Abstract
Objectives:
To examine the temporal trajectory of insurance coverage for next-generation tumor sequencing (sequencing) by private US payers, describe the characteristics of coverage adopters and nonadopters, and explore adoption trends relative to the Centers for Medicare and Medicaid Services’ National Coverage Determination (CMS NCD) for sequencing.
Methods:
We identified payers with positive coverage (adopters) or negative coverage (nonadopters) of sequencing on or before April 1, 2019, and abstracted their characteristics including size, membership in the BlueCross BlueShield Association, and whether they used a third-party policy. Using descriptive statistics, payer characteristics were compared between adopters and nonadopters and between pre-NCD and post-NCD adopters. An adoption timeline was constructed.
Results:
Sixty-nine payers had a sequencing policy. Positive coverage started November 30, 2015, with 1 payer and increased to 33 (48%) as of April 1, 2019. Adopters were less likely to be BlueCross BlueShield members (P < .05) and more likely to use a third-party policy (P < .001). Fifty-eight percent of adopters were small payers. Among adopters, 52% initiated coverage pre-NCD over a 25-month period and 48% post-NCD over 17 months.
Conclusions:
We found an increase, but continued variability, in coverage over 3.5 years. Temporal analyses revealed important trends: the possible contribution of the CMS NCD to a faster pace of coverage adoption, the interdependence in coverage timing among BlueCross BlueShield members, the impact of using a third-party policy on coverage timing, and the importance of small payers in early adoption. Our study is a step toward systematic temporal research of coverage for precision medicine, which will inform policy and affordability assessments.
Keywords: insurance coverage, precision medicine, temporal policy analyses, tumor sequencing
Introduction
Precision medicine—the use of genetics and genomics to inform clinical decisions—is rapidly permeating healthcare, with a soaring number of available tests, increasing spending, expanded disease scope, and transition from single-gene to multigene testing and exome/genome sequencing.1 Given this proliferation, clinical adoption, patient access, and affordability of precision medicine become crucial.2,3 Insurance coverage is a key determinant of clinical adoption and patient access and thus is important to understand.4,5
In the US multipayer system, insurance coverage fora test may vary across payers notonly in substance (what is coveredandhow), but also in time (when individual payers decide to grant coverage). Hence, insurance coverage in the United States is not a single event but a process. Studying it as a dynamic, rather than a static, phenomenon may produce valuable temporal insights relevant in the multipayer environment. These insights may include how long it takes for a test to gain coverage from a certain number of payers, which payers adopt positive coverage earlier than other payers, and what events preceding coverage decisions may have had an impact. These insights could informanalyses of trends in patient access and affordability, which also evolve over time.
Temporal coverage analyses require access to historical policy information, but this is not easily available, as payers typically post only the most current coverage policy on their websites.6 Unsurprisingly, to date, most research on insurance coverage for precision medicine has been cross-sectional, painting a picture of coverage only at a point in time.7–10 Several studies included temporal analyses, but they were constrained by a small number of considered payers or unavailability of the necessary historical policy information.11–13 Thus, temporal trends in insurance coverage for personalized medicine have been unexplored.
Our study used an opportunity to access a proprietary library of historical and current medical policies of US payers by Canary Insights (http://canaryinsights.com/). Using the data we abstracted from the Canary database, we explored temporal trends in insurance coverage for next-generation tumor sequencing (hereafter referred to as sequencing), which is used to genetically interrogate a person’s tumor to guide the selection of oncologic therapies.
We focused on sequencing for several reasons. First, its clinical importance: sequencing is now broadly used across advanced cancers to inform decisions on life-prolonging treatments, with demonstrated survival benefit in some cancers.14 It has been recommended by the National Comprehensive Cancer Network (NCCN)—a leading oncology guideline body—in non–small-cell lung cancer (NSCLC) since 201515 and later in other cancers.16 Second, generalizability: sequencing represents a novel but rapidly growing test category of multigene panels, which is proliferating in many disease areas beyond oncology. And third, the existence of coverage history: since its emergence in clinical practice in 2012,17 sequencing has gained coverage by a number of payers, including private health plans and Centers for Medicare and Medicaid Services (CMS). This provides an opportunity to explore the trends in private payer coverage relative to the decision by CMS, the largest and an influential US payer. This is of particular interest because the CMS’s announcement of sequencing cove rage was unexpected and generated substantial debate.18–21 We previously found that many private payers were considering the CMS decision in their internal coverage decision making, and the current study can now address the question of whether, when, and which private payers have followed CMS in providing coverage.20
The objectives of our study were to examine (1) how adoption of positive coverage for sequencing increased over time among private US payers, (2) what the characteristics were of payers who were adopters of positive coverage and how they compared with characteristics of nonadopters, (3) the characteristics of payers who granted coverage before and after the CMS’s decision, and (4) whether policy features changed over time for individual payers. This study builds on our previous research on coverage for sequencing and other multigene panels, including an interview study with payers examining their perspectives on coverage for sequencing, conducted before sequencing started to gain coverage.22–24 In contrast, the present work examines the actual adoption of positive coverage that occurred thereafter. In addition, we previously examined payers’ coverage policies for sequencing and other multigene panels, but those were cross-sectional analyses.8,9
Our study is an innovative step toward systemic temporal assessment of payer coverage for precision medicine, with a potential to inform future policy, access, and affordability analyses of this fast-developing field.
Methods
Definitions, Conceptual Framework, and Variables
We defined a positive coverage policy as a policy stating coverage of sequencing for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization). A negative coverage policy was a policy stating that sequencing was not covered for any indication or any sequencing test(s). Adopters were defined as payers who adopted positive coverage for sequencing on or before April 1, 2019. Nonadopters were defined as payers who had an explicit negative coverage policy for sequencing as of April 1, 2019.
We focused on private payers because, in total, they cover two-thirds of the insured US population.25 We did not include Medicaid, as coverage policies are not consistently available from state Medicaid agencies and therefore are not consistently cataloged in the Canary Database.
We conceptualized that the following characteristics of private payers would be associated with the timing of sequencing coverage:
Payer size measured in commercial enrollment. Payer size is a factor in payer decision making because larger payers may have more resources to monitor and assess new genomic technologies.26 Prior coverage studies focused only on large or medium to large payers,7,9,10,12 with 1 study reporting that the largest payers provided coverage earlier than others.12 The timing of coverage decisions by smaller payers has not been explored. We defined smaller payers as covering fewer than 1 000 000 lives in commercial enrollment. We used commercial, and not total, enrollment because it is directly affected bya payer’s coverage policies, whereas enrollees in noncommercial plans, such as Medicare Advantage, are affected by CMS policies.
Whether a payer belonged to the BlueCross BlueShield Association. The association offers health technology assessment and coverage policy guidance to its 39 members, but the guidance is not binding, and each member makes its own policy decisions. Prior studies considered BlueCross BlueShield member plans as independent payers but noted that there may be interdependence among them.7,12
Whether a payer serviced a Medicare Advantage plan, a type of Medicare plan administered by private payers. Payers servicing Medicare Advantage must follow the CMS coverage policy for sequencing for these enrollees, which may create a dichotomy with their commercial enrollee policies. It has been suggested that these payers may follow CMS in their commercial coverage sooner than other payers.20
Whether a payer used policies provided by a third party. Payers may use third-party independent organizations offering laboratory benefit management services, which may include coverage policy development for genetic testing.10,27 This trend has increased in recent years,28 but its impact on the timing of coverage decisions is unknown.
Based on our research questions and conceptual framework, we developed study variables (Table 1). Independent variables reflected payer characteristics (1–4 in the aforementioned list). Dependent variables were adopter (whether a payer adopted a positive coverage policy for any sequencing test and any indication on or before April 1, 2019), first coverage date by payer (date of the first positive coverage policy by payer), and pre–National Coverage Determination (NCD) adopter (whether the date of the first coverage policy was before or after November 30, 2017, when CMS released the draft NCD for sequencing29). We chose the date of the draft NCD announcement, rather than the date of the final NCD issuance (March 18, 2018): the draft was largely unexpected, attracted considerable attention, and generated more than 300 public comments,30 whereas the final NCD was imminent, although somewhat different in content.31 The CMS NCD provided coverage for next-generation tumor sequencing for Medicare patients with advanced solid cancers; the covered tests must be approved by the Food and Drug Administration for the indications used and serve as a companion diagnostic to guide the use of targeted therapies.
Table 1.
Study variables and definitions.*
| Variable | Variable description and values | Dependent or independent variable |
|---|---|---|
| Adopter | 1 - Adopter, payer who adopted a positive coverage policy for sequencing on or before April 1, 2019 0 - Nonadopter, payer who had a negative coverage policy for sequencing as of April 1, 2019 |
Dependent |
| First coverage date by payer | Date of payer's first coverage decision for sequencing | Dependent |
| Pre-NCD adopter | 1 - Adopter, payer with the first coverage policy for sequencing adopted before CMS released draft NCD on November 30, 2017 0 - Adopter, payer with the first coverage policy for sequencing adopted after CMS released draft NCD on November 30, 2017 |
Dependent |
| Payer size | Number of covered lives for commercial enrollment: larger (≥1 000 000); smaller (<1 000 00) | Independent |
| BlueCross BlueShield member | 1 - payer is a member of BCBSA 0 - payer is not a member BCBSA |
Independent |
| Offers Medicare Advantage | 1 - Payer offers Medicare Advantage 0 - Payer does not offer Medical Advantage |
Independent |
| Adopted a third-party policy | 1 - payer who used a third-party LBM policy in the coverage decision for sequencing 0 - payer who did not use a third-party LBM policy in the coverage decision for sequencing |
Independent |
BCBSA indicates BlueCross BlueShield Association; CMS, Centers for Medicare and Medicaid Services; LBM, Laboratory Benefit Management company; NCD, National Coverage Determination; sequencing, next-generation tumor sequencing.
Positive coverage policy states coverage of sequencing for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization). Negative coverage policy states that sequencing is not covered for any indication or any sequencing test(s). Third-party policy is by a third-party laboratory benefit management company.
Selection of Policies, Payer Sampling, and Data Abstraction
We used the Canary Insights database to obtain private payers’ coverage policies for sequencing and to identify payer characteristics. Canary is a proprietary database containing more than 40 000 medical policies from more than 200 commercial payers, Medicare, and Medicaid, which publish coverage policies on their websites. It is updated daily based on a proprietary search engine. The policies are stored in a historical fashion in the form published by a payer and are not abstracted or curated by Canary.
We conducted policy selection from Canary between April 15, 2019, and April 30, 2019, using the following search terms: “tumor sequencing,” “tumor molecular profiling,” “tumor biomarkers,” “broad molecular profiling,” “cancer genetic testing,” “sequencing,” and “genetic testing.” In addition, relevant current procedural terminology codes were used as search terms 81445, 81450, and 81455. If a payer had multiple historical policy versions, all of them were selected and downloaded on a local server. We validated our search terms by confirming that we did not find any instances of sequencing coverage determinations within other policies (eg, hereditary cancer genetic testing) or that the policies we identified using our search terms did not refer to other policies that may include a sequencing coverage determination. In addition, we used the publically available information from the website of eviCore (https://www.evicore.com), a third-party laboratory benefit management company whose sequencing policies were used by some payers to confirm the dates of initiation of positive coverage by those payers.
Data were abstracted in several steps. First, for the adopter versus nonadopter analysis, we defined the study cohort as private payers with an explicit sequencing coverage policy (positive or negative, for any indication or test). Payers without a sequencing policy were excluded because the absence of a policy is inconclusive whether they cover sequencing or not. We then abstracted payer characteristics from Canary for all payers in our study cohort. Second, for adopters, we identified the first positive coverage based on policy date; these policies were abstracted for the policy date and whether adopted from a third-party company. In addition, we reviewed the content of positive coverage policies to identify changes between policy versions and cancers covered for sequencing.
Data Analyses
Our unit of analysis was the payer (with a positive or negative decision regarding coverage of sequencing). We described the distribution of payer characteristics and the temporal adoption of positive sequencing coverage. We used the Fisher exact test to examine the associations between payer characteristics and whether positive coverage adopter in addition to payer characteristics and whether pre-versus post-NCD adopter. All analyses were performed using STATA/SE 14.2 software (StataCorp, College Station, TX). We also constructed a visual timeline of coverage decisions as an additional illustration of temporal coverage trends.
Results
Payer Sample Characteristics
Of the 92 private payers in the Canary database, 69 (75%) had an explicit coverage policy for sequencing during the study period and were included in our sample. Each payer had only 1 policy related to sequencing, with several historical versions. Table 2 describes the characteristics of the payer sample. Collectively, the 69 payers in our sample cover 184 045 000 commercial enrollees: 59% of the sample were small payers, 52% were Blue Cross BlueShield members, 72% offered Medicare Advantage, and 22% used a third-party sequencing policy.
Table 2.
Sample characteristics: Private payers with an explicit positive or negative coverage policy for next-generation tumor sequencing.
| Payer characteristic | Percentage (n = 69) |
|---|---|
| Size, in covered lives* | |
| 1 000 000 or more | 41 |
| Less than 1 000 000 | 59 |
| BlueCross BlueShield member | |
| Yes | 52 |
| No | 48 |
| Offer Medicare Advantage | |
| Yes | 72 |
| No | 28 |
| Adopted a third-party policy | |
| Yes | 22 |
| No | 78 |
Size is reported for lives covered by commercial insurance. Third-party policy is provided by a third-party laboratory benefit management company. Positive coverage policy states coverage of sequencing for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization). Negative coverage policy states that sequencing is not covered for any indication or any sequencing test(s).
How the Adoption of Positive Coverage for Sequencing Increased Over Time
The first positive coverage decision for sequencing by a payer occurred in 2015. Thereafter, the number of payers granting first time positive coverage increased every year, with the biggest increment in 2017 (Table 3). By April 1, 2019, 48% (33 of 69)—nearly half of the payer sample—adopted positive sequencing coverage. This adoption occurred over a period of 3.5 years, from November 2015 to April 2019—the end of our study period (Fig. 1). The first adopter was a smaller payer. The combined population of commercial enrollees covered for sequencing increased every year, with the largest increment in 2018. By the end of the study period, the population covered for sequencing reached more than 128 million commercial lives, comprising 70% of the total commercial population in our payer sample (data not shown).
Table 3.
Initiation of positive coverage of next-generation tumor sequencing by year.*
| Year | Percentage of payers who initiated positive coverage policy for sequencing (n = 69) | Commercial enrollee population covered by new coverage in this year† |
|---|---|---|
| 2015 | 1 | 522 570 |
| 2016 | 4 | 23 961 190 |
| 2017‡ | 20 | 38 690 818 |
| 2018 | 17 | 64 014 700 |
| 2019§ | 4 | 910 275 |
| Total | 48 | 128 099 553 |
Sequencing indicates next-generation tumor sequencing.
Positive coverage policy states coverage of sequencing for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization).
Commercial population is reported based on the data available in the Canary Insights database as of 04/01/2019.
Year of Center for Medicare and Medicaid Services (CMS) announcement of the draft National Coverage Determination (NCD).
Includes coverage through April 1, 2019.
Figure 1.
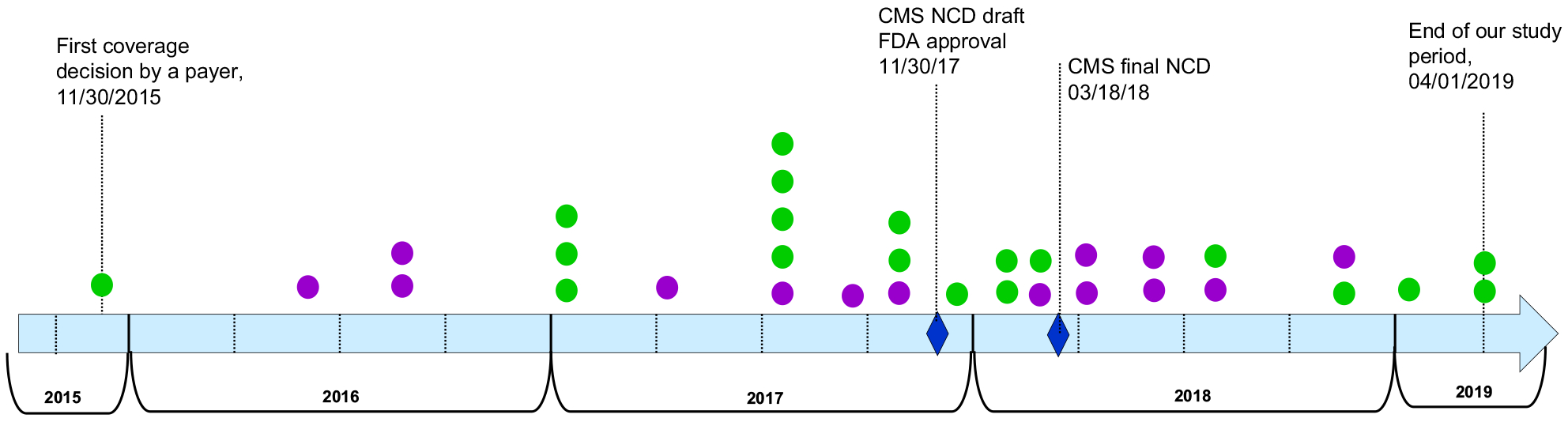
Timing of payer adoption of positive coverage for NGTS Note. Adopters, payers who adopted positive coverage for NGTS on or before 04/01/2019 for any indication, any NGTS test(s) and any policy stipulation (eg, with or without prior authorization); N = 33, total number of payers who adopted a positive coverage for NGTS on or before 04/01/2019. Green circles denote payers with less than 1 000 000 commercial enrollees. Purple circles denote payers with 1 000 000 or more commercial enrollees.
NGTS indicates next-generation tumor sequencing.
Characteristics of Adopters and Nonadopters of Positive Coverage for Sequencing
In comparing the adopter and nonadopter characteristics (Table 4), we found that adopters were less likely to be BlueCross BlueShield members than nonadopters (36% vs 67%, respectively; P = .02), and were more likely to use a third-party policy for sequencing than nonadopters (45% vs 0%, respectively; P < .001). Among adopters, a slightly higher, not statistically significant proportion of payers offered Medicare Advantage than among nonadopters (76% vs 69%). The adopter and nonadopter groups had approximately the same proportion of smaller payers under 1 000 000 in commercial enrollees (58% vs 61%, respectively).
Table 4.
Characteristics of payer adopters and nonadopters of coverage for next-generation tumor sequencing.*
| Payer characteristic | Percentage of adopters, n = 33 | Percentage of nonadopters, n = 36 | P value |
|---|---|---|---|
| Size, in covered lives | |||
| 1 000 000 or more | 42 | 39 | 0.8 |
| Less than 1 000 000 | 58 | 61 | |
| BlueCross BlueShield member | |||
| Yes | 36 | 67 | 0.02† |
| No | 64 | 33 | |
| Offer Medicare Advantage | |||
| Yes | 76 | 69 | 0.6 |
| No | 24 | 31 | |
| Adopted a third-party policy | |||
| Yes | 45 | 0 | 0.0001‡ |
| No | 55 | 100 | |
Sequencing indicates next-generation tumor sequencing.
Adopters are payers who adopted positive coverage for sequencing on or before April 1, 2019, for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization). Nonadopters are payers who had explicit negative coverage for sequencing as of April 1, 2019 (ie, not covering sequencing for any indication or any sequencing test[s]). Size is reported for lives covered by commercial insurance. Offer Medicare Advantage includes payers who service Medicare Advantage plans on behalf of Medicare. Third-party policy is provided by third-party laboratory benefit management company.
P < .05.
P < .001.
Characteristics of Pre-NCD and Post-NCD Adopters of Positive Coverage for Sequencing and Observations of Adoption Over Time
Payer characteristics were not statistically different between the pre-NCD and post-NCD adopters (Table 5). Nevertheless, there were informative numeric and qualitative observations of adoption between the 2 periods. Approximately the same number of payers adopted a positive sequencing coverage pre-NCD (52%) as post-NCD (48%; Table 5). Post-NCD adoption occurred over a shorter time: 17 months for post-NCD adoption versus 25 months for pre-NCD adoption (Fig. 1). Pre-NCD adoption was uneven and sparser in time, whereas post-NCD adoption occurred at a relatively steady pace throughout the 17 months.
Table 5.
Characteristics of pre-NCD and post-NCD adopters of coverage for next-generation tumor sequencing.*
| Payer characteristic | Percentage of pre-NCD adopters, n = 17 | Percentage of post-NCD adopters, n = 16 | P value |
|---|---|---|---|
| Size, in covered lives | |||
| 1 000 000 or more | 41 | 44 | 1.0 |
| Less than 1 000 000 | 59 | 56 | |
| BlueCross BlueShield Member | |||
| Yes | 29 | 44 | 0.5 |
| No | 71 | 56 | |
| Offer Medicare Advantage | |||
| Yes | 65 | 87.5 | 0.2 |
| No | 35 | 12.5 | |
| Adopted a third-party policy | |||
| Yes | 53 | 37.5 | 0.5 |
| No | 47 | 62.5 | |
Sequencing indicates next-generation tumor sequencing.
Adopters are payers who adopted positive coverage for sequencing on or before April 1, 2019, for any indication, any sequencing test(s), and any policy stipulation (eg, with or without prior authorization). Pre-NCD adopters are payers who adopted positive coverage for sequencing before the Centers for Medicare and Medicaid Services (CMS) released draft National Coverage Determination (NCD) on November 30, 2017. Post-NCD adopters are payers who adopted positive coverage for sequencing after CMS released the draft NCD. Size is reported for lives covered by commercial insurance. Offer Medicare Advantage includes payers who service Medicare Advantage plans on behalf of Medicare. Third-party policy is provided by third-party laboratory benefit management company.
Post-NCD adopters had a higher portion of BlueCross Blue Shield members than pre-NCD adopters (44% vs 29%) and proportionally more payers offering Medicare Advantage (87% vs 65%). Among pre-NCD adopters, a higher proportion used a third party policy than among post-NCD adopters (Table 5). Of note, of the 5 payers comprising the “spike” in adoption in 2017 (Fig. 1), 4 used the same third-party company.
The pre-NCD and post-NCD adopter groups contained an approximately equal percentage of smaller payers (59% vs 56%, respectively; Table 5). Four of the 5 payers adopting a positive coverage within 3 months of the draft NCD date were smaller payers (Fig. 1).
Temporal Changes in Features of Adopters’ Policies
We found that none of the adopters changed policy features between their first and last version of the policy (data not shown). Among the 33 adopters of positive coverage, most (67%) adopted a general, guideline-dependent policy: instead of listing specific cancers or biomarkers that they covered for sequencing, they referred to a clinical guideline by NCCN. Among adopters, 79% covered the use of sequencing in multiple cancers (either listing specific cancers or referring to NCCN guidelines), whereas 21% covered it only for NSCLC. In the pre-NCD adopter group, 18% covered sequencing for NSCLC only, as opposed to 25% in the post-NCD adopt group. None of the adopters had a requirement of Food and Drug Administration approval for the test.
Discussion
Our study examined the timing of insurance coverage for next generation tumor sequencing by US private payers on or before April 1, 2019, and explored temporal coverage trends relative to the draft CMS NCD. This study, to our knowledge, is the first temporal examination of payer coverage for precision medicine using a large cohort of US private payers. Our findings have a range of implications for clinical practice, patient access, and health policy for precision medicine and other innovative technologies.
Implications of the Time Horizon of Payers’ Adoption of Positive Coverage
We found that a considerable number of payers—nearly half of our sample—initiated coverage over a 3.5-year period. These payers represented more than 70% of the total commercial enrollment of the payer sample. Nevertheless, the remaining half of our sample, with the total commercial enrollment of more than 55 million lives, had a negative coverage policy at the end of the3.5-year period. Although we do not advocate for or against coverage of sequencing, the variability across payers causes variation in patient care and challenges clinicians with a dichotomy between some clinical guidelines and insurance coverage. We expect that it may take a number of years for this variability to be reduced. Coverage variability across payers is a well-recognized phenomenon,7,9,10 but temporal considerations highlight additional unexplored topics such as the duration and pervasiveness of this variability. These, in turn, pose important questions for the future coverage of sequencing and other precision medicine innovations. Extrapolating our findings into the future, it may take another 3 to 4 years, and in total, 8 years, for most payers in our cohort to grant positive coverage. Before sequencing reaches that level of coverage, new cancer tests and technologies may rise, mature, and compete with sequencing for payer coverage and clinical adoption, only to be then outpaced by another wave of innovations. The incongruence of coverage adoption time frames and innovation cycles may grow with acceleration of new test introduction1 and may further challenge the feasibility of achieving consistency in coverage policy and broad access for a given technology. These considerations should be included in the future policy and research agenda on precision medicine.
CMS and Private Payers’ Coverage Decision-Making
Prior research found that payers consider CMS coverage determinations in their own coverage decisions but only as one of the many factors in their complex decision-making process. Specific to sequencing, we previously reported that some payers intended to review, but not necessarily follow, the CMS NCD.20 We suggested that private payers may not cover sequencing in the shorter term but probably will in the medium to longer term. Contrary to our expectation, the temporal analysis in this study showed that private payers continued initiating coverage for sequencing in the short term after the NCD and at a higher pace than pre-NCD. Although we did not observe a statistical influence of the NCD on private coverage, these data suggest that the NCD played a role in payer decisions to cover sequencing. We also found that a higher proportion of post-NCD adopters were payers that offered Medicare Advantage plans than pre-NCD adopters. This may further support the suggestion that NCD affected coverage decisions, as payers may have wanted to avoid the dichotomy between Medicare and their own commercial policies within 1 payer. Although our findings may suggest that the CMS NCD may have had an impact on the initial coverage decisions, we found a lack of impact on the content of coverage policy of payers who initiated coverage pre-NCD. Future research should continue monitoring whether and when other private payers initiate coverage for sequencing and ascertain the potential impact of the CMS NCD on coverage decisions and content in the medium and longer term.
Trends in Adopter and Nonadopter Characteristics and Implications for Research and Policy Making
Our study discovered other notable trends in coverage policy. First, our findings highlighted an important role played by smaller payers in the coverage adoption process. Previous coverage policy research focused on medium and large payers9,10,12,13 and suggested that the largest payers are the earliest adopters of a precision medicine technology.12 In contrast, we included payers of all sizes and found that most of the adopter group were smaller payers covering in total 4 257 000 commercial lives (data not shown). Likewise, most of the pre-NCD adopter subgroups were smaller payers. Furthermore, we found that the first payer initiating coverage for sequencing, in addition to 4 of the 5 payers initiating positive coverage first after the CMS NCD, were small payers. This challenges a common assumption that smaller payers may not have resources for technology monitoring and assessment and therefore trail in coverage for precision medicine. Our results suggest that smaller payers may be nimbler in their decision-making and some of them may be innovators in coverage policy; therefore, they should be included in policy research and the overall dialogue on policy making related to precision medicine and other novel technologies.
Second, we found that BlueCross BlueShield members were significantly less likely to become adopters of positive coverage for sequencing within our study period, although the 2 largest Blue Cross BlueShield members did initiate coverage during this period. Also, proportionally fewer BlueCross BlueShield members were pre-NCD than post-NCD adopters. BlueCross BlueShield Association provides its members with coverage policy guidance, but it is nonbinding, and every member payer can make their own coverage decisions. Prior coverage policy research considered BlueCross BlueShield payers as independent entities,7,9,10,12 although a potential interdependence in policy content across these payers has been suggested.7,12 Our results suggest that this interdependence may be stronger than previously assumed and manifest itself not only in the content but also in the timing of coverage decisions. BlueCross BlueShield is an important group of payers in the United States, and future research should further elucidate the trends and dynamics of coverage decision making within this payer group.
Third, our results indicate that the adoption of coverage policy from a third-party company has become an important trend. Several prior articles noted this trend,27,28 but our study is the first, to our knowledge, to quantify it in relation to the timing of coverage decisions. We showed that almost half of the payers who adopted a positive policy for sequencing in the 3.5 years of our study used a third-party policy. We also showed that a higher proportion of earlier, pre-NCD adopters were third-party policy users than the post-NCD adopters. Our findings indicate that the use of a third-party company may be becoming a factor in the acceleration of coverage decisions, and its potential impact should be better understood. Future research on coverage policy should include not only payers but also third-party companies providing coverage policy guidance as a service to payers.
Implications for Research, Policy, and Affordability Assessment of Precision Medicine
Our study demonstrated that using temporal analyses in examining complex real-world phenomena may generate unique insights that are otherwise infeasible to obtain. Our approach is instructive to future policy research, which may benefit from temporal analyses of the dynamic field of precision medicine. We believe that this will improve the transparency of policy decision making and its impact on key stakeholders including patients, clinicians, and society as a whole. Such research will also inform affordability assessment and the impact of precision medicine coverage and access on population health, which are dynamic in nature and thus important to study temporally. To enable these efforts, rigorous and systematic registries are needed to capture policy and other data chronologically. The need for systematic policy registries has been previously noted,9,10 but our study underscores the necessity of incorporating the temporal dimension in these registries. Such registries may create larger-scale and broader data sets that would enable not only additional analyses but potentially the development of a temporal statistical model, which could make the coverage landscape more understandable and perhaps more predictable.
Although our study focused on payer coverage within the United States’ unique multipayer system, our findings may be relevant to other countries with single-payer systems but with multiple coverage decision makers at regional or local levels such as Canada and some European countries.32,33 These countries may also experience varied coverage of a specific technology across local authorities, and a temporal view of their respective coverage decisions may be instructive for national assessment and policymaking. A similar assessment may be also be informative for the European Union (EU), where consistency or health policy and technology assessment is being considered at the EU level,33,34 and where coverage decision making is distributed not only across countries but also within some countries and across localities.
Limitations
Our study had several limitations. We considered a limited set of private payers’ characteristics, although there may be other important features of these companies affecting their coverage decisions. Our study explored coverage trajectory relative to one factor—the CMS coverage determination—whereas payers consider a number of factors, including results of clinical evidence studies. Although our payer cohort was relatively large, it was still insufficient to elucidate statistical significance. Future efforts should continue, evolve, and broaden this research to larger, multifactorial data samples; an expanded spectrum of technologies; and longer time horizons. Another limitation of our study is that we conducted only a limited review of the content of coverage policies. Future research should examine similarities and differences in coverage policy features and their consistency with NCCN and other guide lines for sequencing and other genomic testing.
In conclusion, we conducted a temporal examination of private payers’ coverage policies for next-generation tumor sequencing. We found an increase but also continuing variability in coverage over the 3.5-year study period. Using temporal analyses allowed us to identify several important trends, including the possible contribution of the CMS NCD to a faster pace of coverage adoption, the interdependence in coverage timing among payers who are Blue Cross BlueShield members, the impact of using a third-party policy on the timing of coverage initiation, and the importance of smaller payers in early coverage adoption. Our study is a step toward systematic temporal research of coverage policy for precision medicine. Future efforts should evolve this research as well as leverage it in the assessments of affordability of precision medicine.
Acknowledgments
We thank Canary Insights for providing access to its database of coverage policies.
Source of financial support: This study was funded by grants from the National Human Genome Research Institute (U01 HG009599) and from the National Cancer Institutes (R01 CA221870).
Footnotes
Conflict of interest: K.A.P. and M.P.D. receive consulting fees from Illumina. K.A.P. received travel support from Illumina to attend health economist work group (GEECS) meetings. A.W.K. received research funding to Stanford University from Myriad Genetics from 2017–2019 for an unrelated project. R.K.K. receives research funding to her institution for conduction of clinical trials from Adaptimmune, Agios, Astra Zeneca, Bayer, BMS, Eli Lilly, EMD Serono, Exelixis, Merck, Novartis, Partner Therapeutics, QED, and Taiho. R.K.K. also receives consulting income from GNE/Roche and Ipsen. J.R.T. and C.B.W. have no financial disclosures.
REFERENCES
- 1.Phillips KA, Deverka PA, Hooker GW, Douglas MP. Genetic test availability and spending: where are we now? Where are we going? Health Aff (Millwood). 2018;37(5):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger A, Olson S. The Economics of Genomic Medicine: Workshop Summary. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 3.Garrison LP Jr, Towse A. A strategy to support efficient development and use of innovations in personalized medicine and precision medicine. J Manag Care Spec Pharm. 2019;25(10):1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips KA, Trosman JR, Kelley RK, Pletcher MJ, Douglas MP, Weldon CB. Genomic sequencing: assessing the health care system, policy, and big-data implications. Health Aff (Millwood). 2014;33(7):1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff (Millwood). 2018;37(5):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers JD, Chenoweth M, Thorat T, Neumann PJ. Private payers disagree with Medicare over medical device coverage about half the time. Health Aff (Millwood). 2015;34(8):1376–1382. [DOI] [PubMed] [Google Scholar]
- 7.Graf MD, Needham DF, Teed N, Brown T. Genetic testing insurance coverage trends: a review of publicly available policies from the largest US payers. Per Med. 2013;10(3):235–243. [DOI] [PubMed] [Google Scholar]
- 8.Clain E, Trosman JR, Douglas MP, Weldon CB, Phillips KA. Availability and payer coverage of BRCA1/2 tests and gene panels. Nat Biotechnol. 2015;33(9):900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips KA, Deverka PA, Trosman JR, et al. Payer coverage policies for multigene tests. Nat Biotechnol. 2017;35(7):614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu CY, Loomer S, Ceccarelli R, et al. Insurance coverage policies for pharma-cogenomic and multi-gene testing for cancer. J Pers Med. 2018;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trosman JR, Van Bebber SL, Phillips KA. Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract. 2010;6(5):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dervan AP, Deverka PA, Trosman JR, Weldon CB, Douglas MP, Phillips KA. Payer decision making for next-generation sequencing-based genetic tests: insights from cell-free DNA prenatal screening. Genet Med. 2017;19(5):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas MP, Parker SL, Trosman JR, Slavotinek AM, Phillips KA. Private payer coverage policies for exome sequencing (ES) in pediatric patients: trends over time and analysis of evidence cited. Genet Med. 2019;21(1):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan O, Shrestha R, Cunich M, Schofield DJ. Application of next-generation sequencing to improve cancer management: a review of the clinical effectiveness and cost-effectiveness. Clin Genet. 2018;93(3):533–544. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6. 2015. J Natl Compr Canc Netw. 2015;13(5):515–524. [DOI] [PubMed] [Google Scholar]
- 16.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foundation Medicine. History. https://www.foundationmedicine.com/about. Accessed October 20, 2019.
- 18.Prasad V Why the US Centers for Medicare and Medicaid Services (CMS) should have required a randomized trial of Foundation Medicine (F1CDx) before paying for it. Ann Oncol. 2018;29(2):298–300. [DOI] [PubMed] [Google Scholar]
- 19.Quinn B Medicare’s universal NCD for next generation sequencing in cancer patients: understanding its scope and implications. 2018. https://drive.google.com/file/d/1RBsruNDw74-RTaWqa9LVJNL3ReF7wp_g/view. Accessed October 20, 2019.
- 20.Phillips KA, Trosman JR, Weldon CB, Douglas MP. New Medicare coverage policy for next-generation tumor sequencing: a key shift in coverage criteria with broad implications beyond Medicare. JCO Precis Oncol. 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips KA. Evolving payer coverage policies on genomic sequencing tests: beginning of the end or end of the beginning? JAMA. 2018;319(23):2379–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trosman JR, Weldon CB, Kelley RK, Phillips KA. Challenges of coverage policy development for next-generation tumor sequencing panels: experts and payers weigh in. J Natl Compr Canc Netw. 2015;13(3):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trosman JR, Weldon CB, Douglas MP, et al. Payer coverage for hereditary cancer panels: barriers, opportunities, and implications for the precision medicine initiative. J Natl Compr Canc Netw. 2017;15(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trosman JR, Weldon CB, Gradishar WJ, et al. From the past to the present: insurer coverage frameworks for next-generation tumor sequencing. Value Health. 2018;21(9):1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Census Bureau. Health insurance coverage in the United States: 2017. https://www.census.gov/library/publications/2018/demo/p60-264.html. Accessed October 15, 2019.
- 26.Trosman JR, Van Bebber SL, Phillips KA. Health technology assessment and private payers’ coverage of personalized medicine. J Oncol Pract. 2011;7(3 suppl):18s–24s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezalla EJ. Payer view of personalized medicine. Am J Health Syst Pharm. 2016;73(23):2007–2012. [DOI] [PubMed] [Google Scholar]
- 28.Phillips KA, Deverka PA. Policy implicationsof commercial payeruse of third party benefit managers: the example of the emerging role of lab benefit managers for genetic testing. Health Affairs Blog. 2019. https://www.healthaffairs.org/do/10.1377/hblog20191021.563154/full/. Accessed October 24, 2019.
- 29.Centers for Medicare and Medicaid Services. Proposed decision memo for next generation sequencing (NGS) for Medicare beneficiaries with advanced cancer (CAG-00450N). https://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=290&bc=AAAAAAAAAAQAAA%3D%3D. Accessed September 30, 2019.
- 30.Centers for Medicare and Medicaid Services. View public comments for next generation sequencing (NGS) for Medicare beneficiaries with advanced cancer. https://www.cms.gov/medicare-coverage-database/details/nca-viewpublic-comments.aspx?NCAId=290&ExpandComments=n&bc=ACAAAAAACAAAAA%3D%3D&. Accessed September 30, 2019.
- 31.Centers for Medicare and Medicaid Services. Decision memo for next generation sequencing (NGS) for Medicare beneficiaries with advanced cancer (CAG-00450N). https://www.cms.gov/medicare-coverage-database/details/ncadecision-memo.aspx?NCAId=290&bc=AAAAAAAAACAA. Accessed October 5, 2019.
- 32.Martin D, Miller AP, Quesnel-Vallee A, Caron NR, Vissandjee B, Marchildon GP. Canada’s universal health-care system: achieving its potential. Lancet. 2018;391(10131):1718–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido MV, Kristensen FB, Nielsen CP, Busse R. Health Technology Assessment and Health Policy-Making in Europe: Current Status, Challenges and Potential (Public Health). Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 34.Erdos J, Ettinger S, Mayer-Ferbas J, de Villiers C, Wild C. European collaboration in health technology assessment (HTA): goals, methods and outcomes with specific focus on medical devices. Wien Med Wochenschr. 2019;169(11–12):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]


