Abstract
Antioxidant activity, angiotensin I-converting enzyme (ACE) inhibitory activity, and protein profile of crust (the dried surface of dry-aged beef) were evaluated compared to unaged, wet-, and dry-aged beef. Antioxidant activity was determined using 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) radical scavenging activities, ferric reducing antioxidant power, and ferrous ion chelating activity. The crust samples showed the greatest (P < 0.05) ACE inhibitory and antioxidant activity resulting from the three different mechanisms of action (radical scavenging, non-radical redox potential activity, and metal chelating) among the treatments. Small molecular weight protein bands and small peptides (<3 kDa) indicating potent bioactivity were evident in the myofibrillar protein profile of crust samples. The lowest (P < 0.05) ACE inhibitory activity was observed in unaged beef. The results indicate that crust could be utilized in various areas as a functional ingredient possessed antioxidant and ACE inhibitory activity instead of being discarded. In addition, dry aging can use for generation of functional ingredient from beef as the regime.
Subject terms: Peptides, Metabolomics
Introduction
Postmortem aging enhances the tenderness and juiciness of meat through the activation of proteolysis by endogenous proteolytic enzymes1. The two methods of aging include wet aging (stored in vacuum packaging) and dry aging (stored under controlled temperature, relative humidity and airflow with exposure to air)2. During dry aging, air exposure in meat leads to considerable weight loss (20–45%) due to moisture evaporation and the generation of crust (trimming portion) on the meat surface that has intensively concentrated beefy flavor compared to wet-aged meat3,4. During dry aging, protein decomposition or concentration of free amino acids by microbial proteases or moisture evaporation respectively could bestow bioactivities, including antioxidant and angiotensin I–converting enzyme (ACE) inhibitory activities5. Recent studies reported the generation of several bioactive peptides with antioxidant and ACE inhibitory activities from meat and meat products during dry fermentation or aging with protease treatmen6–8. Muscle-based proteins contain unique amino acids with high bioavailability, such as methylhistidine and hydroxymethyl lysine9 and functional dipeptides including carnosine (β-Ala-His) and anserine [β-Als-His(3-Me)], which exhibit antioxidant activity as chelating metal ions or scavenging free radicals10,11. However, functional properties, including antioxidant and ACE inhibitory activity, of the crust produced by the dry aging process, are unknown and not studied. The crust accounts for approximately 35% of dry-aged meat, and is usually discarded.
We hypothesized that the crust which is inevitably produced from dry-aged meat possesses antioxidant and ACE inhibitory activities by the production of peptides during aging. This study determined the antioxidant and ACE inhibitory activities of the crust (trimmed portion of dry-aged beef) compared to unaged (fresh), wet-aged, and dry-aged beef samples. Furthermore, the difference in protein profiles was determined between unaged beef and crust samples and the identification of small peptides was conducted.
Methods
Meat sample preparation
At 24-h postmortem, loins (M. longissimus, n = 4) were obtained from 23-month-old castrated Holstein bulls (quality grade 3, n = 2) slaughtered locally. Each loin was cut into two sections and the six of eight sections randomly assigned to three treatments: (1) unaged (fresh), (2) wet-aged in vacuum packaging for 28 days at 4 °C, or (3) dry-aged for 28 days at 4 °C, RH of 75%, and airflow of 2.5 m/s. Crust was trimmed approximately 1 cm from the external surface of each dry-aged meat by a professional butcher. Pathogens were not detected in the crusts based on 16S rDNA sequencing (data not shown). All samples (crust, flesh of dry-aged, wet-aged, and fresh beef) were trimmed of visible fat and connective tissue, cut into cubes, and minced in a blender (HMF-985, Hanil, Incheon, Korea) for 30 s. The blended meat samples were immediately frozen in liquid nitrogen and lyophilized. The lyophilized samples were ground to a fine powder using a mortar and pestle, screened through a 10-mesh sieve, and kept at −70 °C until use.
Extraction
The whole muscle protein was extracted from the beef samples with a minor modification and used for the further analysis of antioxidant and ACE inhibitory activities12. Briefly, 1 g of each beef sample was homogenized for 30 s in 10 mL of 0.02 M phosphate buffer (pH 7.4). The homogenate was centrifuged (Continent 512R, Hanil) at 1,500 × g for 15 min at 4 °C. The supernatant was collected and used for analysis of antioxidant and ACE inhibitory activities.
Measurement of antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The meat extract was diluted 50 times with deionized distilled water (DDW), and 2 mL of the diluted samples were mixed with 2 ml of 0.2 mM DPPH in methanol13. After vigorous vortexing, the mixture was left in dark for 20 min at room temperature then centrifuged (Continent 512R, Hanil) at 2,268 × g for 10 min at 4 °C. The absorbance of the supernatant was measured at 517 nm using a spectrophotometer (X-ma 3100; Human Corp., Seoul, Korea). Trolox was used as the antioxidant standard. The absorbance was converted to the equivalent activity of Trolox per mL based on the standard curve and expressed as Trolox equivalent antioxidant capacity (TEAC, TEAC/ml extract). DPPH radical scavenging activity (%) was calculated as [1 − (Absorbance of sample at 517 nm/Absorbance of control at 517 nm)] × 100.
2,2′-Azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS) assay
ABTS capacity of each muscle extract was measured by the degree of suppression of ABTS radical cation (ABTS.+) produced by the reaction of ABTS with potassium sulfate14. To obtain the ABTS. + solution, a mixture of 7.0 mM ABTS and 2.45 mM potassium persulfate (final concentration) was prepared and stored in the dark for 16 h. The solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. Fresh ABTS.+ solution was prepared for each analysis. The reaction mixture consisting of 3 mL of the ABTS.+ working solution and 20 μL sample was placed in a cuvette and incubated for 5 min at 30 °C. The mixture was centrifuged (Continent 512 R, Hanil) at 2,268 × g for 5 min. The absorbance of the supernatant was measured at 734 nm using a spectrophotometer (X-ma 3100; Human Corp.). The percentage ABTS.+ inhibition was calculated as:
where AB is the absorbance of the blank and AS is the absorbance of the muscle extract. Trolox was used as the antioxidant standard as described in DPPH assay.
Ferric reducing antioxidant power (FRAP) assay
As a non-radical redox potential-based method, the reducing power of each muscle extract was determined15. Acetate buffer (300 mM, pH 3.6), 10 mM 2,4,6-tris-2,4,6-tripyridyl-2-triazine in 40 mM HCl, and 20 mM ferric chloride solution were prepared in 10:1:1 (v/v/v) portions. The mixture was incubated in a water bath maintained at 37 °C for 30 min. FRAP (3 ml) was added to 100 μl of each sample diluted 4-fold in DDW and incubated for 5 min. The mixture was centrifuged (Continent 512 R, Hanil) at 2,268 ×g for 5 min. The absorbance of the supernatant was measured at 593 nm using a spectrophotometer (X-ma 3100, Human Corp.). Increased absorbance indicated greater reducing power.
Ferrous ion chelating activity assay
The chelating activity of each muscle extract was determined by measuring the ferrous ion-ferrozine complex at 562 nm16. Briefly, 0.5 mL of muscle extract sample was added to 50 μl of FeCl2 (2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.1 ml) and 3.2 ml of ethanol. The mixture was vortexed vigorously and left in dark at room temperature for 10 min and was then centrifuged (Continent 512R, Hanil) at 2,268 g for 5 min. The absorbance of the supernatant was measured at 562 nm using a spectrophotometer (X-ma 3100, Human Corp.). The inhibition of ferrous ion-ferozzine complex formation was calculated as:
where AB′ is the absorbance of the blank and AS′ is the absorbance of the muscle extract.
Measurement of ACE inhibitory activity
ACE inhibitory activity of each sample was determined17. Ten microliters of each muscle extract was incubated with 30 μl hippuryl-L-histidyl-L-leucine (HHL, 12.5 mM in 0.1 M sodium borate buffer) at 37 °C for 10 min. Distilled water instead of sample was used as a blank and control. After incubation, 10 μl of ACE (peptidyldipeptide hydrolase from rabbit lung acetone extract) was added and the mixture incubated at 37 °C for 30 min. The enzymatic reaction was stopped by adding 50 μl of 1 N HCl. The hippuric acid generated by the action of ACE on HHL was extracted from the acidified solution into 300 μl ethyl acetate by vortex mixing for 15 s. The extract was centrifuged at 1,000 ×g for 5 min at 4 °C. Aliquots of 250 μL of each ethyl acetate layer were transferred to clean tubes and evaporated by heating at 70 °C for 1 h in a water bath. The hippuric acid was redissolved in 300 μl of DDW and the amount formed was determined by the absorbance at 228 nm. ACE inhibitory activity (%) was calculated as:
where AS is the absorbance of the sample, ASB is the absorbance of the sample blank, AC is the absorbance of the control, and ACB is the absorbance of the control blank. Enalapril maleate salt (0.3 mg/ml) was the control.
Protein profile using Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Myofibrillar proteins for SDS-PAGE analysis were prepared from unaged and crust sample using 0.03 M phosphate buffer (pH 7.4) and 0.1 M phosphate buffer potassium containing 0.7 M potassium iodide and 0.02% sodium azide as the isolating medium18. The protein concentration of isolated beef samples was determined and analyzed the isolated myofibrillar protein19,20. The stacking gel and separating gel contained 4.5% and 12.5% polyacrylamide, respectively, and 20 μl of meat extract was loaded onto the gel. Protein standards (Precision Plus ProteinTM Unstained Standards, Bio-Rad, Hercules, CA, USA) were included in each electrophoretic run to determine molecular size. Electrophoresis was performed using the AE-6531 Mini-Slab Size Electrophoresis System (Atto Corporation, Tokyo, Japan) at 20 mA for 70 min. Gels were stained for 30 min in 0.1% Coomassie Brilliant Blue R-250 solution containing 30% methanol and 10% acetic acid, then were destained for 90 min in 30% methanol and 10% acetic acid.
Identification of peptides using quadrupole time-of-flight (Q-TOF) liquid chromatography tandem mass spectrometry (LC/MS/MS)
The samples (1 g) were extracted from the unaged and crust samples with 9 ml of 30% acetonitrile in deionized distilled water. The extracts were centrifuged (Combi 514R, Hanil) in Amicon® ultra centrifugal filters (Ultracel® - 3 K, Merck Millipore Ltd., Burlington, MA, USA) to obtain small peptides (<3 kDa). Then, the supernatants were injected to a Triple TOF 5600® Q-TOF LC/MS/MS (AB Sciex, Foster City, CA, USA) using a Ultimate 3000 RSLC HPLC (Thermo Fisher Scientific Inc., Waltham, MA, USA). Mobile phase consisted of solvent A (0.1% formic acid in double-deionized water) and solvent B (0.1% formic acid in 95% acetonitrile) and the gradient programming was as follows: Solvent A and B were set at 95 and 5% from 0 to 1 min, respectively. Solvent A was decreased at 0% from 16 to 18 min. Solvent B was increased at 40% at 16 min and kept increasing at 100% from 18 to 20 min. Solvent A was increased at 95% from 20 to 26 min. Solvent B was decreased at 5% from 21 and 26 min. The flow rate was 0.3 ml/min and the injection volume was 15 μl. The auto-sampler was set at 4 °C. The electrospray ionization (ESI) mass spectra data were acquired under positive ion mode with a source temperature of 500 °C and voltage of 5500 V. The curtain gas, ion source gas 1, and ion source gas 2 were 50, 50, and 25 psi, respectively. Two full-scan mass spectra were acquired over an m/z range of 350–1800 on the MS mode. The data were collected using Analyst TF 1.7 software and analyzed using PeakView 2.2 and ProteinPilot® 4.5 (AB Sciex).
Statistical analysis
The experimental design was completely randomized. Data were from two observations with three replications per observation. The error bars in the figures represent the mean ± standard error values (SE). Statistical analysis was performed using the SAS statistical package (version 9.3, SAS Institute Inc., Cary, NC, USA). Significant differences among the treatments were determined using the Student–Newman–Keul’s multiple comparison test at a level of P < 0.05.
Results and discussion
Antioxidant activity
Radical scavenging activity
DPPH radical scavenging activity was significantly affected (P < 0.05) by the aging methods and beef portion (surface or inner) (Fig. 1(A)). Crust protein extract exhibited the greatest values (44.7% and 0.48 TEAC/ml protein extract; P < 0.05) and wet-aged beef extract showed the lowest values (40.8% and 0.43 TEAC/mL extract; P < 0.05) in DPPH radical scavenging activity, respectively. In this study, protein extract from dry-aged beef (flesh) also showed significantly higher value than wet-aged beef and a lower DPPH radical scavenging activity compared to that from crust (P < 0.05). This result could be assumed that the presence of microorganisms21,22 or moisture evaporation23,24 produced or concentrated free amino acids during dry aging. As many studies agreed, amino acids or peptides produced by proteolytic enzymes of microorganisms present on the surface (crust) during dry aging since dry-aged beef is prone to microbial contamination and mold/yeast growth often occurs on the crust21,22. It is expected that both endogenous enzymes and exogenous proteolytic enzymes produced from microorganism act together, resulting in enhanced degradation of meat protein and production of different peptides in fresh, wet-aged, and dry-aged beef. It was described that naturally generated peptides by microorganisms can induce biological activity by producing peptides of different sizes and various free amino acids25. In addition, microbial fermentation can generate bioactive peptides having antioxidant activity from muscle-based foods26. Different molds and yeasts were found and reported from the crust of dry-aged beef3,27. In addition, during dry aging, moisture evaporation could lead to concentration of free amino acids showing antioxidant activity. Several studies reported that antioxidant activity of protein hydrolysates related to α-amino group content and properties of free amino acids or small peptides such as size, composition, etc.28,29. In addition, previous study reported more abundant aromatic amino acids, including phenylalanine, tyrosine, and tryptophan were present in dry-aged beef compared to wet-aged beef23,24. Aromatic amino acids is known as strong radical scavengers since they donate a proton to electron-deficient radicals and maintain their stability with resonance structure30.
Figure 1.
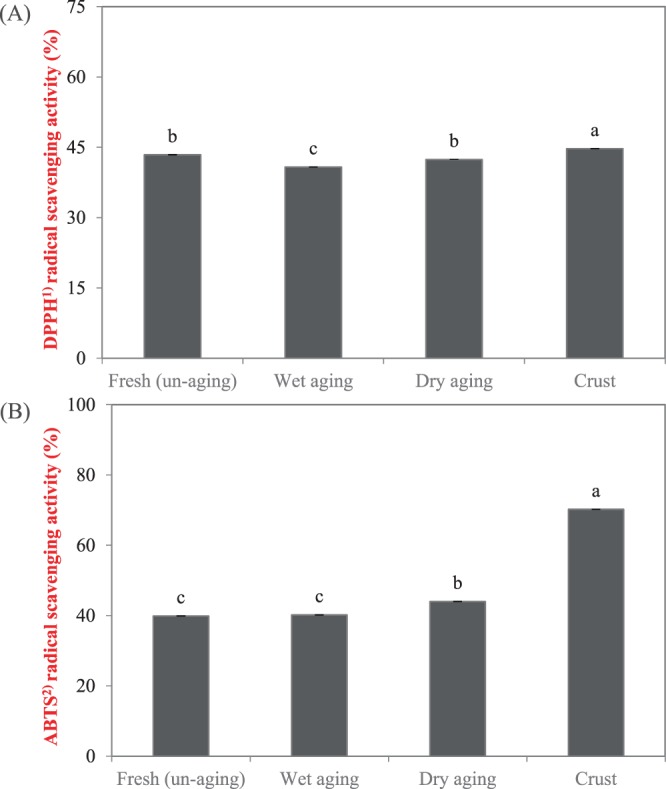
Antioxidant activity determined by radical scavenging activity of unaged, wet-aged, edible portion and crust of dry-aged beef. Error bars indicate the standard errors of means. a–cMeans with different letters on the bar are significantly different (P < 0.05). (1)DPPH (2,2-diphenyl-1-picrylhydrazyl (2)ABTS [2,2′-azino-di-(3-ethylbenzene-thiazoline sulfonate)] radical scavenging activities.
The ABTS radical cation scavenging activity of four protein extracts were similar to the DPPH radical scavenging results (Fig. 1(B)). The greatest ABTS radical cation scavenging activity (69.7% and 43% higher than the lowest value) was observed in crust protein extract, followed by dry-aged beef. This also could be due to microbial growth on the crust previously described. The protein extracts from wet-aged and unaged beef displayed significantly lower ABTS radical cation scavenging activity, with no significant difference between them.
Ferric ion reducing power
Reducing agents act as electron donors to the reduced species and a higher value in absorbance indicates greater reducing capacity. The highest absorbance at 593 nm (0.34; P < 0.05) was observed in crust protein extract (Fig. 2(A)). Previous study presented that protein extracted from fermented sausages exhibited 2-3-time higher antioxidant activity at the end of ripening than at the initial processing stage31. Hence, in this study, we postulated that the direct action of microorganisms on the surface of the dry-aged meat (crust) could contribute to the generation of the reducing power capacity. The difference in absorbance among protein extracts from unaged, wet-aged, and dry-aged beef (ranging from 0.23 to 0.25) seems not to be meaningful, despite the significant difference between wet- and dry-aged beef.
Figure 2.
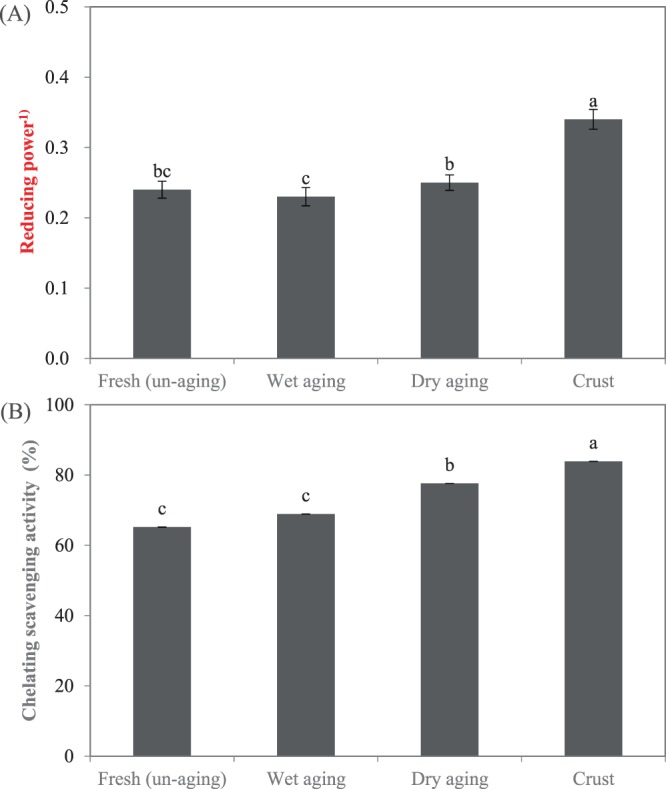
Antioxidant activity determined by (A) ferric ion reducing power and (B) metal chelating activity of unaged, wet-aged, edible portion and crust of dry-aged beef. Error bars indicate the standard errors of means. a–cMeans with different letters on the bar are significantly different (P < 0.05). (1)Reducing power measured by absorbance at 593 nm.
Metal chelating activity
A clear difference in metal chelating activity was evident between the samples (Fig. 2(B)). Chelating activity was greatest for crust protein extract (83.9%; P < 0.05), followed by dry-aged beef (77.6%). Chelating activities were significantly lower for protein extracts from unaged and wet-aged beef (65.2% and 68.9%, respectively). According to previous studies, dry-aged beef exhibited a higher amount of the sulfur-containing amino acid methionine23,24, which perhaps contributed to the increased metal chelating activity in comparison with wet-aged beef. Consequently, the type of amino acid or peptide produced by proteolysis might depend on the endogenous enzymes and those from microorganisms present on the surface of the beef during aging period.
ACE inhibitory activity
ACE inhibitory activity of protein extract was affected by the aging method (wet or dry aging) and the section of dry-aged meat [internal or external (crust) sections] (Fig. 3). Significantly high and low ACE inhibitory activities were observed for protein extract from crust and unaged beef, respectively. Similarly, it was reported that peptides derived from dry-cured ham by the action of microbial endo- and exogenous enzymes presented ACE inhibitory activity32. The protein extract from wet-aged beef showed similar (P > 0.05) ACE inhibitory activity to unaged beef. It was described that the limited generation and/or identification of peptides with ACE inhibitory activity by endogenous proteases due to the lack of control and irregularity of endogenous proteases33. A recent report demonstrated that injection of thermolysin in beef loin during the marination process resulted in approximately 500-times higher ACE inhibitory activity than control (IC50 1,206.0 vs 2.3 μg/mL) with an inhibitory effect on cancer cell viability after 3 days of storage8. Generation of small peptides through proteolysis by various enzymes (endo- and/or exogenous enzymes) increases the potent ACE inhibitory activity due to production of peptides with unspecified cleavage sites34–36. In addition, multi-enzymes hydrolysates derived from protein based ingredients exhibited higher ACE inhibitory activity compared to single-enzyme counterpart37. Generally, crust is more prone to protein decomposition by the aforementioned enzymes because the meat surface layer can be directly affected by microorganisms during dry aging. This assumption should be confirmed in a further study by investigating the effect of microorganisms (microbial enzymes) growing on the surface of beef on the production of ACE inhibitory peptides during dry aging. In addition, relationship between ACE inhibitory activity and the production of small peptide would be more discussed in Section of identification of peptides.
Figure 3.
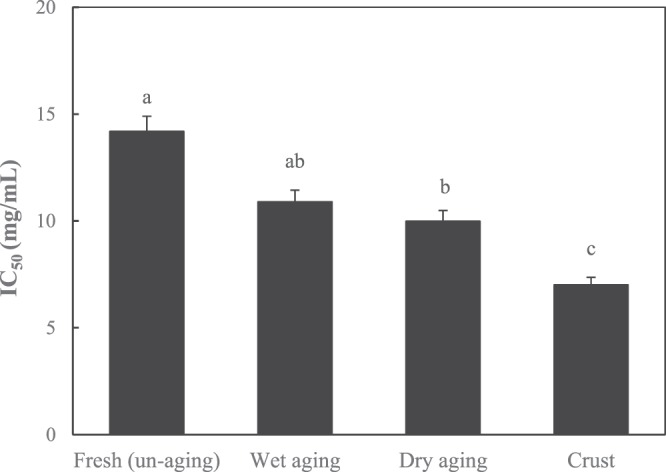
Angiotensin I-converting enzyme (ACE) inhibitory activity (IC50) of unaged, wet-aged, edible portion and crust of dry-aged beef. Error bars indicate the standard errors of means (1.03). a–cMeans with different letters on the bar are significantly different (P < 0.05).
Protein profile by SDS-PAGE
Protein bands with low molecular weight (<15 kDa) were more prominently observed in aged samples compared to unaged one (Fig. 4). Especially, the crust sample had higher intensity in bands with low molecular weight among the treatments. About 5–10 kDa peptides in dry sausages exhibited up to 86% of the DPPH radical scavenging activity38. In addition, low-molecular-weight peptides generated from protein by protease treatment exhibited higher ACE inhibitory activity compared with high-molecular-weight peptides39. Taken together, in this study, the generation of low molecular weight proteins may induce greater antioxidant and/or ACE inhibitory activity of crust and dry aged beef due to the proteolysis that occurs during dry aging. However, in this study, difference in appearance of protein band with <15 kDa in dry- and wet-aged samples was ambiguous. this result would be elucidated plainly based on identification of peptide and discussed in followed section.
Figure 4.
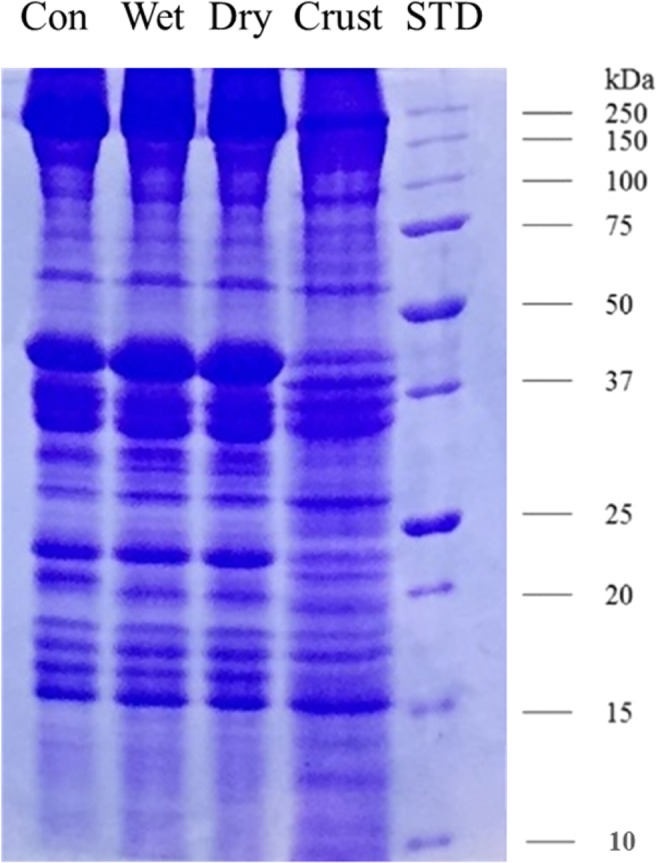
Myofibrillar protein pattern of unaged (Con), wet-aged, edible portion and crust of dry-aged beef. STD, standard molecular bands.
Identification of peptides
Similar to the result from SDS-PAGE, much more number of small peptides (<3 kDa; 143 different types of peptides) from the aged and crust samples than those of unaged beef were detected, regardless of aging method (See Supplementary Table S1). On the other hand, types of peptides depended on aging method and location of dry-aged beef. Before aging, there were only 6 small peptides identified from the samples, while 52 and 64 different peptides from 19 and 25 different protein sources were found on the wet- and dry-aged samples, respectively. Interestingly, peptide containing same amino acid sequence was not generated between unaged and crust samples. This phenomenon was possibly due to presence of aforementioned microbial enzymes in crust which can more affected by microorganisms compared to edible portion. In our previous study, we found different changes in the metabolites of dry-aged beef by different microbial compositions22. Small peptides were more generated in dry-cured ham at final processing time causing by action of endopeptidase and exopeptidase by microorganisms during processing, comparing to those at initial time40. Many studies found that small peptides by enzymatic hydrolysis released and then it can increase functional property including antioxidant and ACE inhibitory activities in food41. In the present study, smaller peptides (<1 kDa) were observed in crust samples among the treatments, which was corresponded with specificity (lysyl-lysine residue; peptide No. 32) of previously reported peptide antioxidants42. According to previous study, peptides composing histidine at the C-terminus exhibited effective free radical scavenging activity43. Total 15 peptides (peptide 22, 27, 43, 51, 53, 61, 120, 137–139, 143–146, and 149) matched to aforementioned specificity were observed in all samples, except for unaged samples. In addition, hydrophobic amino acids [alanine (A), valine (V), leucine (L), and isoleucine (I)] which can effectively act to free radicals from the lipid phase as scavenger were mostly presented in meat samples of this study.
Previous studies found that substrates having N-terminal branched-chain amino acid and C-terminal hydrophobic amino acids can bind to active site of ACE as exhibiting strong affinity44. In this study, total 17 types of potential inhibitory peptides were observed as indicated with asterisk in Table S1. Among the 17 types of peptides, 1 (No. 125), 6 (No. 39, 40, 45, 76, 128, and 141), 7 (No. 12, 21, 38, 39, 42, 45, and 112), and 7 (No. 33, 39, 76, 95, 108, 133, and 135) types of the ACE inhibitory peptides were observed in unaged, wet- and dry-aged, and crust samples with partial overlap, respectively. ACE inhibitor possesses hydrophobic substituents at three C-terminal amino acids which can bind to active site of ACE45. Especially, presence of arginine (R), lysine (K) or proline (P) led to strong ACE inhibition activity in food protein-derived peptides at each of the three C-terminal position46,47. In this study, peptides (peptide 90 and 133) with aforementioned characteristic were observed in crust samples. Hence, the greatest (P < 0.05) ACE inhibition activity in crust samples was exhibited probably due to the presence of lysine and/or proline at the three C-terminal position.
Conclusion
Based on this study, the excellent activities of antioxidant and ACE inhibitory activities from the crust may be due to the increases in these small peptides and sequence position of functional amino acids which were generated endo- and exopeptidases. The effect of microorganisms on proteolysis and lipolysis in the crust and edible portion of meat, and the relationship with the generation of bioactive peptides and characteristic flavors are under investigation in our laboratory. Consequently, the crust could be utilized as a functional ingredient possessing antioxidant and ACE inhibitory activity instead of disuse. In other words, dry aging can be used for generation of bioactive peptides from beef loin.
Supplementary information
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agricultural and Forestry (Project No. 119024-3) and the BK21 Plus Program of the Department of Agricultural Biotechnology, Seoul National University, Seoul, Republic of Korea.
Author contributions
J.C. wrote the main manuscript; J.C., B.P. and H.J.L. analyzed and interpreted data and contributed the general idea of the study; C.J. supervised and conceived the project. All authors discussed the results and contributed to the preparation and critical revision of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64861-0.
References
- 1.Choe J, Stuart A, Kim YHB. Effect of different aging temperatures prior to freezing on meat quality attributes of frozen/thawed lamb loins. Meat Sci. 2016;116:158–164. doi: 10.1016/j.meatsci.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Lee, et al. Determination of salable shelf-life for wrap-packaged dry-aged beef during cold storage. Korean J. Food Sci. An. 2018;38:251–258. doi: 10.5851/kosfa.2018.38.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh H, Lee HJ, Lee J, Jo C, Yoon Y. Identification of microorganisms associated with the quality improvement of dry-aged beef through microbiome analysis and DNA sequencing, and evaluation of their effects on beef quality. J. Food Sci. 2019;84:2944–2954. doi: 10.1111/1750-3841.14813. [DOI] [PubMed] [Google Scholar]
- 4.Khan MI, Jung S, Nam KC, Jo C. Postmortem aging of beef with a special reference to the dry aging. Korean J. Food Sci. An. 2016;36:159–169. doi: 10.5851/kosfa.2016.36.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajapakse, et al. K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005;38:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- 6.Gallego M, Mora L, Escudero E, Toldrá F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018;276:71–78. doi: 10.1016/j.ijfoodmicro.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Ma, et al. Tenderization of yak meat by the combination of papain and high-pressure processing treatments. Food Bioprocess Tech. 2019;12:681–693. doi: 10.1007/s11947-019-2245-3. [DOI] [Google Scholar]
- 8.Seol, et al. Bioactivities of peptide fractions derived from proteolytic enzyme-injected Hanwoo longissimus muscle in a model system. Int. J. Food Prop. 2018;21:220–227. doi: 10.1080/10942912.2018.1440241. [DOI] [Google Scholar]
- 9.Wang D, Shahidi F. Protein hydrolysate from turkey meat and optimization of its antioxidant potential by response surface methodology. Poul. Sci. 2018;97:1824–1831. doi: 10.3382/ps/pex457. [DOI] [PubMed] [Google Scholar]
- 10.Mora L, Sentandreu MA, Toldrá F. Contents of creatine, creatinine and carnosine in porcine muscles of different metabolic types. Meat Sci. 2008;79:709–715. doi: 10.1016/j.meatsci.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Ambigaipalan P, Al-Khalifa AS, Shahidi F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods. 2015;18:1125–1137. doi: 10.1016/j.jff.2015.01.021. [DOI] [Google Scholar]
- 12.Kim YH, Huff-Lonergan E, Sebranek JG, Lonergan SM. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010;85:759–767. doi: 10.1016/j.meatsci.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–120. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 14.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 16.Chung HJ, Jeon IS. Antioxidative activities of methanol extracts from different parts of Chrysanthemum zawadskii. Korean J. Food Preserv. 2011;18:739–745. doi: 10.11002/kjfp.2011.18.5.739. [DOI] [Google Scholar]
- 17.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 18.Grabski AC, Burgess RR. Preparation of samples for SDS-polyacrylamide gel electrophoresis: procedures and tips. Innovations. 2001;13:10–12. [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee, et al. Analysis of low-marbled Hanwoo cow meat aged with different dry-aging methods. Asian Australas. J. Anim. 2017;30:1733–1738. doi: 10.5713/ajas.17.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, et al. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019;153:152–158. doi: 10.1016/j.meatsci.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim YHB, Kemp R, Samuelsson LM. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016;111:168–176. doi: 10.1016/j.meatsci.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Lee, et al. Role of moisture evaporation in the taste attributes of dry- and wet-aged beef determined by chemical and electronic tongue analyses. Meat Sci. 2019;151:82–88. doi: 10.1016/j.meatsci.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Toldrá F, Reig M, Aristoy MC, Mora L. Generation of bioactive peptides during food processing. Food Chem. 2018;267:395–404. doi: 10.1016/j.foodchem.2017.06.119. [DOI] [PubMed] [Google Scholar]
- 26.Kim DC, Chae HJ, In MJ. Existence of stable fibrinclotting inhibitor in salt fermented anchovy sauce. J. Food Compos. Anal. 2004;17:113–118. doi: 10.1016/S0889-1575(03)00106-6. [DOI] [Google Scholar]
- 27.Park B, Yong HI, Choe J, Jo C. Utilization of the crust from dry-aged beef to enhance flavor of beef patties. Korean J. Food Sci. An. 2018;38:1019–1028. doi: 10.5851/kosfa.2018.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- 29.Kittiphattanabawon P, Benjakul S, Vissessanguan W, Shahidi F. Effect of extraction temperature on functional properties and antioxidative activities of gelatin from shark skin. Food Bioprocess Tech. 2012;5:2646–2654. doi: 10.1007/s11947-010-0427-0. [DOI] [Google Scholar]
- 30.Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- 31.Vaštag Ž, Popović L, Popović S, Petrović L, Peričin D. Antioxidant and angiotensin-I converting enzyme inhibitory activity in the water-soluble protein extract from Petrovac sausage (Petrovská Kolbása) Food Control. 2010;21:1298–1302. doi: 10.1016/j.foodcont.2010.03.004. [DOI] [Google Scholar]
- 32.Gallego M, Mora L, Fraser PD, Aristoy MC, Toldrá F. Degradation of LIM domain-binding protein three during processing of Spanish dry-cure ham. Food Chem. 2014;149:121–128. doi: 10.1016/j.foodchem.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 33.Lafarga T, Hayes M. Bioactive peptides from meat muscle and byproducts: Generation, functionality and application as functional ingredients. Meat Sci. 2014;98:227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Choe, et al. Isolation and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from thermolysin-injected Hanwoo M. longissimus. Asian Australas. J. Anim. 2019;32:430–436. doi: 10.5713/ajas.18.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Chen X, Huang J, Huang M, Zhou G. Generation of bioactive peptides from duck meat during post-mortem aging. Food Chem. 2017;237:408–415. doi: 10.1016/j.foodchem.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 36.Toldrá F, Flores M, Sanz Y. Dry-cured ham flavour: Enzymatic generation and process influence. Food Chem. 1997;59:523–530. doi: 10.1016/S0308-8146(97)00013-7. [DOI] [Google Scholar]
- 37.Kong, et al. Optimization of conditions for enzymatic production of ACE inhibitory peptides from collagen. Food Bioprocess Tech. 2011;4:1205–1211. doi: 10.1007/s11947-009-0198-7. [DOI] [Google Scholar]
- 38.Sun, et al. Structural characteristics of peptides extracted from Cantonese sausage during drying and their antioxidant activities Structural characteristics of peptides extracted from Cantonese sausage during drying and their antioxidant activities. Innov. Food Sci. Emerg. 2009;10:558–563. doi: 10.1016/j.ifset.2009.07.006. [DOI] [Google Scholar]
- 39.Aluko RE. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Tech. 2015;6:235–262. doi: 10.1146/annurev-food-022814-015520. [DOI] [PubMed] [Google Scholar]
- 40.Mora L, Sentandreu MA, Toldrá F. Identification of small troponin T peptides generated in dry-cured ham. Food Chem. 2010;123:691–697. doi: 10.1016/j.foodchem.2010.05.035. [DOI] [Google Scholar]
- 41.Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: A review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Suetsuna K. Antioxidant peptides from the protease digest of prawn (Penaeus japonicus) muscle. Mar. Biotechnol. 2000;2:5–10. doi: 10.1007/s101269900002. [DOI] [PubMed] [Google Scholar]
- 43.Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- 44.Toopcham T, Mes JJ, Wichers HJ, Roytrakul S, Yongsawatdigul J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017;220:190–197. doi: 10.1016/j.foodchem.2016.09.183. [DOI] [PubMed] [Google Scholar]
- 45.Choe, et al. Identification of angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of pork loin. Int. J. Food Prop. 2019;22:1112–1121. doi: 10.1080/10942912.2019.1629690. [DOI] [PubMed] [Google Scholar]
- 46.Gu Y, Majumder K, Wu J. QSAR-aided in silico approach in evaluation of food proteins as precursor of ACE inhibitory peptides. Food Res. Int. 2011;44:2465–2474. doi: 10.1016/j.foodres.2011.01.051. [DOI] [Google Scholar]
- 47.Fernández, et al. Influence of starter culture and a protease on the generation of ACE inhibitory and antioxidant bioactive nitrogen compounds in Iberian dryfermented sausage ‘salchichón’. Heliyon. 2016;2:e00093. doi: 10.1016/j.heliyon.2016.e00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


