Abstract
Understanding patterns of pathogen emergence can help identify mechanisms involved in transmission dynamics. Beak and feather disease virus (BFDV) poses a major threat world-wide to wild and captive parrots. Yet data from wild birds on seasonal fluctuations in prevalence and infection intensity, and thereby the potential high-risk times for virus transmission, have been lacking. We screened wild Crimson Rosellas (Platycercus elegans) for BFDV in blood and cloacal swabs. Prevalence in blood samples and cloacal swabs, as well as viral load varied with Julian date and in blood, were highest after the breeding season. Breeding birds had lower viral load and lower BFDV prevalence in blood than non-breeding birds (10.1% prevalence in breeding vs. 43.2% in non-breeding birds). BFDV prevalence was much higher in younger (<3 years) than older (≥3 years) birds for both blood samples (42.9% vs. 4.5%) and cloacal swabs (56.4% vs. 12.3%). BFDV status in blood and cloacal samples was not correlated within individuals. We show that, at least in P. elegans, BFDV infection seems to occur year-round, with seasonal changes in prevalence and load found in our samples. Our analyses suggest that the seasonal changes were associated primarily with the breeding season. We also discuss age and sex as important predictors of BFDV infection.
Subject terms: Ecology, Zoology, Natural hazards, Diseases
Introduction
Emerging infectious diseases of wildlife pose a major threat to the conservation of global biodiversity1,2. Knowledge of temporal patterns of infection allows improved targeting of management strategies to times when pathogens are most prevalent3,4. Pathogens are often present in host populations year-round, but prevalence fluctuates seasonally5. Several mechanisms have been proposed to explain this, including seasonal changes in weather3,6, in pathogen virulence and presence5, and variation in host susceptibility7,8. Seasonal breeding can influence host susceptibility, with lowered antibody production and increased parasite load during the breeding season often typical9 and generally attributed to a weakened immune system during offspring care3. Only a few wildlife studies have investigated the influence of seasonal changes in host biology on pathogen dynamics, with the available studies finding a peak in pathogen prevalence after the breeding season, and explaining this via an influx of susceptible young into the host population6,10.
Among pathogens, viruses have a particularly severe impact on wildlife, as they can adapt quickly to a wide range of potential hosts and are responsible for a high proportion of emerging infectious diseases11. Beak and feather disease virus (BFDV) is an ssDNA circovirus with a global distribution that can infect most, if not all, Psittaciformes (parrots, lorikeets, cockatoos)12. This is concerning, as the Psittaciformes are among the most threatened bird orders13. Accordingly, BFDV has been declared a ‘key threatening process to biodiversity’ by the Australian Government and a Threat Abatement Plan has been established14,15. It has furthermore been found in an increasing number of non-psittacine birds16–18. BFDV can be transmitted horizontally through crop secretions, faeces and feather dander19, and vertically from mother to embryonated egg20. It can cause acute and mostly fatal disease in very young birds, as well as chronic, often fatal disease mostly in older individuals21, and has been shown to persist for up to at least seven months in wild host individuals22. The risk of severe consequences of infection with BFDV is especially high in small populations of endangered species with low genetic diversity23–25. BFDV presence can become a particularly high-risk factor where distributions of abundant hosts overlap with those of vulnerable species12. Data on prevalence and spread in wild populations, even abundant ones which may act as pathogen reservoirs, are rare but urgently needed for species conservation12. Possible seasonal fluctuations in BFDV prevalence have been discussed26, but good data on seasonal prevalence and potential high-risk times are lacking. Such data are however necessary for a targeted, more effective pathogen management approach by conservationists, and could lead to a better understanding of BFDV infection dynamics.
BFDV causes disease with severe signs in many, but not all species of Psittaciformes27. Psittacine Beak and Feather Disease (PBFD) is the most common disease in wild Australian psittacines28. Severity of infection can range from subclinical, with no signs or mild signs, to severe clinical disease with feather dystrophy and abnormal beak and claw growth27,29. Many infected birds seem to stay asymptomatic12; yet asymptomatic birds play an important role as virus shedders30. Individuals that are infected with BFDV usually excrete large amounts of virus in feather dander and faeces19,31, often while giving PCR-negative results for BFDV if only one sample type is tested32.
Crimson Rosellas (Platycercus elegans) are an abundant and wide-spread Australian parrot which may act as a reservoir host for BFDV33. In the P. elegans elegans subspecies, BFDV prevalence of 34.5% has been reported34. P. elegans show no to only mild signs of BFDV infection, which can be challenging to detect in observation-based disease monitoring27. It has been proposed that BFDV prevalence in wild P. elegans might be influenced by time of year or season33, but studies with systematic sampling throughout the year to investigate this have been lacking. Such screening can provide a foundation for targeted pathogen management plans and for studies on host susceptibility12. A previous study showed that within breeding pairs of P. elegans, BFDV prevalence was lower than expected by random mating, and positive assortative mating of individuals without BFDV infection was suggested as a possible mechanism35. However, no study has investigated the patterns of BFDV prevalence between breeding and non-breeding birds, for a better understanding of BFDV prevalence dynamics throughout the year.
We investigated the seasonality of BFDV in breeding (trapped as parental birds in nest boxes) and non-breeding (trapped outside the breeding season) wild P. e. elegans. We tested whether host characteristics including breeding status, age and sex were associated with prevalence and viral load (intensity of infection). We also investigated whether host body mass was related to infection status and viral load, with the aim of elucidating fitness consequences of BFDV infection8. We predicted that prevalence and intensity of infection would be highest in autumn (after the breeding season) due to the influx of susceptible youngsters6,10. Our key aims were to 1) test for seasonal variation in BFDV prevalence and viral load, as well as body mass, to identify potential high-risk times for virus transmission, and 2) test whether host breeding status and age are related to any observed seasonal changes in BFDV prevalence and load.
Results
We collected blood samples from 142 individual P. e. elegans over two years (October 2016 to September 2018) including two breeding seasons (October to January). Of the 123 P. elegans with known breeding status, breeding birds included 36.7% (29 of 79) young birds (<3 years) and 63.3% (50 of 79) older birds (≥3 years), whereas non-breeding birds included 61.4% (27 of 44) young birds and 38.6% (17 of 44) older birds.
BFDV prevalence in blood
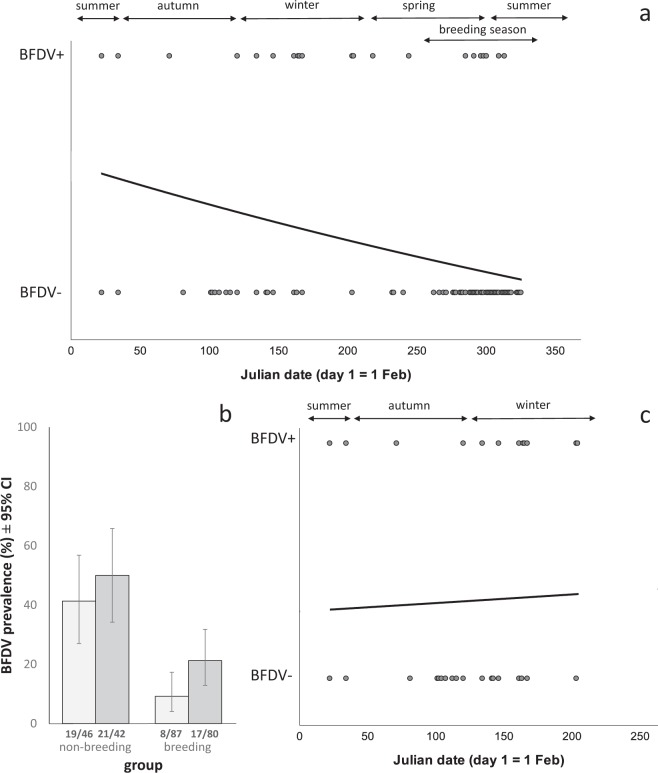
In blood samples, we detected BFDV in all age and sex classes of P. e. elegans, and the overall prevalenceblood (refers to population prevalence of BFDV in blood samples) was 21.8% (31 of 142 individuals, 95% confidence interval (CI) 15.3 – 29.5). When we tested the complete data set (i.e. both breeding and non-breeding birds) we did not find an effect of season on prevalenceblood (see Supplementary Table S3 for statistics). We did, however, find significant linear and quadratic effects of date (p = 0.005 and 0.046 respectively, Supplementary Table S3), indicating fluctuations in BFDV prevalence throughout the year: prevalenceblood appeared to be highest after the breeding season (1st February) and then declined towards the next breeding season (Fig. 1a). Host sex and age class were also consistently related to prevalenceblood, regardless of controlling for season, date, or breeding status. Overall, females (14 of 58, 24.1%, 95% CI 13.9 – 37.2) had a higher prevalence than males (13 of 65, 20.0%, 95% CI 11.1 – 31.8). However, when accounting for season, date, or breeding status, males were more likely to be infected than females (Supplementary Table S3, Supplementary Fig. S2). Young birds (<3 years) had a significantly higher prevalenceblood (24 of 56, 42.9%, 95% CI 29.7 – 56.8) than older birds (≥3 years) (3 of 67, 4.5%, 95% CI 0.9 – 12.5). This was the case when controlling for season, date, or breeding status (Supplementary Table S3). Breeding birds had a lower prevalenceblood (8 of 79, 10.1%, 95% CI 4.5 – 19.0) than non-breeding birds (19 of 44, 43.2%, 95% CI 28.3 – 59.0; p = 0.015, Supplementary Table S3, Fig. 1b). When we analysed non-breeding birds only, we found no relationship between prevalenceblood and date (p > 0.35) or season (p = 0.35), but the effects of age and sex remained (Supplementary Table S3; Fig. 1c). Mean prevalenceblood in young females by season and breeding status is shown in Supplementary Fig. S3 and within young birds by age in months in Supplementary Fig. S4, but sample sizes did not permit further statistical analysis for these age and sex cohorts.
Figure 1.
BFDV prevalence versus (a,c) date and (b) breeding status. ‘BFDV+’ indicates BFDV-positive birds, ‘BFDV−’ indicates BFDV-negative birds. Prevalence versus date is represented as a logistic regression curve. (a) Prevalenceblood of birds caught throughout the year, including outside the breeding season (‘non-breeding’) and during (‘breeding’) the breeding season. (b) Mean prevalence (with 95% confidence intervals) by breeding status, where light grey bars indicate population prevalence of BFDV in blood samples, and dark grey bars indicate population prevalence of BFDV in cloacal swabs. (c) Prevalenceblood for non-breeding birds only. In (a,b) we show only prevalenceblood and not prevalencecloacal, because cloacal swabs were not subject to the same level of validation as blood samples. Date is shown as Julian date, with the 1st February, which marks the end of the breeding season, set as day 1, and so the 31st January is day 365. Fractions at the base of bars indicate the number of infected birds out of the number of trapped individuals. Nine birds which were caught in walk-in traps during the breeding season are not shown in (b,c) as we could not determine whether or not they were breeding.
BFDV prevalence in cloacal swabs
The overall prevalencecloacal (refers to population prevalence of BFDV in cloacal swabs) was 33.3% (40 of 120, 95% CI 24.9 – 41.8). An individual’s BFDV status in blood was independent of its BFDV status in cloacal swabs (p = 0.20, Supplementary Table S4). Of the 51 P. e. elegans with BFDV prevalence data for both blood samples and cloacal swabs, and that were also BFDV positive (BFDV+) in at least one sample type, 21.0% (11 of 51, 95% CI 10.3 – 32.9) were only BFDV+ in blood (BFDV+ blood), 37.3% (19 of 51, 95% CI 23.0 – 50.5) were only BFDV+ in cloacal swabs (BFDV+ cloacal), and 41.2% (21 of 51, 95% CI 27.7 – 54.7) were BFDV+ in both sample types.
In cloacal samples, we found a cubic relationship between date and BFDV prevalence (with prevalence increasing after the breeding season, then decreasing, then increasing again throughout the year, similar to a sinusoidal curve; p = 0.017, Supplementary Table S4). When accounting for year of study, there was no significant relationship between date and BFDV prevalence (Supplementary Table S9). In the same model, prevalencecloacal (refers to population prevalence of BFDV in cloacal swabs) did not differ significantly between males and females (males: 16 of 66, 24.2%, 95% CI 14.5 – 36.4; females: 23 of 54, 42.6%, 95% CI 29.2 – 56.8; p = 0.296, Supplementary Table S4), but prevalencecloacal was higher in younger than in older birds (young, <3 years: 31 of 55, 56.4%, 95% CI 42.3 – 69.7; old, ≥3 years: 8 of 65, 12.3%, 95% CI 5.5 – 22.8; p < 0.001, Supplementary Table S4). Prevalencecloacal was lower in breeding (17 of 80, 21.2%, 95% CI 12.9 – 31.8) than in non-breeding birds (21 of 42, 50%, 95% CI 34.2 – 65.8; Fig. 1), but this effect was not significant when accounting for sex and age (p = 0.159, Supplementary Table S4). We could not test for an effect of season on prevalencecloacal when including breeding birds, as we did not have any BFDV-negative, young, breeding males. When we confined our analysis to non-breeding birds, we found a significant quadratic effect of date on prevalencecloacal (p = 0.033, Supplementary Table S4), with an increase in prevalence after the breeding season and a subsequent decrease towards the next breeding season. In non-breeding birds, when we tested season instead of date, it was non-significant (p = 0.615, Supplementary Table S4). The effect of date became non-significant, and the model testing season instead of date did not converge, when we included year in the model (Supplementary Table S9).
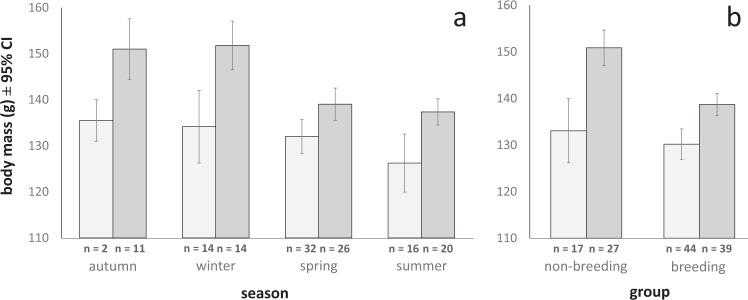
For additional analysis of age effects on sample type, we used only non-breeding birds in order to exclude any possible confounding effect of breeding status (Fig. 2). In non-breeding birds, BFDV prevalence in blood samples was still significantly higher in young birds (<3 years, 16 of 27, 59.3%, 95% CI 38.3 – 77.6) than in older individuals (≥3 years, 3 of 17, 17.7%, 95% CI 3.8 – 43.4; p = 0.01, Supplementary Table S3). We found a trend towards the same difference in cloacal swabs (p = 0.053, Supplementary Table S4).
Figure 2.
BFDV prevalence (± 95% confidence intervals) shown separately for age classes (<3 years, ≥3 years) and sample types (blood: light grey bars, cloacal swabs: dark grey bars). Shown here: Birds with known age, sampled outside the breeding season, to exclude possible confounding effects of breeding status on BFDV prevalence. Fractions at the base of bars indicate the number of infected birds out of the total number of trapped individuals.
Viral load in blood
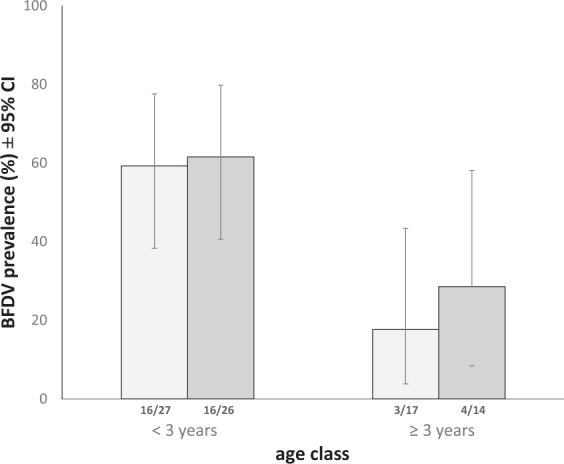
Amongst those birds that were BFDV+blood, we found that breeding birds had a significantly lower viral load than non-breeding birds (n = 21, t = -3.766, df = 16.976, p = 0.002, standard error SE = 0.284; Fig. 3). There was no difference in viral load between sexes in birds that were BFDV+ blood (n = 22, t = -0.483, df = 19.643, p = 0.634, SE = 0.46). Our sample size for viral load was insufficient for statistical analysis of seasonal variation and differences between age classes, but we show mean viral load for these categories (Supplementary Fig. S5).
Figure 3.
Mean BFDV load, shown separately for non-breeding and breeding birds (‘non-breeding’ denotes birds caught outside the breeding season). Viral load is shown as mean relative gene expression, log10 transformed with 95% confidence intervals. Viral load could not be determined from cloacal swabs, due to low DNA yield. Non-breeding birds shown here consist of 14 young (<3 years) and two older (≥3 years) birds, breeding birds were all young birds (<3 years).
Seasonal variation in body mass
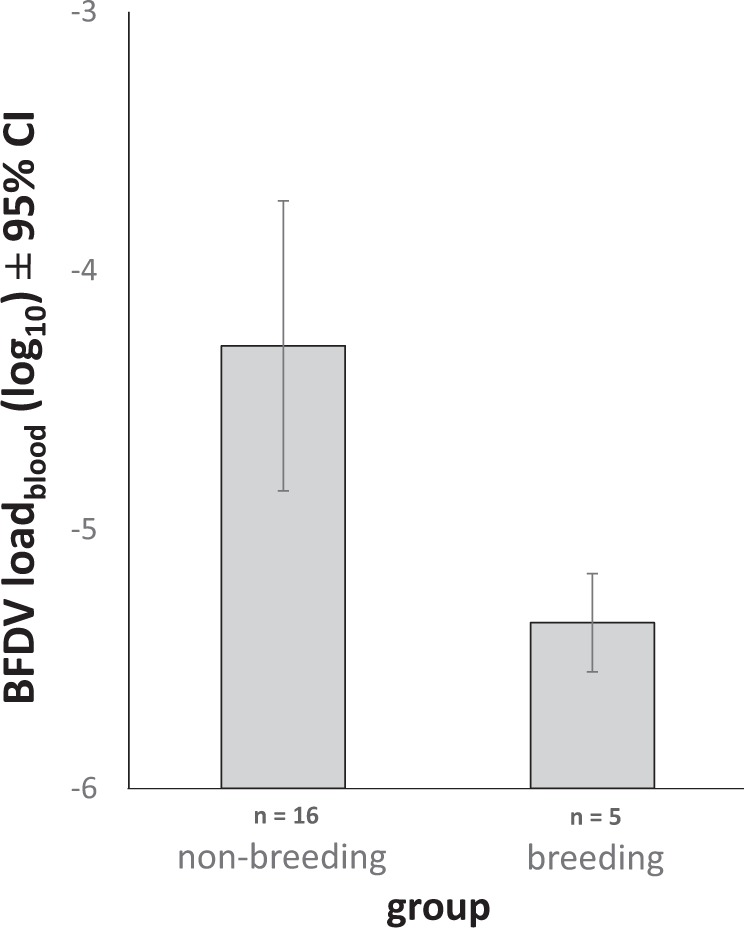
We found significant seasonal and linear date effects on body mass (Supplementary Table S6), with birds being lightest in spring and summer and heaviest in autumn (Fig. 4). Breeding birds were lighter than non-breeding birds (breeding: n = 77, mean m = 134.06 g, 95% CI 131.69 – 136.43, non-breeding: n = 42, m = 144.38 g, 95% CI 139.97 – 148.79; p < 0.001, Supplementary Table S6). We found this also when analysing only males (p < 0.001, Supplementary Table S6), but not in females (Fig. 4, p = 0.056, Supplementary Table S6). Using only non-breeding birds, to exclude the possible confounding effect of breeding status, we found no significant effect of BFDV infection (blood: p = 0.271, cloacal swabs: p = 0.055, Supplementary Table S6) or viral load on body mass (p = 0.288, Supplementary Table S6).
Figure 4.
Mean body mass ± 95% confidence intervals shown separately by sex (light grey: females, dark grey: males), season and breeding status (‘non-breeding’ denotes birds caught outside the breeding season).
Discussion
Disease outbreaks commonly show seasonal variation3,5, yet although BFDV is of major concern for the conservation of psittacines world-wide12 and has been studied for decades, no study had tested for seasonal changes in prevalence of BFDV. In wild P. elegans, we show that BFDV appears to be present in the host population year-round. Prevalence and viral load varied across the year, and with breeding status and age. In breeding birds, we found a lower BFDV prevalence in blood samples, and lower viral load in blood samples, compared to birds that we caught outside the breeding season. Previous work by our group found a lower than expected BFDV prevalence within breeding P. elegans pairs; one possible explanation may be that infected individuals might be less likely to breed35. In addition, adult P. elegans may be more likely to become infected with BFDV outside of the breeding season because they may be more susceptible after breeding3. We provide comparisons of BFDV prevalence during and outside the breeding season, which may suggest, among other explanations, a possible link between infection and the likelihood of breeding.
In our study, we found that BFDV prevalence in both sample types varied with date (i.e. quadratic or cubic date terms, with the cubic date term included where it improved the fit of the model). Prevalence in blood appeared to be highest after the breeding season, in autumn. In cloacal swabs, prevalence seemed to increase after the breeding season and peak in early winter. This later peak in prevalence in cloacal swabs than in blood samples may possibly be due to a delayed onset of viral shedding after a short viraemia32. The higher prevalence after the breeding season might be driven by the influx of young birds into the host population following seasonal breeding32,36,37. In support of this interpretation, we found that young P. elegans had a higher BFDV prevalence in blood than older birds, and a trend towards higher BFDV prevalence in cloacal swabs than the older individuals. Previous studies have also shown that birds under one year of age are particularly likely to get infected with BFDV33,38,39. Nestlings may be especially likely to contract BFDV because they may get infected by their parents or by contaminated nesting material27; alternatively, they may become infected by conspecifics or interspecific reservoir hosts after the breeding season37. In P. elegans, nestlings fledge after five weeks and usually accompany the parents for one to two months40. They then form post-breeding flocks until the next breeding season41, whereas adult P. elegans usually stay together in pairs and only rarely form large groups with conspecifics41,42. In House Finches (Carpodacus mexicanus), flocking behaviour of young birds has been shown to elevate pathogen prevalence, caused by high contact rates with infected individuals6 and increased density of susceptible hosts8. Young P. elegans might be more susceptible to infections, as their immune system is naïve to the virus. Such an effect, with young birds showing lower seroprevalence, higher pathogen prevalence and more intense shedding of pathogens, and being more likely to be infected, has been shown in several avian host-pathogen systems, for example in young C. mexicanus that were infected with the bacteria-like Mycoplasma gallisepticum6, and for infection of wild birds with avian influenza virus43. We accounted for host age when testing the effect of date and season, also because our ‘non-breeding birds’ group contained a higher proportion of young birds than the ‘breeding birds’ group, as can be seen in Fig. S1 and Table S1. Our findings reinforce the potential importance of targeted management strategies in threatened species infected with BFDV, in order to protect young birds, because they appear particularly susceptible to BFDV infection. We realise that it would be preferable to enter site and year into the analysis of seasonal variation, to validate the seasonal effect across years and geographical regions. In our study, however, BFDV prevalence for some age or sex groups was so low that the models often did not converge, which is why we could only show some of this information in the Supplementary Material. The bigger sample size we have for one of the years of our study could result in stochastic seasonal variation in that single year leading to an interpretation of overall, consistent seasonal effects. Future studies should include year and site when possible.
In blood samples, we found a BFDV prevalence of 20.0% for males and 24.1% for females. In comparison, previous studies have documented a slightly higher prevalence (males 28.3%, females 30.8%)35, although those included other subspecies of P. elegans. In P. e. elegans alone, 45%33 and 34.5%34 BFDV prevalence was detected previously in blood, which is again higher than the 21.8% we report in this study. One explanation for the differences between studies may be the effect of breeding status: based on the findings reported here, studies would be expected to find a higher prevalence of BFDV if a higher proportion of birds were sampled outside of the breeding season. In addition to the effects of breeding status, flocking behaviour and age (discussed above), seasonal fluctuations in prevalence of wildlife disease may also be influenced by other variables, including weather factors3, which can vary across years, so leading to strong inter-annual fluctuations in prevalence. In contrast to the higher prevalence we detected in females, we found males to be more likely to be infected than females when controlling for season, date, breeding status or age, and when testing non-breeding birds only. We think that our analysis of non-breeding birds may be the most reliable, as subadult male P. elegans rarely breed35, which can lead to models including breeding birds being quite unbalanced. Including breeding status and age in particular could change the direction of the observed sex effect (females or males more likely to be infected), which should be taken into account when analysing the influence of host sex on BFDV infection. While further research would be desirable, our findings suggest that males may be more likely to be infected with BFDV than females, at least in P. elegans.
We found a higher BFDV prevalence in cloacal swabs (33.3%) than in blood samples (21.8%), and this also applied to all subsets of birds we tested (e.g. subsets of breeding, young, old birds). This may indicate that a high proportion of hosts may shed BFDV into the environment, which could have important implications for conservation management. Cloacal swabs are a commonly used method to estimate viral shedding32,44, as BFDV and other pathogens are often transmitted via the faecal-oral route19,45. Additionally, in birds that were BFDV positive in at least one sample type, we found no association between BFDV presence in blood samples and BFDV presence in cloacal swabs. Some viral infections, such as infection with herpes simplex virus46, can become latent, usually after an active phase, and the viruses are then only detectable in certain tissues where they persist until re-activation. BFDV can be undetectable in blood during latent phases of infection, but may still be detectable in the cloaca32,47. Positive cloacal swab results in birds with BFDV-negative blood may result from viral persistence within the Bursa of Fabricius, which sits near the cloaca and is one of the main sites of BFDV replication32,48,49. On the other hand, birds that are BFDV positive in blood only, but not in cloacal swabs, may be in the early stages of infection, before occurrence of viral shedding47. Additionally, a small percentage of the BFDV positives in blood may represent remnant viral DNA from previous infections. It is however thought that the majority of BFDV positives in blood represent active viral infection35, and the qPCR detection assay we used is a very widely used method for investigations of BFDV prevalence33,50. Cloacal swabs have not been subject to the same level of validation as blood samples for BFDV detection, and cloacal results should therefore be interpreted more cautiously than blood samples. To confirm that the BFDV DNA detected in cloacal swabs is indeed viable virus, and not viral DNA fragments excreted after ingestion, future studies considering shedding of BFDV would benefit from including haemagglutination assays for antigen detection in faecal or feather samples31, or sequencing of BFDV-positive swabs, as has been done for blood samples33. Our results are, however, in accordance with other studies showing that BFDV32 and other circoviruses48 can be detected in the cloaca even while not being detectable in blood. As BFDV is thought to be highly stable in the environment27, prolonged periods of virus shedding may lead to high levels of environmental contamination. While we cannot fully exclude false negative results due to the much lower DNA yield from swabs than from blood, or false positives due to contamination from feathers surrounding the cloaca, our results suggest that BFDV presence can be missed in subjects if one only tests for active infection in blood.
Lastly, we found no effect of either BFDV infection or viral load on body mass. We did however find seasonal variation in body mass when pooling data from breeding and non-breeding birds. Males in our study had their lowest body mass during the breeding season. Decreased body mass due to chick rearing has been reported in many other avian species51, often hypothesised to occur due to increased energy expenditure during the breeding season3,52.
Conclusion
Knowledge of seasonal fluctuations in prevalence and severity of infections, and understanding of their causes, can lead to fundamentally better control of disease risks in wild host populations. Here we discuss that BFDV prevalence in natural P. elegans populations can show seasonal variation, with a possible peak prevalence in blood samples occurring after breeding. Our data suggest that this variation may be mainly influenced by changes in host breeding status and the influx of young birds into the population, a finding consistent with studies on other avian pathogens. We detected BFDV in a large proportion of the cloacal swabs we analysed, suggesting that wild hosts might shed the virus over extended periods of time, although swabs should be subject to further validation. Based on our and other data, which show highest prevalence and load in young birds, as well as a higher prevalenceblood outside of the breeding season, we conclude that, in P. elegans at least, the high-risk time for BFDV infection and transmission might be during and after the breeding season, when young, susceptible host individuals enter the population and subsequently flock together. Accordingly, our findings suggest consideration of targeted BFDV management aimed at the protection of young hosts, at least in threatened or vulnerable species. Future studies should test whether the pattern we find in P. elegans is found in other avian hosts of BFDV, and across additional geographical regions and years.
Methods
Study species
P. elegans is widely distributed throughout south-eastern and eastern Australia40. It is classified by the IUCN as of least concern due to its large range and high numbers, but is thought to be declining due to ongoing habitat destruction53. P. elegans use nest hollows for reproduction and show high nest site fidelity, but low social pair fidelity54. Female P. elegans can start breeding in their first year, whereas males typically only start breeding when they are at least two years old55. The species has been shown to be an excellent model system for BFDV research33,35,56.
Sample collection
We conducted this study under Deakin University animal ethics approval (B31-2015 and B37-2016) and Australian Bird and Bat Banding authority 2319, and it complied with the laws of Victoria (research permit 10007969). We collected samples from P. e. elegans in southern Victoria, Australia, from October 2016 until September 2018. We captured breeding birds during two breeding seasons using nest box traps57, from October 2016 to January 2017, and from October 2017 to January 2018. We also caught P. elegans in baited walk-in cage traps year-round (see Supplementary Table S1 and Supplementary Fig. S1 for information on numbers trapped, by age, sex, season and trap type). We used three field sites: Bellbrae (S38°19′ E144°11′, 110 nest boxes and two walk-in traps), Meredith/She Oaks (S37°51′ E144°06′, 50 nest boxes and two walk-in traps), and Steiglitz (S37°52′ E144°18′, 80 nest boxes and one walk-in trap).
We collected blood samples to study patterns of active infection (prevalence and intensity of infection)35, as well as cloacal swabs to estimate viral shedding32, and recorded body mass and tarsus length to estimate host condition51,58,59. We took ~100 µl of blood from the brachial vein and stored it in ethanol at room temperature. We took cloacal swabs and stored them at 3 °C in the field, then froze them at −80 °C upon return to the laboratory on the same day. To avoid virus transmission between sampled birds, the cotton bags we used to hold birds in were autoclaved after each use, blood sampling equipment was single-use and banding and measuring tools were sprayed with F10 SC Veterinary Disinfectant (Health and Hygiene Pty Ltd, South Africa) after each use.
We assigned birds to one of three age classes based on distinct plumage colouration: subadult (<1 year), young adult (1 – 3 years) and adult (≥3 years)56. Nestlings were not included in the study reported here. For analysis, subadults and young adults were combined into the age class ‘young bird’ (<3 years) to ensure an adequate sample size for comparison of breeding and non-breeding birds. To analyse changes in prevalence at a finer scale, we calculated age in months for these ‘young birds’, by calculating the estimated mean fledging time for the 2017 breeding season (n = 29 nests, mean 14 Dec, range 12 Nov – 17 Jan). We then correlated capture date with plumage colouration and wing stripe40, and assigned age in months accordingly (Supplementary Table S2). For example, we estimated that green subadults with full wing stripe on most or all primaries caught in January were one month old.
DNA extraction and PCR
To extract BFDV and host DNA from blood and swab samples, we used an ammonium acetate DNA extraction method that is commonly used in BFDV studies and gives high DNA yields12,33,60. The extracted DNA was stored in low Tris-EDTA buffer (10 mM Tris.HCL, 0.1 mM EDTA; pH 7.5 – 8.0) at -20 °C34. We determined DNA quality and quantity using a DU 640B spectrophotometer (Beckman Coulter, CA, U.S.A.) with a 1: 200 dilution. We sexed birds using a modified PCR protocol by Griffiths, et al.61. For BFDV detection, we diluted DNA to the same concentration (200 ng/µl), and then used a probe-based quantitative real-time PCR (qPCR) method34. We ran the assay using a PikoReal Real-Time PCR System (Thermo Fisher Scientific Inc., MA, U.S.A.). We added positive and no-template controls to each qPCR plate, and all samples were run in duplicate. Duplicate samples with Cq values (cycle at which probe fluorescence crosses the arbitrarily set detection baseline) differing by more than one cycle were run again. Although the qPCR method amplifies a shorter fragment of viral DNA than conventional PCR, it has been shown to deliver comparable results, while being more sensitive than the conventional assay34. qPCR is a widely used method for BFDV surveillance50. As most positives detected by qPCR represent active infection with viable virus as confirmed by sequencing33, but some can be non-active remnant viral DNA, we define prevalence as the percentage of individuals positive for BFDV viral DNA35. BFDV-positive (BFDV+) birds are termed either BFDV+blood or BFDV+cloacal, depending on which sample type was positive for BFDV presence. Samples that were BFDV-negative are termed BFDV−blood or BFDV−cloacal.
For comparative analysis of viral load across individuals, we re-ran BFDV+blood samples we used for comparative viral load analysis on the same qPCR plate, again all at a concentration of 200 ng/µl, to avoid possible slight variation of results across plates. We then used a comparative method to calculate viral load using the Cq values of each BFDV-positive sample33,62: Viral load = 2(−ΔCq). The resulting data were then log10-transformed to achieve normality34. Diluting cloacal swab DNA to the same concentration was not possible due to much lower DNA yield. We therefore only report prevalence for cloacal swabs, and not viral load. We re-tested a subset of walk-in-trapped P. elegans blood samples (subset with the highest BFDV prevalence) which were BFDV−blood, to estimate the likelihood of false negatives. None of the samples which were initially BFDV−blood came up BFDV+blood in the repeat run.
Statistical analyses
We carried out statistical analyses using SPSS 25.01 (IBM, Armonk NY, U.S.A.). To ensure independence of cases, we used only data from the first capture if individuals were recaptured. We pooled the data from our two years of study and from the three field sites, because we found overlapping confidence intervals between years and sites during initial data analysis (Supplementary Fig. S6). Additionally, Eastwood, et al.33 found that in P. elegans, geographic location, host density and parrot community diversity and composition do not explain differences in BFDV prevalence or load. A very low BFDV prevalence in some sex/age groups did not permit us to include year and trapping site as random intercepts, as well as age and sex in the same model, as the models did not converge. When we ran site and year separately as main effects in the full models, they were not significant, while we still found effects of age, date and sex (Supplementary Tables S7 – S10). We combined two of our three field sites, namely Meredith/She Oaks and Steiglitz, for statistical analysis. The two sites are located within 10 km of each other. Data from previous studies show that most recaptured P. elegans were trapped or resighted within approximately 10 km of their banding site54,63. We therefore defined Meredith/She Oaks and Steiglitz as one P. elegans population. We are aware that due to different and sometimes low sample sizes of infected birds trapped in some years and sites, and the resulting grouping of years and sites for most of our analyses, some of the observed seasonal patterns may in fact be stochastic effects. We address this in the discussion.
Initial calculations of prevalence included birds trapped during the breeding season (September – January, caught as parental birds in nest box, hereafter referred to as ‘breeding’) and birds trapped outside the breeding season (February – August, caught in walk-in traps, hereafter referred to as ‘non-breeding’), to represent an average prevalence across the population35. We report prevalence by sample type tested, as prevalenceblood (refers to population prevalence in blood samples) and prevalencecloacal (refers to population prevalence in cloacal swabs).
We analysed BFDV prevalence and host body mass using generalized linear models (GLM), which are commonly used as a flexible method for analysis of seasonal variation64. For binary data, we report Binomial (Clopper-Pearson) ‘exact’ CI as a widely used, conservative method recommended for small sample sizes65. We used a binomial distribution with a logit link to analyse effects on prevalence and a Gaussian distribution with identity link for the analysis of body condition and body mass. Where applicable, we checked the residuals to confirm that they conformed to the model assumptions. We report model outputs in Supplementary Tables S3–S11. In all figures and the main body of the text, we show raw data, i.e. means with 95% confidence intervals (CI). To report model fit values for binary data (e.g. infection status) we used the Nagelkerke R2. For continuous data (e.g. body mass), we show the overall R2 created by univariate analysis of variance.
We analysed temporal changes in BFDV prevalence using two predictors: season (spring, summer, autumn, winter) and Julian date (‘date’; with the 1st February set as day 1). We analysed date as a continuous variable in addition to season, because grouping samples by season can result in samples that were in fact collected close together being assigned to different seasons, potentially leading to unreliable results (for example, a bird trapped on the 31st May would be in the autumn group, but a bird trapped one day later would be in the winter group). Both season (as a categorical predictor) and date (as a continuous predictor) are common methods for investigating seasonal effects64,66. Seasons were defined as follows: Spring: 1st September to 30th November; summer: 1st December to 28th February; autumn: 1st March to 31st May; winter: 1st June to 31st August. Season was included as an ordinal variable (season 1 = spring, 2 = summer, 3 = autumn, 4 = winter). During exploratory data analysis we ran the same models using season as either ordinal or nominal variable, which led to identical results. Julian date was set to day one on the 1st February, to represent a biologically significant date67, i.e. the end of the breeding season of P. elegans in our study area35. During exploratory data analysis, we also tested 1st January (start of the year) and 1st September (beginning of the breeding season) as Julian day 1. Between the three examples of Julian day 1, the resulting R2, AICc and p-values were very similar (Supplementary Table S11); we therefore only report results based on 1st February as day 1. We centred Julian date to avoid collinearity, and tested linear, quadratic and cubic date predictors. For blood samples, models with quadratic date terms showed better fit (based on AICc and Nagelkerke R2) than models with cubic as well as quadratic date terms (data not shown). For body condition and body mass, models had almost identical fit with and without the cubic date terms. We therefore chose to use the most parsimonious models and for blood samples, body condition and body mass, we report model outputs without the cubic date term. For cloacal swabs and when testing our complete data set of P. elegans, models including cubic as well as quadratic date terms showed better fit than models that included only quadratic terms. We thus report results based on these models (Supplementary Table S4). Additional predictors we used were host traits that have previously been shown to influence BFDV infection (age class and sex)56. After performing GLMs on the full data set, we also tested seasonal effects separately in subsets of breeding and non-breeding birds, as date and breeding status were correlated. Nine birds which were caught in walk-in traps during the breeding season were excluded from analyses comparing breeding and non-breeding birds, as we could not determine whether or not they were breeding at the time of capture. Sample size thus varies between models, and also because of missing information for some predictors for some individual birds.
We repeated models analysing body mass by including tarsus length as a predictor, to estimate body condition by accounting for individual size differences. Body condition results are only shown in Supplementary Table S5, as they are very similar to results with body mass which did not include tarsus length (Supplementary Table S6). To compare viral load between groups (males vs females, breeding vs non-breeding) we conducted t-tests35.
Supplementary information
Acknowledgements
We are most grateful to the private landowners for their cooperation and Shane Raidal, Soren Alexandersen, Michael Magrath and Justin Eastwood for advice with methodologies. We thank Briana Spolding for laboratory assistance and our field volunteers for their help with sample collection. We thank Raoul Ribot and Andrea Crino for their feedback on earlier versions of this manuscript. This study was funded by the Australian Research Council (grants LP140100691; DP180103494), Deakin University, BirdLife Australia and the Holsworth Wildlife Research Endowment.
Author contributions
J.M.M., M.L.B. and A.T.D.B. designed the study. J.M.M., H.S.S. and M.L.B. conducted fieldwork. J.M.M., H.S.S. and K.W. conducted the molecular lab work. J.M.M., M.L.B. and A.T.D.B. performed the statistical analysis. All authors contributed to the interpretation of the results and the writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64631-y.
References
- 1.Daszak P, Cunningham AA, Hyatt AD. Emerging Infectious Diseases of Wildlife - Threats to Biodiversity and Human Health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 2.Preece ND, et al. A guide for ecologists: Detecting the role of disease in faunal declines and managing population recovery. Biological Conservation. 2017;214:136–146. doi: 10.1016/j.biocon.2017.08.014. [DOI] [Google Scholar]
- 3.Altizer S, et al. Seasonality and the dynamics of infectious diseases. Ecology Letters. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 4.Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2541–2550. doi: 10.1098/rspb.2006.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerging Infectious Diseases. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini PR, Dhondt AA, Dobson A. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proceedings of the Royal Society B: Biological Sciences. 2004;271:2569–2577. doi: 10.1098/rspb.2004.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell SF, Ho MS. Seasonality of infectious diseases and severe acute respiratory syndrome–what we don’t know can hurt us. The Lancet Infectious Diseases. 2004;4:704–708. doi: 10.1016/S1473-3099(04)01177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altizer S, Davis AK, Cook KC, Cherry JJ. Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Canadian Journal of Zoology. 2004;82:755–763. doi: 10.1139/z04-050. [DOI] [Google Scholar]
- 9.Norris K, Anwar M, Read AF. Reproductive Effort Influences the Prevalence of Haematozoan Parasites in Great Tits. Journal of Animal Ecology. 1994;63:601–610. doi: 10.2307/5226. [DOI] [Google Scholar]
- 10.Lord RD. Seasonal Reproduction of Vampire Bats and Its Relation to Seasonality of Bovine Rabies. Journal of Wildlife Diseases. 1992;28:292–294. doi: 10.7589/0090-3558-28.2.292. [DOI] [PubMed] [Google Scholar]
- 11.Fogell DJ, et al. Hygiene and biosecurity protocols reduce infection prevalence but do not improve fledging success in an endangered parrot. Scientific Reports. 2019;9:4779. doi: 10.1038/s41598-019-41323-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogell DJ, et al. Trade and conservation implications of new beak and feather disease virus detection in native and introduced parrots. Conservation Biology. 2018;32:1325–1335. doi: 10.1111/cobi.13214. [DOI] [PubMed] [Google Scholar]
- 13.Olah G, et al. Ecological and socio-economic factors affecting extinction risk in parrots. Biodiversity and Conservation. 2016;25:205–223. doi: 10.1007/s10531-015-1036-z. [DOI] [Google Scholar]
- 14.Australian Department of the Environment and Heritage. Threat Abatement Advice for the key threatening process. Commonwealth of Australia, Canberra ACT (2016).
- 15.Australian Department of the Environment and Heritage. Threat abatement plan for beak and feather disease affecting endangered psittacine species. Commonwealth of Australia, Canberra ACT (2005).
- 16.Amery-Gale J, et al. A high prevalence of beak and feather disease virus in non-psittacine Australian birds. Journal of Medical Microbiology. 2017;66:1005–1013. doi: 10.1099/jmm.0.000516. [DOI] [PubMed] [Google Scholar]
- 17.Sarker S, Lloyd C, Forwood J, Raidal SR. Forensic genetic evidence of beak and feather disease virus infection in a Powerful Owl (Ninox strenua) Emu - Austral Ornithology. 2016;116:71–74. doi: 10.1071/mu15063. [DOI] [Google Scholar]
- 18.Sarker S, et al. Evidence of a deep viral host switch event with beak and feather disease virus infection in rainbow bee-eaters (Merops ornatus) Scientific Reports. 2015;5:14511. doi: 10.1038/srep14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie BW, et al. Routes and prevalence of shedding of psittacine beak and feather disease virus. American Journal of Veterinary Research. 1991;52:1804–1809. [PubMed] [Google Scholar]
- 20.Rahaus M, et al. Detection of beak and feather disease virus DNA in embryonated eggs of psittacine birds. Veterinarni Medicina. 2008;53:53–58. doi: 10.17221/1932-VETMED. [DOI] [Google Scholar]
- 21.Studdert MJ. Circoviridae: new viruses of pigs, parrots and chickens. Australian Veterinary Journal. 1993;70:121–122. doi: 10.1111/j.1751-0813.1993.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 22.Martens JM, et al. Persistence of beak and feather disease virus (BFDV) infection in wild Crimson Rosellas (Platycercus elegans) Emu - Austral Ornithology. 2019;119:1–5. doi: 10.1080/01584197.2019.1640069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters A, et al. Evidence of psittacine beak and feather disease virus spillover into wild critically endangered Orange-bellied Parrots (Neophema chrysogaster) Journal of Wildlife Diseases. 2014;50:288–296. doi: 10.7589/2013-05-121. [DOI] [PubMed] [Google Scholar]
- 24.Sarker, S., Ghorashi, S. A., Forwood, J. K. & Raidal, S. R. Whole-Genome Sequences of Two Beak and Feather Disease Viruses in the Endangered Swift Parrot (Lathamus discolor). Genome Announcements1, 10.1128/genomeA.00842-13 (2013). [DOI] [PMC free article] [PubMed]
- 25.Kundu S, et al. Tracking viral evolution during a disease outbreak: the rapid and complete selective sweep of a circovirus in the endangered Echo parakeet. Journal of Virology. 2012;86:5221–5229. doi: 10.1128/JVI.06504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson B, et al. Emerging infectious disease or evidence of endemicity? A multi-season study of beak and feather disease virus in wild red-crowned parakeets (Cyanoramphus novaezelandiae) Archives of Virology. 2015;160:2283–2292. doi: 10.1007/s00705-015-2510-3. [DOI] [PubMed] [Google Scholar]
- 27.Raidal SR, Peters A. Psittacine beak and feather disease: ecology and implications for conservation. Emu - Austral Ornithology. 2018;118:80–93. doi: 10.1080/01584197.2017.1387029. [DOI] [Google Scholar]
- 28.Paré, J. A. & Robert, N. In Infectious Diseases of Wild Birds (eds N. J. Thomas, D. B. Hunter, & C. T. Atkinson) (Wiley Online Library, 2007).
- 29.Pass DA, Perry RA. The pathology of psittacine beak and feather disease. Australian Veterinary Journal. 1984;61:69–74. doi: 10.1111/j.1751-0813.1984.tb15520.x. [DOI] [PubMed] [Google Scholar]
- 30.Jackson B, et al. Clinical beak and feather disease virus infection in wild juvenile eastern rosellas of New Zealand; biosecurity implications for wildlife care facilities. New Zealand Veterinary Journal. 2014;62:297–301. doi: 10.1080/00480169.2014.909750. [DOI] [PubMed] [Google Scholar]
- 31.Raidal SR, Sabine M, Cross GM. Laboratory diagnosis of psittacine beak and feather disease by haemagglutination and haemagglutination inhibition. Australian Veterinary Journal. 1993;70:133–137. doi: 10.1111/j.1751-0813.1993.tb06104.x. [DOI] [PubMed] [Google Scholar]
- 32.Hess M, Scope A, Heincz U. Comparitive sensitivity of polymerase chain reaction diagnosis of psittacine beak and feather disease on feather samples, cloacal swabs and blood from budgerigars (Melopsittacus undulates, Shaw 18005) Avian Pathology. 2004;33:477–481. doi: 10.1080/03079450400003619. [DOI] [PubMed] [Google Scholar]
- 33.Eastwood JR, et al. Phylogenetic analysis of beak and feather disease virus across a host ring-species complex. Proceedings of the National Academy of Sciences USA. 2014;111:14153–14158. doi: 10.1073/pnas.1403255111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastwood JR, et al. Prevalence of beak and feather disease virus in wild Platycercus elegans: comparison of three tissue types using a probe-based realtime qPCR test. Australian Journal of Zoology. 2015;63:1–8. doi: 10.1071/zo14052. [DOI] [Google Scholar]
- 35.Eastwood JR, et al. Prevalence of BFDV in wild breeding Platycercus elegans. Journal of Ornithology. 2019;160:557–565. doi: 10.1007/s10336-019-01639-w. [DOI] [Google Scholar]
- 36.Cosgrove CL, Wood MJ, Day KP, Sheldon BC. Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. Journal of Animal Ecology. 2008;77:540–548. doi: 10.1111/j.1365-2656.2008.01370.x. [DOI] [PubMed] [Google Scholar]
- 37.Pathak AK, Boag B, Poss M, Harvill ET, Cattadori IM. Seasonal breeding drives the incidence of a chronic bacterial infection in a free-living herbivore population. Epidemiology and Infection. 2011;139:1210–1219. doi: 10.1017/S0950268810002311. [DOI] [PubMed] [Google Scholar]
- 38.Perry RAA. A psittacine combined beak and feather disease syndrome with particular reference to the Australian Cockatoos Cacatua galerita (Sulphurcrested Cockatoo), Cacatua leadbeateri (Major Mitchell or Pink Cockatoo), Cacatua roseicapella (Galah or Rose-breasted Cockatoo) and Cacatua sanguinea (Little Corella) Refresher Course on Aviary and Caged Birds. The Post-graduate Committee in Veterinary Science. Proceedings. 1981;55:81–108. [Google Scholar]
- 39.Jacobson ER, et al. Feather and beak dystrophy and necrosis in cockatoos: clinicopathologic evaluations. Journal of the American Veterinary Medical Association. 1986;189:999–1005. [PubMed] [Google Scholar]
- 40.Higgins, P. J. Handbook of Australian, New Zealand and Antarctic Birds. Volume 4:Parrots to Dollarbird. Vol. 4 25–29, 192–195, 321–341, plates 15 & 16 (Oxford University Press, 1999).
- 41.Magrath RD, Lill A. Age-related Differences in Behaviour and Ecology of Crimson Rosellas, Platycercus elegans, during the Non-Breeding Season. Australian Wildlife Reasearch. 1985;12:299–306. doi: 10.1071/WR9850299. [DOI] [Google Scholar]
- 42.Juniper, T. & Parr, M. Parrots - a guide to the parrots of the world. (Pica Press, 1998).
- 43.van Dijk JG, et al. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. Journal of Animal Ecology. 2014;83:266–275. doi: 10.1111/1365-2656.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umar S, et al. Variation in Viral Shedding Patterns between Domestic and Wild Terrestrial Birds Infected Experimentally with Reassortant Avian Influenza Virus (H9N2) Avian Biology Research. 2019;9:200–206. doi: 10.3184/175815516x14667741490471. [DOI] [Google Scholar]
- 45.Gopinath S, Carden S, Monack D. Shedding light on Salmonella carriers. Trends in Microbiology. 2012;20:320–327. doi: 10.1016/j.tim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Cook ML, Stevens JG. Latent Herpetic Infections Following Experimental Viraemia. Journal of General Virology. 1976;31:75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- 47.Khalesi B, Bonne N, Stewart M, Sharp M, Raidal S. A comparison of haemagglutination, haemagglutination inhibition and PCR for the detection of psittacine beak and feather disease virus infection and a comparison of isolates obtained from loriids. Journal of General Virology. 2005;86:3039–3046. doi: 10.1099/vir.0.81275-0. [DOI] [PubMed] [Google Scholar]
- 48.Todd D, et al. Detection of pigeon circovirus in cloacal swabs: implications for diagnosis, epidemiology and control. Veterinary Record. 2006;159:314–317. doi: 10.1136/vr.159.10.314. [DOI] [PubMed] [Google Scholar]
- 49.Smyth, J. A. et al. Identification of circovirus infection in three species of gull. The Veterinary Record159, 10.1136/vr.159.7.212 (2006). [DOI] [PubMed]
- 50.Fogell DJ, Martin RO, Groombridge JJ. Beak and feather disease virus in wild and captive parrots: an analysis of geographic and taxonomic distribution and methodological trends. Archives of Virology. 2016;161:2059–2074. doi: 10.1007/s00705-016-2871-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christe P, Møller AP, González G, De Lope F. Intraseasonal variation in immune defence, body mass and hematocrit in adult house martins Delichon urbica. Journal of Avian Biology. 2002;33:321–325. doi: 10.1034/j.1600-048X.2002.330317.x. [DOI] [Google Scholar]
- 52.Schrader MS, Walters EL, James FC, Greiner EC. Seasonal Prevalence of a Haematozoan Parasite of Red-Bellied Woodpeckers (Melanerpes carolinus) and its Association With Host Condition and Overwinter Survival. The Auk: Ornithological Advances. 2003;120:130–137. doi: 10.1093/auk/120.1.130. [DOI] [Google Scholar]
- 53.BirdLife International. Platycercus elegans. The IUCN Red List of Threatened Species2018, 10.2305/IUCN.UK.2018-2.RLTS.T22733483A132181501.en (2018).
- 54.Eastwood JR, et al. Pair fidelity in long-lived parrots: genetic and behavioural evidence from the Crimson Rosella (Platycercus elegans) Emu – Austral Ornithology. 2018;118:369–374. doi: 10.1080/01584197.2018.1453304. [DOI] [Google Scholar]
- 55.Forshaw, J. M. & Cooper, W. T. Australian Parrots. 3rd revisededition., (Avi-Trader Publishing, 2002).
- 56.Eastwood JR, et al. Host heterozygosity and genotype rarity affect viral dynamics in an avian subspecies complex. Scientific Reports. 2017;7:13310. doi: 10.1038/s41598-017-13476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg ML, Ribot RF. A simple, inexpensive trap for capturing parrots and other cavity nesting birds. Corella. 2008;32:78–79. [Google Scholar]
- 58.Labocha MK, Hayes JP. Morphometric indices of body condition in birds: a review. Journal of Ornithology. 2012;153:1–22. doi: 10.1007/s10336-011-0706-1. [DOI] [Google Scholar]
- 59.Bowers EK, et al. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruford, M. W., Hanotte, O., Brookfield, J. F. Y. & Burke, T. In Molecular Genetic Analysis of Populations: A Practical Approach (ed A. R. Hoelzel) 287–336 (IRL Press, 1998).
- 61.Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Molecular Ecology. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 62.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 63.Higgins, P. J. Handbook of Australian, New Zealand and Antarctic Birds. Volume 4: Parrots to Dollarbird. (Oxford University Press, 1999).
- 64.Christiansen CF, Pedersen L, Sørensen HT, Rothman KJ. Methods to assess seasonal effects in epidemiological studies of infectious diseases—exemplified by application to the occurrence of meningococcal disease. Clinical Microbiology and Infection. 2012;18:963–969. doi: 10.1111/j.1469-0691.2012.03966.x. [DOI] [PubMed] [Google Scholar]
- 65.Brown LD, Cat TT, DasGupta A. Interval Estimation for a proportion. Statistical Science. 2001;16:101–133. [Google Scholar]
- 66.Naumova EN, et al. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiology and Infection. 2007;135:281–292. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans SR, Summers AGR, Sheldon BC. Seasonality of carotenoid-based plumage coloration: modelling wavelength-specific change through spectral reconstruction. Journal of Avian Biology. 2012;43:234–243. doi: 10.1111/j.1600-048X.2012.05654.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.