Abstract
New treatments have transformed multiple myeloma into a chronic disease. Hence, optimal management of treatment and disease-related complications remains a critical component of survivorship care. Survivorship care models in cancers requiring a fixed-duration therapy may not be applicable to myeloma as patients are exposed to multiple lines of continuous therapy along the disease trajectory. Infections and secondary cancers, which are the most common therapy-related causes of death in myeloma, need special consideration. Identifying patients at a high risk of toxicities will facilitate individualized treatment selection and designing clinical trials for protective strategies targeting those patients, for example, prophylactic antibiotic or immunoglobulin replacement for primary prevention of infections in high-risk patients. Long-term follow up of ongoing trials and epidemiologic data will be help identify the nature and trajectory of rare toxicities with a long latency like secondary cancers. Patients who are frail, have persistent renal insufficiency, and refractory to multiple lines of therapy need special attention. In this review, we discuss the incidence, risk-factors, and management of treatment and disease-related complications in myeloma, discuss knowledge gaps and research priorities in this area, and propose a survivorship care model to improve health-care delivery to a growing pool of myeloma survivors.
Introduction
Multiple Myeloma is a clonal plasma cell neoplasm, characterized by anemia, renal insufficiency, hypercalcemia, and bone destruction. The incidence of myeloma has increased globally by 126% between 1990 and 20161. Furthermore, the age-standardized mortality rate has been steadily declining1, likely due to new drugs including proteasome inhibitors [PIs], immunomodulatory drugs [IMiDs], and monoclonal antibodies [mAbs] as well as upfront autologous hematopoietic cell transplantation [AHCT] in eligible patients. Although myeloma is predominantly a disease of older adults with a median age at diagnosis of 69 years, incidence in younger adults aged 20–49 years is steadily rising in the United States2. Recent data from the Swedish national registry showed a roughly three-fold increase in myeloma prevalence from 1980 to 20143. Furthermore, modification of the International Myeloma Working Group [IMWG] diagnostic criteria by addition of disease-defining biomarkers4 will lead to re-classification of some patients with high-risk smoldering myeloma to newly diagnosed myeloma, further increasing the pool of myeloma survivors on active treatment.
New treatments have converted myeloma into a chronic disease, with the treatment goal being to prevent end-organ damage, improve or maintain quality of life [QoL], and achieve long-term disease free survival. Notably, most patients remain on continuous treatment for extended time-periods. Hence, management of immediate and late complications from disease and treatment is a critical component of survivorship care in myeloma. In this review, we summarize the incidence, risk-factors, and management of common treatment and disease-related complications [Figure I] and discuss implications for survivorship care in this population. Since transplant-related late effects have been well-described in literature5, we will focus mainly on complications from non-transplant therapies. However, we acknowledge that exposures and risk factors of systemic complications in myeloma are often difficult to separate and must be considered collectively. The incidence and risk factors of major complications is summarized in Table I. Strategies for prevention and management is summarized in Table II.
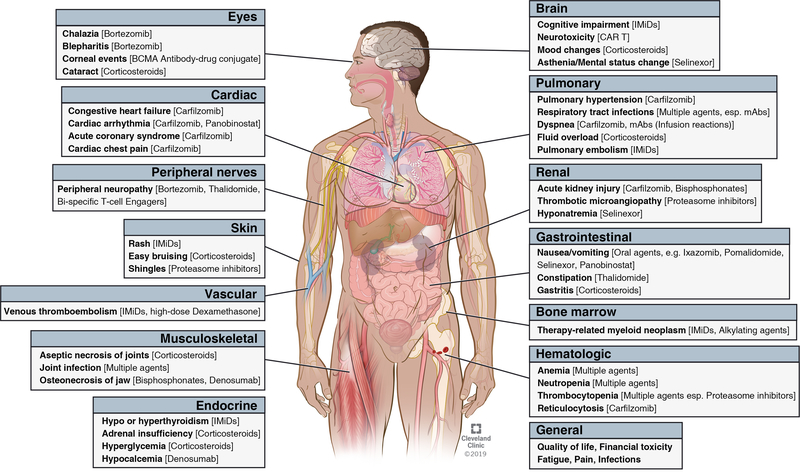
Figure I.
Major treatment-related toxicities in multiple myeloma.
Abbreviations: BCMA: B-cell maturation antigen. ADC: Antibody-drug conjugate. IMiDs: Immunomodulatory drugs. PIs: Proteasome inhibitors. CAR T: Chimeric Antigen Receptor T-cell therapy
Table I.
Incidence and Risk-Factors for Common Treatment-related Toxicities in MM
| Toxicities/Late Effects | Incidence | Risk Factors | ||
|---|---|---|---|---|
| Patient-related | Disease-related | Treatment-related | ||
| Peripheral Neuropathy | ➢ Bortezomib [IV]: All-grade: 31–44% [≥grade 3: 8–13%]10–12 ➢Bortezomib [SC]: All-grade: 35% [≥grade 3: 5%]9 ➢Carfilzomib: All-grade: 5–15% [≥ grade 3: 0.2–1.5%]8,17 ➢Ixazomib: All-grade: 19–27% [≥grade 3: 1–2%]13,18 ➢Thalidomide: All-grade: Up to 70% [≥grade 3: 7–11%, with ≥grade 3 motor neuropathy in 3%]14,15 ➢Lenalidomide: All-grade: 22% [≥grade 3: 0.75–2%]18,19 ➢Pomalidomide: All-grade: 4–15% [≥Grade 3: 0–1%]16,20 |
➢ Pre-exiting PN125 ➢Comorbidities [Diabetes Mellitus, Nutritional deficiencies, Alcohol abuse, and Hypothyroidism] ➢Age/Sex7 |
➢ Concomitant Monoclonal gammopathy-associated PN, AL Amyloidosis, or POEMS syndrome | ➢Use of PIs or IMiDs [specifically, bortezomib or thalidomide] ➢Intravenous9 or twice-weekly21 administration of bortezomib ➢Thalidomide daily dose >200 mg or treatment duration >6–12 months22. ➢Previous treatment with potentially neurotoxic drugs [e.g. vincristine or thalidomide] |
| Secondary Malignancy | ➢ Actuarial incidence of secondary hematologic malignancies: 0.4–9.8%37–41,45 ➢ Standardized Incidence Ratio [Observed: Expected] for secondary hematologic malignancies: 2.2–8.239,41 ➢Actuarial incidence of secondary solid tumors: 3.5–5.3%38,41 ➢Standardized Incidence Ratio [Observed: Expected] for secondary solid tumors: 0.75–1.0938,41 |
➢Older Age47,48 ➢Germline predisposition ➢Prior malignancies40 ➢Obesity48 ➢Male sex48 |
➢Underlying Plasma Cell Disorder [including precursor conditions like MGUS] ➢Immunophenotypic abnormalities characteristic of MDS in the bone marrow at diagnosis of plasma cell disorder50 |
➢Treatment with alkylating agents [especially oral melphalan]37,47 ➢Duration of therapy [oral melphalan]37. Risk: 3% with each year of melphalan therapy ➢Treatment with lenalidomide46,47 ➢Combined treatment with lenalidomide and oral melphalan47 ➢HDM-ASCT48,49 |
| Venous Thromboembolism | ➢Lenalidomide-based combination regimens in Newly Diagnosed MM: Rd-7–13.5%19,57; VRd- 13%57 ➢Thalidomide-based combination regimens in Newly Diagnosed MM: VTd [Newly diagnosed]: 3% grade 3–458 ➢Carfilzomib-based combination regimens: 1.7–10.2%8,17,64 |
➢Personal or family history of VTE59 ➢Known hypercoagulable state59 ➢Central venous catheter or pacemaker60,62 ➢Neurologic disease with extremity paresis60 ➢Immobilization60 ➢Surgery60 ➢Trauma60 ➢Superficial vein thrombosis60 ➢Obesity62 ➢Asian race, Alaskan native or Native American [Low risk]61,62 ➢Cardiac disease ➢Chronic kidney disease ➢Acute infection ➢Diabetes mellitus |
➢Diagnosis of MM per se53 ➢Hyperviscosity56 |
➢Concomitant use of IMiDs with high-dose dexamethasone, anthracycline, or multi-agent cytotoxic chemotherapy55,56 ➢Use of erythropoietin stimulating agents56 ➢Carfilzomib-based combination regimens [with lenalidomide or dexamethasone]8,64 |
| Therapy-related Cardiac Toxicities | ➢Carfilzomib-based combination regimens: All-grade: 18–51%; ≥Grade 3: 8%71,72 | ➢Chronic Kidney Disease ➢Anemia ➢Traditional cardiac risk-factors: Family history, HTN, HLD, DM, Tobacco use71 ➢Elevated baseline BNP or NT-pro-BNP71 ➢Normal baseline BNP/NT-Pro-BNP that becomes elevated mid-first cycle of treatment71 |
➢Underlying AL amyloidosis or cardiac light chain deposition disease ➢Underlying hypercalcemia [Risk of arrhythmias] |
➢Carfilzomib-based regimens70 ➢Carfilzomib dose ≥45 mg/m270 ➢High-dose Dexamethasone63 |
| Infections | ➢Bortezomib-based combination regimens: All-grade 28–48% and ≥grade 3 6–15%57,78,79 ➢Carfilzomib-based combination regimens: All-grade 28–29% and ≥grade 3 9–13%*8,17 ➢Lenalidomide-based combination regimens: All-grade 27–34% and ≥grade 3 14–25%19,57,80 ➢Daratumumab-based combination regimens: All-grade 46–86% and ≥grade-3 23–32%78,80,81 |
➢Male sex74 ➢Older age74 ➢ECOG PS82 |
➢Baseline serum β-2 microglobulin82 ➢Baseline LDH82 ➢Baseline hemoglobin82 ➢Extramedullary disease and low ALC [Risk factors for CMV infection]75 ➢Suppression of polyclonal immunoglobulins [Immunoparesis] |
➢Treatment with anti-CD-38 monoclonal antibody78,80 ➢Treatment with PIs [Increased risk of VZV infections] |
| Therapy-related renal toxicities | ➢Carfilzomib-All-grade: 14–37% and ≥grade 3: 4–19% ➢Zoledronic Acid-All-grade: 5–6% and ≥grade 3: 3% for AKI91,92 |
➢CrCl 30–60 ml/min for patients receiving Zoledronic Acid91 | ➢Not known | ➢Use of carfilzomib-based combination regimens ➢Use of zoledronic acid as bone modifying therapy |
| Bone Health | ➢Incidence of SREs after treatment initiation for newly diagnosed MM in the setting of BMA use: 27–45% | ➢Baseline SRE98 | ➢Diagnosis of MM per se | ➢Prolonged use of corticosteroids100 |
Abbreviations: IV: Intravenous. SC: Subcutaneous. POEMS: Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal plasma proliferative disorder, and Skin changes, PN: peripheral Neuropathy, PIs: Proteasome Inhibitors, IMiDs: Immunomodulatory drugs. MGUS: Monoclonal Gammopathy of Undetermined Significance. MDS: Myelodysplastic Syndrome. HDM-ASCT: High Dose Melphalan-Autologous Stem Cell Transplantation. ND: Newly Diagnosed. Rd: Lenalidomide-dexamethasone. VRd: Bortezomib-Lenalidomide-Dexamethasone. VTd: Bortezomib-Thalidomide-Dexamethasone. VTE: Venous Thromboembolism. IMiDs: Immunomodulatory Drugs. HTN: Hypertension. HLD: Hyperlipidemia. DM: Diabetes Mellitus. BNP: Brain Natriuretic Peptide. NT-pro-BNP: N-Terminal Pro-Brain Natriuretic Peptide. ECOG: Eastern Co-operative Oncology group. PS: Performance Status. LDH: Lactate Dehydrogenase. HZV: Herpes Zoster Virus. AKI: Acute Kidney Injury. SREs: Skeletal-Related Events [Typically defined as a composite of pathologic fractures, spinal cord compression, and need for surgery or palliative radiotherapy to the affected bone]. BMA: Bone-modifying Agent
Estimates of pulmonary infection only [including upper respiratory tract infection, pneumonia, and lung infection in primary publication]
Table II.
Prevention and Management of Common Toxicities and Complications in MM
| Toxicity/ Late Effects | Prevention | Management |
|---|---|---|
| Peripheral Neuropathy | ➢ Active management of common comorbidities like DM, nutritional deficiencies, hypothyroidism, and alcohol abuse ➢ Choice of optimal treatment regimen considering baseline risk-factors for PN [e.g. considering DRd for induction instead of D-VMP or VRd in a transplant-ineligible patient with pre-existing PN] |
➢ Assessment of severity ➢ Dose modification of the offending agent ➢ Pharmacotherapy: Duloxetine24, Venlafaxine25 [Supported by RCT data]. Others used off-label: Gabapentin, pregabalin, tramadol, opiate analgesics. ➢ Assessment of fall risk and frailty in older adults. ➢ Physical therapy |
| Secondary Malignancy | ➢ Avoid using oral alkylating agents for a prolonged duration [>1 year] ➢ Avoid using alkylating agent-based induction therapy prior to ASCT, if PIs, IMiDs, and mAbs are available49 ➢ Avoid using lenalidomide and oral melphalan combinations [e.g. MPR] ➢ If alkylating agent required for disease control, consider cyclophosphamide over melphalan50 ➢ Risk assessment for development of secondary malignancies prior to choosing a treatment regimen ➢ Baseline bone marrow examination prior to initiating lenalidomide maintenance51 ➢ Consider age-appropriate cancer screening for patients, especially for those in MRD-negative remission who are expected to have prolonged survival ➢ Consider therapy-related myeloid neoplasm in differential diagnosis for patients with persistent unexplained cytopenias |
➢ Management according to underlying tumor type |
| Venous Thromboembolism | ➢ Avoid using high-dose dexamethasone with IMiD-based regimens [except in rare circumstances like cast nephropathy, when benefit might outweigh risk] ➢ Avoid using doxorubicin or multi-agent cytotoxic chemotherapy [except bortezomib] with IMiD-based regimens ➢ Meticulous assessment of patient-related, disease-related, and treatment-related risk factors as well as bleeding risk prior to choosing the optimal treatment regimen and thromboprophylaxis strategy |
➢ Therapeutic anticoagulation with LMWH, warfarin, or DOACs [Lack of studies comparing above agents in the context of myeloma] ➢ Avoid treatment regimens with a high VTE risk in subsequent lines of therapy for patients with prior VTE unless benefits outweigh risk ➢ Address modifiable risk factors like obesity |
| Cardiac Complications | ➢ Pre-treatment cardiovascular assessment for addressing traditional risk-factors like HTN, HLD, tobacco abuse, and DM. ➢ Pre-treatment BNP or NT-Pro-BNP to assess the risk of subsequent cardiac toxicities with PIs71 ➢ Consider cardiology evaluation and co-management for high-risk patients ➢ Careful consideration of CV risk factors prior to choosing a treatment regimen |
➢ Hold offending agent if patient develops signs and symptoms of CHF until initial evaluation is completed ➢ Consider evaluation and co-management by cardiooncology clinic ➢ If patient is re-challenged with carfilzomib, consider lower dose and lower IV fluid volume ➢ Aggressive risk factor modification ➢ Optimization of blood pressure control |
| Infections and Immunity | ➢ Careful assessment of frailty status at baseline and critical decision points [e.g. initiation of subsequent lines of treatment] to anticipate severe treatment-related toxicities including infection ➢ Ensuring that patients are up-to-date with recommended vaccinations, especially, annual inactivated influenza and pneumococcal polysaccharide vaccine [PCV13→PPV23]87 ➢ Infectious disease consultation to complete specific vaccination schedules after hematopoietic cell transplantation ➢ Consider daily oral levofloxacin in the first 12 weeks of induction therapy126 ➢ Consider IVIG in patients with recurrent infections or secondary hypogammaglobulinemia85–87 ➢ HZV prophylaxis for patients receiving PIs [until at least 2 months post-treatment], anti-CD38 MoABs, high-dose dexamethasone, and post hematopoietic cell transplantation87 ➢ Consider PJP and anti-fungal prophylaxis in patients receiving high-dose dexamethasone87 |
➢ Treatment according to the site of infection and/or causative organism |
| Renal Complications | ➢ Careful assessment of baseline renal function, comorbidities and risk of renal toxicity with the treatment regimen ➢ Consider using denosumab instead of zoledronic acid for bone-modifying therapy if CrCl<60 ml/min ➢ Monitoring of serum calcium, vitamin D, parathyroid hormone, and iron level [for anemic patients] in patients with CKD ➢ Follow dose modification guidelines for chemotherapeutic agents in patients with baseline renal dysfunction |
➢ Follow general dose modification guidelines if renal toxicity develops with treatment ➢ Consider alternative causes like disease progression, renal amyloidosis, MGRS, or TMA if renal function does not improve after holding the offending agent [kidney biopsy should be considered in selected cases after consultation with nephrologist] |
| Skeletal-Related Events and Bone Health | ➢ Initiation of BMAs [Bisphosphonates or RANKL inhibitors] at diagnosis and relapse in MM irrespective of the presence of baseline bone disease ➢ Advantage of zoledronic acid over denosumab: treatment can be de-escalated to every 3 months, data on overall survival benefit over clodronic acid, lower cost, and no signal for rebound fracture after discontinuation ➢ Advantage of denosumab over zoledronic acid: Subcutaneous route of administration and lower risk of AKI, especially in patients with baseline renal impairment. ➢ High index of suspicion for spinal cord compression in patients with concerning signs and symptoms ➢ Strategies to prevent ONJ in patients on BMAs: Comprehensive dental evaluation at baseline [including treatment of active dental infection and eliminating sites at a high risk of infection]; Avoiding invasive dental procedures and maintaining excellent oral hygiene while on BMAs; Holding bisphosphonates 90 days before and after invasive dental procedures [e.g. tooth extraction, dental implants, and surgery to the jaw but not root canals]; Limiting cumulative duration of BMAs [e.g. discontinuing zoledronic acid after 2 years from diagnosis if patient is in a deep hematologic remission] ➢ Lifestyle modifications: Weight loss, exercise, smoking cessation, and limitation of alcohol consumption ➢ Consider strategic discontinuation of corticosteroids from the treatment backbone once a deep hematologic remission [≥VGPR] has been achieved. Do not administer corticosteroids with lenalidomide in the maintenance setting104 |
➢ Consider balloon kyphoplasty over vertebroplasty for vertebral compression fractures in MM ➢ Consider orthopedics, radiation oncology, and pain management or palliative care evaluation in patients with SREs |
| Hematologic Complications | ➢ Consider G-CSF for secondary prophylaxis in patients with febrile neutropenia on prior chemotherapy cycle at physician’s discretion ➢ No role of prophylactic growth factors for red blood cells and platelets |
➢ Consider ESAs if hemoglobin persistently <10 g/dl after treatment with anti-MM agent(s), with the goal of reducing transfusion needs |
| Endocrine Complications | ➢ Consider baseline thyroid function assessment prior to initiating IMiDs ➢ Consider strategic discontinuation of dexamethasone from treatment backbone once deep hematologic remission is achieved ➢ Avoid high-dose dexamethasone except in special circumstances like myeloma cast nephropathy ➢ Consider assessment for hypogonadism in males and pre-menopausal females |
➢ Obtain thyroid function panel and initiate appropriate treatment if symptoms of hypo or hyperthyroidism develop during therapy ➢ Co-management with endocrinology in patients with adrenal insufficiency from long-term steroid use |
| Ocular Complications | ➢ Ocular hygiene and patient education regarding potential ocular complications with therapy ➢ Consider annual ophthalmologic evaluation to assess for toxicities from long-term corticosteroid use e.g. cataract |
➢ Bortezomib-induced eyelid complications [Chalazia or Blepharitis]: Ocular therapy [1st line]:Warm compresses, topical antibiotic [+/− steroids], or oral antibiotic [Doxycycline]113 Dose modification of offending agent [2nd line] |
Abbreviation: DM: Diabetes mellitus. PN: Peripheral neuropathy. RCT: Randomized controlled trial. DRd: Daratumumab-Lenalidomide-Dexamethasone. D-VMP: Daratumumab-Bortezomib-Melphalan-Prednisone. ASCT: Autologous Stem Cell Transplantation. MPR: Melphalan-Prednisone-Lenalidomide. LMWH: Low Molecular Weight Heparin. DOACs: Direct Oral Anti-Coagulants. PIs: Proteasome Inhibitors. HTN: Hypertension. HLD: Hyperlipidemia. DM: Diabetes Mellitus. CV: Cardiovascular. IV: Intravenous. HZV: Herpes Zoster Virus. MoABs: Monoclonal Antibodies. PI: Proteasome Inhibitors. PJP: Pneumocystis jiroveci pneumonia. CrCl: Creatinine Clearance. MGRS: Monoclonal Gammopathy of Renal Significance. TMA: Thrombotic Microangiopathy. BMA: Bone Modifying Agent. RANKL: Receptor Activator of Nuclear factor-κB Ligand. ONJ: Osteonecrosis of Jaw. VGPR: Very Good Partial Response. SRE: Skeletal-related events. G-CSF: Granulocyte Colony Stimulating Factor. CKD: Chronic Kidney Disease. PCV: Pneumococcal Conjugate Vaccine. PPV: Pneumococcal Polysaccharide Vaccine.
1. Neurologic Complications
1.1. Peripheral Neuropathy
Treatment-induced Peripheral Neuropathy [PN] is a common and a potentially debilitating toxicity in myeloma. Apart from pre-existing PN6, which is an established risk-factor for severe treatment-induced PN in myeloma, literature on risk-factors is mostly borrowed from traditional cytotoxic chemotherapies7. The incidence of PN with commonly used agents and risk factors has been summarized in Table I8–20.
PIs and IMiDs are the two major drug classes that cause PN in myeloma. Among PIs, bortezomib has the highest incidence of PN, with approximately one-third of patients developing this toxicity. Notably, subcutaneous9 and once-weekly administration21 of bortezomib leads to a lower incidence of severe PN without compromising efficacy. The time-to-onset of bortezomib-induced PN is around 2 months, with a plateau in incidence after 5–6 months6. With optimal dose modification, bortezomib-induced PN is completely reversible in 60% of patients at a median of 6 months, with initial improvement seen at a median of 2 months6. The primary target of bortezomib is small nerve fibers and dorsal root ganglion, leading to sensory polyneuropathy22. Involvement of large fibers and motor neurons causing areflexia, proprioception loss, and muscle weakness is rare and typically happens in the backdrop of severe sensory PN. Among second generation PIs, carfilzomib is associated with a significantly lower incidence of PN compared to bortezomib8,17. Notably, there is a lack of longitudinal toxicity data with newer PIs. The incidence of PN with the first-generation IMiD thalidomide is higher, seen in up to two-thirds of patients14,15. An increased incidence is noted beyond a daily dose of 200 mg and with a longer treatment duration22. Furthermore, motor and autonomic neuropathy is more common with thalidomide than with bortezomib. Thalidomide-induced PN is reversible in only one-fourth of patients and takes around 4–6 years22. Controlled studies of newer IMiDs [lenalidomide and pomalidomide] did not demonstrate an increased signal of PN16,18–20. In the first-in-human study of bispecific T-cell engager [BiTE] targeting B-cell maturation antigen [BCMA], two out of 42 patients developed grade 3 polyneuropathy, with both resolving by 2–3 months after treatment discontinuation23.
Management
The first step in management of PN is severity assessment and dose modification of the offending agent22. Other causes like metabolic or inflammatory neuropathy should be considered in the differential diagnosis, especially if the clinical course is atypical for treatment induced PN. Neurophysiologic studies are not sensitive to small-fiber involvement and may not mirror symptoms. However, they are helpful when other causes [e.g. demyelinating neuropathy related to underlying monoclonal gammopathy] are in the differential diagnosis. Regarding pharmacotherapy, duloxetine has RCT data on efficacy in chemotherapy-induced PN24. Although patients with bortezomib-induced PN were not included, neurotoxic targets of bortezomib are similar to taxanes and platinum7. Venlafaxine is also effective for neuropathic pain, demonstrated in a small RCT in the context of oxaliplatin-induced PN25. Drugs like gabapentin, pregabalin, and tricyclic antidepressants are commonly used off-label26. For poorly controlled neuropathic pain, opiates, tramadol, or inhaled cannabis could be considered, however, robust efficacy data are lacking.
Future Directions
Studies on predictive biomarkers for development of treatment induced-PN with PIs and IMiDs are urgently needed. Since PN is a symptomatic adverse event which is prone to under-reporting by clinicians27, incorporating patient-reported outcomes [PROs] in clinical trials and practice can help identify symptoms early. Quantifying the burden of PN using toxicity over time [ToxT] framework28 in clinical trials will help better estimate the toxicity burden with individual therapies. RCTs of non-pharmacological measures [e.g. acupuncture] should be conducted to mitigate symptoms and improve physical functioning.
1.2. Cognitive Impairment
Cognitive impairment has been reported with the use of IMiDs in myeloma29,30. In the phase III TOURMALINE-MM1 trial comparing ixazomib-lenalidomide-dexamethasone to lenalidomide-dexamethasone in relapsed/refractory myeloma, a decline in cognitive functioning from baseline was noted in both arms29. AHCT can also lead to short-term deterioration in cognitive functioning, which resolves by 6-month follow-up31. Cognitive impairment caused by IMiDs is mostly reversible within days to weeks after dose discontinuation30. High-dose dexamethasone can also lead to mood changes and cognitive impairment. Selinexor, which has received accelerated approval by the FDA for triple-class refractory myeloma, causes mental status changes in 17% of patients [6% with grade 3]32.
Management
Risk factors such as prior dementia, cognitive impairment, frailty, cerebrovascular disease, and polypharmacy should be meticulously assessed before initiating a new treatment regimen. Once cognitive impairment is detected on anti-myeloma therapy, search for alternate etiologies like hypothyroidism, vitamin B12 deficiency, and vascular dementia should be considered. For patients with persistent cognitive impairment, central nervous system stimulants like methylphenidate, anti-dementia drugs like donepezil, memantine, and gingko biloba, and bone marrow stimulants like erythropoietin have been used33, although robust evidence-base is lacking in this population.
Future Directions
Cognitive behavioral therapy is effective in cancer survivors with cognitive impairment34, and further studies are warranted in myeloma survivors. Incorporating frailty assessment tools like the one developed by the IMWG35 or Revised Myeloma Comorbidity Assessment [R-MCI]36 at critical time-points [e.g. diagnosis, relapse or initiating a new line of therapy] should be prospectively studied.
2. Secondary Malignancies
The association between myeloma and therapy-related myeloid neoplasms [t-MN] was initially described in the era of alkylating agent-based therapies37. Notably, there is marked heterogeneity in estimates of cumulative incidence across databases37–42, likely due to variable follow-up durations and ascertainment of myelodysplastic syndrome [MDS]. Prior to the era of PIs and IMiDs [1958–1996], the Swedish group showed a standardized incidence ratio [SIR] of 8.2 for secondary acute myeloid leukemia [AML] in patients with myeloma39. Another study on Finnish patients treated with alkylating agents with a median follow-up of 16 years revealed a cumulative risk of acute leukemia of approximately 10% at 9 years, with the risk being 45-fold compared to the general population38. Data from the SEER registry showed an increase in cumulative incidence of secondary malignancies from 4.7% in the 1995–99 to 6.3% in the 2005–09 cohort [p<0.001], with a progressive increase in the risk of secondary hematologic malignancies. Studies have also demonstrated an increased risk of acute lymphoblastic leukemia [ALL] in myeloma survivors, with SIR of 5.4843. A recent study on therapy-related ALL [t-ALL] identified myeloma as the second most common cancer preceding t-ALL, after breast cancer44. The median time to development of secondary malignancies from myeloma diagnosis is around 3–5 years38,45. Most population studies do not demonstrate an increase in the risk of therapy-related solid tumors in myeloma38,41.
Among currently used anti-myeloma therapies, there is robust evidence on increased risk of secondary malignancy with oral melphalan and lenalidomide. In a British RCT, duration of melphalan but not cyclophosphamide was associated with an increased risk of t-MN37. Post-transplant maintenance with lenalidomide increases the risk of both secondary hematologic [6% vs 3%] and solid neoplasms [7% vs 4%] compared to controls46. Furthermore, combined exposure to lenalidomide and oral melphalan [but not lenalidomide with cyclophosphamide] is associated with a five-fold higher risk of secondary hematologic malignancies compared to melphalan alone47. The incremental risk of developing secondary malignancies with AHCT in the era of PIs and IMIDs is unclear. A CIBMTR study on patients undergoing AHCT between 1990 and 2010 did not find an increased risk of second malignancies in general. However, subgroup analysis showed increased risk of AML [SIR 5.2] and melanoma [SIR 3.6]48. Risk factors for t-MN risk after AHCT are older age48, male sex48, obesity48, and duration of pre-transplant alkylator therapy49.
Management
Careful consideration of risk-factors for development of secondary malignances is warranted prior to initiating treatment in myeloma. Age-appropriate cancer screening per guidelines should be considered, since an increasing proportion of standard-risk myeloma patients may achieve long-term survival. Combined exposure to lenalidomide and oral melphalan should be avoided50. The duration of alkylator-based therapy, if used, should not exceed 6–12 months. The IMWG guidelines recommend performing a baseline bone marrow examination to rule out overt dysplasia or clonal cytogenetic abnormalities at initiation of lenalidomide maintenance51. Since transplant-eligible myeloma patients are known to have a high incidence of CHIP [Clonal Hematopoiesis of Indeterminate Potential] at approximately 30%52, long-term follow-up is needed to assess whether CHIP can be a predictor of subsequent t-MN. Survival in patients developing t-MN after myeloma is similar to those with secondary AML/MDS45 and allogeneic HCT is the only curative option in this setting.
Future Directions
Most of the data on characterization of secondary cancers in myeloma survivors include patients treated prior to the novel agent era. Hence, more data is needed on incidence and timeline for development of secondary cancers exclusively in the era of PIs and IMiDs with long follow-up. Research on genetic determinants of second cancers like damage in DNA repair genes and CHIP will help individualize therapy and surveillance. The impact of AHCT on development of second cancers in the current era also needs to be clarified.
3. Cardiovascular and Thrombotic Complications
3.1. Venous Thromboembolism
The diagnosis of MM per se is associated with a high risk of venous thromboembolism [VTE], with a relative risk of 9.2 [95% CI, 7.9–10.8] compared to matched general population53. A signal for increased VTE risk was identified with both thalidomide and lenalidomide54,55, which led to the initiation of routine risk-stratified thromboprophylaxis with IMiDs56.
The incidence of VTE with currently used IMiD-based combination regimens and risk factors are summarized in Table I19,53,55–61. The strongest patient-specific risk-factors of VTE include previous VTE, presence of central venous catheter, and prior surgery or immobilization56,60–62. Treatment-related risk factors include use of IMiDs with high-dose dexamethasone, doxorubicin, or multi-agent chemotherapy55,56,63. The incidence of VTE with IMiDs is highest in the first 6 months of therapy and higher in newly diagnosed compared to relapsed/refractory setting56. The risk of VTE with IMiD maintenance is extremely low and routine thromboprophylaxis is not recommended in this setting56. Notably, two RCTs of carfilzomib have demonstrated a signal for higher VTE risk with carfilzomib-based combination regimens compared to control8,64. Hence, the FDA label recommends thromboprophylaxis with carfilzomib-based regimens. A new risk model called “SAVED” score has been developed and validated, which has the following variables: Surgery within 90 days, Asian race, VTE history, age ≥80 years, and dexamethasone dose [c-index of 0.61]65. This model could be considered for identifying high-risk patients in clinical practice and designing thromboprophylaxis trials targeting such patients.
Management
As per IMWG consensus guidelines, patients with one or no risk factor should take aspirin [ASA] 81–325 mg daily and those with more than one risk-factor should take low molecular weight heparin [LMWH] or therapeutic dose warfarin56. Treatment should follow ASCO guidelines for cancer-related VTE66.
Future Directions
The incidence of VTE with contemporary IMiD-based regimens despite thromboprophylaxis is 6–7%67,68. Around 70% of VTE events happen on ASA67. Hence, well- designed clinical trials on thromboprophylaxis strategies targeting patients at a high risk of VTE are urgently needed. Currently, efforts are underway to test direct oral anticoagulants like apixaban for thromboprophylaxis in myeloma [NCT02066454]. Identifying novel biomarkers like differentially expressed genes or pathways for myeloma-associated VTE will also facilitate risk stratification and treatment.
3.2. Therapy-related Cardiac Toxicities
Patients with myeloma have a high incidence of baseline cardiovascular co-morbidities69. Furthermore, 10–20% of patients also have concomitant light-chain amyloidosis which requires a high index of suspicion during diagnostic workup.
Carfilzomib has demonstrated a signal for increased cardiac toxicities, with the incidence of all-grade and ≥grade 3 toxicities being 18.1% and 8.2% respectively and the risk ratio for high-grade cardiac toxicities being 2.270. Potential mechanisms include endothelial toxicity, inhibition of the ubiquitin proteasome system in cardiac myocytes, and cardiorenal syndrome71,72. The most common cardiac toxicities with carfilzomib are heart failure [systolic or diastolic], cardiac chest pain, hypertension, arrhythmia, acute coronary syndrome, and pulmonary hypertension70,71. Approximately 90% of cardiac toxicities happen in the first 3 months of treatment, with the median time to first event being 31 days and a plateau in the incidence curve beyond 5 months71. Elevated natriuretic peptide at baseline but not echocardiogram or electrocardiography findings are predictive for development of subsequent cardiac toxicities with PIs. Furthermore, normal baseline natriuretic peptide level which becomes elevated mid-first cycle is also an independent predictor of cardiotoxicity71. Although cardio-toxicity is considered to be a class effect of PIs, the incidence is lower with bortezomib, as shown in the ENDEAVOR trial, where the incidence of grade 3 hypertension and dyspnea was 9% and 5% in carfilzomib arm versus 3% and 2% in bortezomib arm respectively8.
Management
Management of therapy-related cardiotoxicity depends on the type of event and severity. In patients who develop signs and symptoms of congestive heart failure on carfilzomib-based regimens, initial evaluation should include an echocardiogram and cardio-oncology consultation. Carfilzomib should be held at this time until evaluation is completed. After signs and symptoms subside, re-challenge with carfilzomib at a lower dose and lower fluid volume could be considered after risk-benefit assessment. Notably, majority of cardiac toxicities are transient, with natriuretic peptide levels returning to near-baseline value at a median of 3.5 weeks after the event71. Transient dyspnea after carfilzomib administration has been well-described and is usually self-limiting, however, pulmonary hypertension, infection, and pulmonary embolism should be in the differential diagnosis.
Future Directions
Prospective studies with cardio-toxic agents to identify the trajectory and predictive biomarkers for specific cardiac events are needed to inform clinical practice and surveillance. Pre-clinical studies show that carfilzomib has off-target effects on the AMPKα/mTORC1 pathway and metformin can be potentially cardio-protective by restoring the AMPKα phosphorylation in mouse models, leading to reduced fractional shortening of ventricles73. This lays the groundwork for a clinical trial of metformin in patients at a high risk of cardiotoxicity with carfilzomib.
4. Infections and Immunity
Myeloma is associated with a high risk of infections, both due to the underlying disease and treatment-induced immunosuppression. A Swedish study on 9,253 patients treated prior to the novel agent-era showed a cumulative incidence of any infection of 41% at 2.6 years median follow-up, with the risk being seven-fold compared to controls74. Around 90% of patients had bacterial and 15% had viral infections. The most commonly reported bacterial infections were pneumonia, septicemia, cellulitis, pyelonephritis, meningitis, osteomyelitis, and endocarditis, in decreasing order of incidence. Herpes zoster and influenza were the most commonly reported viral infections. Notably, the risk of infection was highest in the first year following diagnosis. Furthermore, the risk ratio of infection increased progressively from six-fold in 1988–1993 cohort to 12-fold in 2000–2004 cohort compared to matched controls. A retrospective study has shown higher risk of cytomegalovirus infection in patients with extramedullary disease and low absolute neutrophil count75. Treatment with daratumumab is also a risk factor for Listeria infection, likely due to CD38 expression on activated macrophages, which play a role in Listeria defense76. The cumulative incidence of hepatitis B virus [HBV] reactivation, as described in a Japanese study, is 8% and 14% at 2 and 5 years respectively, among which, 17% developed clinical hepatitis77. On multivariable analysis, receipt of AHCT was associated with a significantly higher odds of HBV reactivation. The incidence of infections with currently used anti-myeloma agents are summarized in Table I8,17,19,57,74,75,78–82.
A validated predictive model for early ≥grade 3 infections has been developed in the context of treatment with IMiDs82. The scoring system includes 4 variables: ECOG Performance Status, ᵦ−2 microglobulin, hemoglobin, and lactate dehydrogenase. The model divided patients into high and low risk groups, with the risk of early serious infection being 24% vs 7% respectively [p< 0.0001; c-index, 0.66]. Notably, an initial grade ≥ 3 infection in the first 4 months of treatment was associated with a worse OS.
Management
One of the key clinical questions in this area is the optimal strategy for infection prevention. An RCT on patients receiving induction therapy prior to the novel agent-era did not demonstrate risk reduction of serious bacterial infections with prophylactic ciprofloxacin or trimethoprim-sulfamethoxazole [TMP-SMZ] over placebo83. However, another UK trial demonstrated a significant reduction in the risk of febrile episode or death [primary endpoint] in the first 12 weeks with daily oral levofloxacin compared to placebo [27% vs 19% respectively; P=0.002]84 in the context of cyclophosphamide and PI/IMiD-based therapy. Notably, levofloxacin significantly reduced the incidence of invasive gram-negative but not gram-positive infections. Based on available data, levofloxacin prophylaxis in the first 12 weeks can be considered in newly diagnosed patients. A small RCT in the 1990s had demonstrated a decrease in the risk of pneumonia or septicemia with use of intravenous immunoglobulin [IVIG] in the plateau phase [0% vs 24% respectively]85. The maximal benefit from IVIG was derived by those who had a suboptimal IgG antibody response to pneumococcal polysaccharide vaccine before therapy. Another small Italian RCT in the era of PIs and IMiDs has also shown that monthly subcutaneous immunoglobulin replacement in myeloma patients with secondary hypogammaglobulinemia leads to a lower infection rate and superior QoL86. However, given the cost and potential toxicities of IVIG, it is not used routinely for primary prophylaxis in newly diagnosed patients. However, IVIG should be considered in patients with recurrent infections and IgG level persistently below 400 mg/dl. Vaccinations including inactivated influenza and pneumococcal polysaccharide should be administered to all patients. For those receiving PIs, anti-CD-38 mAbs, or high-dose dexamethasone, herpes zoster virus prophylaxis is mandatory. Pneumocystis jiroveci pneumonia and anti-fungal prophylaxis should be considered in patients on high-dose dexamethasone87. Screening and prophylaxis for HBV reactivation should follow general ASCO guidelines, which recommends starting antiviral therapy in patients who are HBsAg-positive/anti-HBc–positive before or during anti-cancer treatment.
Future Directions
Whether all newly diagnosed myeloma patients treated with combinations of PIs, IMiDs, mAbs, and low-dose dexamethasone need infection prophylaxis still remains an open question. Furthermore, whether antibiotics [levofloxacin and/or TMP-SMZ] or immunoglobulin replacement is better for primary prophylaxis also needs to be tested in well-designed RCTs in the context of current treatment landscape. Designing these studies as a companion to therapeutic trials run by cooperative groups might be feasible and facilitate faster accrual. The validated tool for infection prediction, as mentioned earlier82, can be used to target high-risk patients for such trials.
5. Renal Complications
Renal health is an important survivorship issue in patients with myeloma. Approximately one in five patients with myeloma present with renal impairment at diagnosis88. Complete renal response can be seen in 40% of these patients treated in the era of PIs and IMiDs, which leads to a superior OS compared to non-responders88. In patients with cast nephropathy, instituting prompt bortezomib-based systemic therapy along with intravenous hydration and treating hypercalcemia aggressively are key to renal recovery89. Management of myeloma-related renal dysfunction in the novel agent-era is beyond the scope of this review and has been described elsewhere90.
Among current treatments, signal for therapy-related renal toxicity has been seen with the second generation PI carfilzomib and with bisphosphonates8,17,91,92. RCTs have shown a higher rate of all-grade and ≥grade 3 renal toxicities in carfilzomib arms compared to controls8,17,64,93. Majority of renal toxicities were classified as acute kidney injury [AKI]. However, there is a lack of prospective data on risk factors and trajectory of renal toxicities with carfilzomib. A study on real-world experience with carfilzomib-based regimens has shown the risk of all-grade AKI and creatinine increase to be 28% and 37% respectively, with majority being grade 1–2. Most toxicities were transient, similar to cardiac toxicities94. The incidence of renal toxicity is higher in patients with eGFR<508,93. Endothelial toxicity, cardiorenal syndrome, and thrombotic microangiopathy [TMA] are some of the proposed mechanism for renal adverse events with carfilzomib72. Zoledronic acid also increases the risk of AKI compared to denosumab, with the risk being more in patients with eGFR<60 ml/min91. Notably, zoledronic acid should be avoided in patients with eGFR<30 ml/min. The incidence and risk-factors of therapy-related renal toxicities is summarized in Table I.
Management
There is a lack of data on risk factors and dose modification strategy specifically for renal toxicities. Hence, general dose modification guidelines should be followed. Notably, lenalidomide can be administered safely in a once-daily dosing regimen in patients with creatinine clearance <30 ml/min at doses of at least 15 mg, whether or not on dialysis95. For patients on bisphosphonates, a spot urine sample should be checked every 3–6 months, and if positive, should be followed by 24-hour urine protein analysis to look for the presence and degree of albuminuria96. If patients develop AKI on anti-myeloma therapy, causes such as progression of underlying disease, hypercalcemia, development of renal amyloidosis, and sepsis should be ruled out. Supportive care should be initiated, including adequate hydration, avoidance of nephrotoxic agents, and optimal blood pressure control. TMA should be in the differential diagnosis if AKI develops on carfilzomib. Case reports have suggested that carfilzomib-induced TMA is associated with heterozygous deletion of complement pathway genes CFHR3-CFHR1, and eculizumab may be effective in this setting97.
Future Directions
Prospective studies are needed to identify the nature, trajectory, and risk factors of renal toxicity with carfilzomib, especially with widespread use in frontline setting. Pre-clinical studies to identify the molecular mechanism of renal toxicity may help devise preventative strategies.
6. Musculoskeletal Complications [Bone Health]
Patients with myeloma are at risk of skeletal-related events [SREs], either as an end-organ damage from underlying disease or from prolonged administration of corticosteroids. In clinical trials, SREs are typically defined as a composite of pathologic fracture, spinal cord compression, and need for surgery or palliative radiotherapy to the affected bone. Even with routine use of bone-modifying agents [BMAs], the incidence of SREs after treatment initiation in newly diagnosed myeloma remains substantial at 27–45% in recent clinical trials [Table I]91,92. Approximately 80% of SREs happen in the first 6 months after diagnosis91. Baseline SRE at diagnosis is a risk-factor for subsequent SREs within 1 year98. Corticosteroids form the backbone of most combination regimens used in newly diagnosed and relapsed/refractory MM. A prednisolone equivalent of more than 30 mg per day or a cumulative dose of more than 5g is associated with 14-fold increase in the risk of vertebral fracture and a 3-fold increase in the risk of hip fracture99. However, the fracture risk decreases dramatically after discontinuation of corticosteroids100.
Management
All patients with MM should be initiated on BMAs, either bisphosphonates or denosumab, at diagnosis irrespective of the presence of baseline bone disease96,101. BMAs may be discontinued after two years at at physician’s discretion if a patient is in deep hematologic remission. However, denosumab has a reversible mechanism of action, with reports of rebound fractures after discontinuation in the setting of osteoporosis102. Hence, patients should be switched to bisphosphonates in case denosumab is discontinued. In patients with renal impairment at baseline, denosumab should be preferred over zoledronic acid due to a lower incidence of AKI96. Furthermore, denosumab can be administered subcutaneously unlike bisphosphonates, which are administered intravenously. For patients on zoledronic acid, treatment burden can be reduced by de-escalating frequency from every 4-week to every 12-week without compromising efficacy103. Both bisphosphonates and denosumab leads to comparable rates of jaw osteonecrosis [≈4%]91. Strategies to prevent ONJ are summarized in Table II. In the maintenance setting, adding corticosteroids to lenalidomide does not improve PFS or OS, both in transplant-eligible and ineligible patients104. Hence, corticosteroid should be strategically discontinued once a deep hematologic remission is achieved to avoid toxicities. If BMAs are discontinued in first remission after 2 years of treatment, they should be resumed at subsequent relapse96.
Future Directions
Further investigation on serum and urinary biomarkers of bone resorption in myeloma survivors can be potentially used to personalize the intensity and duration of BMAs. Long-term follow up data from clinical trials on denosumab should be investigated to identify the incidence of rebound fractures, if any, after dose discontinuation. Clinical trials on novel BMAs like romosozumab [monoclonal antibody targeting sclerostin] and sotatercept [a fusion protein of the extracellular domain of high affinity activin receptor IIA and human immunoglobulin G Fc domain] should be conducted in patients developing SREs on currently used BMAs.
7. Hematologic Complications
Anemia is one of the most common hematologic toxicities in myeloma and can be a result of anti-myeloma therapy or poorly controlled disease. A Cochrane review of more than 20,000 cancer patients enrolled in RCTs of erythropoietin stimulating agents [ESAs] showed significantly lower transfusion requirement but higher risk of thromboembolic events and death in patients receiving ESAs105. In non-myeloid hematologic malignancies like myeloma, current ASCO/ASH guidelines recommend waiting for hematologic response of treatments prior to initiating ESAs106. Red blood cell transfusions can be considered in the interim. If hemoglobin is persistently <10 g/dl, ESAs can be administered after considering alternate causes of anemia, like nutritional deficiency or therapy-related myeloid neoplasm. The target hemoglobin should be individualized to the lowest level needed to decrease transfusion requirements and should not exceed 12 g/dl. In patients who do not respond within 6 to 8 weeks, ESAs should be discontinued. Iron supplementation should be considered in patients receiving ESAs both with and without iron deficiency.
Neutropenia has been reported with several combination regimens in myeloma, especially those including traditional cytotoxic agents and newer IMiDs107. ASCO recommends using granulocyte-colony stimulating factor [G-CSF] as primary prophylaxis with chemotherapy regimens having a febrile neutropenia risk of ≥20%108. Secondary G-CSF prophylaxis can be considered if dose modification is thought to compromise treatment outcome. G-CSF should be administered after AHCT to reduce the duration and severity of neutropenia108.
8. Endocrine Complications
Thyroid function abnormalities have been reported with the use of IMiDs. The incidence of new thyroid dysfunction after lenalidomide initiation is 6%109. A prospective study revealed grade 2 thyroid abnormality in 10% of patients, with the median time to thyroid dysfunction being 4 months [range, 2–8]110. Hypothyroidism is also a late effect of AHCT111. Patients should be assessed for baseline thyroid dysfunction prior to initiating IMiDs and a high index of suspicion for hypo- and hyperthyroidism should be maintained during the treatment course.
Prolonged administration of corticosteroids, which forms the backbone of most combination regimens in myeloma, can lead to adrenal insufficiency [AI]. A retrospective study showed a cumulative median dexamethasone dose of 960 mg prior to developing AI in myeloma patients112. Patients experiencing signs and symptoms of AI [e.g. hypotension, weight loss, diarrhea, and fatigue] should have a serum cortisol level and ACTH stimulation test done to establish diagnosis. Co-management with endocrinologist should be considered.
9. Ocular Complications
Eyelid complications, including chalazia and blepharitis, has been reported with bortezomib113. Time-to-ocular complication from first bortezomib exposure is around 3 months. Rare cases of eyelid complications have been reported after carfilzomib as well113. Treatment includes ocular therapy or dose modification/omission of the offending agent. Average time to resolution with ocular therapy alone is around 2 months. Ocular therapy consists of warm compresses, topical antibiotics [+/− steroids], and oral antibiotic. Prolonged oral doxycycline can be used to treat severe bortezomib-induced blepharitis114. Combination of ocular therapy and discontinuation of the offending agent leads to a higher rate of resolution than ocular therapy alone113.
A novel antibody-drug conjugate targeting B-cell maturation antigen [Belantamab mafadotin; GSK2857916], which has a high single-agent activity [60%] in relapsed MM, leads to ocular toxicity in the form of corneal events115. Corneal toxicity was noted in 63% of patients with 9% being grade 3 or higher. The most common finding on ophthalmologic examination was superficial punctate keratitis, with decrease in visual acuity by Snellen method noted in most patients during treatment. Around one-third of patients had complete resolution, with the median duration of toxicity being 30 days. Management included dose modification or omission, and supportive care with artificial tears and steroid eye drops. Further data on long-term follow up and phase 3 trials will establish the trajectory and late effects of corneal toxicities with GSK2857916.
10. Frailty, Symptomatic AEs, and Quality of Life
Myeloma is a disease of older adults, with approximately one-third of patients more than 75 years of age. The IMWG frailty index incorporates age, activities of daily living [ADL], instrumental ADLs, and Charlson Comorbidity Index to identify three categories of patients: fit, intermediate fit, and frail. Baseline frailty status is predictive of treatment discontinuation rate, severe non-hematologic toxicities, and overall survival35. Gait speed and grip strength, which can be easily assessed in the clinic, also identifies frail patients and predicts for higher mortality, unplanned hospitalization, and emergency department visits116. Assessment of frailty status at baseline and decision points [e.g. initiation of subsequent lines of therapy] is important for treatment decision-making and supportive care delivery.
Several symptoms including pain, fatigue, anxiety, depression, and decreased mobility are independently associated with global QoL in patients with myeloma117. A systematic review of QoL findings from myeloma clinical trials showed clinically meaningful QoL improvements with effective first-line therapy118. However, in relapsed myeloma, no clinically meaningful improvement or deterioration was observed, with maintenance of QoL on treatment in most studies. Gastrointestinal symptomatic adverse events including nausea, vomiting, diarrhea, or constipation can be seen with several oral agents including thalidomide, lenalidomide, pomalidomide, ixazomib, and panobinostat. Diarrhea can happen in up to 44% of patients receiving lenalidomide maintenance119. Further studies are needed on the value of assessing PROs in clinical practice and developing survivorship care plans in this patient population.
Survivorship Care in Multiple Myeloma
In cancer survivorship, the term “survivor” applies to individuals with cancer, anywhere along the disease trajectory from diagnosis till end-of-life120. Much of the work in cancer survivorship has focused on curable cancers e.g. early stage breast cancer, Hodgkin’s lymphoma, and survivors of allogeneic HCT. However, the survivorship care needs of patients with myeloma is unique due to several factors. First, myeloma remains an incurable disease despite advances in therapy and marked improvement in overall survival. Second, the treatment paradigm has shifted from a fixed duration-therapy to continuous therapy until disease progression or unacceptable toxicity. Hence, implementation of survivorship care plan [SCP] at a single time-point may not be meaningful since the exposure profile changes along the disease trajectory. Third, despite myeloma being an incurable disease, around one-tenth of transplant-eligible patients are able to achieve an overall survival comparable to demographically matched healthy population121. The proportion of patients achieving long-term survival will likely increase due to current advances in therapy. Hence, further research on non-cancer comorbidities, QoL, and myeloma- or therapy-related late adverse effects in this population will be needed to optimize survivorship care. Fourth, fear of relapse and prognostic uncertainty remains a challenge for patients and caregivers, given the rapidly changing treatment landscape and new insights into biology and risk stratification. Longitudinal conversations with patients regarding their prognosis, treatment options, and goals of care are important to facilitate informed treatment decisions. Finally, patients with myeloma can experience financial burden due to high drug costs122, missed work, and difficulty obtaining or maintaining health insurance. Further research on the prevalence of financial toxicity, impact on QoL, and identifying opportunities for intervention [e.g. financial navigator programs] is urgently needed in this population.
Survivorship care is best delivered under the framework of patient-centric care models. There are several patient, provider, and system level factors that are important in this context. Important patient-level factors include distance from myeloma treatment center, disease biology, complexity of ongoing therapy, and depth of disease control. Provider-level factors include expertise and comfort in providing survivorship care, knowledge of survivorship care needs, and relationship with patient. System-level concerns include payer requirements and access to subspecialties for multidisciplinary care. Since myeloma patients are on continuous therapy, the treating hematologist/oncologist is often the primary clinician driving survivorship care with the assistance of primary care providers [PCP] and other subspecialties depending on patients’ unique needs [Figure II]. PCPs should be educated about common side-effects of medications [e.g. hypertension with carfilzomib] and roles of different members in the care team should be clearly defined. Co-management with subspecialty teams may be needed in patients developing specific toxicities [e.g. endocrinology consultation for management of adrenal insufficiency or uncontrolled diabetes exacerbated by corticosteroids]. Ancillary services like physical therapy, social workers, financial navigator, clinical psychologist, and dietician should be utilized as needed.
Figure II.
Survivorship care model for patients living with multiple myeloma
Primary responsibility for cancer-related care and complications from treatment or underlying cancer
Primary responsibility for managing non-cancer co-morbidities
Co-management with multiple myeloma specialist or primary care physician on special situations e.g. co-management with cardiooncology for carfilzomib-induced cardiotoxicity
Communication time-points between multiple myeloma specialist, primary care physician, and other subspecialists:
a) Discuss multiple myeloma diagnosis, planned induction and consolidation therapy, and anticipated adverse events.
b) Discuss maintenance strategy, adverse effects of maintenance therapy, and plan for post-transplant immunization in transplant-eligible patients.
c) Discuss disease relapse, treatment strategy, and anticipated adverse events of treatment
d) Communication between multiple myeloma specialist, primary care physician, and subspecialist during periods of active subspecialty care needs.
The role of SCPs in myeloma is not well-defined. In patients remaining in a stable remission post-transplant, individualized SCP has been shown to reduce distress and improve mental QoL in an RCT123. However, due to constantly changing exposure profile in most patients, SCPs at several time-points along the disease trajectory [e.g. Figure II; time-points a, b, c, and d] may facilitate better care-coordination and further studies on the utility of SCPs are warranted.
Conclusion and Future Directions
With transformation of myeloma into a chronic disease, focus on immediate and late complications from treatment and underlying disease is critical to deliver optimal survivorship care. The knowledge gap and agenda for future research is summarized in Table III. Long-term follow-up data from clinical trials is needed to assess the incidence of late complications like secondary malignancies and major adverse cardiovascular events. Large databases or tumor registries can also be helpful in identifying rare adverse events. In a study from the UK with 1,843 myeloma patients who have survived one year after diagnosis, the incidence of coronary artery disease, arrhythmia, heart failure, valvular heart disease, pericarditis, and stroke was higher compared to demographically matched-controls124. Since infection-related morbidity and mortality is one of the major non-malignant late effect in myeloma survivors, further research is needed to identify patients at high risk of infection and design evidence-based strategies to mitigate risk. Reduction of SREs along the disease trajectory is another area of unmet need in this population as the risk remains substantial [27–45%] despite currently available agents. With an increasing prevalence of myeloma survivors, the community is responsible for appropriately monitoring and managing both early and late complications. Further research to investigate the biology and management of treatment-related complications alongside drug development will continue to improve the quantity and quality of life in patients with myeloma.
Table III.
Overview of Knowledge Gaps and Research Agenda for Management of Toxicities in MM
| Toxicity/ Late Effects | Knowledge Gaps and Research Priorities |
|---|---|
| Peripheral Neuropathy | ➢ Identify risk-factors and predictive biomarkers [including pharmacogenomic markers] for development of PN with PIs and IMiDs ➢ Assessment of longitudinal persistence of PN-related symptoms using Toxicity over Time [ToxT] models28 ➢ Implementation of PRO instruments in clinical trials and practice to quantify symptom burden and potential early intervention ➢ Conduct RCTs on pharmacological as well as non-pharmacological interventions for management of therapy related PN in MM |
| Secondary Malignancy | ➢ Conduct population studies using large databases to estimate the risk of secondary hematologic and solid malignancies exclusively in the era of PIs and IMiDs ➢ Long-term follow up of ongoing RCTs in MM [Median time to development of secondary malignancies from MM diagnosis is around 3–5 years38,45], with incorporation of secondary malignancies as an additional endpoint ➢ Investigate the trajectory of incidence, genetic makeup, and treatment outcomes of secondary malignancies after MM diagnosis ➢ Identify predictive biomarkers [e.g. CHIP or inherited damage in DNA-damage repair genes] to ascertain the risk of developing subsequent secondary malignancies ➢ Investigate the impact of duration of lenalidomide maintenance on risk of secondary malignancies ➢ Long-term follow up of patients undergoing HDM-ASCT without exposure to alkylating agents or anthracycline during induction therapy to ascertain the risk of second malignancies with auto-transplant in the context of novel-agent based induction and subsequent therapies |
| Venous Thromboembolism | ➢ Develop and validate risk –stratification models for predicting VTE with contemporary combination regimens. ➢ Externally validate SAVED and IMPEDE-VTE scores in datasets with contemporary IMiD-based combination regimens [e.g. IMiDs in combination with PIs, MoABs, or both] ➢ Conduct RCTs of novel oral anticoagulants for thromboprophylaxis in MM ➢ Secondary analysis of clinical trials to identify the cumulative incidence and trajectory of VTE events with new IMiD-based combination regimens |
| Therapy-related Cardiac Toxicity | ➢ Conduct prospective multi-center studies on CVAEs with specific agents of interest like carfilzomib. ➢ Secondary analysis of individual patient-level clinical trial data to identify predictors of cardiac toxicities ➢ Preclinical studies to identify the mechanistic basis of cardiotoxicity with proteasome inhibitors ➢ Perform RCTs of cardio-protective strategies [e.g. using ACEi or beta-blockers] in high-risk patients ➢ Clinical trial of metformin to prevent carfilzomib-induced cardiotoxicity based on promising pre-clinical data |
| Infections and Immunity | ➢ Develop a validated predictive model for severe infections in the context of treatment with combination of novel agents [PIs, IMiDs, and mAbs] ➢ Perform RCTs on prophylactic antibiotics in patients at a high risk of severe infections and infection-related mortality ➢ Perform RCTs on IVIG in the context of currently used mAb-based combination regimens ➢ Periodic investigation of population-based registries and databases to identify risk and epidemiology of infections with changing treatment patterns ➢ Translational studies on immune reconstitution in MM upon treatment with different combination regimens |
| Therapy-related Renal Toxicity | ➢ Conduct prospective multi-center studies to assess the nature and trajectory of renal toxicities with carfilzomib-based combination regimens. ➢ Develop and validate predictive model for identifying patients at a high risk of therapy-related renal toxicity |
| Bone Health | ➢ Incorporation and reporting of PROs in clinical trials of bone-modifying agents in MM ➢ Conduct correlative studies to identify biomarkers for bone resorption which can be potentially used to tailor therapy ➢ Conduct clinical trials on novel bone modifying agents like romosozumab [monoclonal antibody targeting sclerostin] and sotatercept [a fusion protein of the extracellular domain of high affinity activin receptor IIA and human immunoglobulin G Fc domain] in MM ➢ Conduct randomized trials on structured physical activity and impact on bone health in MM survivors ➢ Identify patient-related, disease-related, and treatment-related risk factors of SREs in MM |
| Survivorship Care | ➢ Conduct quantitative and qualitative research on financial toxicity with anti-cancer therapy in MM ➢ Conduct RCTs on palliative care intervention to optimize symptom management |
Abbreviations: PN: Peripheral Neuropathy. PI: Proteasome Inhibitors. IMiDs: Immunomodulatory drugs. ToxT: Toxicity over Time. PRO: Patient-reported outcome. HRQoL: Health-related quality of life. PN: Peripheral Neuropathy. RCT: Randomized Controlled Trial. MM: Multiple Myeloma. CHIP: Clonal Hematopoiesis of Undetermined Significance. ASCT: Autologous Stem Cell Transplantation. t-MN: Therapy-related Myeloid Neoplasm. MoABs: Monoclonal Antibodies. HDACi: Histone Deacetylase Inhibitors. IVIG: Intravenous Immunoglobulins. CVAE: Cardiovascular adverse events. ACEi: Angiotensin Converting Enzyme inhibitors. PROs: Patient-reported outcomes
Acknowledgments
Funding Source: Navneet Majhail is partially supported by an NIH grant R01-CA215134.
Footnotes
Conflicts of Interest: No relevant financial conflicts of interest.
Ethics Committee Approval: Not Applicable
References
- 1.Cowan AJ, Allen C, Barac A, et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA oncology. September 1 2018;4(9):1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. The Lancet. Public health. March 2019;4(3):e137–e147. [DOI] [PubMed] [Google Scholar]
- 3.Turesson I, Bjorkholm M, Blimark CH, Kristinsson S, Velez R, Landgren O. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. European journal of haematology. April 20 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet. Oncology. November 2014;15(12):e538–548. [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. March 2012;18(3):348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Mateos MV, Richardson PG, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. European journal of haematology. January 2011;86(1):23–31. [DOI] [PubMed] [Google Scholar]
- 7.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nature reviews. Neurology. December 2010;6(12):657–666. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. The Lancet. Oncology. January 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. The Lancet. Oncology. May 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Barlogie B, Berenson J, et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. New England Journal of Medicine. 2003;348(26):2609–2617. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. The New England journal of medicine. June 16 2005;352(24):2487–2498. [DOI] [PubMed] [Google Scholar]
- 12.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. The New England journal of medicine. August 28 2008;359(9):906–917. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (London, England). January 19 2019;393(10168):253–264. [DOI] [PubMed] [Google Scholar]
- 14.Prince HM, Mileshkin L, Roberts A, et al. A multicenter phase II trial of thalidomide and celecoxib for patients with relapsed and refractory multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. August 1 2005;11(15):5504–5514. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III Clinical Trial of Thalidomide Plus Dexamethasone Compared With Dexamethasone Alone in Newly Diagnosed Multiple Myeloma: A Clinical Trial Coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2006;24(3):431–436. [DOI] [PubMed] [Google Scholar]
- 16.Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. The Lancet. Oncology. October 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 17.Facon T, Lee JH, Moreau P, et al. Randomized phase 3 study of carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible, NDMM patients. Blood. 2019:blood-2018–2009-874396. [DOI] [PubMed] [Google Scholar]
- 18.Moreau P, Masszi T, Grzasko N, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. New England Journal of Medicine. 2016;374(17):1621–1634. [DOI] [PubMed] [Google Scholar]
- 19.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine. September 4 2014;371(10):906–917. [DOI] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. New England Journal of Medicine. 2018;379(19):1811–1822. [DOI] [PubMed] [Google Scholar]
- 21.Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. April 22 2010;115(16):3416–3417. [DOI] [PubMed] [Google Scholar]
- 22.Delforge M, Blade J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. The Lancet. Oncology. November 2010;11(11):1086–1095. [DOI] [PubMed] [Google Scholar]
- 23.Topp MS, Duell J, Zugmaier G, et al. Anti–B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. Journal of Clinical Oncology.0(0):JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 24.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. April 3 2013;309(13):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Annals of oncology : official journal of the European Society for Medical Oncology. January 2012;23(1):200–205. [DOI] [PubMed] [Google Scholar]
- 26.Delforge M, Ludwig H. How I manage the toxicities of myeloma drugs. Blood. April 27 2017;129(17):2359–2367. [DOI] [PubMed] [Google Scholar]
- 27.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nature reviews. Clinical oncology. May 2016;13(5):319–325. [DOI] [PubMed] [Google Scholar]
- 28.Thanarajasingam G, Minasian LM, Baron F, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. The Lancet. Haematology. November 2018;5(11):e563–e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leleu X, Masszi T, Bahlis NJ, et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. American journal of hematology. May 4 2018. [DOI] [PubMed] [Google Scholar]
- 30.Morgan AE, Smith WK, Levenson JL. Reversible dementia due to thalidomide therapy for multiple myeloma. The New England journal of medicine. May 1 2003;348(18):1821–1822. [DOI] [PubMed] [Google Scholar]
- 31.Ahmedzai SH, Snowden JA, Ashcroft AJ, et al. Patient-Reported Outcome Results From the Open-Label, Randomized Phase III Myeloma X Trial Evaluating Salvage Autologous Stem-Cell Transplantation in Relapsed Multiple Myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. July 1 2019;37(19):1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. The New England journal of medicine. August 22 2019;381(8):727–738. [DOI] [PubMed] [Google Scholar]
- 33.Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. The Lancet. Oncology. February 2019;20(2):e92–e102. [DOI] [PubMed] [Google Scholar]
- 34.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: a cancer journal for clinicians. March 2015;65(2):123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelhardt M, Domm AS, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. May 2017;102(5):910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuzick J, Erskine S, Edelman D, Galton DA. A comparison of the incidence of the myelodysplastic syndrome and acute myeloid leukaemia following melphalan and cyclophosphamide treatment for myelomatosis. A report to the Medical Research Council’s working party on leukaemia in adults. British journal of cancer. May 1987;55(5):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acute leukaemia and other secondary neoplasms in patients treated with conventional chemotherapy for multiple myeloma: a Finnish Leukaemia Group study. European journal of haematology. August 2000;65(2):123–127. [DOI] [PubMed] [Google Scholar]
- 39.Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. British journal of cancer. September 28 2001;85(7):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonsdottir G, Lund SH, Bjorkholm M, et al. The impact of prior malignancies on second malignancies and survival in MM patients: a population-based study. Blood advances. November 28 2017;1(25):2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa LJ, Godby KN, Chhabra S, Cornell RF, Hari P, Bhatia S. Second primary malignancy after multiple myeloma-population trends and cause-specific mortality. British journal of haematology. August 2018;182(4):513–520. [DOI] [PubMed] [Google Scholar]
- 42.Gertz MA, Terpos E, Dispenzieri A, et al. Therapy-related myelodysplastic syndrome/acute leukemia after multiple myeloma in the era of novel agents. Leukemia & lymphoma. June 2015;56(6):1723–1726. [DOI] [PubMed] [Google Scholar]
- 43.Chakraborty S, Hauke RJ, Bonthu N, Tarantolo SR. Increased incidence of a second lymphoproliferative malignancy in patients with multiple myeloma--a SEER based study. Anticancer research. October 2012;32(10):4507–4515. [PubMed] [Google Scholar]
- 44.Saygin C, Kishtagari A, Cassaday RD, et al. Therapy-related acute lymphoblastic leukemia is a distinct entity with adverse genetic features and clinical outcomes. Blood advances. December 23 2019;3(24):4228–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonsdottir G, Lund SH, Bjorkholm M, et al. Survival in multiple myeloma patients who develop second malignancies: a population-based cohort study. Haematologica. April 2016;101(4):e145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. October 10 2017;35(29):3279–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. The Lancet. Oncology. March 2014;15(3):333–342. [DOI] [PubMed] [Google Scholar]
- 48.Mahindra A, Raval G, Mehta P, et al. New cancers after autotransplantations for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. April 2015;21(4):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Govindarajan R, Jagannath S, Flick JT, et al. Preceding standard therapy is the likely cause of MDS after autotransplants for multiple myeloma. British journal of haematology. November 1996;95(2):349–353. [DOI] [PubMed] [Google Scholar]
- 50.Chung A, Liedtke M. Therapy-related myeloid neoplasms after treatment for plasma-cell disorders. Best practice & research. Clinical haematology. March 2019;32(1):54–64. [DOI] [PubMed] [Google Scholar]
- 51.Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Annals of oncology : official journal of the European Society for Medical Oncology. February 1 2017;28(2):228–245. [DOI] [PubMed] [Google Scholar]
- 52.Mouhieddine TH, Park J, Redd RA, et al. The Role of Clonal Hematopoiesis of Indeterminate Potential (CHIP) in Multiple Myeloma: Immunomodulator Maintenance Post Autologous Stem Cell Transplant (ASCT) Predicts Better Outcome. Blood. 2018;132(Supplement 1):749–749. [Google Scholar]
- 53.Kristinsson SY, Pfeiffer RM, Bjorkholm M, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. June 17 2010;115(24):4991–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. The New England journal of medicine. June 21 2001;344(25):1951–1952. [DOI] [PubMed] [Google Scholar]
- 55.Menon SP, Rajkumar SV, Lacy M, Falco P, Palumbo A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer. April 1 2008;112(7):1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. February 2008;22(2):414–423. [DOI] [PubMed] [Google Scholar]
- 57.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet (London, England). February 4 2017;389(10068):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet (London, England). December 18 2010;376(9758):2075–2085. [DOI] [PubMed] [Google Scholar]
- 59.Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. August 1 2004;101(3):558–566. [DOI] [PubMed] [Google Scholar]
- 60.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of internal medicine. March 27 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 61.Li A, Wu QV, Warnick G, et al. The HAS-RISC Score for Venous Thromboembolism (VTE) Risk Stratification in Newly Diagnosed Multiple Myeloma Patients Starting Immunomodulatory Drugs (IMID). Blood. 2018;132(Suppl 1):144–144. [Google Scholar]
- 62.Sanfilippo KM, Wang T-F, Luo S, Carson KR, Thomas TS, Gage B. Prediction of venous thromboembolism (VTE) in multiple myeloma (MM): Myeloma clot score (MCS). Journal of Clinical Oncology. 2018;36(15_suppl):6585–6585. [Google Scholar]
- 63.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. The Lancet. Oncology. January 2010;11(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. New England Journal of Medicine. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 65.Li A, Wu Q, Luo S, et al. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients With Multiple Myeloma. Journal of the National Comprehensive Cancer Network : JNCCN. July 1 2019;17(7):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. August 5 2019:JCO1901461. [DOI] [PubMed] [Google Scholar]
- 67.Leleu X, Rodon P, Hulin C, et al. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thrombosis and haemostasis. October 2013;110(4):844–851. [DOI] [PubMed] [Google Scholar]
- 68.Chakraborty R, Bin Riaz I, Malik SU, et al. Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis. Cancer. January 8 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson D Jr., Esseltine DL, Regnault A, Meunier J, Liu K, van de Velde H. The influence of baseline characteristics and disease stage on health-related quality of life in multiple myeloma: findings from six randomized controlled trials. British journal of haematology. August 2016;174(3):368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waxman AJ, Clasen S, Hwang WT, et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA oncology. March 8 2018;4(3):e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornell RF, Ky B, Weiss BM, et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. June 12 2019:JCO1900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Garcia D, Cornell RF, et al. Cardiovascular and Thrombotic Complications of Novel Multiple Myeloma Therapies: A Review. JAMA oncology. July 1 2017;3(7):980–988. [DOI] [PubMed] [Google Scholar]
- 73.Efentakis P, Kremastiotis G, Varela A, et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood. February 14 2019;133(7):710–723. [DOI] [PubMed] [Google Scholar]
- 74.Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. January 2015;100(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa T, Aisa Y, Shimazaki K, Ito C, Nakazato T. Cytomegalovirus reactivation in patients with multiple myeloma. European journal of haematology. January 2016;96(1):78–82. [DOI] [PubMed] [Google Scholar]
- 76.Khan S, Vaisman A, Hota SS, et al. Listeria Susceptibility in Patients With Multiple Myeloma Receiving Daratumumab-Based Therapy. JAMA oncology. November 27 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsukune Y, Sasaki M, Odajima T, et al. Incidence and risk factors of hepatitis B virus reactivation in patients with multiple myeloma in an era with novel agents: a nationwide retrospective study in Japan. Blood cancer journal. November 23 2017;7(12):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. The New England journal of medicine. February 8 2018;378(6):518–528. [DOI] [PubMed] [Google Scholar]
- 79.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The New England journal of medicine. April 6 2017;376(14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. The New England journal of medicine. October 6 2016;375(14):1319–1331. [DOI] [PubMed] [Google Scholar]
- 81.Facon T, Kumar S, Plesner T, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. New England Journal of Medicine. 2019;380(22):2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dumontet C, Hulin C, Dimopoulos MA, et al. A predictive model for risk of early grade >/= 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial. Leukemia. June 2018;32(6):1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vesole DH, Oken MM, Heckler C, et al. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. December 2012;26(12):2517–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drayson MT, Bowcock S, Planche T, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. The Lancet. Oncology. October 23 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapel HM, Lee M, Hargreaves R, Pamphilon DH, Prentice AG. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma. Lancet (London, England). April 30 1994;343(8905):1059–1063. [DOI] [PubMed] [Google Scholar]
- 86.Vacca A, Melaccio A, Sportelli A, Solimando AG, Dammacco F, Ria R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: a randomized trial. Clinical immunology (Orlando, Fla.). June 2018;191:110–115. [DOI] [PubMed] [Google Scholar]
- 87.Network NCC. Multiple Myeloma. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma_blocks.pdf Accessed 6/26/2019.
- 88.Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood cancer journal. March 20 2015;5:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonsalves WI, Godby K, Kumar SK, Costa LJ. Limiting early mortality: Do’s and don’ts in the management of patients with newly diagnosed multiple myeloma. American journal of hematology. January 2016;91(1):101–108. [DOI] [PubMed] [Google Scholar]
- 90.Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. May 1 2016;34(13):1544–1557. [DOI] [PubMed] [Google Scholar]
- 91.Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. The Lancet. Oncology. March 2018;19(3):370–381. [DOI] [PubMed] [Google Scholar]
- 92.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet (London, England). December 11 2010;376(9757):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hajek R, Masszi T, Petrucci MT, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. Jan 2017;31(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood advances. February 28 2017;1(7):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mikhael J, Manola J, Dueck AC, et al. Lenalidomide and dexamethasone in patients with relapsed multiple myeloma and impaired renal function: PrE1003, a PrECOG study. Blood cancer journal. August 29 2018;8(9):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson K, Ismaila N, Flynn PJ, et al. Role of Bone-Modifying Agents in Multiple Myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. March 10 2018;36(8):812–818. [DOI] [PubMed] [Google Scholar]
- 97.Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygous CFHR3-CFHR1 deletion. Blood advances. December 11 2018;2(23):3443–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim C, Bhatta S, Cyprien L, Fonseca R, Hernandez RK. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: Observations from real-world data. Journal of bone oncology. February 2019;14:100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis and rheumatism. January 2007;56(1):208–214. [DOI] [PubMed] [Google Scholar]
- 100.Buckley L, Humphrey MB. Glucocorticoid-Induced Osteoporosis. New England Journal of Medicine. 2018;379(26):2547–2556. [DOI] [PubMed] [Google Scholar]
- 101.Chakraborty R, Majhail NS, Anwer F. Denosumab vs Zoledronic Acid for Bone-Targeted Therapy in Multiple Myeloma: What Are the Unanswered Questions? JAMA oncology. June 27 2019. [DOI] [PubMed] [Google Scholar]
- 102.Cummings SR, Ferrari S, Eastell R, et al. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. February 2018;33(2):190–198. [DOI] [PubMed] [Google Scholar]
- 103.Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. Jama. January 3 2017;317(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. The Lancet. Oncology. December 2015;16(16):1617–1629. [DOI] [PubMed] [Google Scholar]
- 105.Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. The Cochrane database of systematic reviews. December 12 2012;12:CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bohlius J, Bohlke K, Castelli R, et al. Management of Cancer-Associated Anemia With Erythropoiesis-Stimulating Agents: ASCO/ASH Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. May 20 2019;37(15):1336–1351. [DOI] [PubMed] [Google Scholar]
- 107.Leleu X, Gay F, Flament A, Allcott K, Delforge M. Incidence of neutropenia and use of granulocyte colony-stimulating factors in multiple myeloma: is current clinical practice adequate? Annals of hematology. March 2018;97(3):387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. October 1 2015;33(28):3199–3212. [DOI] [PubMed] [Google Scholar]
- 109.Figaro MK, Clayton W Jr., Usoh C, et al. Thyroid abnormalities in patients treated with lenalidomide for hematological malignancies: results of a retrospective case review. American journal of hematology. June 2011;86(6):467–470. [DOI] [PubMed] [Google Scholar]
- 110.Adam Z, Pour L, Pelcova J, Mayer J. Combination Of Lenalidomide, Dexamethasone and Cyclophosphamide as Treatment For The First Relapse Of Multiple Myeloma: Efficacy, Toxicity and Occurance Of Thyroid Abnormalities. Blood. 2013;122(21):3233–3233. [Google Scholar]
- 111.Burns LJ. Late effects after autologous hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. January 2009;15(1 Suppl):21–24. [DOI] [PubMed] [Google Scholar]
- 112.Tremblay D, Choudhury N, Ravikumar A, Levine AC, Chari A. The characterization of adrenal insufficiency and identification of its risk factors in patients with plasma cell dyscrasias. American journal of hematology. October 2015;90(10):E202–203. [DOI] [PubMed] [Google Scholar]
- 113.Sklar BA, Gervasio KA, Leng S, Ghosh A, Chari A, Wu AY. Management and outcomes of proteasome inhibitor associated chalazia and blepharitis: a case series. BMC ophthalmology. May 14 2019;19(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Veys MC, Delforge M, Mombaerts I. Treatment With Doxycycline for Severe Bortezomib-Associated Blepharitis. Clinical lymphoma, myeloma & leukemia. July 2016;16(7):e109–112. [DOI] [PubMed] [Google Scholar]
- 115.Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. The Lancet. Oncology. December 2018;19(12):1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. July 25 2019;134(4):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramsenthaler C, Osborne TR, Gao W, et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC cancer. July 7 2016;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nielsen LK, Jarden M, Andersen CL, Frederiksen H, Abildgaard N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. European journal of haematology. July 2017;99(1):3–17. [DOI] [PubMed] [Google Scholar]
- 119.Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. The Lancet. Haematology. September 2017;4(9):e431–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nekhlyudov L, Ganz PA, Arora NK, Rowland JH. Going Beyond Being Lost in Transition: A Decade of Progress in Cancer Survivorship. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. June 20 2017;35(18):1978–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Usmani SZ, Hoering A, Cavo M, et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma - an IMWG Research Project. Blood cancer journal. November 23 2018;8(12):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajkumar SV, Harousseau JL. Next-generation multiple myeloma treatment: a pharmacoeconomic perspective. Blood. December 15 2016;128(24):2757–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Majhail NS, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica. May 2019;104(5):1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet (London, England). September 21 2019;394(10203):1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, Characteristics, and Reversibility of Peripheral Neuropathy During Treatment of Advanced Multiple Myeloma With Bortezomib. Journal of Clinical Oncology. 2006;24(19):3113–3120. [DOI] [PubMed] [Google Scholar]
- 126.Drayson MT, Bowcock S, Planche T, et al. Tackling Early Morbidity and Mortality in Myeloma (TEAMM): Assessing the Benefit of Antibiotic Prophylaxis and Its Effect on Healthcare Associated Infections in 977 Patients. Blood. 2017;130(Suppl 1):903–903.28637661 [Google Scholar]