Abstract
Hydrogen sulfide (H2S) is considered as a novel second-messenger molecule associated with the modulation of various physiological and pathological processes. In the field of antitumor research, endogenous H2S induces angiogenesis, accelerates the cell cycle and inhibits apoptosis, which results in promoting oncogenesis eventually. Interestingly, high concentrations of exogenous H2S liberated from donors suppress the growth of various tumors via inducing cellular acidification and modulating several signaling pathways involved in cell cycle regulation, proliferation, apoptosis and metastasis. The selective release of certain concentrations of H2S from H2S donors in the target has been considered as an alternative tumor therapy strategy. Triple-negative breast cancer (TNBC), an aggressive subtype with less than one year median survival time, is known to account for approximately 15–20% of all breast cancers. Due to the lack of approved targeted therapy, the clinical treatment of TNBC is still hindered by metastasis as well as recurrence. Significant efforts have been spent on developing novel treatments of TNBC, and remarkable progress in the control of TNBC by H2S donors and their derivatives have been made in recent years. This review summarizes various pathways involved in antitumor and anti-metastasis effects of H2S donors and their derivatives on TNBC, which provides novel insights for TNBC treatment.
Keywords: Triple-negative breast cancer, Hydrogen sulfide, Antitumor effect
1. Introduction
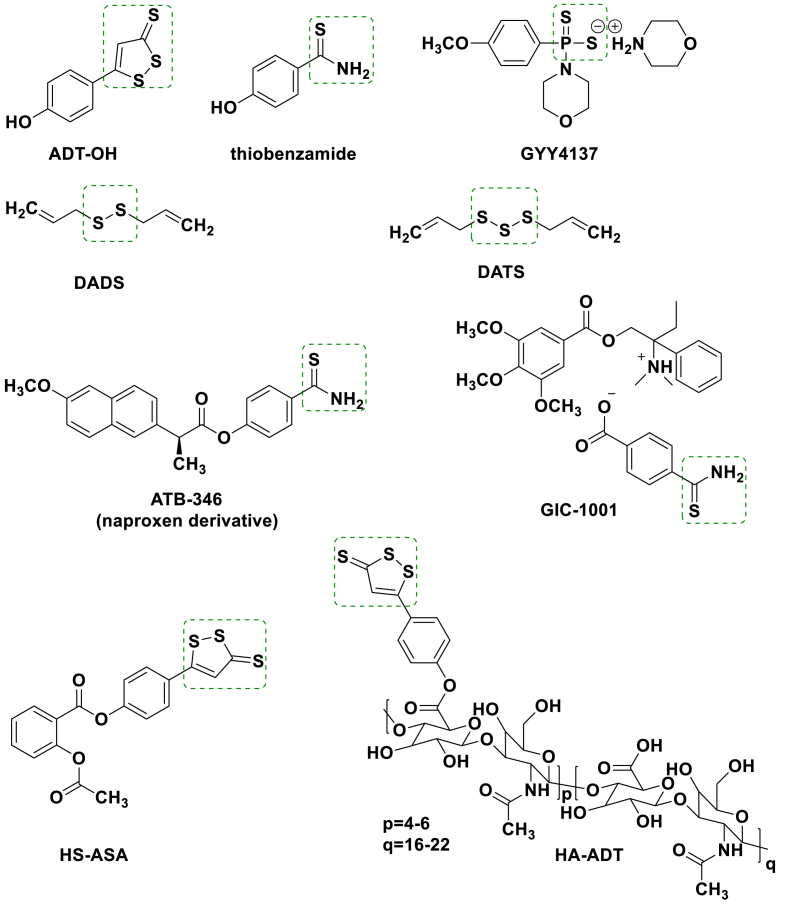
H2S has been discovered as a novel endogenous signaling gaseous transmitter along with nitric oxide (NO) and carbon monoxide (CO) [1,2] since Abe and Kimura revealed the endogenous production and intracellular signaling cascades of H2S [3]. In general, taking l-cysteine as a substrate, endogenous H2S liberation is mainly attributed to cystathionine-β-synthase (CBS), cystathionine-β-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) (Fig. 1) [4]. The liberated H2S participates in modulating physiological and pathological processes, including intestinal disorders [5], cardiovascular diseases [[6], [7], [8], [9]], diseases caused by oxidative stress [[10], [11], [12]] and inflammation [13,14]. Sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) were generally used as the H2S donors in early studies. Although sulphide inorganic salts exhibited cytotoxic effects on several tumor cells and therapeutic potential on the cardiovascular system [[15], [16], [17]], rapid oxidation of NaHS and Na2S solution and uncontrolled release of H2S lead to the discrepancies in curative effect [18]. Recently, some organic H2S donors (ADT-OH, thiobenzamide, GYY4137, DADS and DATS, Fig. 2) are used for endogenous H2S production in a sustained fashion [[19], [20], [21], [22]]. Several H2S donor-based therapeutics have entered Phase II clinical trials, such as ATB-346 and GIC-1001 (Fig. 2) [23,24]. Besides cardiovascular diseases and chronic diseases [[25], [26], [27], [28], [29], [30], [31], [32]], H2S donors show significantly different roles in neoplasia compared with endogenous H2S. Endogenous H2S and low levels of exogenous H2S induce angiogenesis, accelerate cell cycle, inhibit apoptosis, and promote oncogenesis eventually [33,34], whereas H2S donors trigger high concentrations of exogenous H2S production to prevent tumor development. The donors selectively inhibit progression of tumors by inducing intracellular acidification and inhibiting proliferation and metastasis of tumor cells through EGFR/ERK/MMP-2, PTEN/Akt, PI3K/Akt/mTOR and NF-κB pathways with no obvious adverse effects on animal health [[35], [36], [37], [38], [39], [40], [41], [42]]. Thus, H2S donors are instrumental for developing novel antitumor therapies with less side effects [43].
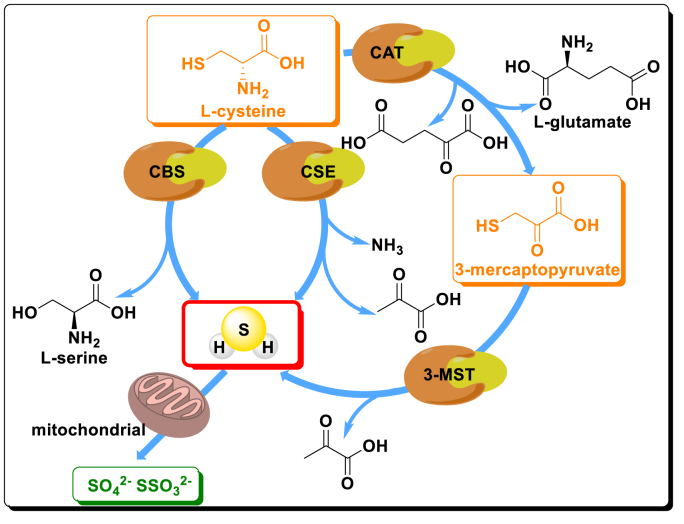
Fig. 1.
Main mechanisms of endogenous H2S production in mammalian cells. Endogenous H2S can be produced mainly through three pathways. Among which, l-cysteine directly generates H2S through the catalysis of CBS or CSE. H2S is also biosynthesized by the synergistic effects of CAT and 3-MST. Ultimately, H2S is metabolized in mitochondria in the form of thiosulfate or sulfate.
Fig. 2.
Structural components of H2S donors and H2S generating drug candidates. Potential antitumor mechanism of H2S donors against TNBC.
Rising mortality highly features cancer as a global health problem [44]. In terms of women's health, breast cancer has been prescribed as the most common cause of cancer death. Among them, around 15%–20% are diagnosed as TNBC, an aggressive subtype which is implicated in the lack of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) expression [45,46]. Patients can hardly ameliorate symptoms through endocrine therapy and no other treatments for TNBC have established except adjuvant chemotherapy or radiotherapy after surgery [47]. The chemotherapeutic drugs for advanced TNBC are mainly taxanes or anthracyclines. Although chemotherapy prolongs the survival of patients, the effects are relatively limited due to the poor prognosis, and high-intensity chemotherapy will impair the living quality of patients [[48], [49], [50]]. In contrast to other breast cancers, TNBC is frequently accompanied by the recurrence and higher probability of metastasis to the central nervous system as well as lungs [51,52]. The aggressive metastatic behaviors lead to the limited median survival which is less than one year for TNBC patients. Even if TNBC has been deeply studied in the past several decades and many targeted therapeutic agents including poly(ADP-ribose) polymerase (PARP) inhibitors [53], vascular endothelial growth factor (VEGF) inhibitors [54], epidermal growth factor receptor (EGFR) inhibitors [55] and tyrosine kinases (TKs) inhibitors [56] have been tried, none of them have completed all clinical trials. Elevated mortality raises the urgency of novel agents against TNBC, especially metastatic TNBC.
The progression of ER-negative breast cancer cells is related to the constitutively activated NF-κB [[57], [58], [59]]. Matrix metalloproteinases (MMPs), the key causes that account for the invasion and metastasis of tumor cells, could be regulated by the NF-κB signaling pathway and are over-expressed during breast cancer cells growth [60,61]. H2S donors have been shown to inhibit NF-κB activation among other tumor cells [35]. They might have potential effects on metastatic tumors.
In terms of TNBC, H2S donors exhibited potent therapeutic potential. As early as 2011, Chattopadhyay et al. verified the antitumor effect of HS-ASA (a novel H2S donating aspirin, Fig. 2) on TNBC MDA-MB-231 cells in vitro and in vivo. HS-ASA was shown to inhibit proliferation accompanied by cell cycle arrest at G0/G1 phase in a dose-dependent manner. It markedly restrained the translocation of NF-κB (p65) into the nucleus via inhibiting the phosphorylation status of IKKα and IKKβ to decrease dissociation between NF-κB and IκB. Furthermore, after HS-ASA treatment, thioredoxin reductase-1 (TrxR) inhibition induced reactive oxygen species (ROS) accumulation also disrupted redox homeostasis in MDA-MB-231 cells. The subsequent decline of tumor mass and volume in mouse xenograft model indicated potent antitumor effects of HS-ASA on TNBC through inhibiting NF-κB pathway and TrxR activity, and elevating ROS levels [62].
Besides eliminating tumors, Liu et al. demonstrated that DATS (an extract of garlic) resulted in sharp attenuation of MDA-MB-231 cell migration, and metastasis phenotype inhibition was observed in zebrafish xenograft model. After the treatment with DATS, MDA-MB-231 cells with smooth surfaces and decreasing pseudopodia were observed, which indicated cell migration inhibitory effect. In addition, by blocking the NF-κB pathway, DATS significantly inhibited the mRNA levels, protein expression, and enzyme activity of MMP-2/9, the proteinases over-expressed during invasion, oncogenesis and metastasis, to degrade and reshape the dynamic balance of extracellular matrix. After DATS treatment, phosphorylation of ERK level was declined in a dose-dependent manner, which proved that the ERK/MAPK pathway was also associated with TNBC [63]. It has become a consensus that breast cancer stem cells play an important role in tumor proliferation and metastasis. As an important transcription regulator, the expression of FoxQ1 is related to the growth and metastasis of breast cancer. The down-regulation of FoxQ1 expression in DATS-treated SUM159 TNBC cells was discovered. As one of the markers of tumor stem cells, ALDH1 activity was completely inhibited after FoxQ1 knockdown and was further inhibited in SUM159 xenograft model. These studies showed that FoxQ1 might be a new target for TNBC treatment by DATS [64].
As a volatile extract of garlic oil, DADS also endowed with potent inhibitory effects on TNBC. Like DATS, Huang et al. confirmed the inhibitory effect of DADS on migration and invasion through wound-healing, transwell migration and invasion assays. The expression and activity of MMP-9 were significantly declined after the incubation with DADS. Besides, the potent antimetastatic effect of DADS was also revealed based on the observed reversal of the epithelial-mesenchymal transition (EMT). As an AU-rich RNA-binding protein, tristetraprolin (TTP) involves in the degradation of urokinase type plasminogen activator (uPA), an upstream gene of MMP-9, and inhibits tumor metastasis by mediating the expression of MMP-9. As reported by Xiong et al., decline of uPA and upregulation of TTP were detected after DADS treatment, which could be completely counteracted by TTP siRNA. Therefore, targeting TTP might be capable of exerting inhibitory effect on TNBC metastasis. By ameliorating aberrant activation of the β-catenin pathway, DADS improved the activity of caspase-3/9 and the expression of pro-apoptotic factor Bax, while it down-regulated anti-apoptotic factor Bcl-2 levels in MDA-MB-231 and BT-549 TNBC cell lines. These findings highlight the pro-apoptotic effect of DADS on TNBC cells. Consistent with the aforesaid in vitro results, the volume and weight of DADS-treated MDA-MB-231 xenograft tumors in mice were obviously declined [65,66].
ADT-OH, a commonly used H2S donor that exhibited prominent cytoprotective properties [67], was conjugated with hyaluronic acid (HA) to improve the retention time and water solubility. Similarly, Dong et al. found that HA-ADT (Fig. 2) induced MDA-MB-231 cells apoptosis by elevating the ratio of Bad/Bcl-xl and Bax/Bcl-2 as well as the expression of cleaved caspase-3/9 and cleaved PARP. Further preliminary mechanism studies found PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways were suppressed in HA-ADT group compared with the control (PBS), NaHS and GYY4137 groups. The markedly shrunken xenograft tumors were also found in HA-ADT-treated nude mice, accompanied with reduced CD31 expression that demonstrated angiogenesis inhibition [68].
In summary, on the one hand, H2S donors and their derivatives effectively modulated the proliferation and apoptosis of TNBC cells by inhibiting the phosphorylation or expression of proteins associated with NF-κB, PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways. On the other hand, they also inhibited the expression of MMP-2/9 and EMT to resist TNBC metastasis by inhibiting aberrant activation of the β-catenin pathway. Other pathways that H2S may be involved in TNBC prevention deserve further investigation. The mechanism of H2S donors and their derivatives against TNBC is summarized in Fig. 3. As for sulfide salts, although NaHS administration alone exhibits little inhibitory effect on tumor growth in mice, it decreases O2 consumption and increases O2 delivery to alleviate tumor hypoxia, eventually radiosensitized MDA-MB-231 tumors. The results provided insights for H2S donors in combination therapy [69].
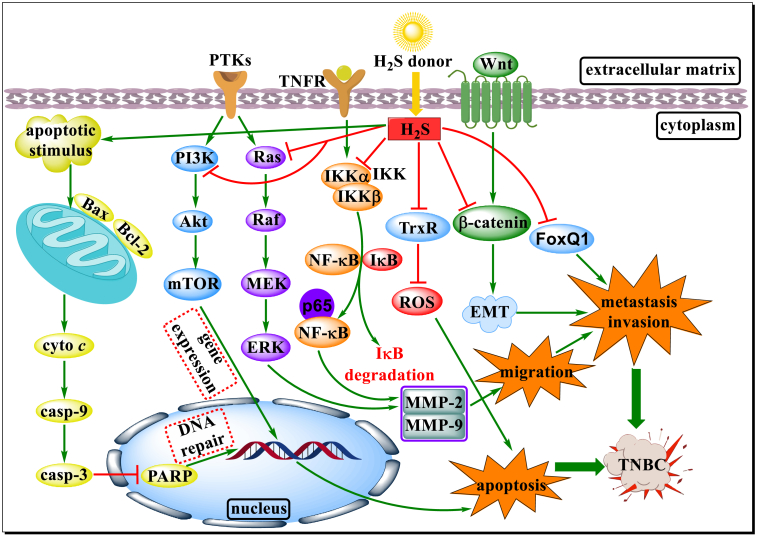
Fig. 3.
Schematic diagram of partial action pathways of H2S donors and their derivatives on TNBC. H2S donors and their derivatives participate in the regulation of multiple pathways to induce apoptosis and block the invasion, proliferation and metastasis of TNBC cells by triggering the release of H2S in response to specific stimuli. They not only promote TNBC cells apoptosis by activating mitochondrial apoptosis pathway and inhibiting phosphorylation or expression of related proteins involved in NF-κB, PI3K/Akt/mTOR, Ras/Raf/MEK/ERK signaling pathways, but also ameliorate aberrant activation of the β-catenin pathway, followed by MMP-2/9 activity inhibition and EMT reversal.
2. Conclusions and perspectives
Since the current therapies for TNBC are mainly adjuvant chemotherapy or radiotherapy after surgery, patients can hardly bear side effects of long-term chemotherapy or radiotherapy, and they still face the risks of metastasis and relapse due to the lack of targeted drug molecules. Recently, H2S donors and their derivatives have made attractive development in TNBC therapy. Although H2S donors liberate high concentrations of H2S, there is no research on actual impact of the H2S itself in TNBC treatment, and it is unclear whether the remaining structural fragments after H2S generation are related to the potent antitumor activity. The precise antitumor mechanism of H2S deserves further study. Moreover, it is worth noting that the regulation mechanism of the expression and activity of CBS, CSE and 3-MST is remained ambiguous and argued. Given the evidence that inhibition of CBS induces apoptosis in several tumor cells (colon cancer, ovarian cancer, breast cancer, etc.), perhaps inhibition of H2S biosynthesis through targeted inhibition of H2S-producing enzymes exhibits comparable antitumor effects to H2S donors. At present, investigations also elaborated on the connection between H2S and NO which participated in modulating multiple physiological and pathological processes [70,71], such second messenger molecules provide novel insights for TNBC treatment. In light of the bell-shaped model effect of H2S has been verified and widely accepted, the selective release of certain concentrations of H2S from H2S donors in the target to minimize the adverse effects of H2S is essential. To cope with the special tumor microenvironment, H2S donors that sensitive to definite pH, enzymes, NIR light or free radicals are being studied [[72], [73], [74], [75]]. Nanosized delivery systems have also been tried for targeted delivery of exogenous H2S and hope to endow it with improved antitumor effect through intratumoral conversion of nanostructures to microstructures [76,77]. These attempts may help researchers to study the precise mechanisms of H2S in the therapy of cancers, especially TNBC. We hope that H2S donating compounds can greatly improve the survival rate of TNBC patients in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This paper was financially supported by Fok Ying Tung Education Foundation (171035), General Scientific Research Projects of Department of Education in Liaoning Province (2017LQN05), High-level Innovative Project in Shenyang (Young and Middle-aged Technological Innovative Support Plan, RC190483) and Career Development Support Plan in Shenyang Pharmaceutical University.
References
- 1.Kolluru G.K., Shen X., Yuan S., Kevil C.G. Gasotransmitter heterocellular signaling. Antioxidants Redox Signal. 2017;26:936–960. doi: 10.1089/ars.2016.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 3.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z.J., Ng M.Y., Lee Z.W., Dai W., Hagen T., Moore P.K., Huang D., Deng L.W., Tan C.H. Hydrogen sulfide donors in research and drug development. Med. Chem. Commun. 2014;5:557–570. [Google Scholar]
- 5.Distrutti E., Sediari L., Mencarelli A., Renga B., Orlandi S., Antonelli E., Roviezzo F., Morelli A., Cirino G., Wallace J.L., Fiorucci S. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Therapeut. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 6.Mani S., Untereiner A., Wu L., Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis, Antioxid. Redox Signal. 2014;20:805–817. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 7.Wu D.D., Wang J., Li H., Xue M.Z., Ji A.L., Li Y.Z. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2015:1–16. doi: 10.1155/2015/186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng B., Yang J., Qi Y., Zhao J., Pang Y., Du J., Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 9.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carballal S., Trujillo M., Cuevasanta E., Bartesaghi S., Moller M.N., Folkes L.K., Garcia-Bereguiain M.A., Gutierrez-Merino C., Wardman P., Denicola A., Radi R., Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radical Biol. Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 12.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M. Hydrogen sulfide and substance P in inflammation, Antioxid. Redox Signal. 2010;12:1191–1202. doi: 10.1089/ars.2009.2927. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X.Y., Liu S.J., Liu Y.J., Wang S., Ni X. Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell. Mol. Life Sci. 2010;67:1119–1132. doi: 10.1007/s00018-009-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D., Li J., Zhang Q., Tian W., Zhong P., Liu Z., Wang H., Wang H., Ji A., Li Y. Exogenous hydrogen sulfide regulates the growth of human thyroid carcinoma cells. Oxid. Med. Cell. Longev. 2019:6927298. doi: 10.1155/2019/6927298. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao A.Y., Maynard M.R., Piett C.G., Nagel Z.D., Alexander J.S., Kevil C.G., Berridge M.V., Pattillo C.B., Rosen L.R., Miriyala S., Harrison L. Sodium sulfide selectively induces oxidative stress, DNA damage, and mitochondrial dysfunction and radiosensitizes glioblastoma (GBM) cells. Redox Biol. 2019;26:101220. doi: 10.1016/j.redox.2019.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T.S., Zhao B., Wang C., Wang H.Y., Liu Z.W., Li W., Jin H.F., Tang C.S., Du J.B. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp. Biol. Med. 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Wang H., Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.H., Moore P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 20.Busquet M., Calsamiglia S., Ferret A., Carro M.D., Kamel C. Effect of garlic oil and four of its compounds on rumen microbial fermentation. J. Dairy Sci. 2005;88:4393–4404. doi: 10.3168/jds.S0022-0302(05)73126-X. [DOI] [PubMed] [Google Scholar]
- 21.Lai K.C., Hsu S.C., Kuo C.L., Yang J.S., Ma C.Y., Lu H.F., Tang N.Y., Hsia T.C., Ho H.C., Chung J.G. Diallyl sulfide, diallyl disulfide, and diallyl trisulfide inhibit migration and invasion in human colon cancer colo 205 cells through the inhibition of matrix metalloproteinase-2, -7, and -9 expressions. Environ. Toxicol. 2013;28:479–488. doi: 10.1002/tox.20737. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa U., van der Vlies A.J. Design and synthesis of polymeric hydrogen sulfide donors. Bioconjugate Chem. 2014;25:1290–1300. doi: 10.1021/bc500150s. [DOI] [PubMed] [Google Scholar]
- 23.Wallace J.L., Caliendo G., Santagada V., Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br. J. Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cenac N., Castro M., Desormeaux C., Colin P., Sie M., Ranger M., Vergnolle N. A novel orally administered trimebutine compound (GIC-1001) is anti-nociceptive and features peripheral opioid agonistic activity and hydrogen sulphide-releasing capacity in mice. Eur. J. Pain. 2016;20:723–730. doi: 10.1002/ejp.798. [DOI] [PubMed] [Google Scholar]
- 25.Nagpure B.V., Bian J.S. Brain, learning, and memory: role of H2S in neurodegenerative diseases. Handb. Exp. Pharmacol. 2015;230:193–215. doi: 10.1007/978-3-319-18144-8_10. [DOI] [PubMed] [Google Scholar]
- 26.Levinn C.M., Cerda M.M., Pluth M.D. Activatable small molecule H2S donors. Antioxidants Redox Signal. 2020;32:96–109. doi: 10.1089/ars.2019.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M., Sparatore A., Del Soldato P., McGeer E., McGeer P.L. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia. 2010;58:103–113. doi: 10.1002/glia.20905. [DOI] [PubMed] [Google Scholar]
- 28.Lee M., Tazzari V., Giustarini D., Rossi R., Sparatore A., Del Soldato P., McGeer E., McGeer P.L. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: potential for treating Parkinson disease. J. Biol. Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang S.C., Sohn E.H., Lee S.R. Hydrogen sulfide as a potential alternative for the treatment of myocardial fibrosis. Oxid. Med. Cell. Longev. 2020;2020:4105382. doi: 10.1155/2020/4105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo W., Cheng Z., Zhu Y. Hydrogen sulfide and translational medicine. Acta Pharm. Sin. 2013;34:1284–1291. doi: 10.1038/aps.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang N., Liu Y., Li T., Tuo Q. Role of hydrogen sulfide in chronic diseases. DNA Cell Biol. 2020;39:187–196. doi: 10.1089/dna.2019.5067. [DOI] [PubMed] [Google Scholar]
- 32.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D., Si W., Wang M., Lv S., Ji A., Li Y. Hydrogen sulfide in cancer: friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Kashfi K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxidants Redox Signal. 2014;20:831–846. doi: 10.1089/ars.2013.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reis A., Stern A., Monteiro H.P. S-nitrosothiols and H2S donors: potential chemo-therapeutic agents in cancer. Redox Biol. 2019;27:101190. doi: 10.1016/j.redox.2019.101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbari M., Sogutdelen E., Juriasingani S., Sener A. Hydrogen sulfide: emerging role in bladder, kidney, and prostate malignancies. Oxid. Med. Cell. Longev. 2019;2019:2360945. doi: 10.1155/2019/2360945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellmich M.R., Szabo C. Hydrogen sulfide and cancer. Handb. Exp. Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu D., Li M., Tian W., Wang S., Cui L., Li H., Wang H., Ji A., Li Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017;7:5134. doi: 10.1038/s41598-017-05457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S.S., Chen Y.H., Chen N., Wang L.J., Chen D.X., Weng H.L., Dooley S., Ding H.G. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017;8:e2688. doi: 10.1038/cddis.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Citi V., Piragine E., Pagnotta E., Ugolini L., Di Cesare Mannelli L., Testai L., Ghelardini C., Lazzeri L., Calderone V., Martelli A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1) Phytother Res. 2019;33:845–855. doi: 10.1002/ptr.6278. [DOI] [PubMed] [Google Scholar]
- 42.Gong Q.H., Wang Q., Pan L.L., Liu X.H., Xin H., Zhu Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-κB pathway in rats. Brain Behav. Immun. 2011;25:110–119. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Lee Z.W., Deng L.W. Role of H2S donors in cancer biology. Handb. Exp. Pharmacol. 2015;230:243–265. doi: 10.1007/978-3-319-18144-8_13. [DOI] [PubMed] [Google Scholar]
- 44.Boyle P. The globalisation of cancer. Lancet. 2006;368:629–630. doi: 10.1016/S0140-6736(06)69225-8. [DOI] [PubMed] [Google Scholar]
- 45.Kumar P., Aggarwal R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016;293:247–269. doi: 10.1007/s00404-015-3859-y. [DOI] [PubMed] [Google Scholar]
- 46.Denkert C., Liedtke C., Tutt A., Minckwitz G.V. Molecular alterations in triple-negative breast cancer–the road to new treatment strategies. Lancet. 2017;389:2430–2442. doi: 10.1016/S0140-6736(16)32454-0. [DOI] [PubMed] [Google Scholar]
- 47.Yao H., He G.C., Yan S.C., Chen C., Song L.J., Rosol T.J., Deng X.Y. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8:1913–1924. doi: 10.18632/oncotarget.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 49.Fadare O., Tavassoli F.A. Clinical and pathologic aspects of basal-like breast cancers. Nat. Clin. Pract. Oncol. 2008;5:149–159. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- 50.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Dieras V., Hegg R., Im S.A., Shaw Wright G., Henschel V., Molinero L., Chui S.Y., Funke R., Husain A., Winer E.P., Loi S., Emens L.A., Investigators I.M.T. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 51.Lin N.U., Claus E., Sohl J., Razzak A.R., Arnaout A., Winer E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sihto H., Lundin J., Lundin M., Lehtimaki T., Ristimaki A., Holli K., Sailas L., Kataja V., Turpeenniemi-Hujanen T., Isola J., Heikkilä P., Joensuu H. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13:R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Audeh M.W. Novel treatment strategies in triple-negative breast cancer: specific role of poly(adenosine diphosphate-ribose) polymerase inhibition. Pharmgeno. Pers. Med. 2014;7:307–316. doi: 10.2147/PGPM.S39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miles D.W., Dieras V., Cortes J., Duenne A.A., Yi J., O'Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 2013;24:2773–2780. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 55.Nabholtz J.M., Chalabi N., Radosevic‐Robin N., Dauplat M.M., Mouret‐Reynier M.A., Van Praagh I., Servent V., Jacquin J.P., Benmammar K.E., Kullab S., Bahadoor M.R.K., Kwiatkowski F., Cayre A., Abrial C., Durando X., Bignon Y.J., Chollet P., Penault‐Llorca F. Multicentric neoadjuvant pilot Phase II study of cetuximab combined with docetaxel in operable triple negative breast cancer. Int. J. Canc. 2016;138:2274–2280. doi: 10.1002/ijc.29952. [DOI] [PubMed] [Google Scholar]
- 56.Yadav B.S., Sharma S.C., Chanana P., Jhamb S. Systemic treatment strategies for triple-negative breast cancer. World J. Clin. Oncol. 2014;5:125–133. doi: 10.5306/wjco.v5.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sovak M.A., Bellas R.E., Kim D.W., Zanieski G.J., Rogers A.E., Traish A.M., Sonenshein G.E. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biswas D.K., Cruz A.P., Gansberger E., Pardee A.B. Epidermal growth factor-induced nuclear factor κB activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8542–8547. doi: 10.1073/pnas.97.15.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakshatri H., Bhat-Nakshatri P., Martin D.A., Goulet R.J., Jr., Sledge G.W., Jr. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su B., Su J., He H., Wu Y., Xia H., Zeng X., Dai W., Ai X., Ling H., Jiang H., Su Q. Identification of potential targets for diallyl disulfide in human gastric cancer MGC-803 cells using proteomics approaches. Oncol. Rep. 2015;33:2484–2494. doi: 10.3892/or.2015.3859. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y., Wang X., Zhou Q., Lu Y., Zhang H., Chen Q., Zhao M., Su S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015;33:338–346. doi: 10.3892/or.2014.3585. [DOI] [PubMed] [Google Scholar]
- 62.Chattopadhyay M., Kodela R., Nath N., Barsegian A., Boring D., Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-κB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 2012;83:723–732. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y.P., Zhu P.T., Wang Y.Y., Wei Z.H., Li T., Zhu Z.J., Sheng X.B., Wang S.L., Ruan J.S., Liu Z.G., Cao Y.Z., Shan Y.L., Sun L.H., Wang A.Y., Chen W.X., Lu Y. Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PloS One. 2015;10 doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S.H., Kaschula C.H., Priedigkeit N., Lee A.V., Singh S.V. Forkhead Box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J. Biol. Chem. 2016;291:13495–13508. doi: 10.1074/jbc.M116.715219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang J., Yang B., Xiang T., Peng W., Qiu Z., Wan J., Zhang L., Li H., Li H., Ren G. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the β-catenin signaling pathway. Mol. Nutr. Food Res. 2015;59:1063–1075. doi: 10.1002/mnfr.201400668. [DOI] [PubMed] [Google Scholar]
- 66.Xiong T., Liu X.W., Huang X.L., Xu X.F., Xie W.Q., Zhang S.J., Tu J., Tristetraprolin A novel target of diallyl disulfide that inhibits the progression of breast cancer. Oncol. Lett. 2018;15:7817–7827. doi: 10.3892/ol.2018.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasegawa U., Tateishi N., Uyama H., van der Vlies A.J. Hydrolysis-sensitive dithiolethione prodrug micelles. Macromol. Biosci. 2015;15:1512–1522. doi: 10.1002/mabi.201500156. [DOI] [PubMed] [Google Scholar]
- 68.Dong Q., Yang B., Han J.G., Zhang M.M., Liu W., Zhang X., Yu H.L., Liu Z.G., Zhang S.H., Li T., Wu D.D., Ji X.Y., Duan S.F. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Canc. Lett. 2019;455:60–72. doi: 10.1016/j.canlet.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 69.De Preter G., Deriemaeker C., Danhier P., Brisson L., Cao Pham T.T., Gregoire V., Jordan B.F., Sonveaux P., Gallez B. A fast hydrogen sulfide-releasing donor increases the tumor response to radiotherapy. Mol. Canc. Therapeut. 2016;15:154–161. doi: 10.1158/1535-7163.MCT-15-0691-T. [DOI] [PubMed] [Google Scholar]
- 70.Youness R.A., Assal R.A., Abdel Motaal A., Gad M.Z. A novel role of sONE/NOS3/NO signaling cascade in mediating hydrogen sulphide bilateral effects on triple negative breast cancer progression. Nitric Oxide. 2018;80:12–23. doi: 10.1016/j.niox.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J. Mol. Biol. 2017;429:543–561. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen W., Chen M., Zang Q., Wang L., Tang F., Han Y., Yang C., Deng L., Liu Y.N. NIR light controlled release of caged hydrogen sulfide based on upconversion nanoparticles. Chem. Commun. 2015;51:9193–9196. doi: 10.1039/c5cc02508g. [DOI] [PubMed] [Google Scholar]
- 73.Kang J., Li Z., Organ C.L., Park C.M., Yang C.T., Pacheco A., Wang D., Lefer D.J., Xian M. pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 2016;138:6336–6339. doi: 10.1021/jacs.6b01373. [DOI] [PubMed] [Google Scholar]
- 74.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y., Henthorn H.A., Pluth M.D. Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. J. Am. Chem. Soc. 2017;139:16365–16376. doi: 10.1021/jacs.7b09527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaur K., Carrazzone R.J., Matson J.B. The benefits of macromolecular/supramolecular approaches in hydrogen sulfide delivery: a review of polymeric and self-assembled hydrogen sulfide donors. Antioxidants Redox Signal. 2020;32:79–95. doi: 10.1089/ars.2019.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y., Yang F., Yuan C., Li M., Wang T., Chen B., Jin J., Zhao P., Tong J., Luo S., Gu N. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics. ACS Nano. 2017;11:1509–1519. doi: 10.1021/acsnano.6b06815. [DOI] [PubMed] [Google Scholar]