Abstract
The biofilms of Enterobacteriaceae are fortified by assembly of curli amyloid fibres on the cell surface. Curli not only provides structural reinforcement, but also facilitates surface adhesion. To prevent toxic intracellular accumulation of amyloid precipitate, secretion of the major curli subunit, CsgA, is tightly regulated. In this work, we have employed solution state NMR spectroscopy to characterise the structural ensemble of the pre-fibrillar state of CsgA within the bacterial periplasm, and upon recruitment to the curli pore, CsgG, and the secretion chaperone, CsgE. We show that the N-terminal targeting sequence (N) of CsgA binds specifically to CsgG and that its subsequent sequestration induces a marked transition in the conformational ensemble, which is coupled to a preference for CsgE binding. These observations lead us to suggest a sequential model for binding and structural rearrangement of CsgA at the periplasmic face of the secretion machinery.
Subject terms: Biochemistry, Microbiology, Structural biology
Introduction
The amyloid fibre is a unique biopolymer, in that it is comprised of protein subunits, and is stabilized through non-covalent interactions. Each subunit characteristically forms a stacked β-sheet that coalesces perpendicular to the fibril axis. Almost all residues in the core of the fibre contribute to its stabilisation, giving it a tensile strength similar to steel and other inorganic materials1. These remarkable material properties make the turnover of amyloid in biological systems a challenge, with its accumulation a causative factor in a number of pathologies, particularly neurodegeneration2. Moreover, studies have also shown that early oligomeric intermediates formed during amyloidogenesis are often particularly cytotoxic3. Despite the inherent risk of cytoxicity, many organisms have evolved mechanisms to utilise the amyloid fold. These ‘functional amyloids’ exist in all domains of life, and operate in a host of diverse roles4. Several bacteria utilise these fibres as a major component of their biofilm matrix, and have evolved distinct apparatus to co-ordinate secretion5–7, The first identified of such biofilm functional amyloids, and the best studied, is that of Escherichia coli8,9, The amyloid produced by this system, termed curli fibres, has been identified in a wide range of Enterobacteriaceae, with homologous systems identified in many Gram negative phyla10,11, Whilst providing bacteria with a structurally re-enforced scaffold, curli has also been implicated in pathogenic infection12, triggering host auto-immune responses13, and even accelerating neurodegeneration14. As a consequence of its extensive study, curli is a model functional amyloid system, driving research forward in a diverse range of applications, including the generation of small molecule inhibitors15 and implementation in novel biomaterials16.
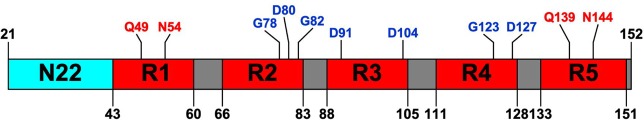
Curli is assembled and regulated by the seven curli-specific genes (csg), found on two divergently transcribed operons, csgBAC and csgDEFG. The master regulator of biofilm formation, transcription factor CsgD, provides tight regulation of amyloid production in response to environmental conditions17,18, CsgA is the main component of the curli fibre, composed of 5 repeat regions (R1-R5) that are predicted to form the amyloid fold19 (Fig. 1). These repeats are 19–23 residues long and possess the consensus sequence Ser-X5-Gln-X4-Asn-X5-Gln. CsgA is exported into the periplasm through the SecYEG complex, as a 13.1 kDa mature peptide, and is maintained as an intrinsically disordered protein (IDP) by the chaperone protein CsgC20. It also possesses an N-terminal sequence (N22) that targets it for secretion across the outermembrane21. This pre-fibril state of CsgA is shuttled to the secretion complex, where it binds to CsgG, the outermembrane pore. CsgG forms a nonameric, 36-stranded β-barrel structure, with a > 1 nm pore that is gated at the periplasmic face22–24, CsgE and CsgF help co-ordinate CsgA translocation at either side of the membrane. CsgE localizes at the periplasmic face of the CsgG secretion pore, also forming a nonameric ring, which functions to prevent non-specific subtrate secretion25,26, and chaperones CsgA through the CsgG pore22. CsgF forms a tight complex with the extracellular face of CsgG, where it co-ordinates templating of curli fibres via an interaction with the nucleator CsgB27–29,
Figure 1.
Schematic representation of the mature CsgA polypeptide. The amyloid core repeat regions R1-R5 (red) are flanked by linker regions (grey). Residues annotated in red are located in the most amyloidogenic repeats (R1 and R5), and have been reported to be critical for curli formation40. Residues annotated in blue act as gatekeeper residues that act to reduce the rate of CsgA aggregation prior to templating at the cell surface41. Numbering includes the signal sequence that is cleaved after perisplamic translocation.
The amyloid fold of CsgA within the curli fibre has been extensively characterized, using data from a wide range of biophysical techniques30–32, Complementing experimental work on the fibrous state of CsgA, a model of CsgA nucleation and assembly has been proposed using ThT-binding studies33, and H/D exchange experiments have identified the most important repeats for driving amyloid formation34. Whilst these have provided valuable insights into fibre assembly, structural analysis of the pre-amyloid state of CsgA present within the perisplam has been more difficult. This is largely due to its disordered and transient nature. Indeed, much of our understanding of CsgA as an IDP comes from investigating other csg-encoded proteins and their relationship with CsgA. Previous work revealed a transient electrostatic mechanism for CsgC inhibition of CsgA aggregation35. Similarly, studies from the CsgE perspective have also highlighted the importance of transient electrostatic interfaces in controlling CsgA polymerization36. Studies have also shown that CsgA can cross seed homologs from other bacterial species, which raises the notion that curli fibers may facilitate multispecies biofilms37. The seeding specificity of curli is a more general phenomenon, as CsgA is able to alter the fibrillation kinetics of several human amyloidogenic proteins38.
This study provides new structural insights into the pre-amyloid CsgA ensemble and its targeting to the curli secretion system using solution-state nuclear magnetic resonance (NMR) spectroscopy. Structural features adopted by CsgA in solution were characterised by gleaning information from chemical shifts and transverse relaxation. These highlight the importance of prion-like motifs as points of conformational exchange in the repeat regions, and the abundance of polyproline II (PPII) in the disordered ensemble. Removal of the N-terminal targeting region (N22) causes a significant conformational redistribution within the repeat regions of CsgA, particularly near important regions that gate-keep amyloid formation, suggesting a regulatory role. Furthermore, we show that CsgE recognises the truncate in preference to the mature protein that includes N22. While the extrapolation of our conclusions to the bacterial cell has limitations, as we ignore the influence of other cellular factors, our work supports a model in which a step-wise progression of structural re-arrangements in CsgA occur during export and secretion. First, intramolecular interactions involving N22 together with CsgC assist in preventing inappropriate amyloid formation and premature binding of CsgE. Then, N22 is bound specifically by the periplasmic face of outermembrane CsgG where it is sequestered from pre-fibrillar CsgA. Finally, this alters the conformation distribution of the CsgA ensemble and primes CsgA for interaction with CsgE. Subsequent interaction with of the CsgE ring with the base of the CsgG pore drives encapsulation of CsgA and entropic release through the pore.
Results
Backbone assignment of the disordered, pre-fibril CsgA
Prior characterisation of CsgA by solution-state NMR had been hampered by low signal intensity, spectral overlap, and limited life-time of samples. To address these issues, we optimised several aspects of sample and experimental conditions. Low sensitivity is largely a consequence of low sample concentrations, typically sub 20 μM. We chose to use C-terminally His-tagged CsgA for rapid purification and increased yields. Previous studies have shown that his-tagged and untagged CsgA behave similar in amyloid aggrateion assays39.As efforts to increase the sample concentration by centrifugal methods promotes amyloid formation and precipitation, induction times for expression were lengthened in order to produce protein at higher intracellular concentrations. To abate fibril formation and also mimic the periplasmic state of CsgA, the curli inhibitory chaperone CsgC was added to samples at a 1:40 ratio. 1H-15N HSQC spectra were recorded in the presence and absence of CsgC, revealing no prominent chemical shift perturbations, consistent with previous work revealing the transient nature of the CsgC-CsgA encounter35 (Supplementary Figure S1). Further improvements were obtained by the reduction of sample temperature to 10 °C, which also favourably reduced the rate of unwanted amyloidogenesis. Use of higher strength magnetic fields (950 MHz) improved resolution for regions with the limited dispersion that is typical of IDPs. Final samples were 150 μM and stable for several days.
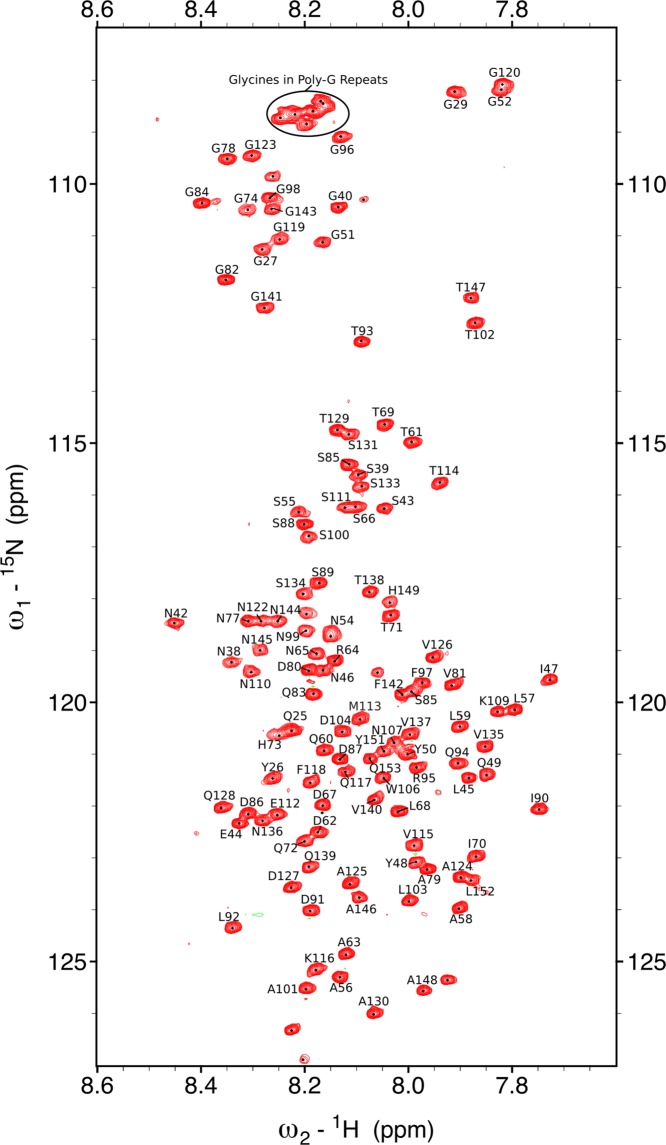
Assignment experiments were carried out using triple resonance methodology, with the hNcocaNNH pulse sequence particularly useful for overcoming ambiguity in overlapped regions. Despite narrow 1H chemical shift dispersion (7.6–8.4 ppm), 1H-15 N correlations were assigned for 118/134 (88.1%) of residues (Fig. 2). The C-terminal His-tag is excluded from this count. A majority of the unassigned residues are located in, or proximal to, the glycine-rich repeats within the N22. Prolines, P24 and P41, are also unassigned, as are residues located after the N-terminal methionine (G21-V23).
Figure 2.
15N-1 H HSQC spectrum of 150 μM CsgA in 20 mM Na2 HPO4, pH 7.2, 10 °C. Amide resonances are labelled with one-letter code. Spectra recorded at 950 MHz. Overlapped glycine residues in glycine-rich repeats are highlighted at the top of the spectra. Chemical shifts are displayed in parts-per-million (ppm) units.
The N22 region influences the conformational ensemble of CsgA
Next, using our backbone assignment, we sought to characterize the curli pathway from the perspective of CsgA. The binding affinities of the export complex were assessed using surface plasmon resonance (SPR). In this experiment, an NTA sensor chip was activated using Ni+ and the CsgG-His complex was immobilized via the His-tag, with a peptide comprising N22 from CsgA being injected. Experiments with full length CsgA were not successful, due to the aggregation of CsgA on the SPR chip. Our N22 peptide data showed a specific interaction with a dissociation constant in the micromolar range (0.53 μM; Fig. 3). This is broadly consistent with a value of 28.3 μM, recently measured for the shorter N6 peptide using isothermal calorimetry. The CsgG-N22 interaction likely reflects the specific interaction with full-length CsgA, as it has been shown the N22 peptide is not part of the amyloid fold and it is sufficient to direct secretion of heterologous proteins to the cell surface21. The interaction between the N22 and CsgG would therefore sequester the N-terminal region from the dynamic solution state ensemble. Therefore, to probe differences in the conformational preference of CsgA between sequestered and un-sequestered N22, we produced a truncation of CsgA lacking this region (CsgAΔN22).
Figure 3.
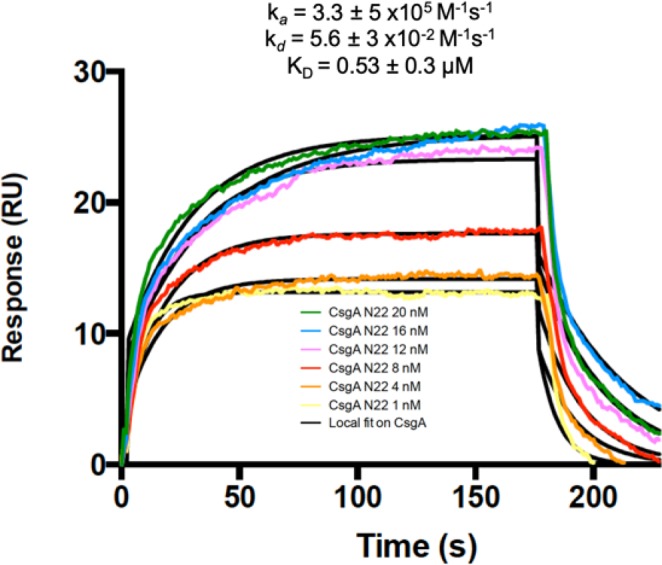
SPR sensorgrams obtained for CsgA N22 peptide indicate binding to CsgG. Binding of the CsgA N22 peptide was assessed in the range of 20 − 1 nM concentrations using a non-activated flow cell as a control. The binding curves were fitted based on 1:1 binding model and the parameters calculated locally. Final value of the affinity constant obtained was 0.53 μM.
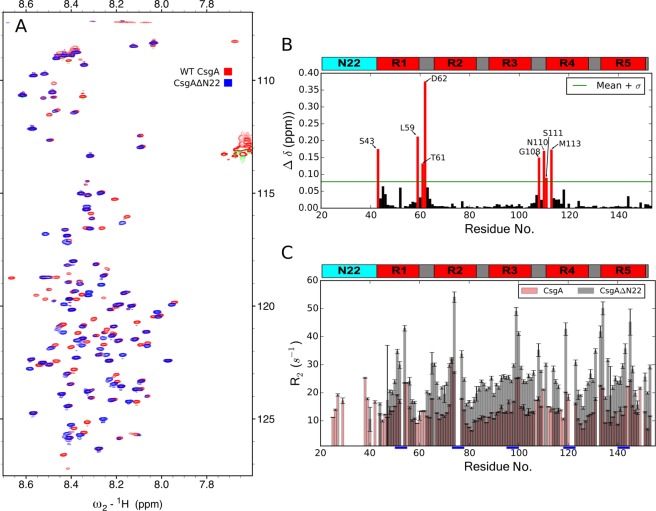
The 1H-15 N HSQC spectra of CsgAΔN22 reveals significant chemical shift differences (Fig. 4A), which indicates changes in the conformational distribution of the disordered ensemble. In order to map these changes to the CsgA sequence, independent assignment experiments were carried out for CsgAΔN22 and chemical shift perturbations (CSPs) plotted against the CsgA sequence (Fig. 4B). Outside of shifts proximal to the N22 region, which would be a direct result of the truncation, two main clusters are observed where the largest changes occur. Residues L59, T61, and D62 are located within repeat R1, which is essential for amyloid formation40. Interestingly, G108, N110, S111, and M113 are located in the linker between the amyloidogenic R3 and the non-amyloidogenic R440,19, and are flanked by gatekeeper residues D91, D104, G123 and D127, which prevent the repeat regions from participating in nucleation events41.
Figure 4.
Truncation of the N22 region shifts dynamics in key amyloidogenic regions. (A) Overlay of 15N-1 H HSQC spectra of CsgA (red) and CsgAΔN22 (blue) highlights widespread CSPs. (B) Mapping of these CSPs to the sequence reveals two prominent clusters that are proximal to key gate-keeper residues. The threshold (green line) for selection of most significant shifts (Δ δ) was calculated as the mean of all CSPs plus the standard deviation. (C) Transverse relaxation rates are elevated across the sequence of the truncation. The highest relaxation rates in both truncate and full-length are noted proximal to prion-like repeats (underlined blue). Error bars represent the estimated errors from fitting exponential decay from experiments performed in triplicate. Bar plots are transparently overlapped according to colouring in the legend to illustrate elevated relaxation in CsgAΔN22.
To further investigate differences between the molecular ensembles of CsgA and CsgAΔN22, transverse relaxation rates (R2) were measured for the amide resonances. This parameter is sensitive to conformational dynamics on the fast time scale (ps-ns), and also influenced by slower exchange processes (μs-ms)42. It is often used to identify areas of backbone flexibility and conformational exchange processes. The R2 rates of CsgA are generally low across the sequence, in the range of 10 s−1, typical of an IDP due to the inherent and high flexibility (Fig. 4C). In contrast, CsgAΔN22 has significantly elevated R2 values across the whole sequence, approximately twice that for CsgA, suggesting that the truncate exhibits conformational exchange on a μs-ms time scale. In order to probe for μs-ms dynamics further, CPMG experiments were conducted on both samples, however no dispersion was detected. The dynamics indicated by the increased R2 values suggest they may arise from intramolecular conformational exchange or self association on the faster end of the μs timescale. A few short sequence regions of CsgA, proximal to the glycine-rich motifs of repeats, exhibit elevated transverse relaxation (as denoted by the blue bars in Fig. 4C). A previous study identified these hexapeptide motifs (Q-X-G-G/F-G-N) as similar to to the highly amyloidogenic peptide regions of animal and yeast prion proteins, and demonstrated they are vital for curli formation43. The R2 values observed here appear to support their findings, and highlight that important structural transitions appear to occur in these motifs. Although it has been shown in vitro that both CsgAΔN22 and CsgA readily formed amyloid fibrils on broadly similar timescales, the aggregation kinetics display different protein concentration dependencies44.
To better gauge how differences in the chemical shifts and R2 relaxation rates related to the conformational distribution in the CsgA/CsgAΔN22 ensembles, secondary structure propensities (SSPs) were calculated. We used the δ 2D method to calculate propensities from chemical shift data, as it is optimally parameterized for disordered proteins. As would be expected for IDPs, the major SSP in CsgA is random coil, however there are noticeable sub-populations of other motifs (Fig. 5A). In full-length CsgA, the major non-coil propensities are PPII helices. This type of secondary structure has high conformational flexibility, providing a low energy barrier for conversion to other structural motifs, and has been observed as an important intermediate in transitions from disordered states into the amyloid fold45,46, Truncation of the N22 causes a transition away from PPII, particularly in the linker regions between the repeats (Fig. 5B). Furthermore and most notably, a helix propensity emerges between R1 and R2, providing a structural basis for these the prominent CSP clusters.
Figure 5.
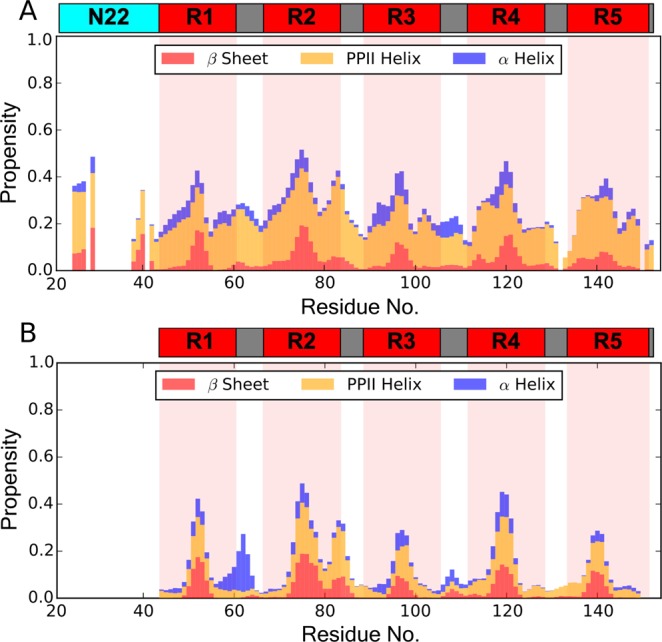
Residual secondary structure propensities (SSPs) in CsgA transition away from PPII helices upon truncation of N22. (A) Outside of random coil, full-length CsgA displays a predominantly PPII helix structure. Small β-sheet propensities are noted in the repeat regions. (B) CsgAΔN22 has a reduced PPII helix density, with a significant α-helix propensity appearing between R1 and R2. SSPs are represented by stacked bar plots, with colours showing the relative propensity at each residue, as in the legend. Random coil propensity, the dominant structure in IDPs, is not actively displayed. Regions highlighted red on the plots align with the repeat regions in the sequence schematic above.
CsgE interactions with CsgA
We proceeded to monitor the interaction between both CsgA constructs and CsgE, the periplasmic cap to the CsgG pore, from the perspective of CsgA. Previous studies have a identified weak and transient interactions between CsgA and CsgE when studying CSPs from the CsgE perspective36. Our rationale was that, because the N22 region binds to CsgG, and we have identified conformational re-arrangement in CsgAΔN22, mimicking sequestration, CsgE may differentially bind to the two CsgA species. We adopted the same approach by employing the oligomerization-disrupted W48A/F79A CsgE mutant as it gives markedly better NMR spectra, while retaining is functional chaperoning effect on CsgA26. The W48A/F79A CsgE mutant was incubated at a four-fold excess to CsgA prior to recording spectra. Small but significant chemical shift changes were noted for CsgAΔN22 (~0.015 ppm) (Supplementary Figure S2).
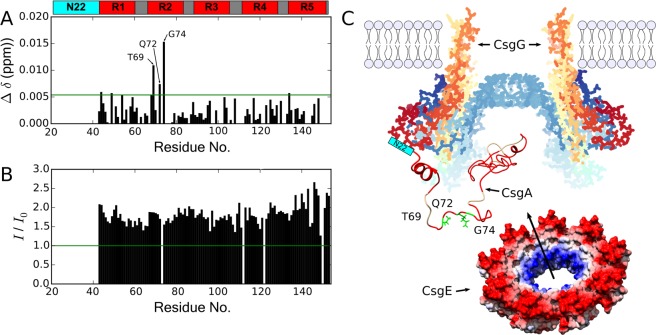
Strikingly, the significant shifts (T69/Q72/G74) are located at the start of R2 (Fig. 6A). As this cluster is proximal to where the α-helix propensity appears in CsgAΔN22, it suggests this conformational transition upon CsgG binding to N22 may be important for CsgE recognition. Further supporting this, no significant shifts of this magnitude were observed in the incubation of CsgE with full-length CsgA (Supplementary Figure S3).
Figure 6.
CsgE interactions are only observed with CsgAΔN22 truncate, which stabilises the pre-fibril state prior to secretion through into the extracellular space. (A) CsgAΔN22 CSPs when incubated with a four times excess of W48A/F79A CsgE. Despite observed differences being relatively small, no significant shift differences were noted when CsgE was incubated with full-length CsgA. This suggests a transient interaction in the region adjacent to the α-helix propensity that emerged in the truncate. (B) Differences in signal intensity between CsgAΔN22, incubated overnight at 4 °C, with a four times excess of CsgE (I), and without (I0). Signal loss likely a result of transition to an amyloid state. CsgE reduces the rate of this signal loss. (C) Model of key interactions during the periplasmic export of CsgA. The N22 region binds to CsgG, causing a structural re-arrangement of CsgA. CsgE acts as a nonameric cap, preventing amyloid formation and facilitating export through transient interactions with CsgA in the region of T50/Q53/G55. This then results in CsgA being capped in the pore by the CsgE ring, with release of N22 and secretion through CsgG. Sequence motifs of CsgA coloured as in the sequence schematic in (A), CsgE surface coloured according to electrostatic potential - positive (red) through to negative (blue). PDB accession codes = CsgE: 2NA4, CsgG: 4UV3.
Previous work has shown the CsgE has a stabilising effect of the disordered CsgA monomer and this effect is driven by charge-charge interactions36, akin to the effect of CsgC on amyloidogenic proteins35. Notably, charge swap mutations of the E31 and E85 in CsgE increased CsgE-mediated stabilization of CsgA. We also observed the stabilising effect of CsgE on the unfolded state of CsgAΔN22. After 24 hours incubation at 4 °C, samples with CsgE present produced greater signal intensity (Fig. 6B). As the amyloid fibre is not visible in solution state NMR, higher signal intensity relates to a higher population of soluble pre-amyloid CsgA species. This is consistent with the hypothesis that CsgE has a similar function to CsgC in preventing premature fibre formation in the periplasm.
Discussion
The rapid polymerization of CsgA into the functional curli amyloid is critical for construction of the E. coli biofilm. However, whilst the time-scale for this process favours the organism, it presents a challenge for structural studies. In this work, we addressed this challenge by optimising solution conditions and present an NMR characterisation of the CsgA pre-amyloid state, both prior to and after capture by the secretion machinery. The utilisation of the NMR assignment has allowed us to observe structural motifs within the intrinsically disordered monomer. Non-coil secondary structure propensities in free CsgA favour the PPII conformation that has been found important in amyloid transitions45,46, As expected, there are minimal β-sheet propensities that are concentrated in the core-forming repeat regions. Transverse relaxation rates are elevated in the prion-like motifs of the repeat regions, suggesting these are important sites of conformational exchange en route to amyloid precipitation. Our work has shown that the N-terminal N22 region binds specifically to CsgG, and the structural details of this has been recently illuminated with the cryo-electron microscopy structure of the CsgG-CsgF-CsgAN22 complex47. A six residue stretch (V22-G27) is bound in a surface channel on the periplasmic face of CsgG, with conserved residues V23, Q25, and Y26 making the most important contacts. We sought to characterize the conformation upon recruitment to the secretion pore, and therefore produced a truncate - CsgAΔN22. Widespread chemical shift differences in CsgAΔN22 suggested a switch in the disordered ensemble, concentrated in clusters of residues previously identified as gatekeepers for prevention of amyloidosis. Further characterisation of the truncate revealed transverse relaxation was approximately twice the rate compared with the full-length protein, and also that PPII propensity collapses, coupled with the emergence of a prominent α-helical motif between R1 and R2. These conformational perturbations suggest that the N22 has a dual-purpose, first directly targeting CsgA to CsgG and once bound by CsgG inducing a conformation transition in CsgA that facilitates secretion through the CsgG pore.
It is important to note that the N22 region is not cleaved from CsgA, therefore a mechanism must exist for its release from the periplasmic face of CsgG prior to secretion. Indeed, the structural transitions observed in our truncate provide new insight into how this may occur. Interactions with CsgE, which regulates periplasmic substrate export, were observed from CsgAΔN22, but was not detectable with full length CsgA. Therefore, combining results from this study with the structure-function analysis of CsgE36, we present a model for CsgA recognition and the interplay with the CsgE cap (Fig. 6C). In short, we propose that CsgA is kept in it monomeric state with in the perisplasm by freely diffusing CsgC, until it is recruited to CsgG via the specific interaction with its N-terminal N22 region. Subsequent sequestration of N22 induces a conformational re-distribution of the disordered CsgA ensemble, which promotes interactions with CsgE and could also assist in stabilising the CsgE nonamer. This notion is also consistent with recent pull-down assays showing an interaction between CsgE and CsgAΔN22, but not CsgA47. The CsgE oligomer then plugs the periplasmic aperture of the CsgG pore and releases the N22 region. Transient electrostatic interactions from CsgE stabilise the unfolded state of CsgA in its captured state preventing premature amyloid accumulation. Furthermore, oligomerised CsgE encloses the CsgA substrate within the CsgG-CsgE vestibule, reducing its conformational flexibility and the entropy. The release of this high energy intermediate to the extracellular milieu contributes to the driving force for secretion.
A persistent feature of pre-amyloid in curli and other functional amyloid systems is the role of weak transient interactions in preventing amyloid formation and guiding the protein for export and subsequent templating35,36,48–50, The transience of interactions between CsgC/CsgE and CsgA, as observed by fast exchange behaviour in NMR titration experiments35,36,49, is likely important for the progressive handling and delicate manipulation of the dynamic pre-amyloid ensemble. Such fine tuning of this early stage of amyloid formation enables the bacteria to deliver subunits in the appropriate state for efficient and homogeneous fibre format at the surface. It also explains why knock-out mutants of AgfE and AgfC in Salmonella typhi do not abrogate secretion but assemble mixed fibre morphology51.
Methods
Protein expression and purification
The gene encoding the mature CsgA polypeptide (Uniprot P28307, residues 22–151) was obtained by PCR amplification of E. coli BL21 (DE3) genomic DNA. An expression construct with a C-terminal His-tag was obtained by ligation into a pET-28a vector using NcoI/XhoI sites. The CsgAΔN22 truncation was cloned using the Q5 Site-Directed Mutagenesis kit (NEB). CsgA variants were transformed into BL21 (DE3) E. coli expressions strains, inoculated into 800 mL of labelled M9 minimal media (15NH4Cl/13 C6 D-Glucose), and grown to 1.0 OD600. Temperature was decreased to 22 °C, IPTG (isopropyl-BD-thiogalactoside) added to a final concentration of 1 mM, and expression induced for 16 hours. Harvested cell pellets were stored at −80 °C. The pTrc99A expression vector for oligomerization-inhibited W48A/F79A CsgE mutant was kindly provided by Professor Matthew Chapman. W48A/F79A CsgE was purified as outlined previously52. CsgC-6xHis and CsgG-6xHis were expressed and purified as described previously53.
Denaturing purification of recombinant pre-amyloid CsgA
Cell pellets were resuspended in 15 mL g−1 of denaturation buffer (50 mM Tris- HCl, 6 M guanidine hydrochloride, pH 8). Lysis was achieved using sonication, and cell debris removed by centrifugation at 30,000 RCF for 30 minutes. Supernatant was loaded onto a 5 mL FF HisTrap column (GE Life Sciences) that was pre-equilibrated in denaturation buffer. Immobilized metal affinity chromatography (IMAC) was conducted in a step-wise manner with increasing imidazole concentrations (30/60/120/300 mM imidazole, 50 mM Tris-HCl, 6 M guanidine hydrochloride, pH 8). Fractions containing pure amyloid monomer were determined using SDS-PAGE, then pooled, concentrated to 2.5 mL using 10 kDa cut-off Vivaspin 20 device (Sartorius Biotech), and buffer-exchanged by passing through a PD-10 desalting column (GE Life Sciences) equilibrated in low salt buffer (20 mM Na2PO4, pH 7.2). Samples were either immediately used, or flash frozen in liquid N2 and lyophilised.
NMR data collection, assignments, and analysis
Samples of 150 μM CsgA, [U-15N] or [U-13C, 15N], were prepared in low salt buffer (20 mM Na2 PO4, 10% D2 O, pH 7.2. To prevent CsgA from transitioning to amyloid in the time course of assignment experiments (several days), curli-inhibitory chaperone was added at a concentration of 3.75 μM.
All spectra were recorded at 283.2 K. Data were collected on either a Bruker Avance III HD 950 MHz spectrometer, or a Bruker Avance III HD 800 MHz spectrometer. Both were equipped with a 5 mm TCI cryoprobe. Assignment of C α, C β, HN, and N chemical shifts were achieved using the following triple resonance spectra: HNCACB54, HNcoCACB54, hNcocaNNH55. Non-uniform sampling (NUS) methodologies were implemented in data collection. All spectra were processed with NMRPipe56, using SMILE to process NUS data57. Spectra were visualised and analysed using SPARKY58. Semi-automated, iterative backbone assignment was achieved using the algorithm MARS59. Secondary structure populations were determined from assigned chemical shifts, using the δ 2D methodology that is optimized for analysing disordered proteins60. 15N transverse relaxation parameters were acquired using Carr-Purcell-Meiboom-Gill (CPMG) experiments61. These experiments were recorded with 14 different CPMG frequencies, ranging from 50 to 1600 Hz with three points repeated in duplicate, with a 20 ms mixing time per CPMG block. Values of R2 were derived from line shape integration of peaks and fitting using the software package NMRPINT62. For interaction experiments, CsgA constructs (100 μM) were incubated with W48A/F79A CsgE (400 μM) for 24 hours at 4 °C prior to recording spectra. No CsgC was included in these samples. Receiver gains were set to the same value. CsgE was buffer exchanged into low salt buffer immediately before incubation. Chemical shift perturbations were calculated as a euclidean distance (d), and weighted as previously published63.
Surface plasmon resonance
The experiments were performed on a Biacore 3000 using an NTA sensor chip (GE Healthcare, USA). The running buffer (10 mM HEPES, 150 mM NaCl, 0.36 mM DDM, pH 8.0) and the remaining buffers were prepared according to manufacturer’s instructions, using 0.36 mM DDM as surfacant substitute. The senor chip was activated with 30 μL of 0.5 mM NiSO4 at 10 μl/min. 30 μL of 200 nM CsgG were immobilized at 10 μl/min. 150 μl and 250 μl of N22 peptide was injected at 50 μl/min at varying concentration. The reference flow cell was a non-activated surface, as recommended by GE Healthcare. Curve fitting was done based on a 1:1 binding model.
Supplementary information
Acknowledgements
This work was supported by the Wellcome Trust (Senior Investigator Award 100280 and multi-user equipment grant 104833 to SM) and a BBSRC PhD studentship to LS. We thank Professor Matthew Chapman for providing CsgE expression constructs and the MRC Biomedical NMR Centre within the Crick Institute for access to 950 MHz NMR spectrometer time.
Author contributions
S.M. and L. Sewell conceived the experiment(s), L.Sewell, F.S., Y.X., L. Sefer. and J.T. conducted the experiment(s), S.M. and L.Sewell analysed the results. All authors reviewed the manuscript.
Data availability
Accession codes CsgA chemical shift assignment have been deposited under Biological Magnetic Resonance Bank [BMRB] ID codes 50138.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64135-9.
References
- 1.Knowles TPJ, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nature Nanotechnology. 2011;6:469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- 2.Dobson CM. Protein misfolding, evolution and disease. Trends in Biochemical Sciences. 1999;24:329–332. doi: 10.1016/S0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 3.Salahuddin P, Fatima MT, Abdelhameed AS, Nusrat S, Khan RH. Structure of amyloid oligomers and their mechanisms of toxicities: Targeting amyloid oligomers using novel therapeutic approaches. European Journal of Medicinal Chemistry. 2016;114:41–58. doi: 10.1016/j.ejmech.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 4.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid–from bacteria to humans. Trends in biochemical sciences. 2007;32:217–24. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Römling U, Bian Z, Hammar M, Sierralta WD, Normark S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. Journal of Bacteriology. 1998;180:722–731. doi: 10.1128/JB.180.3.722-731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dueholm MS, et al. Functional amyloid in Pseudomonas. Molecular Microbiology. 2010;77:1009–1020. doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- 7.Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2230–4. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsén A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–5. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 9.Evans ML, Chapman MR. Curli biogenesis: order out of disorder. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2014;1843:1551–1558. doi: 10.1016/j.bbamcr.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zogaj X, Bokranz W, Nimtz M, Römling U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infection and immunity. 2003;71:4151–8. doi: 10.1128/iai.71.7.4151-4158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dueholm MS, Albertsen M, Otzen D, Nielsen PH, Schuster S. Curli Functional Amyloid Systems Are Phylogenetically Widespread and Display Large Diversity in Operon and Protein Structure. PLoS ONE. 2012;7:e51274. doi: 10.1371/journal.pone.0051274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung, C., Marschall, J., Burnham, C. A. D., Byun, A. S. & Henderson, J. P. The bacterial amyloid curli is associated with urinary source bloodstream infection. PLoS ONE9, 10.1371/journal.pone.0086009 (2014). [DOI] [PMC free article] [PubMed]
- 13.Tursi SA, et al. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLOS Pathogens. 2017;13:e1006315. doi: 10.1371/journal.ppat.1006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harach T, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Scientific Reports. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cegelski L, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nature Chemical Biology. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorval Courchesne NM, Duraj-Thatte A, Tay PKR, Nguyen PQ, Joshi NS. Scalable Production of Genetically Engineered Nanofibrous Macroscopic Materials via Filtration. ACS Biomaterials Science and Engineering. 2017;3:733–741. doi: 10.1021/acsbiomaterials.6b00437. [DOI] [PubMed] [Google Scholar]
- 17.Gerstel U, Park C, Römling U. Complex regulation of csgD promoter activity by global regulatory proteins. Molecular Microbiology. 2004;49:639–654. doi: 10.1046/j.1365-2958.2003.03594.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogasawara H, Yamamoto K, Ishihama A. Role of the biofilm master regulator CsgD in cross-regulation between biofilm formation and flagellar synthesis. Journal of Bacteriology. 2011;193:2587–2597. doi: 10.1128/JB.01468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Smith DR, Jones JW, Chapman MR. In vitro polymerization of a functional escherichia coli amyloid protein. Journal of Biological Chemistry. 2007;282:3713–3719. doi: 10.1074/jbc.M609228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans M, et al. The Bacterial Curli System Possesses a Potent and Selective Inhibitor of Amyloid Formation. Molecular Cell. 2015;57:445–455. doi: 10.1016/j.molcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivanathan V, Hochschild A. Generating extracellular amyloid aggregates using e. coli cells. Genes & development. 2012;26:2659–2667. doi: 10.1101/gad.205310.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson LS, Ashman EM, Hultgren SJ, Chapman MR. Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Molecular Microbiology. 2006;59:870–881. doi: 10.1111/j.1365-2958.2005.04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal P, et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature. 2014;516:250–253. doi: 10.1038/nature13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao B, et al. Structure of the nonameric bacterial amyloid secretion channel. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5439–44. doi: 10.1073/pnas.1411942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nenninger AA, et al. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Molecular Microbiology. 2011;81:486–499. doi: 10.1111/j.1365-2958.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu Q, et al. Solution nmr structure of csge: Structural insights into a chaperone and regulator protein important for functional amyloid formation. Proceedings of the National Academy of Sciences. 2016;113:7130–7135. doi: 10.1073/pnas.1607222113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubeis, T. et al. Structural and functional characterization of the Curli adaptor protein CsgF, 10.1002/1873-3468.13002 (2018). [DOI] [PubMed]
- 28.Shu Q, et al. The E. coli CsgB nucleator of curli assembles to β-sheet oligomers that alter the CsgA fibrillization mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6502–6507. doi: 10.1073/pnas.1204161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammar M, Bian Z, Normark S. Nucleator-dependent intercellular assembly of adhesive curli organelles in escherichia coli. Proceedings of the National Academy of Sciences. 1996;93:6562–6566. doi: 10.1073/pnas.93.13.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian P, et al. Structure of a functional amyloid protein subunit computed using sequence variation. Journal of the American Chemical Society. 2015;137:22–5. doi: 10.1021/ja5093634. [DOI] [PubMed] [Google Scholar]
- 31.DeBenedictis EP, Ma D, Keten S. Structural predictions for curli amyloid fibril subunits CsgA and CsgB. RSC Adv. 2017;7:48102–48112. doi: 10.1039/C7RA08030A. [DOI] [Google Scholar]
- 32.Perov S, et al. Structural insights into curli csga cross-β fibril architecture inspire repurposing of anti-amyloid compounds as anti-biofilm agents. PLoS pathogens. 2019;15:e1007978. doi: 10.1371/journal.ppat.1007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreasen, M. et al. Physical determinants of amyloid assembly in biofilm formation. mBio10, 10.1128/mBio.02279-18 (2019). [DOI] [PMC free article] [PubMed]
- 34.Wang H, Shu Q, Rempel DL, Frieden C, Gross ML. Understanding curli amyloid-protein aggregation by hydrogen–deuterium exchange and mass spectrometry. International Journal of Mass Spectrometry. 2017;420:16–23. doi: 10.1016/j.ijms.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JD, et al. Electrostatically-guided inhibition of Curli amyloid nucleation by the CsgC-like family of chaperones. Scientific Reports. 2016;6:24656. doi: 10.1038/srep24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein RD, et al. Structure-Function Analysis of the Curli Accessory Protein CsgE Defines Surfaces Essential for Coordinating Amyloid Fiber Formation. mbio.asm.org. 2018;9:1349–1367. doi: 10.1128/mBio.01349-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, et al. Promiscuous cross-seeding between bacterial amyloids promotes interspecies biofilms. Journal of Biological Chemistry. 2012;287:35092–35103. doi: 10.1074/jbc.M112.383737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartman K, et al. Bacterial curli protein promotes the conversion of pap248-286 into the amyloid sevi: cross-seeding of dissimilar amyloid sequences. PeerJ. 2013;1:e5. doi: 10.7717/peerj.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, Y., Smith, D. R., Hufnagel, D. A. & Chapman, M. R. Experimental manipulation of the microbial functional amyloid called curli. In Bacterial cell surfaces, 53–75 (Springer, 2013). [DOI] [PMC free article] [PubMed]
- 40.Wang X, Chapman MR. Sequence Determinants of Bacterial Amyloid Formation. Journal of Molecular Biology. 2008;380:570–580. doi: 10.1016/j.jmb.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhou Y, Ren JJ, Hammer ND, Chapman MR. Gatekeeper residues in the major curlin subunit modulate bacterial amyloid fiber biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:163–168. doi: 10.1073/pnas.0908714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay LE, Torchia DA, Bax A. Backbone dynamics of proteins as studied by nitrogen-15 inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry. 1989;28:8972–8979. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- 43.Cherny I, et al. The formation of Escherichia coli curli amyloid fibrils is mediated by prion-like peptide repeats. Journal of Molecular Biology. 2005;352:245–252. doi: 10.1016/j.jmb.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 44.Zimmer, A. Structural Characterisation of Fungal and Bacterial Amyloids by Hydrogen/Deuterium Exchange NMR Spectroscopy. Ph.D. thesis (2011).
- 45.Blanch EW, et al. Is polyproline II helix the killer conformation? a raman optical activity study of the amyloidogenic prefibrillar intermediate of human lysozyme. Journal of Molecular Biology. 2000;301:553–563. doi: 10.1006/JMBI.2000.3981. [DOI] [PubMed] [Google Scholar]
- 46.Adzhubei AA, Anashkina AA, Makarov AA. Left-handed polyproline-II helix revisited: proteins causing proteopathies. Journal of Biomolecular Structure and Dynamics. 2017;35:2701–2713. doi: 10.1080/07391102.2016.1229220. [DOI] [PubMed] [Google Scholar]
- 47.Yan, Z., Yin, M., Chen, J. & Li, X. Assembly and substrate recognition of curli biogenesis system. Nature Communications11 (2020). [DOI] [PMC free article] [PubMed]
- 48.Swasthi HM, Mukhopadhyay S. Electrostatic lipid–protein interactions sequester the curli amyloid fold on the lipopolysaccharide membrane surface. Journal of Biological Chemistry. 2017;292:19861–19872. doi: 10.1074/jbc.M117.815522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chorell, E. et al. Bacterial chaperones csge and csgc differentially modulate human α-synuclein amyloid formation via transient contacts. PloS one10 (2015). [DOI] [PMC free article] [PubMed]
- 50.Dunbar, M., DeBenedictis, E. & Keten, S. Dimerization energetics of curli fiber subunits CsgA and CsgB. npj Computational Materials5, 10.1038/s41524-019-0164-5 (2019).
- 51.Gibson DL, White AP, Rajotte CM, Kay WW. Agfc and agfe facilitate extracellular thin aggregative fimbriae synthesis in salmonella enteritidis. Microbiology. 2007;153:1131–1140. doi: 10.1099/mic.0.2006/000935-0. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Shu Q, Rempel DL, Frieden C, Gross ML. Continuous and pulsed hydrogen-deuterium exchange and mass spectrometry characterize CsgE oligomerization. Biochemistry. 2015;54:6475–81. doi: 10.1021/acs.biochem.5b00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor JD, et al. Atomic resolution insights into curli fiber biogenesis. Structure. 2011;19:1307–1316. doi: 10.1016/j.str.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muhandiram DR, Kay LE. Gradient-Enhanced Triple-Resonance Three-Dimensional NMR Experiments with Improved Sensitivity. Journal of Magnetic Resonance, Series B. 1994;103:203–216. doi: 10.1006/jmrb.1994.1032. [DOI] [Google Scholar]
- 55.Sun Z-YJ, Frueh DP, Selenko P, Hoch JC, Wagner G. Fast Assignment of 15N-HSQC Peaks using High-Resolution 3D HNcocaNH Experiments with Non-Uniform Sampling. Journal of Biomolecular NMR. 2005;33:43–50. doi: 10.1007/s10858-005-1284-4. [DOI] [PubMed] [Google Scholar]
- 56.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. Journal of Biomolecular NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 57.Ying, J., Delaglio, â. F., Torchia, D. A. & Ad Bax, Sparse multidimensional iterative lineshape-enhanced (SMILE) reconstruction of both non-uniformly sampled and conventional NMR data. 10.1007/s10858-016-0072-7. [DOI] [PMC free article] [PubMed]
- 58.Lee W, Tonelli M, Markley JL. NMRFAM-SPARKY: Enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung Y-S, Zweckstetter M. Mars- robust automatic backbone assignment of proteins. Journal of Biomolecular NMR. 2004;30:11–23. doi: 10.1023/B:JNMR.0000042954.99056.ad. [DOI] [PubMed] [Google Scholar]
- 60.Camilloni, C., De Simone, A., Vranken, W. F. & Vendruscolo, M. Determination of Secondary Structure Populations in Disordered States of Proteins Using Nuclear Magnetic Resonance Chemical Shifts. 10.1021/bi3001825 (2012). [DOI] [PubMed]
- 61.Tollinger M, Skrynnikov NR, Mulder FAA, Forman-Kay JD, Kay LE. Slow dynamics in folded and unfolded states of an SH3 domain. Journal of the American Chemical Society. 2001;123:11341–11352. doi: 10.1021/ja011300z. [DOI] [PubMed] [Google Scholar]
- 62.Niklasson M, et al. Comprehensive analysis of NMR data using advanced line shape fitting. Journal of Biomolecular NMR. 2017;69:93–99. doi: 10.1007/s10858-017-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson MP. Using chemical shift perturbation to characterise ligand binding. Progress in Nuclear Magnetic Resonance Spectroscopy. 2013;73:1–16. doi: 10.1016/J.PNMRS.2013.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession codes CsgA chemical shift assignment have been deposited under Biological Magnetic Resonance Bank [BMRB] ID codes 50138.