Abstract
The development of novel paediatrics formulations is critical towards achieving the UNAIDS 90-90-90 targets. According to the latest UNAIDS reports, the availability of antiretrovirals (ARVs) for children has increased significantly, from 49% in 2015 to 53% in 2017. However, this percentage is considerably lower than the 80% for pregnant women that are currently on treatment. Therefore, there is still an urgent need for an alternative child-friendly delivery system. Lopinavir (LPV) is a protease inhibitor first-line HIV treatment drugs but suffers from low aqueous solubility, bitter state, short half-life leading to a limited dissolution and variable bioavailability upon oral administration. This work focused on the fabrication and characterization of a delivery system entailing Eudragit RSPO-LPV nanoparticles loaded suppositories in two different bases to improve the bioavailability and overcome the problem encountered through oral administration emanating from poor solubility. The prepared nanoparticles by nanoprecipitation method were characterized and compounded into suppositories in fattibase and polyethylene glycol (PEG) bases using a melt fusion method. The suppositories were stored at 5 and 25 °C, and were sampled at 0, 4, 8, 12 weeks. The samples were assessed by particle size, entrapment efficiency (EE), zeta potential and polydispersity index (PDI) variations. The preliminary in vitro release studies were analysed by HPLC. The nanoparticles have an average particle size of 191 nm with spherical morphology, entrapment efficiency, polydispersity index and zeta potential of 79.0 ± 0.5%, 0.224, and 25.87 ± 0.41 mV respectively. The surface analysis of the nanoparticles with FTIR, SEM, PXRD and TGA indicated that the drug was truly encapsulated without any interaction. The in vitro release studies showed that a better release was observed in suppositories formulated with PEG than the fattibase by having higher drug concentration released. Hence, this rectal formulation might serve as an alternative for paediatric HIV treatment upon further investigation.
Keywords: Eudragit, Nanoparticles, Suppositories, Melt fusion method, HIV paediatric, Chemistry, Analytical chemistry, Organic chemistry, Pharmaceutical chemistry, Health sciences
Eudragit, nanoparticles, suppositories, melt fusion method, HIV paediatric; Chemistry; Analytical chemistry; Organic chemistry; Pharmaceutical chemistry; Health Sciences
1. Introduction
Human immunodeficiency virus (HIV) is regarded as a major global epidemic and a serious threat to public health, especially in many developing countries (Lloyd-Sherlock et al., 2014). The recent statistics showed that 36.9 million people were living with HIV, 35.1 million adults and 1.8 million children, respectively (UNAIDS, 2018). Paediatric HIV infection in children remains a significant health issue globally (Newell et al., 2004). Although, the survival of HIV-infected children has improved with increased access of 51% receiving treatment at the end of 2017 when compared to 80% of HIV-infected pregnant women on treatments (UNAIDS, 2018). These populations of children on treatment were still not acceptable when compared to the ratio of infected children, which is high. Therefore, drastic actions must be taken into cognizance in improving paediatric HIV treatment in order to meet the millennium development goal of 90–90–90 target of eradicating HIV prevalence by 2030 (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2017).
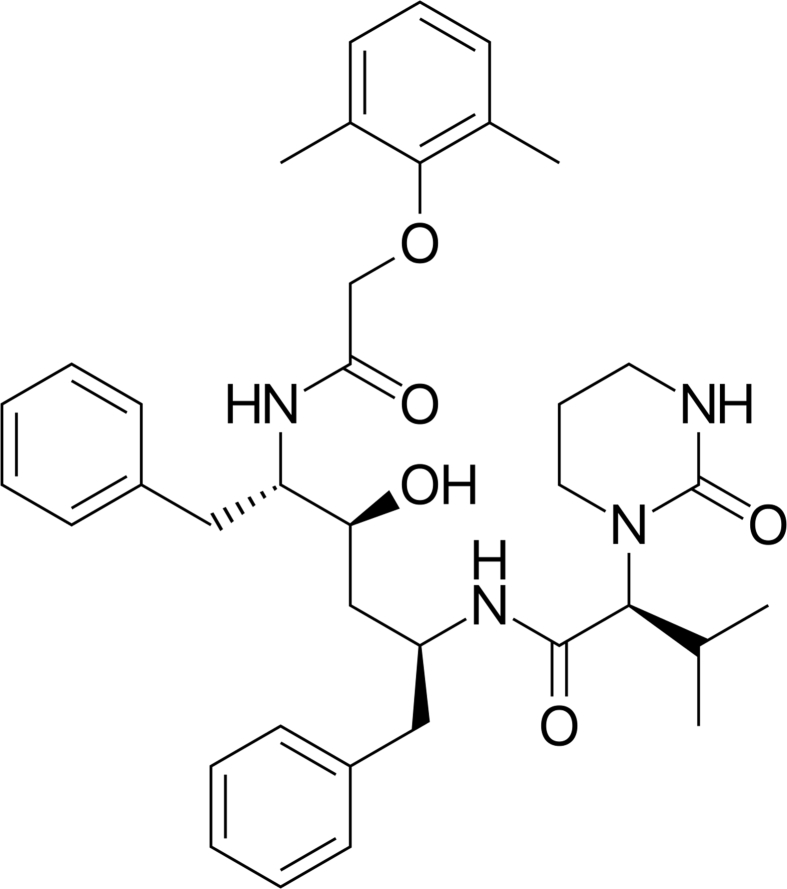
One of the foremost leading components of highly active antiretroviral therapy (HAART) applied for the treatment of HIV infections in both adults and children is LPV. LPV, as depicted in Figure 1, is a potent protease inhibitor (Maartens et al., 2014; Khan et al., 2019). It is currently being used, as a combination therapy with ritonavir (RTV) with limited oral route therapy challenges (Pham et al., 2016). This current treatment which is based on oral administrations, has been shown to suffer from limited bioavailability when administered orally with low aqueous solubility (0.01 mg/mL) (Pham et al., 2016). In particular, most of these drugs are unbearable due to their bitter taste, high alcoholic concentration, hepatic first-pass effect and gastrointestinal side effects (Van Riet-Nales et al., 2012; Lallemant et al., 2011). Therefore, various studies have been done where nanotechnology techniques have been explored extensively to improve specific issues associated with LPV solubility, bioavailability and half-life (Abou-El-Naga et al., 2017; Joshi et al., 2016).
Figure 1.
The chemical structure of lopinavir.
Garima and co-worker prepared LPV-loaded poly lactic-co-glycolic acid (PLGA) nanoparticles (NPs) using nanoprecipitation method, and their results showed an improved bioavailability and control drug release potential without co-administration with ritonavir (Joshi et al., 2016). However, oral administration is mostly suitable for adults when considering the formulations on the market with all the shortcomings which are an indicator that children are not in cognizance consideration when they are prepared (DNDI, 2018). Thereby, arises the call for formulations that will overcome these challenges encountered using oral and intravenous administration which will be highly acceptable to the children and the caregivers through other routes. The rectal route is proposed as alternative routes. This is due to its ease of administration (Van Riet-Nales et al., 2012), which resulted in paediatric clinical studies that proposed its equivalence as a promising alternative route for the treatment of children (Sarmento and das Neves, 2012).
Few studies have been reported on the usage of the rectal delivery system especially for antiretroviral (ARV), such as zidovudine (Priya et al., 2015b), dapivirine (Das Neves et al., 2013a), stavudine, lamivudine and nevirapine (Padmavathi et al., 2015) which demonstrated a controlled release of the ARVs. It is worth noting that no studies have been carried out on formulating LPV nanoparticles (LPV-NPs) into suppositories. Likewise, there is no LPV suppository in the market, and no information exists on their rectal availability. There is an urgency to formulate a child-friendly delivery system, with well tolerable properties as a crucial need for children living with HIV (Ham et al., 2017; Schlatter et al., 2016). This proposed formulation is to overcome the challenges of poor palatability, swallowing, regimen complexity, storage and transportation exhibited by oral formulated ARVs. In the present study, a modified nanoprecipitation method was used to prepare Eudragit RSPO-LPV nanoparticles (Nagavarma et al., 2012a; Lepeltier et al., 2014). The fabricated nanoparticles were then characterized and loaded into suppositories by fusion method (Akin-Ajani et al., 2019) and analyzed for invitro released studies.
2. Materials and methods
2.1. Materials
Eudragit RSPO was donated by Evonik Rohm, GmbH, (Germany). Lopinavir with 98 % purity was purchased from DB Fine chemicals (South Africa). The Pluronic F-127 and the polyethylene glycol (PEG 1500 & 3350) were purchased from Sigma Aldrich USA. Pharmaceutical excipients, namely, Fattibase™ was purchased from Paddocks Laboratories, Minneapolis, (USA). Potassium hydrogen phosphate (KH2PO4), sodium hydrogen phosphate (Na2HPO4), potassium chloride (KCl) and sodium chloride (NaCl) were purchased from Sigma Aldrich (South Africa). Acetonitrile and methanol were purchased from Merck (South Africa), and hydrochloric acid was purchased from Glassworld (South Africa). All other chemicals and reagents were of analytical grade.
2.2. Preparation of lopinavir nanoparticles
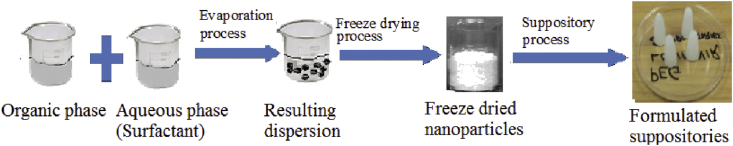
Eudragit RSPO nanoparticles containing LPV were prepared by a modified nanoprecipitation method (Nagavarma et al., 2012a; Lepeltier et al., 2014). The drug and polymer were accurately weighed and dissolved in 5 mL mixture of ethanol and acetone (1:1) respectively at room temperature. The drug and polymer 25 mg and 50 mg (1:2) respectively were mixed using a magnetic stirrer for an hour (Nagavarma et al., 2012a; Lepeltier et al., 2014). Thereafter, the organic phase (drug and polymer) was added dropwise into the 1% surfactant (Pluronic F-127) by a syringe under the high stirring speed of 15000 rpm using Omni PDH (USA) homogenizer for 10 min. After evaporation of the organic solvent from the internal phase in a fume hood for 12 h at 700 rpm using IKA RT10 Power 10-position magnetic hot plate (Germany). The precipitated loaded nanoparticles were isolated by using a centrifuge at 13500 rpm for 15 min and washed three times with distilled water using Hermle Z 326 K (Germany). This is to remove the surfactant in order to obtain surfactant free nanoparticles, the supernatant from the first cycle was taken and measured the absorbance at 259 nm to calculate the encapsulation efficiency (EE). This is calculated by determining the amount of non-encapsulated LPV in the aqueous surfactant solution, against the total amount of drug added to the formulation using a standard calibration curve. The produced suspension was freeze dried for 48 h at -40°C to obtain a fine powder nanoparticle using Christ Alpha plus 1–4 (Germany) freeze dryer. This method is indicated in the schematic diagram in (Figure 2).
Figure 2.
Diagrammatic representation of the preparation of Eudragit RSPO-LPV suppositories.
2.3. Preparation of suppositories
The Eudragit RSPO-LPV suppositories were prepared using fattibase and PEG mixtures (1500 & 3350) as two bases. It was prepared by the fusion method using metal moulds (Akin-Ajani et al., 2019). The displacement value of each base was determined, and the amount of Eudragit RSPO-LPV required for each suppository formulation was calculated (Akin-Ajani et al., 2019). The mixture of the melted bases and the nanoparticles were thoroughly mixed to ensure homogenous mixture before pouring them into the moulds, which resulted in uniform distribution with uniform appearances. Therefore, two different types of formulation (F1 & F2) were made with four ingredients and each suppository containing an equivalent amount of the drug in each one-gram suppository as shown in Table 1.
Table 1.
Eudragit RSPO-LPV nanoparticles suppositories formulation compositions.
| S/N | Ingredients | F1 (%) | F2 (%) |
|---|---|---|---|
| 1 | LPV NPs | 100 | 100 |
| 2 | PEG 1500 | 25 | |
| 3 | PEG 3350 | 75 | |
| 4 | Fattibase | 100 |
2.4. Particle size, particle size distribution and zeta potential
The Eudragit RSPO-LPV nanoparticles were characterized in terms of mean particle size diameter, the polydispersity index (PDI) and the zeta potential using Malvern Zetasizer Nano ZS (Malvern instrument, United Kingdom) installed with DTS software by a dynamic light scattering (DLS)-based method (Salatin et al., 2017a, Salatin et al., 2017b). The nanoparticles formulations were sonicated in distilled water to minimize the interparticle interactions before analysing. It was analysed three times with the Nano ZS instruments to obtained average results.
2.5. Encapsulation efficiency
The encapsulation efficiency (EE) was determined by UV-vis spectrophotometer (Merck Spectroquant® Prove 300, Germany). The prepared Eudragit RSPO-LPV nanoparticles were isolated using a centrifuge at 13500 rpm for 10 min through the supernatant obtained, which was analysed. The EE of the nanoparticles was calculated using the following equation.
Wt is the total amount of pure LPV and Wf is the total amount of free LPV in the supernatant (Zhang et al., 2019b; Matlhola et al., 2015b).
2.6. Fourier transmission infra-red spectroscopy
The drug excipients compatibility studies of the Eudragit RSPO-LPV nanoparticles, Eudragit RSPO and LPV were obtained by using an Agilent Technologies Cary 600 Series FTIR Spectrometer (USA). The samples were dispersed in dry potassium bromide (KBr). The spectra were run between the range 4000 cm−1 – 500 cm−1 (Song et al., 2008).
2.7. Scanning electron microscopy
The morphological studies of the Eudragit RSPO-LPV nanoparticles, Eudragit RSPO and LPV, were examined by scanning electron microscope (FEI Quanta 250 FEG SEM, UK) operating at 10 kV. The nanoparticles were dusted onto double-sided tape on an aluminium stub and coated with gold using a cold sputter coater prior to imaging. Coated samples were then scanned, and photomicrographs were taken (Esmaeili et al., 2015).
2.8. X-ray diffraction
Crystallinity phase identification of Eudragit RSPO-LPV nanoparticles, Eudragit RSPO and LPV were carried out on PANalyticalX'pert Pro (PANalytical, Almelo, Netherlands). The sample was placed in a glass sample holder, irradiated and measured under the following conditions: Anode, Cu; Kα1, 1.5405 Å; Kα2, 1.54443 Å; K-Beta, 1.39225 Å; Kα1/Kα2 ratio, 0.5; Generator settings, 40 mA, 45 kV; divergence slit, 0.957o, fixed; step size, 0.017o in 2θ; scan step times, 19.685 s; temperature, 25 °C. The data was analyzed using X'Pert Data Collector software version 4.0A.
2.9. Differential scanning calorimetry
Thermal characteristics of Eudragit RSPO-LPV nanoparticles, Eudragit RSPO and LPV were carried out using a differential scanning calorimeter (DSC with software star ‘e’ Mettler Toledo, Greifensee, Switzerland) at a heating rate of 10 °C/min over a temperature range of 30–400 °C under an inert atmosphere with a nitrogen gas flow of 35 mL/min.
2.10. Thermogravimetric
Thermogravimetric studies of Eudragit RSPO-LPV nanoparticles, Eudragit RSPO and LPV were characterized using a Mettler DTG 3+ (Mettler Toledo, Greifensee, Switzerland) instrument. Powder samples, weighing about 5–8 mg was placed in aluminium crimp cells, open or sealed (100 μl) and heated to an end temperature range of 30–400 °C, at a heating rate of 10 °C/min, with a nitrogen gas flow of 35 mL/min.
2.11. Stability studies
Stability studies of prepared Eudragit RSPO-LPV nanoparticles were assessed according to International Conference on Harmonization (ICH) Q1A (R2) guidelines (ICH Harmonized Tripartite Guideline, 2003). Briefly, Eudragit RSPO-LPV nanoparticles were stored in sealed glass vials at 25 °C/60 ± 5% relative humidity in the stability chamber (Remi, Mumbai, India). Control samples were stored at 5 °C in a refrigerator. This was carried out on the basis of particle size, entrapment efficiency (EE), zeta potential and polydispersity index (PDI) variations for three months. For products, adequate shelf-life, dosage forms and active ingredients must be stable chemically and physically for extended periods.
2.12. In vitro drug release
The in vitro drug release of Eudragit RSPO-LPV suppositories were carried out by USP rotating basket dissolution apparatus (Ramadan, 2013a). Each suppository was placed in the basket and was lowered to a height 5 mm from the bottom of the vessel containing 100 mL, citric acid/phosphate buffer (50 mM) solution pH 7.4 at a temperature range 37 °C and rotation of 75 rpm. At the predetermined time interval of, 0, 5, 10, 15, 30, 45, 60 and 90 min, 3 mL aliquot was withdrawn and analysed by HPLC for the concentration of drug released. The dissolution medium was replaced by an equal volume of fresh buffer dissolution media after each withdrawal to maintain the total volume. The drug release data were normalized by converting drug concentration in solution to a percentage of the cumulative drug release. An HPLC system Agilent 1200 series; Agilent Technologies Inc., Santa Clara, CA (USA) composes of quaternary pump, degasser, autosampler, Phenomenex C18 RP column (5 ìm packing, 4.6 × 150 mm), Phenomenex C18 RP guard column, and diode array detector was employed. The mobile phase was composed of a mixture of 0.1 M methanol and phosphate buffer in the ratio of 85:15. The method was developed and stabilized for 1 h with the mobile flow rate maintained at 1.0 mL/min, with baseline monitoring prior to actual analysis. The column temperature was maintained at 25 °C, and the detection was performed at 259 nm.
3. Results and discussion
3.1. Particle size, particle size distribution and zeta potential
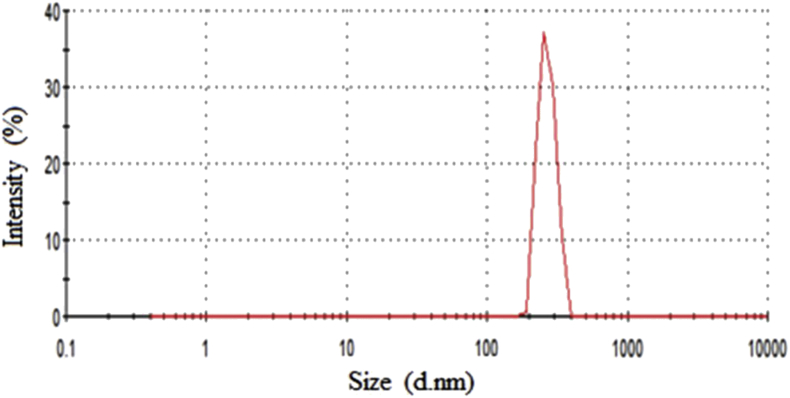
Eudragit RSPO-LPV exhibited a uniform size distribution and the average particle diameters of approximately 190.9 ± 0.21 nm and polydispersity index 0.224 (Figure 3), which was found to be almost similar to the value of drug-loaded nanoparticles of 180.6 ± 2.32 nm obtained through LPV-loaded polylactic-co-glycolic acid (PLGA) nanoparticles (NPs) (Joshi et al., 2016). Eudragit RSPO being a positively charged polymer imparts cationic nature with values ranging from 25.6 to 26.0 mV, similar to the value obtained in RHT-Eudragit nanoparticles (Salatin et al., 2017a, Salatin et al., 2017b). It is a well-established fact that a zeta potential with greater absolute value is an indicator of higher and better stability of the colloidal systems (Hans and Lowman, 2002; Wang and Keller, 2009). Therefore, symbolising a stable formulation developed.
Figure 3.
Particle size distribution by intensity as a function of particle size for Eudragit RSPO-nanoparticles.
3.2. Encapsulation efficiency
The entrapment efficiency of 79.0 ± 0.5% was produced, which is similar to the results obtained in other LPV nanoparticles researches carried out (Patel et al., 2016; Ravi et al., 2014). It is a significant parameter in clinical applications and predominantly depends on the ability of the compound to dissolve in the matrix material or polymer (dissolution or solid dispersal) (Kumari et al., 2010a). The entrapment efficiency obtained, which is probably due to the lipophilic nature of the drug results in the high interaction between the drug and the polymer (Varshney and Tanwar, 2010b; Mandal, 2010).
3.3. Scanning electron microscopy
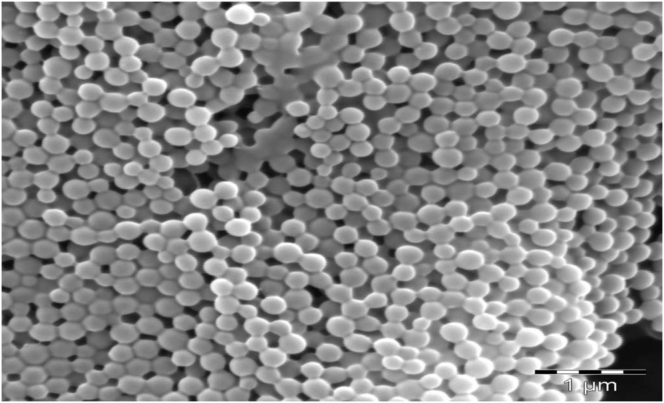
Scanning electron microscopy studies in Figure 4, revealed that Eudragit RSPO-LPV nanoparticles were spherical when compared with the morphology of LPV with irregular crystals indicative that the drug was entrapped within the carrier (Ravi et al., 2015). The size distribution has average particles mean diameter of 190.9 ± 0.21 nm.
Figure 4.
SEM micrograph of Eudragit RSPO-LPV nanoparticles.
3.4. Fourier transform infrared spectroscopy
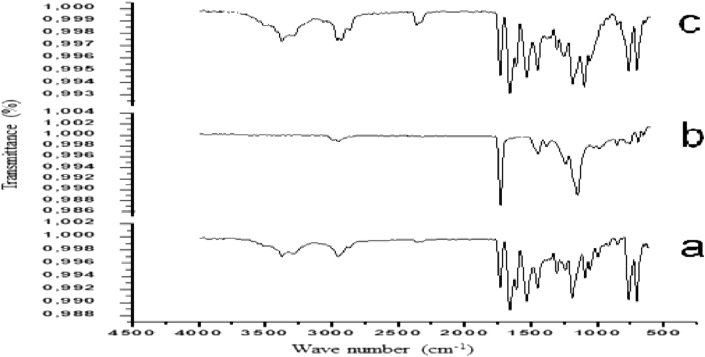
Infrared spectroscopy spectra, as shown in Figure 5, was performed to identify the compatibility of LPV with Eudragit RSPO. The FTIR spectra of LPV shows major characteristic absorption peaks at 3373.02 cm−1 (O–H stretching, 2953.11 cm−1 (C–H2 stretching) and 1658.62 cm−1(amide bond C=O stretching) and 1531.68 cm−1 (urea C=O stretching) and 1352.68 cm−1 (C–N) which are in agreement with the reported values from preparation and evaluation of metastable solid-state forms of LPV (Lemmer and Liebenberg, 2013). All physical characteristics absorption peaks of LPV were retained in Eudragit RSPO-LPV nanoparticles without a major shift in the structure of Eudragit polymer as a result of ionic electrostatic interaction between the drug and the polymer during the formation of nanoparticles.
Figure 5.
FTIR spectrum of (a) Eudragit RSPO-LPV nanoparticles, (b) LPV, (c) Eudragit RSPO.
The red shift in the peak of the drug carbonyl from 1658.62 cm−1 to 1731.84 cm−1 may be attributed to the hydrogen bonding interaction between LPV O–H group and the Eudragit C=O groups in the Eudragit RSPO-LPV nanoparticles, which confirmed the compatibility of LPV with Eudragit polymers. These peaks confirmed that there was no interaction between the drug and other components.
3.5. X-ray diffraction
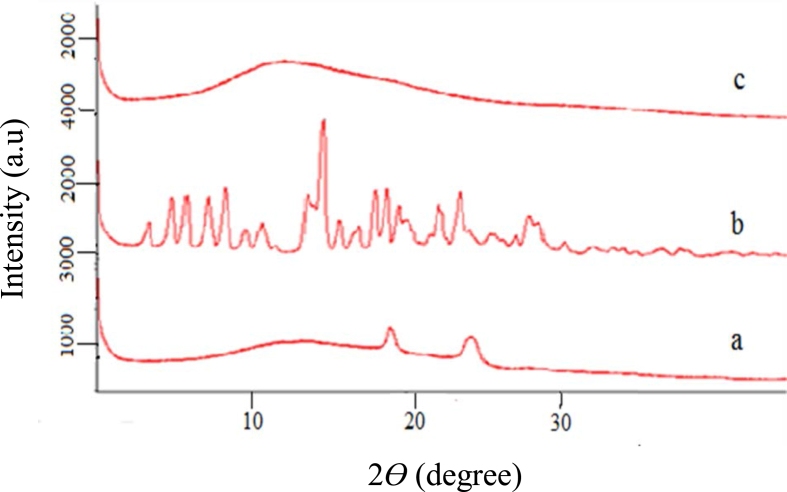
X-ray diffractogram pattern of the LPV, Eudragit RSPO, Eudragit RSPO-LPV nanoparticles is shown in Figure 6. LPV has shown several diffraction crystal peaks at 2θ 7 to 29.5o, indicating crystallinity (Khan et al., 2019) while the Eudragit RSPO-LPV nanoparticles showed a broad, amorphous peak intensifying that there were molecular miscibility and interaction between the components. This confirmed that the drug was molecularly encapsulated in the polymer, and converted from the crystalline form into the amorphous peaks on encapsulating (Khan et al., 2019). Hence, it is assumed that the poor solubility of LPV must be addressed by preparing amorphous forms of the drug and it is confirmed that the high internal energy and specific volume of the amorphous state have already been reported to enhance dissolution, solubility, and bioavailability (Hancock and Parks, 2000).
Figure 6.
XRD results where the y-axis represents counts per second and x-axis is theta degree; (a) Eudragit RSPO-LPV nanoparticles, (b) LPV, (c) Eudragit RSPO.
3.6. Thermogravimetric
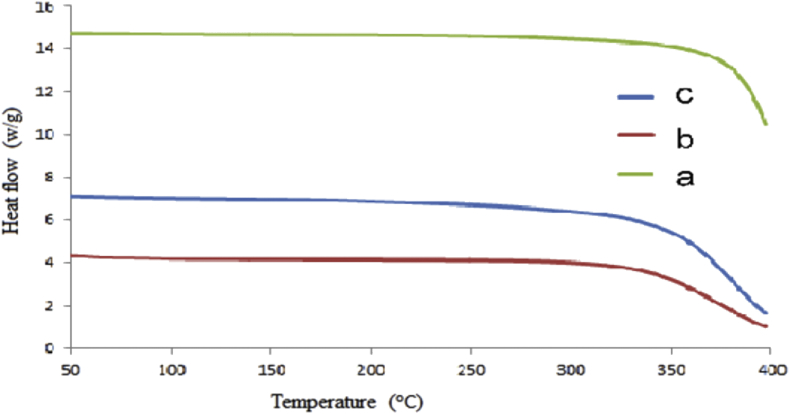
The thermal stability analysis in Figure 7 indicated that Eudragit RSPO-LPV nanoparticles showed better stability as compared to the polymer and the drug. The weight loss occurred between 314 and 300C for LPV, 321 and 400 °C for Eudragit RSPO while it is between 349 and 400 °C for the Eudragit RSPO-LPV nanoparticles. Any increment in the moisture contents of a polymer will result in melting temperature reduction (Cao and Bhoyro, 2001). The maximum weight loss occurs the temperature range of 314–400 °C and the results showed that the Eudragit RSPO-LPV nanoparticles were thermally stable (Lemmer and Liebenberg, 2013).
Figure 7.
TGA curves where the y-axis represents heat flow (w/g) and the x-axis is the temperature (oC); (a) Eudragit RSPO-LPV nanoparticles, (b) LPV, (c) Eudragit RSPO.
3.7. Differential scanning calorimetry
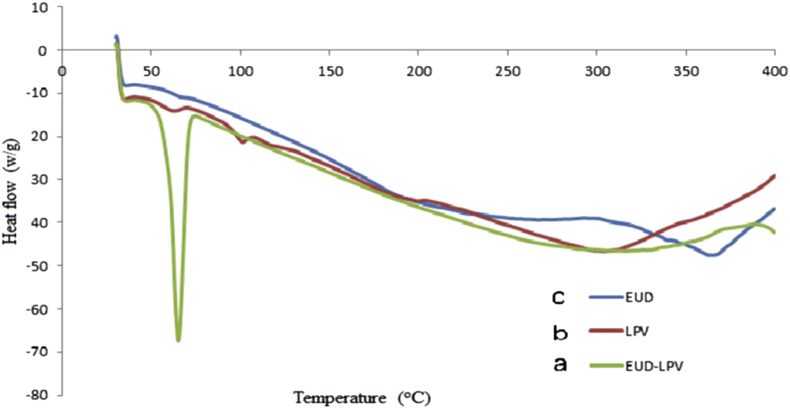
Compatibility studies to investigate the crystallinity in Figure 8 shows endothermic peaks at 66.3C and 64.5C for Eudragit RSPO and Eudragit RSPO-LPV nanoparticles, respectively. LPV has two peaks at 56.7C and 96.8C, which suggest its crystallinity nature (Khan et al., 2019). The product crystallinity depends on temperature and melting point; any reduction in these leads to a decrease in crystallinity and amorphous formation, resulting in increased solubility. There were no significant differences in the individual endotherm and respective endotherm observed in the Eudragit RSPO-LPV loaded in suppositories, which indicates that there was no interaction between excipient and the drug.
Figure 8.
DSC thermograms where y-axis represents heat flow (w/g) and x-axis is the temperature (oC); (a) Eudragit RSPO-LPV nanoparticles, (b) LPV, (c) Eudragit RSPO.
3.8. Stability studies
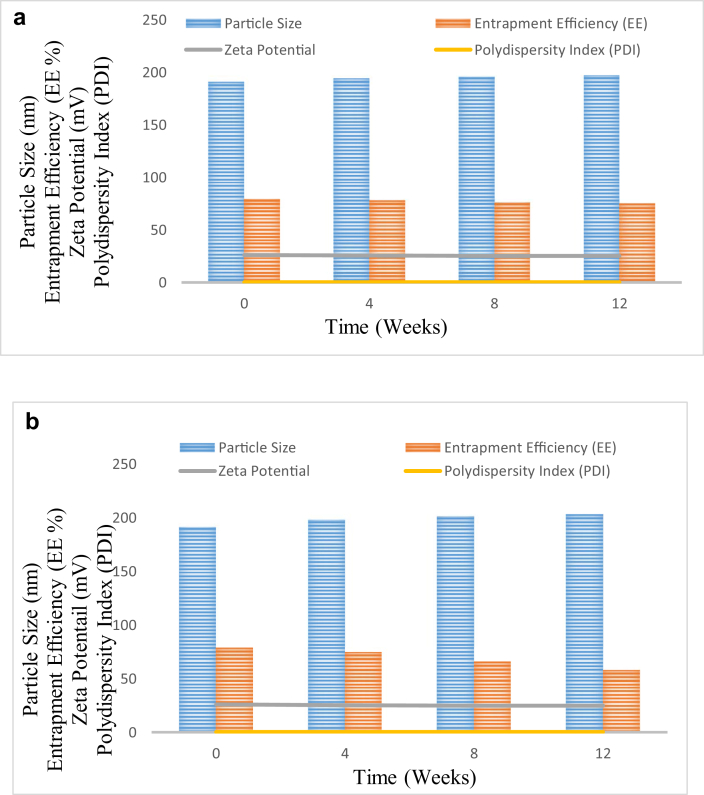
Stability studies for Eudragit RSPO-LPV nanoparticles were done based on particle size, EE, zeta potential and PDI variations for three months. The results in Table 2a and Table 2b and Figure 9 showed that there was no significant (p < 0.05) change in assessed parameters when samples are stored at 5 °C. Similarly, Eudragit RSPO-LPV nanoparticles stored at 25 °C showed no significant change in particle size and zeta potential. However, in these samples, statistically significant (p < 0.05) reduction in EE was observed. The EE of Eudragit RSPO-LPV nanoparticles at the end of 3 months was 73.4% of the initial formulation. Hence, storage under refrigerated condition is recommended for Eudragit RSPO-LPV nanoparticles.
Table 2a.
Stability results of the Eudragit RSPO- LPV NPs suppositories at 5 °C.
| Parameters/weeks | 0 | 4 | 8 | 12 |
|---|---|---|---|---|
| Particle size (nm) | 190.9 | 194 | 196 | 197 |
| EE (%) | 79.0 | 78 | 76 | 75 |
| Zeta potential (mv) | 26.0 | 25.6 | 25 | 25 |
| PDI | 0.224 | 0.203 | 0.215 | 0.241 |
Table 2b.
Stability results of Eudragit RSPO- LPV NPs suppositories at 25 °C.
| Parameters/weeks | 0 | 1 | 8 | 12 |
|---|---|---|---|---|
| Particle size (nm) | 190.9 | 198 | 201 | 203 |
| EE (%) | 79.0 | 75 | 66 | 58 |
| Zeta potential (mV) | 26.0 | 25.3 | 25 | 25 |
| PDI | 0.224 | 0.212 | 0.220 | 0.246 |
Figure 9.
a. Stability results of Eudragit RSPO- LPV NPs in terms of mean particle size, entrapment efficiency (EE), zeta potential and polydispersity index (PDI) stored at 5 °C for 12 weeks. b. Stability results of the Eudragit RSPO- LPV NPs in terms of mean particle size, entrapment efficiency (EE), zeta potential and polydispersity index (PDI) stored at 25 °C and 60% ± 5% RH for 12 weeks.
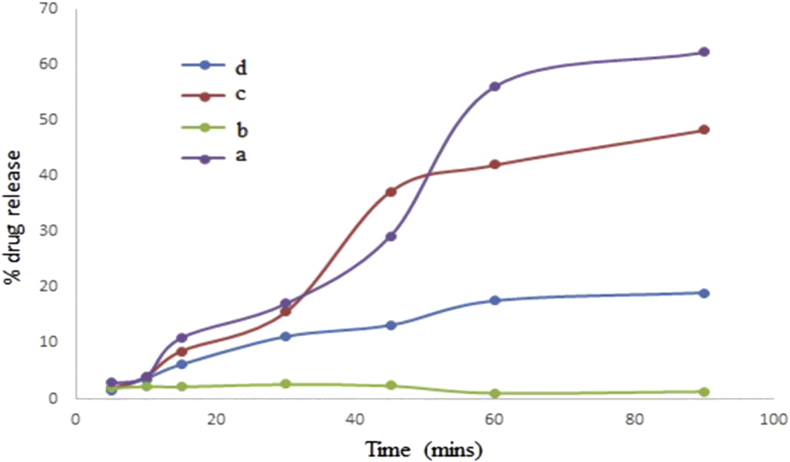
3.9. In-vitro drug release studies
The release mechanism of the suppositories depends on its rate of dissolution (Figure 10). The in vitro drug release was performed to investigate the suppository's ability to release the drug Eudragit RSPO-LPV nanoparticles in two different bases. The results show suppositories prepared with a PEG mixture having a prominent higher release of almost 72 % increase between 30-90 min. The Eudragit RSPO-LPV nanoparticles show 68 % release, and the pure drug had a release of 41 % with the fattibase release declining. The release was as a result of the difference in the two bases, one was oleaginous, and the other was water soluble. Meanwhile, the drug is hydrophobic which resulted in a higher release in the water soluble base, but the oil base withheld the release since drugs with high affinity for the base will not be quickly released into the rectal fluid for absorption. It could also be attributed to the rapid softening and solubilizing properties of the hydrophilic base. This confirmed that hydrophobic drug would exhibit a higher affinity for the lipophilic base while the fattibase would entrap the drug and hinder the migration of the drug out into the rectum for absorption (Abass et al., 2012b). In addition, the drug released depend on the diffusion rate of the drug from the matrix of the Eudragit RSPO polymer reported by Vandenberg et al., 2000).
Figure 10.
The release studies profile where the y-axis represents percentage drug release and the x-axis is time (minutes); (a) Fattibase suppositories (b) LPV, (c) Eudragit RSPO-LPV nanoparticles, (d) PEG suppositories.
4. Conclusion
Eudragit RSPO-LPV nanoparticles were successfully prepared with nanoprecipitation and loaded into suppositories by the fusion method. The results obtained when characterised indicated that the drug was indeed encapsulated and could be a potential carrier for controlled drug delivery. This result is supported by the preliminary in vitro release study of suppositories using two different bases. Suppositories formulated in PEG bases gave better release properties than those in the fattibase, an indication of a good released which can be investigated further as a better alternative route of drug administration for children paediatric HIV treatment.
Declarations
Author contribution statement
L.M. Katata-Seru, B.M. Ojo, O. Okubanjo, R. Soremekun and O.S. Aremu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Sasol Foundation, South Africa and National Research Foundation (NRF Thuthuka UID 87987, NRF UID:106379) in South Africa.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to send our special gratitude to the Chemistry Department and School of Physical and Chemical Sciences at North-West University (Mafikeng Campus) for providing the research work facility.
References
- Abass H., Kamel R., Abdelbary A. Metronidazole bioadhesive vaginal suppositories: formulation, in vitro and in vivo evaluation. Int. J. Pharm. Pharmaceut. Sci. 2012;4:344–355. [Google Scholar]
- Abou-El-Naga I.F., El Kerdany E.D., Mady R.F., Shalaby T.I., Zaytoun E.M. The effect of lopinavir/ritonavir and lopinavir/ritonavir loaded PLGA nanoparticles on experimental toxoplasmosis. Parasitol. Int. 2017;66(6):735–747. doi: 10.1016/j.parint.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Akin-Ajani O.D., Odeku O.A., Babalola Y. Formulation of paediatric paracetamol suppositories using shea butter and dika fat as suppository bases. Trop. J. Nat. Prod. Res. February 2019;3(2):31–36. [Google Scholar]
- Cao J., Bhoyro A.Y. Structural characterization of wool by thermal mechanical analysis of yarns. Tex. Res. J. 2001;71:63–66. [Google Scholar]
- Das Neves J., Amiji M., Bahia M.F. Assessing the physical–chemical properties and stability of dapivirine-loaded polymeric nanoparticles. Int. J. Pharm. 2013;456:307–314. doi: 10.1016/j.ijpharm.2013.08.049. [DOI] [PubMed] [Google Scholar]
- DNDi Ending the Neglect of Paediatric HIV. DNDi; 2018. https://www.dndi.org/wp-content/uploads/2018/08/DNDi_Paediatric-HIV_2018.pdf Available at: [Google Scholar]
- Esmaeili F., Atyabi F., Dinarvand R. Preparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion method. DARU J. Pharm. Sci. 2015;16:196–202. [Google Scholar]
- Ham A.S., Robert W., Buckheit R.W., Jr. Designing and developing suppository formulations for anti-HIV drug delivery. Ther. Deliv. 2017;8(9):805–817. doi: 10.4155/tde-2017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock B.C., Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000;17:397–404. doi: 10.1023/a:1007516718048. [DOI] [PubMed] [Google Scholar]
- Hans M., Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Cur.Opin. Sol. St. Mat. Sc. 2002;6:319–327. [Google Scholar]
- ICH Harmonized Tripartite Guideline . 2003. Stability Testing of New Drug Substances and Drug Products, Q1A(R2), Geneva, Switzerland. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2017. Ending AIDS—Progress towards the 90-90-90 Targets.https://reliefweb.int/report/world/ending-aids-progress-towards-90-90-90-targets [Google Scholar]
- Joshi G., Kumar A., Sawant K. Bioavailability enhancement, Caco-2 cells uptake and intestinal transport of orally administered lopinavir-loaded PLGA nanoparticles. Drug Deliv. 2016;23:3492–3504. doi: 10.1080/10717544.2016.1199605. [DOI] [PubMed] [Google Scholar]
- Khan A.,A., Mudassir J., Akhtar S., Murugaiyah V., Darwis Y. Freeze-dried lopinavir-loaded nanostructured lipid carriers for enhanced cellular uptake and bioavailability: statistical optimization, in vitro and in vivo evaluations. Pharmaceutics. 2019 Feb 25;11(2):E97. doi: 10.3390/pharmaceutics11020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., Yadav S.K., Pakade Y.B. Development of biodegradable nanoparticles for delivery of quercetin. Col. Sur. B: Bio. 2010;80:184–192. doi: 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Lallemant M., Chang S., Cohen R. Pediatric HIV—a neglected disease? N. Engl. J. Med. 2011;365:581–583. doi: 10.1056/NEJMp1107275. [DOI] [PubMed] [Google Scholar]
- Lemmer H., Liebenberg W. Preparation and evaluation of metastable solid-state forms of lopinavir. Die Pharmazie Int. J. Pharm. Sci. 2013;68:327–332. [PubMed] [Google Scholar]
- Lepeltier E., Bourgaux C., Couvreur P. Nanoprecipitation and the “Ouzo effect”: application to drug delivery devices. Adv. Drug Deliv. Rev. 2014;71:86–97. doi: 10.1016/j.addr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Lloyd-Sherlock P., Ebrahim S., Grosskurth H. Oxford University Press; 2014. Is Hypertension the New HIV Epidemic? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maartens G., Celum C., Lewin S.R. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- Mandal B. University of Toledo; 2010. Preparation and Physicochemical Characterization of Eudragit® RL100 Nanosuspension with Potential for Ocular Delivery of Sulfacetamide. Master of Science in Pharmaceutical Sciences. [PubMed] [Google Scholar]
- Matlhola K., Katata-Seru L., Tshweu L. Formulation and optimization of Eudragit RS PO-tenofovir nanocarriers using Box-Behnken experimental design. J. Nanomater. 2015:1–11. [Google Scholar]
- Nagavarma B., Yadav H.K., Ayaz A. Different techniques for preparation of polymeric nanoparticles-a review. Asian J. Pharmaceut. Clin. Res. 2012;5:16–23. [Google Scholar]
- Newell M.-L., Coovadia H., Cortina-Borja M. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- Padmavathi Y., Reddy B., Renuka M. Formulation and evaluation of fixed dose combination suppositories containing stavudine, lamivudine and nevirapine for pediatric applications. J. Sci. Res. 2015;7:87–96. [Google Scholar]
- Patel G., Shelat P., Lalwani A. Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment. Drug Deliv. 2016;23:3027–3042. doi: 10.3109/10717544.2016.1141260. [DOI] [PubMed] [Google Scholar]
- Pham K., Li D., Guo S., Penzak S., Dong X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Contr. Release. 2016;226:88–97. doi: 10.1016/j.jconrel.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Priya N.S., Reddy C.S., Sai C.G. Formulation development and release studies of zidovudine suppositories. J. Drug Deliv. Therapeut. 2015;5:72–75. [Google Scholar]
- Ramadan A.A. Formulation and evaluation of bioadhesive vaginal suppositories containing miconazole nitrate. Int. J. Pharm. Biol. Sci. 2013;4:455–472. [Google Scholar]
- Ravi P.R., Vats R., Dalal V. A hybrid design to optimize preparation of lopinavir loaded solid lipid nanoparticles and comparative pharmacokinetic evaluation with marketed lopinavir/ritonavir coformulation. J. Pharm. Pharmacol. 2014;66:912–926. doi: 10.1111/jphp.12217. [DOI] [PubMed] [Google Scholar]
- Ravi P.R., Vats R., Dalal V. Design, optimization and evaluation of poly-ϵ-caprolactone (PCL) based polymeric nanoparticles for oral delivery of lopinavir. Drug Dev. Ind. Pharm. 2015;41:131–140. doi: 10.3109/03639045.2013.850710. [DOI] [PubMed] [Google Scholar]
- Salatin S., Barar J., Barzegar-Jalali M., Adibkia K., Kiafar F., Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res. Pharm. Sci. 2017;12(1):1–14. doi: 10.4103/1735-5362.199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salatin S., Barar J., Barzegar-Jalali Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res. Pharm. Sci. 2017;12:1–14. doi: 10.4103/1735-5362.199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento B., Das Neves J. Nanosystem formulations for rectal microbicides: a call for more research. Ther. Deliv. 2012;3:1–4. doi: 10.4155/tde.11.139. [DOI] [PubMed] [Google Scholar]
- Schlatter A.F., Deathe A.R., Vreeman R.C. The need for pediatric formulations to treat children with HIV. AIDS Res. Treat. 2016;8 doi: 10.1155/2016/1654938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Zhao Y., Wu W. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int. J. Pharm. 2008;350:320–329. doi: 10.1016/j.ijpharm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- UNAIDS . 2018. Face Sheet: Latest Statistics on the Status of the AIDS Epidemic.http://www.unaids.org/en/resources/fact-sheet Available at. [Google Scholar]
- Van Riet-Nales D.A., Wang S., Saint-Raymond A. The EMA quality guideline on the pharmaceutical development of medicines for paediatric use. Int. J. Pharm. 2012;2:132–134. doi: 10.1016/j.ijpharm.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Vandenberg C.M., Kazmi Y., Stewart J. Pharmacokinetics of three formulations of ondansetron hydrochloride in healthy volunteers: 24-mg oral tablet, rectal suppository, and iv infusion. Am. J. Health Syst. Pharm. 2000;57:1046–1050. doi: 10.1093/ajhp/57.11.1046. [DOI] [PubMed] [Google Scholar]
- Varshney H., Tanwar Y. Formulation, physicochemical characterisations and in vitro evaluation of flurbiprofen. J. Pharm. Res. 2010;3:561–565. [Google Scholar]
- Wang P., Keller A.A. Natural and engineered nano and colloidal transport: role of zeta potential in prediction of particle deposition. Langmuir. 2009;25:6856–6862. doi: 10.1021/la900134f. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xia Q., Li Y. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: a new strategy for clustering drug in inflammatory skin. Theranostics. 2019;9:48–64. doi: 10.7150/thno.29715. [DOI] [PMC free article] [PubMed] [Google Scholar]