CD133: a stem cell biomarker and beyond, Exp Hematol Oncol 2 (2013) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Olive PL, Aquino-Parsons C, MacPhail SH, Liao SY, Raleigh JA, Lerman MI, et al. , Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer, Cancer Res 61 (2001) 8924–8929. [PubMed] [Google Scholar]
- [29].Patard JJ, Fergelot P, Karakiewicz PI, Klatte T, Trinh QD, Rioux-Leclercq N, et al. , Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma, Int J Cancer 123 (2008) 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sergeant G, Lerut E, Ectors N, Hendrickx T, Aerts R, Topal B, The prognostic relevance of tumor hypoxia markers in resected carcinoma of the gallbladder, Eur J Surg Oncol 37 (2011) 80–86. [DOI] [PubMed] [Google Scholar]
- [31].Hasegawa K, Kato R, Torii Y, Ichikawa R, Oe S, Udagawa Y, The relationship between ERCC1 expression and clinical outcome in patients with FIGO stage I to stage II uterine cervical adenocarcinoma, Int J Gynecol Cancer 21 (2011) 1479–1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.