Graphical abstract
Keywords: COVID-19, Hypertension, Cardiovascular disease, Outcome
Abstract
The coronavirus disease (COVID-19) has spread all around the world in a very short period of time. Recent data are showing significant prevalence of arterial hypertension and cardiovascular diseases (CVD) among patients with COVID-19, which raised many questions about higher susceptibility of patients with these comorbidities to the novel coronavirus, as well as the role of hypertension and CVD in progression and the prognosis of COVID-19 patients. There is a very limited amount of data, usually obtained from a small population, regarding the effect of the underlying disease on the outcome in patients with COVID-19. The evaluation of the treatment of these comorbidities at baseline and during COVID-19 is scarce and the results are conflicting. Hypertension and CVD, after the adjustment for other clinical and demographic parameters, primarily age, did not remain independent predictors of the lethal outcome in COVID-19 patients. Some investigations speculated about the association between the renin-angiotensin-aldosterone system (RAAS) and susceptibility to COVID-19, as well as the relationship between RAAS inhibitors and the adverse outcome in these patients. Withdrawing or switching RAAS inhibitors would have uncertain benefits, but it would definitely have many disadvantages such as uncontrolled hypertension, cardiac function deterioration and renal function impairment, which could potentially induce more complications in patients with COVID-19 than the infection of coronavirus itself. The aim of this review article was to summarize the prevalence of hypertension and CVD in patients with COVID-19, their influence on the outcome and the effect of treatment of hypertension and CVD in COVID-19 patients.
1. Introduction
The outbreak of the infection of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused a pandemic of disease called COVID-19 at the beginning of 2020 [1]. There is almost no country in the world that is not affected by COVID-19 and the healthcare systems around the globe have never worked under this enormous pressure. Tens of thousands of newly infected patients and thousands of deaths have been reported each day. Everything started in Wuhan, China, but it has rapidly spread around the world. Europe and the United States are currently the center of the pandemic.
COVID-19 is a respiratory infection caused by the novel coronavirus with high virulence and considerable high mortality, which is difficult to estimate before the pandemic is over. However, the currently available data show that the mortality rate in China is 4.1 %, Italy 12.8 %, Spain 10.2 %, USA 3.9 % and Germany 2.3 % [2], which is significantly higher than for influenza (0.1 %), comparable or lower than for SARS (10 %), but significantly lower than for MERS (34 %) [3,4].
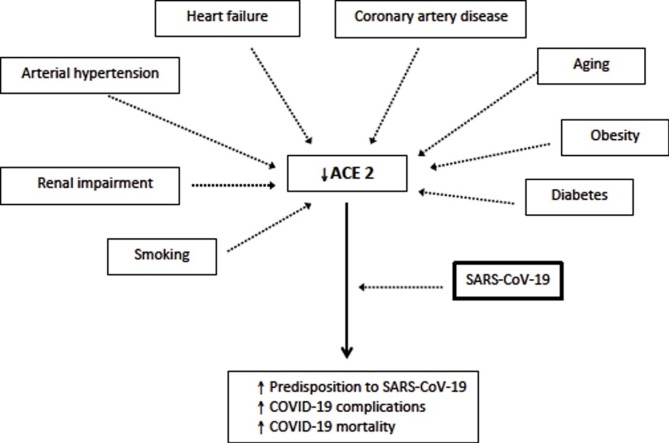
The data coming predominantly from China are showing a significant percentage of arterial hypertension and cardiovascular diseases (CVD) among patients with COVID-19, which raised many questions about higher susceptibility of patients with these comorbidities to the novel coronavirus, as well as the role of hypertension and CVD in progression and the prognosis of COVID-19 patients [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. Recent findings showed an important role of the renin-angiotensin-aldosterone system (RAAS) in these patients, which was explained by the fact that SARS-CoV-2 is using angiotensin-converting-enzyme 2 (ACE2) for binding to the surface of epithelial cells [28]. There are conflicting data on the effect of RAAS inhibitors in COVID-19 patients [9,13,29]. However, it seems that first studies about the adverse impact of RAAS inhibitors were premature because the newly published data even showed a positive effect of RAAS on survival in patients with COVID-19 [29]. It is obvious that more data on this topic are necessary, considering the high prevalence of hypertension and CVD in patients with COVID-19 and regular use of RAAS inhibitors in treatment of both - hypertension and CVD.
The aim of this article was to provide an overview of prevalence of hypertension and CVD in patients with COVID-19, their influence on the outcome and the effect of treatment of hypertension and CVD in COVID-19 patients.
1.1. Epidemiology of hypertension and CVD in COVID-19
The prevalence of hypertension ranged from 4.5 % in non-critically ill patients to >30 % in older patients with COVID-19, whereas the prevalence of CVD was significantly lower and varied from 1 % to 18 % (Table 1 ). It is expected that the prevalence was significantly higher in older patients and those with other comorbidities such as diabetes and kidney impairment. One should notice that different studies used various definitions of CVD. Some included coronary heart disease and heart failure, whereas others also included cerebrovascular disease. Therefore, one should be cautious in the interpretation of these results.
Table 1.
Demographic parameters and cardiovascular diseases in COVID-19 patients.
| Reference | Sample size | Age | Women (%) | Hypertension (%) | CVD (%) | ACEI/ARB (%) | Other important findings |
|---|---|---|---|---|---|---|---|
| Guan et al. [5] | 1590 | 48.9 ± 16.3 | 674 (43) | 269 (17) | 59 (4) | ― | COPD, diabetes, hypertension and malignancy were risk factors for admission to intensive care unit, invasive ventilation and mortality. The risk increased with higher number of comorbidities. |
| Guan et al. [6] | 1099 | 47 (35−58) | 459 (42) | 165 (15) | 25 (2.5) | ― | Epidemiological study, which did not concern the effect of hypertension or CVD on outcome. |
| Wang et al. [7] | 1012 | 50 (39−58) | 488 (48) | 46 (4.5) | 15 (1.5) | ― | CVD, but not hypertension, was more prevalent in COVID-19 patients with aggravation of disease. However, prevalence of risk factors was significantly lower in non-critically than in critically ill patients. |
| Lian et al. [8] | 788 | 46 | 381 (48) | 126 (16) | 11 (1) | ― | Older COVID-19 patients showed significantly higher female gender, rate of comorbidities and rate of severe/critical disease. |
| Feng et al. [9] | 476 | 53 (40−64) | 205 (43) | 113 (24) | 38 (8) | 33 (29) | Incidence of comorbidities was higher in severe and critical groups than in moderately severe group. More patients taking RAAS inhibitors in moderate group than in other two groups. Advanced age (>75 years) was the most responsible for mortality. |
| Shi et al. [10] | 416 | 64 (21−95) | 211 (50) | 127 (31) | 61 (15) | ― | Cardiac injury is common (19.7 %) in patients with COVID-19. |
| Chen et al. [11] | 274 | 62 (44−70) | 103 (38) | 93 (34) | 24 (9) | ― | Acute respiratory distress syndrome and respiratory failure, sepsis, acute cardiac injury, and heart failure were the most common critical complications during exacerbation of COVID-19. |
| Wu et al. [12] | 201 | 51 (43−60) | 73 (36) | 39 (19) | 8 (4) | ― | Older age was associated with increased risk of ARDS and lethal outcome. |
| Guo et al. [13] | 187 | 58.5 ± 14.7 | 96 (51) | 61 (33) | 29 (16) | 19 (10) | Myocardial injury is significantly associated with fatal outcome of COVID-19. The prognosis of patients with underlying CVD without myocardial injury is significantly better. RAAS inhibitors were more prevalent among patients with cardiac injury (5.9% vs. 21%). |
| Guo et al. [14] | 174 | 59 (49−67) | 98 (56) | 43 (25) | 32 (18) | ― | CVD, but not hypertension, was more prevalent among COVID-19 patients with diabetes than in those without diabetes. |
| Zhou et al. [15] | 191 | 56 (46−67) | 72 (38) | 58 (30) | 15 (8) | ― | Older age, higher sequential organ failure assessment and D-dimer were predictors of mortality in COVID-19 patients. |
| Wang et al. [16] | 138 | 56 (22−92) | 63 (46) | 43 (31) | 20 (14) | ― | Study did not investigate the effect of hypertension or CVD. |
| Liu et al. [17] | 137 | 57 (20−83) | 76 (56) | 13 (10) | 10 (7) | ― | Epidemiological study, which did not investigate the effect of hypertension or CVD. |
| Yang et al. [18] | 52 | 59.7 ± 13.3 | 17 (33) | No data | 5 (10) | ― | Patients older than 65 years with comorbidities and ARDS had higher mortality risk. |
| Huang et al. [19] | 41 | 49 (41−58) | 11 (27) | 6 (15) | 6 (15) | ― | Epidemiological study, which did not investigate the effect of hypertension or CVD. |
| Li et al. [38] | 362 | 66 (59–73) | 173 (48) | 362 (100) | 140 (39) | 115 (32) | ACEI/ARBs were not associated with severity of disease or mortality in COVID-19 patients. |
| Mancia et al. [39] | 6272 | 68 ± 13 | 2303 (37) | ― | 1891 (30) | 2896 (46) | ACEI/ARBs did not show any association with COVID-19, although they were more frequently used in these patients in comparison with controls. Higher prevalence is explained by higher frequency of CVD in COVID-19 patients. |
ACEI = angiotensin converting enzyme inhibitor - ARB = angiotensin receptor blocker- ARDS – acute respiratory distress syndrome, COPD – chronic obstructive pulmonary disease, CVD – cardiovascular disease (coronary heart disease, heart failure, with/without cerebrovascular disease).
Meta-analyses confirmed considerable prevalence of hypertension and CVD among patients with COVID-19 [[20], [21], [22], [23], [24]] (Table 2 ). This indicated the importance of hypertension and CVD in COVID-19 patients and raised the question about susceptibility of CVD and hypertensive patients to SARS-CoV-2. Nevertheless, other risk factors, such as smoking and obesity, should not be underestimated and, in most studies, the effect of obesity is not possible to be estimated due to the lack of data.
Table 2.
Summary of the meta-analyses that provided findings on cardiovascular diseases in COVID-19 patients.
| Reference | Sample size | Age | Women (%) | Hypertension (%) | CVD (%) | Other important findings |
|---|---|---|---|---|---|---|
| Emami et al. [20] | 76,993 | – | – | – | – | The most prevalent comorbidities were hypertension, CVD, smoking, and diabetes. COPD, cancer, and chronic kidney disease were also prevalent among patients. |
| Yang et al. [21] | 46,248 | – | – | – | – | The most prevalent comorbidities were hypertension, diabetes, CVD and respiratory disease. Similar results were obtained in severe patients. |
| Wang et al. [22] | 1558 | – | 667 (43) | – | – | Hypertension, diabetes, COPD, CVD, and cerebrovascular disease were independent risk factors associated with COVID-19 patients |
| Li et al. [23] | 1527 | No data | No data | 261 (17) | 250 (16) | Hypertension, CVD and diabetes are the most prevalent comorbidities in COVID-19 patients. |
| Rodriguez-Morales et al. [24] | 656 | 52 | 289 (44) | 122 (18.6) | 78 (11.9) | 36.8 % of patients had 1 or more comorbidities. The most significant were hypertension, CVD, and diabetes. |
ARDS – acute respiratory distress syndrome, COPD – chronic obstructive pulmonary disease, CVD – cardiovascular disease (coronary heart disease, heart failure with/without cerebrovascular disease).
1.2. The outcome of patients with hypertension and CVD in COVID-19
A limited number of studies provided follow-up data, which is the consequence of rapid publication and large interest of healthcare professionals around the world in all information regarding COVID-19 (Table 3 ). Most of these investigations confirmed a significantly higher percentage of hypertension and CVD among patients with the adverse outcome. However, only few studies performed an adequate multivariate analysis including several risk factors with the adjustment for possible confounding factors.
Table 3.
Hypertension and CVD in studies that investigated fatal outcome of COVID-19.
| Reference | Progression/Outcome | Number of patients | Age | Women (%) | Hypertension (%) | CVD (%) |
|---|---|---|---|---|---|---|
| Chen et al. [11] | Non-survivors | 113 | 68 (62−77) | 30 (27) | 54 (48) | 20 (18) |
| Survivors | 161 | 51 (37−66) | 73 (45) | 39 (24) | 7 (4) | |
| Yang et al. [18] | Non-survivors | 32 | 64.6 ± 11.2 | 11 (34) | No data | 10 (31) |
| Survivors | 20 | 51.9 ± 12.9 | 6 (30) | No data | 2 (10) | |
| Zhou et al. [15] | Non-survivors | 54 | 69 (63−76) | 16 (30) | 58 (30) | 13 (24) |
| Survivors | 137 | 52 (45−58) | 56 (41) | 32 (23) | 2 (1) | |
| Du et al. [25] | Non-survivors | 85 | 65.8 ± 14.2 | 23 (27) | 32 (38) | 17 (20) |
| Deng et al. [26] | Non-survivors | 109 | 69 (62−74) | 36 (33) | 40 (37) | 13 (12) |
| Survivors | 116 | 40 (33−57) | 65 (56) | 18 (16) | 4 (3) | |
| Wang et al. [7] | Patients with aggravation | 100 | 56 (47−62) | 38 (38) | 6 (6) | 5 (5) |
| Patients without aggravation | 912 | 50 (38−58) | 450 (49) | 40 (4.4) | 10 (1.1) | |
| Wu et al. [27] | Non-survivors with ARDS | 44 | 68.5 (59−75) | 15 (34) | 16 (36) | 4 (9) |
| Survivors with ARDS | 40 | 50 (40−57) | 9 (23) | 7 (18) | 4 (10) | |
| Feng et al. [9] | Moderate disease | 352 | 51 (37−63) | 162 (46) | 73 (21) | 21 (6) |
| Severe disease | 54 | 58 (48−67) | 21 (39) | 15 (28) | 5 (9) | |
| Critical disease | 70 | 61 (49−68) | 22 (31) | 25 (36) | 12 (17) | |
| Li et al. [38] | Non-survivors Survivors | 77 285 |
72 (65–82)65 (58 –71) |
27 (35) 146 (51) |
77 (100) 285 (100) |
63 (82) 77 (27) |
| Mehra et al. [41] | Non-survivors Survivors | 515 8395 |
56 ± 15 49 ± 17 |
179 (35) 3392 (40) |
130 (25)2216 (26) | 167 (32)1336 (16) |
Guan et al. included 1590 patients and reported that those with hypertension, diabetes, COPD and malignancy were more likely to reach the composite endpoints (admission to intensive care unit, or invasive ventilation, or death) after adjusting for age and smoking status [5]. Patients with ≥2 comorbidities had a significantly increased risk of the adverse outcome. In a small study by Wu et al. hypertension and diabetes were predictors of the acute respiratory distress syndrome (ARDS), but not the lethal outcome in COVID-19 patients [12].
A recently published study that involved 1012 patients with COVID-19 demonstrated that CVD, but not hypertension, was more prevalent in patients with aggravation of disease comparing with those who did not experience deterioration of COVID-19 [7]. However, the analysis of the predictors of disease deterioration was not performed. Another investigation revealed that the incidence of all comorbidities was higher in the COVID-19 patients with a severe and critical clinical course than in the patients with a moderately severe clinical course [9].
Chen et al. reported that hypertension, CVD and diabetes were more prevalent among the COVID-19 patients with the lethal outcome comparing to the survivors [11] (Table 3). However, there was a large difference in age and sex distribution between the groups and the authors did not investigate the effect of comorbidities on the outcome in this population [11]. Zhou et al. reported that hypertension, diabetes, coronary heart disease, chronic renal disease and COPD were more frequent among non-survivors than survivors [15]. The authors found that hypertension, diabetes, and coronary heart disease were predictors of mortality in COVID-19 patients. Nevertheless, after adjustment for age, none of these comorbidities remained significant predictors of mortality.
1.3. RAAS in COVID-19
ACE2 has gained a widespread interest as the cellular receptor of SARS-CoV-2 cause of the COVID-19 pandemic. ACE2 is a master regulator of RAAS, and both reduced or increased function of ACE2 can induce systemic and pulmonary hypertension, heart failure, myocardial infarction, and diabetic cardiovascular complications [30]. ACE2 is highly expressed in the heart, lungs, kidney, and gastrointestinal tract, and it plays an important role in several cardiovascular and immune pathways. The binding affinity of the novel coronavirus with ACE2 appears to be stronger than the virus that causes SARS, which may explain the significantly higher global influence of COVID-19 than the initial SARS. Potential therapeutic strategies may include preventing the binding of human ACE2 and SARS-CoV-2.
In the post-mortem autopsy of the heart tissues from the patients with SARS, about 30 % had a detectable viral SARS-CoV genome and their myocardium was characterized by increased myocardial fibrosis, inflammation, and reduced myocardial ACE2 expression [31,32]. These patients had a much more aggressive disease associated with earlier mortality. These data are still not available in COVID-19 patients, but the studies that investigated cardiac injury by elevation of cardiac enzymes showed that the prevalence of cardiac injury is frequent and more prevalent among patients with the lethal outcome [10,13]. Chen et al. recently revealed that human cardiac pericytes have high expression of ACE2, which could be the target cell of SARS-CoV-2 [33]. The virus-induced pericytes damage can provoke capillary endothelial cells dysfunction and further microvascular dysfunction. Patients with heart failure disease at baseline demonstrated elevated ACE2 expression at both mRNA and protein levels, which further increases the risk of heart attack and the critically ill condition in patients infected by this coronavirus [33].
The biological effects of ACE2 are focused on the formation of angiotensin-(1–7) from angiotensin II. ACE2, unlike ACE, does not convert angiotensin I to angiotensin II, nor do ACE inhibitors block its activity, which is crucial for understanding of its biological properties and the effect of RAAS inhibitors.
1.4. RAAS inhibitors in COVID-19
The influence of RAAS inhibitors on ACE2 activity and expression is known from animal and human studies. The large confusion and panic regarding usage of angiotensin-converting-enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) erupted for two reasons: (i) confusion between ACE and ACE2 inhibitors, and (ii) some studies reported a significantly higher percentage of RAAS inhibitors usage among COVID-19 patients with the lethal outcome.
ACE and ACE2 are two different enzymes with two different active sites and any effect of ACEI on ACE2 activity must, therefore, be an indirect one, through their corresponding substrates. SARS-CoV-2 binding is unlikely to have any relationship with ACE or ACEI. Nevertheless, there are limited data that ACEIs affect the expression of ACE2 in the heart and the kidney [34]. On the other hand, there is more evidence that ARBs modify ACE2 expression [35,36]. Upregulation has been primarily found in the cardiac tissue and in the renal vasculature. However, the results are inconsistent, require high ARBs dosages and vary according to ARB and the organ. Additionally, most investigations measured blood ACE2 activity that reveals the soluble ACE2 protein circulating at very low levels, which is not the same as membrane-bound ACE2. If ARBs upregulated membrane-bound ACE2, it is reasonable to hypothesize that this would be the consequence of AT1 receptor blockade. ACEIs would have a similar effect as ARBs, even though there are only limited data about ACEI-induced upregulation of ACE2. However, the currently available data are mostly obtained in animal studies and could not be directly applied to humans. Therefore, there is no sufficient evidence that ARBs or ACEIs could facilitate SARS-CoV-2 entry and cause COVID-19.
In the recent state-of-art review, Sanchis-Gomar et al. suggested that ARBs might be a better treatment option in COVID-19 patients at higher risk of severe forms of disease due to the equal efficacy but fewer side effects than ACEIs [37]. Nevertheless, the existing studies that included a large number of patients did not give advantage to any group of medications within RAAS inhibitors.
Feng et al. investigated 476 patients with COVID-19 and reported a significantly higher usage of ARBs in hypertensive patients with moderate COVID-19 disease than those with a severe and critical course of disease [9]. There was no difference in usage of ACEIs between COVID-19 patients with different severity of disease. There are no data regarding the association between RAAS inhibitors and the outcome. However, the investigators studied the use of RAAS inhibitors only in hypertensive patients, but not in the patients with other indications for ACEI and ARB (heart failure, diabetes, renal impairment). Moreover, the number of patients who were taking ACEIs and ARBs was very low, 7 % and 24 %, respectively [9]. Interestingly, the percentage of ARBs was significantly higher than ACEIs.
Guo et al. included only 187 patients with COVID-19 and their use of RAAS inhibitors was significantly higher in the patients with detected cardiac injury than in the patients without this injury (21.1 % vs. 5.9 %) [13]. Underlying diseases such as hypertension, coronary artery disease, cardiomyopathy and chronic renal disease were also significantly more prevalent in the patients with cardiac injury [13]. These comorbidities may have largely contributed to cardiac injury in these patients, but these diseases are also the reason for taking RAAS inhibitors. This explains the higher prevalence of RAAS inhibitors in COVID-19 patients with cardiac injury, defined by the elevated serum level of troponin.
There was a great confusion in the social media and among healthcare professionals after these preliminary studies due to uncertainties whether ACEIs and ARBs should be switched to another antihypertensive medications. Several large studies, therefore, concentrated on the effect of ACEI/ARB on morbidity and mortality in COVID-19 patients and they obtained encouraging results. Li et al. included 1178 patients with COVID-19, out of which 362 had hypertension and reported that ACEIs/ARBs are not associated with the severity or mortality of COVID-19 patients [38]. A recent large study from Italy that involved 6272 patients with COVID-19 and 30,759 non−COVID patients showed that the use of ACEIs and ARBs was more frequent among the COVID-19 patients than in the control group due to the higher prevalence of cardiovascular disease [39]. Nevertheless, there was no association between ACEI/ARB use and the risk of COVID-19 [39]. An American study that included 5894 patients with COVID-19 found no association between any single medication class, including RAAS inhibitors, and the positive test for COVID-19 [40]. Mehra et al. involved 8910 patients with COVID-19 from 169 hospitals in Asia, Europe, and North America and revealed no association between the use of ACEI/ARB and the increased in-hospital mortality [41].
A study that investigated COVID-19 patients with hypertension treated with RAAS inhibitors showed that the patients receiving ACEI or ARB therapy had a lower rate of severe diseases and a trend toward a lower level of IL-6 in peripheral blood [29]. Moreover, ACEI or ARB treatment increased the level of CD3 and CD8 T cell in peripheral blood and reduced the peak viral load compared to other antihypertensive drugs. These findings were the first evidence which supports the benefit of ACEIs or ARBs in COVID-19 patients with hypertension because they can potentially improve clinical outcomes in these patients. The authors hypothesized that RAAS inhibitors did not directly inhibit the viral replication, but had an indirect antiviral role by regulation of the immune function and inhibition of the inflammatory response. Nevertheless, the potential mechanisms need to be clarified in the future large human studies. A large study that included 1128 hypertensive patients with COVID-19 showed that inpatient use of ACEI/ARB, after adjustment for age, gender and comorbidities, was related with lower all-cause mortality compared with ACEI/ARB non-users [42]. Further analysis revealed that the use of RAAS inhibitors was also associated with reduced mortality in COVID-19 patients with hypertension in comparison to the use of other antihypertensive drugs [42].
RAAS inhibitors have an important role in the treatment of heart failure and renal impairment (particularly with albuminuria), not only in hypertension. Withdrawing these medications or switching therapy would have uncertain benefits, but definitely many disadvantages such as uncontrolled hypertension, cardiac decompensation and renal function impairment, which could potentially induce more complications in patients with COVID-19 than the infection of SARS-CoV-2 itself. This is why several specialty societies recommended that COVID-19 patients continue therapy with RAAS inhibitors [[43], [44], [45], [46]]. This is in agreement with the latest studies that did not find any association between RAAS inhibitors and increased morbidity and/or mortality in the COVID-19 patients.
1.5. Limitations of the available data
It is important to discuss evident limitations of the available data. One of the most important limitations is the self-reporting of comorbidities on hospital admission, which can cause significant underestimation of comorbidities because of the lack of awareness and/or the lack of diagnostic testing. The follow-up period was short and some patients remained in hospital when the data were being analyzed, meaning that the actual outcome is unknown, which can interfere with the published results. Obesity was not reported in any of these studies and the influence of BMI could not be investigated or included in the adjustment, which would be of great importance.
The data about the therapy at baseline and during the therapy are poor, which is an important potentially confounding factor in evaluation of hypertension and CVD as independent predictors of susceptibility to SARS-CoV-2 or the outcome of COVID-19. The investigations that reported negative association between RAAS inhibitors and COVID-19 included only a limited number of COVID-19 patients, which is an additional limitation for reaching statistical significance.
2. Conclusion
The prevalence of hypertension and CVD is clinically relevant in patients with COVID-19, and particularly in the elderly. The impact of hypertension and CVD on the outcome is still uncertain. There are some concerns that RAAS inhibitors, particularly ARBs, can affect the expression of ACE2 based on animal models. However, there is no clinical evidence that RAAS inhibitors should be restricted or temporarily discontinued in COVID-19 patients. Large longitudinal studies with the comprehensive analysis of all CV risk factors and possible confounders, as well as the evaluation of the treatment on progression and the prognosis of COVID-19, are warranted.
Declaration of Competing Interest
The paper "COVID-19 and cardiovascular diseases: Should we change therapy?" has not been submitted elsewhere, it is not under review, or published previously. There is no possible conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2020. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd402994 Accessed April 9, 2020. [Google Scholar]
- 3.Chan J.W.M., Ng C.K., Chan Y.H., Mok T.Y.W., Lee S., Chu S.Y.Y., Law W.L., Lee M.P., Li P.C.K. Short-term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Liang W.H., Zhao Y., Liang W.H.R., Chen Z.S., Li Y.M. China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020;(March 26) doi: 10.1183/13993003.00547-2020. pii: 2000547 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui DSC Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.L.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;(February 28) doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., Ge L., Wang F., Chen Q., Zhang Y., Zhao Q. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin. Microbiol. Infect. 2020;(April 3) doi: 10.1016/j.cmi.2020.03.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., Jia H., Hu J., Gao J., Zhang Y., Zhang X., Yu G., Wang X., Gu J., Ye C., Jin C., Lu Y., Yu X., Yu X., Ren Y., Qiu Y., Li L., Sheng J., Yang Y. Analysis of epidemiological and clinical features in older patients with Corona Virus Disease 2019 (COVID-19) out of Wuhan. Clin. Infect. Dis. 2020;(March 25) doi: 10.1093/cid/ciaa242. pii: ciaa242 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., Xiong W., Yang D., Chen R., Lu F., Lu Y., Liu X., Chen Y., Li X., Li Y., Summah H.D., Lin H., Yan J., Zhou M., Lu H., Qu J. COVID-19 with different severity: a multi-center study of clinical features. Am. J. Respir. Crit. Care Med. 2020;(April 10) doi: 10.1164/rccm.202002-0445OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;(March 25) doi: 10.1001/jamacardio.2020.0950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;26(March 368):m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 March 13. doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 13.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;(March 27) doi: 10.1001/jamacardio.2020.1017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., Zhao L., Fan H., Luo S., Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020;31(March):e3319. doi: 10.1002/dmrr.3319. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(March 28 (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;(February 7) doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;(February 7) doi: 10.1097/CM9.0000000000000744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;(February 24) doi: 10.1016/S2213-2600(20)30079-5. pii: S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(March 24 (1)):e35. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;(March 12) doi: 10.1016/j.ijid.2020.03.017. pii: S1201-9712(20)30136-3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(April 8) doi: 10.18632/aging.103000. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;(March 11) doi: 10.1007/s00392-020-01626-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y. Escalera-Antezana JP, et al; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;13 doi: 10.1016/j.tmaid.2020.101623. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;(April 3) doi: 10.1164/rccm.202003-0543OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., Wang K., Leng F., Wei S., Chen L., Liu H.G. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;(March 20) doi: 10.1097/CM9.0000000000000824. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 March 13 doi:10.1001/jamainternmed.2020.0994 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 28.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;(March 6) doi: 10.1016/j.cell.2020.02.058. pii: S0092-8674(20)30262-2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ace2/angiotensin 1-7 axis of the renin angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;(March 25) doi: 10.1161/HYPERTENSIONAHA.120.15082. 10.1161/HYPERTENSIONAHA.120.15082.HYPERTENSIONAHA12015082 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am. J. Physiol. Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 37.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin. Proc. 2020;(April 4) doi: 10.1016/j.mayocp.2020.03.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors with Severity or Risk of patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;(April 23) doi: 10.1001/jamacardio.2020.1624. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-Aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;(May 1) doi: 10.1056/NEJMoa2006923. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;(May 1) doi: 10.1056/NEJMoa2008975. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020;(May 1) doi: 10.1056/NEJMoa2007621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu Y.M., Chen M.M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang X., Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;(April 17) doi: 10.1161/CIRCRESAHA.120.317134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Simone G. 2020. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [Google Scholar]
- 44.Iaccarino G., Borghi C., Cicero AFG Ferri C., Minuz P., Muiesan M.L., Mulatero P., Mulè G., Pucci G., Salvetti M., Savoia C., Sechi L.A., Volpe M., Grassi G. Renin-angiotensin system inhibition in cardiovascular patients at the time of COVID19: much ado for nothing? A statement of activity from the directors of the board and the scientific directors of the italian society of hypertension. High Blood Press. Cardiovasc. Prev. 2020;(April 7) doi: 10.1007/s40292-020-00380-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.2020. ESH Update on COVID-19.https://www.eshonline.org/spotlights/esh-stabtement-on-covid-19-2/ [Google Scholar]
- 46.2020. BSH & BCS Joint Statement on ACEi or ARB in Relation to COVID-19.https://www.britishcardiovascularsociety.org/news/ACEi-or-ARB-and-COVID-19 [Google Scholar]