Case report
A 59-year-old man with a history of atrial fibrillation and treated obstructive sleep apnea presented to the emergency department on March 26, 2020 with fever, dry cough, dyspnea and headache. He had no history of personal or familial seizures. A first reverse-transcriptase–polymerase-chain-reaction (RT-PCR) of nasopharyngeal swab test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was negative but SARS-CoV-2 infection was subsequently confirmed by RT-PCR assay performed on tracheal secretions and by computed tomography scan of the chest. The illness rapidly progressed to hypoxemic respiratory failure warranting the initiation of invasive mechanical ventilation on March 28. Lung bacterial overinfection by Hafnia alveii and Proteus vulgaris was diagnosed and sequentially treated with amoxicillin-clavulanic acid/rovamycine (7 days), tazobactam-piperacillin (5 days) and finally with cefepime (7 days). Deep sedation with midazolam was stopped on April 8 and the patient was extubated the following day.
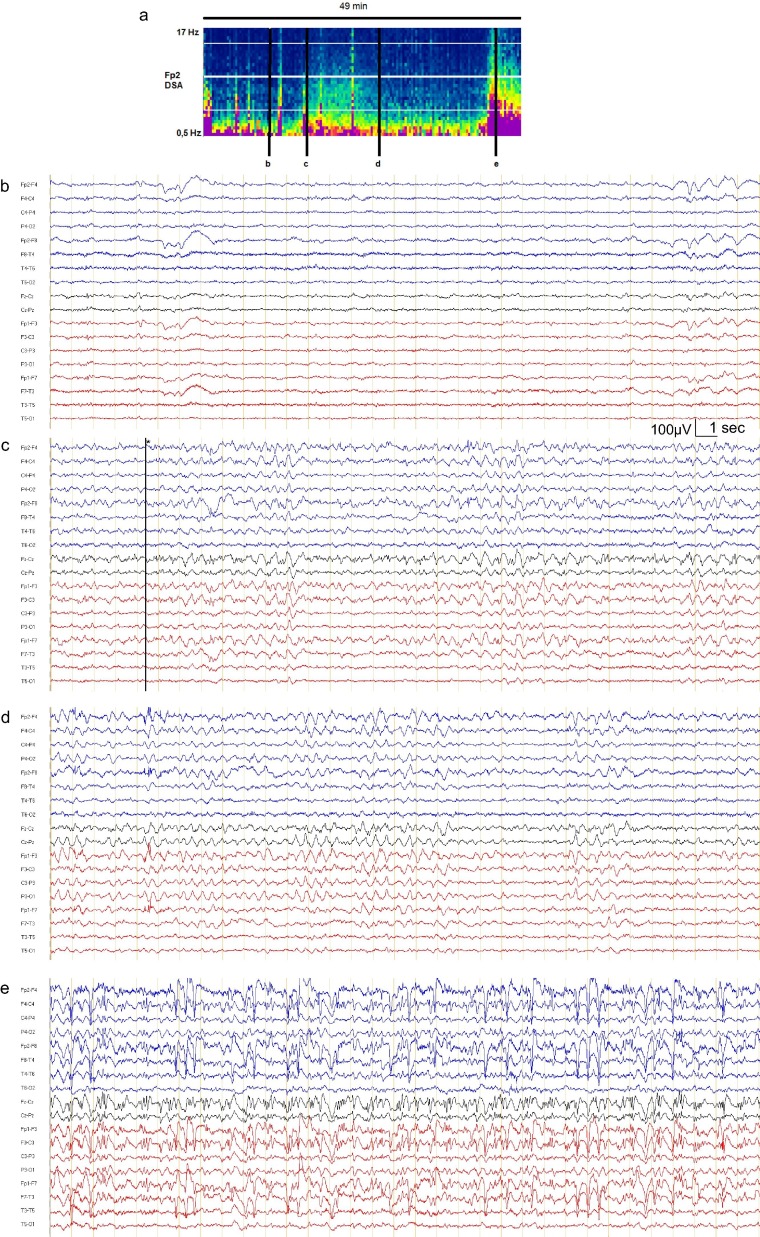
From April 10, the patient started to exhibit short episodes of impaired consciousness together with confusion and behavioral disturbances. Forty-nine minute electroencephalogram (EEG), performed when the patient was awake but confused, showed background fluctuating alertness with preserved responses to simple orders (Fig. 1 b) and two widespread long rhythmic delta discharges with superimposed spikes in predominantly frontal localization simultaneous with impaired awareness (Fig. 1c), highly suggestive of epileptic seizures. The first seizure lasted 6 minutes (Fig. 1c), followed by a moderate interictal frontal activity (Fig. 1d) and the second seizure lasted at least 5 minutes (Fig. 1e), leading to the diagnosis of non-convulsive status epilepticus. Laboratory blood tests and brain MRI were normal; plasma cefepime concentrations were within the normal range. Routine cerebrospinal fluid (CSF) analysis was unremarkable and CSF SARS-CoV2 RT-PCR was negative. Clobazam (30 mg/day) and levetiracetam (1.5 g/day) were introduced. On April 14, the patient had only one brief episode of impaired consciousness and his long-term EEG monitoring was normal. He is now clinically stable and discharged from ICU.
Fig. 1.
(a) Density spectral array (DSA) of the 49-min EEG from electrode Fp2. Representative EEG recordings in bipolar longitudinal montage (b) showing theta-delta background rhythm; (c, e) focal seizures starting with rhythmic spikes and sharp-waves widespread discharge that predominate in the frontal lobes, (d) postictal pattern with slow delta waves predominantly in frontal localization.
To the best of our knowledge, this is the first case of seizures associated with coronavirus disease 2019 without any underlying meningitis or encephalitis (Moriguchi et al., 2020).
Neurotropism of human coronaviruses is supported by the presence of angiotensin converting enzyme 2 (ACE2), the SARS-CoV2 ligand, in brain tissue (Hamming et al., 2004). Local inhibition of brain ACE2 induces a reduction in baroreflex sensitivity (Xia and Lazartigues, 2008). The activation of such a receptor could be responsible for cerebral blood flow dysfunction and sensitize seizure threshold. Moreover, the absence of brain lesion in our case raises the possibility of a cytokine storm mechanism through the ACE2 signaling pathway as hypothesized for confusion (Mao et al., 2020). Our observation not only broadens the clinical spectrum of neurological manifestations that are associated with coronavirus disease 2019 (Helms et al., 2020) but also suggests that EEG should be performed in any SARS-CoV-2-infected patients with alteration of consciousness.
Declaration of Competing Interest
Authors reported no disclosures relevant to the manuscript.
References
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]