Highlights
-
•
Attention-deficit/hyperactivity disorder (ADHD) is one of the most frequent comorbid conditions of internet gaming disorder (IGD).
-
•
The NBS identified a network-level connectivity alteration in comorbid IGD and ADHD.
-
•
PLS regression reveals that the strength of the altered network predicts addiction severity in IGD patients with comorbid ADHD.
Keywords: Adhd, Igd, Diffusion tensor imaging, Brain structural connectivity, Fractional anisotropy, Partial least square regression
Abstract
Background
Internet gaming disorder (IGD) is commonly comorbid with attention-deficit/hyperactivity disorder (ADHD). Although the addiction is more severe when comorbid with ADHD, little is known about the neural correlates of the association. This study aimed to identify whether an ADHD-related structural brain network exists in IGD patients with comorbid ADHD (IGDADHD+) by comparing them with those without comorbid ADHD (IGDADHD-) and elucidating how the sub-network is associated with addiction severity.
Methods
Brain structural networks were constructed based on streamline tractography with diffusion tensor imaging in a cohort of 46 male IGDADHD+ patients, 48 male IGDADHD− patients, and 34 healthy controls (HC). We used network-based statistics (NBS) to identify the sub-network differences between the two IGD groups. Furthermore, the edges in the sub-network that significantly contributed to explaining the Young Internet Addiction Scale (YIAS) score were delineated using partial least square (PLS) regression analyses in IGD patients.
Results
The YIAS score was higher in the IGDADHD+ group than in the IGDADHD- group and was correlated with the Korean Dupaul's ADHD scale score (r = 0.42, p <0.01). The NBS detected a sub-network with stronger connectivity in the IGDADHD+ group than in the IGDADHD−group. The PLS regression model showed that the sub-network is associated with the YIAS score in the IGDADHD+ group (q2 = 0.019). Edges connecting the left pre- and postcentral gyri, bilateral superior frontal gyri, medial orbital parts, and left fusiform to the inferior temporal gyrus were most important predictors in the regression model.
Conclusion
Our results suggest that an aberrant increase in some structural connections within circuits related to inhibitory function or sensory integration can indicate how comorbid ADHD is associated with addiction severity in IGD.
1. Introduction
Internet gaming disorder (IGD), defined as the pathological use of internet gaming, is a rapidly emerging addictive disorder because of the increasing accessibility and use of digital technologies (Karaca et al., 2017, Chou et al., 2005). IGD has been included in section III of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), as a condition that requires further studies. IGD has been commonly associated with attention deficit/hyperactivity disorder (ADHD) (Association, 2013, Carli et al., 2013). ADHD and IGD share some core features such as impulsivity, seeking immediate reward, motivation deficit, and hostility (Yen et al., 2017–Ko et al., 2012). Moreover, significant associations have been found between the level of ADHD symptoms and the severity of internet addiction in children (Yoo et al., 2004). Previous studies indicated that the features of ADHD are not only a significant predictor of comorbidity but also a mediating factor for IGD (Yen et al., 2017). Thus, to prevent the possible mediating effect of ADHD on the severity of IGD, the underlying neurobiological mechanisms associated with the comorbidity itself and its contribution to the aggravation of IGD severity should be investigated (Ko et al., 2012).
In addition to the overlapping core symptoms, previous neuroimaging studies on IGD or ADHD have suggested the common involvement of some neural circuits. In IGD, altered structural connectivity in diffusion tensor imaging (DTI) studies revealed a broadly increased fractional anisotropy (FA) in white matter (WM) tracts within the forceps minor, anterior thalamic radiation, inferior fronto-occipital fasciculus, and inferior longitudinal fasciculus, which are the tracts linking the reward circuitry and the sensory and motor control systems (Dong et al., 2012–Jeong et al., 2016). ADHD is increasingly viewed as a disorder with disrupted wiring of large-scale brain networks during the neurodevelopmental process (Konrad and Eickhoff, 2010–Whelan et al., 2012). A recent meta-analysis found areas of increased FA in the cingulum, posterior corpus callosum, and left inferior fronto-occipital fasciculus, and decreased FA in the anterior cingulate, right orbitofrontal area, and left orbitofrontal area (Aoki et al., 2018). Network-based graph theoretical analysis also discovered that patients with both disease entities share a reduced global efficiency and differences in connectivity compared with healthy controls (Wen and Hsieh, 2016, Zhai et al., 2017, Cao et al., 2013).
In sum, comorbid ADHD leads to increase in the severity of behavioral addiction. Addictive behavior also leads to change in their brain and it may promote the alteration of structural network due to ADHD. Although previous neuroimaging studies have discovered sub-network alterations in ADHD (Hong et al., 2014, Beare et al., 2017, Cao et al., 2013) and the involved areas in ADHD overlap with the areas affected by IGD, no neuroimaging study has addressed the neural correlates regarding the ADHD-comorbidity and their association with the addiction severity.
To investigate, we hypothesized that 1) ADHD comorbidity-related network alteration may exist as a sub-network in the IGD patients with comorbid ADHD (IGDADHD+) when compared to those without comorbid ADHD (IGDADHD-) and 2) the delineated sub-network, named ‘ADHD-related’ network in the present study, would include edges that can explain variance of the addiction severity in IGD patient groups. In detail, the network-based statistic (NBS) method (Zalesky et al., 2010) was used to delineate the ‘ADHD-related’ network which is a cluster of edges that are spatially interconnected. Then, PLS regression analyses were performed to investigate the second hypothesis and determine which edges are important contributors. Specifically, we expect to determine whether the altered connections in the fronto-striatal area may also relate to the severity of addiction, since the two conditions share a common pathophysiology of reward processing and cue suppression (Frodl, 2010). Another candidate besides the fronto-striatal circuit is the sensory motor information processing area, because game-playing involves the cooperation of multiple systems, aberrant connections in these areas in ADHD may make patients more prone to develop internet addiction.
2. Materials and methods
2.1. Participants
Individuals with problematic online game play who visited the On-line Game Clinic Center at the Chung Ang University Hospital were recruited. The study population consisted of 94 young male, right-handed IGD patients of whom 46 had IGDADHD+ and 48 had IGDADHD−. In addition, 34 game time-matched healthy controls were recruited for validation purposes to determine if the sub-network is related to YIAS and K-ARS-P scores in healthy control group (see supplementary results section). Both IGDADHD+ and IGDADHD−groups were assessed for presence of ADHD and other psychiatric diagnoses using the Korean Kiddie Schedule for Affective Disorders and Schizophrenia – Present and Lifetime version (Kim et al., 2004). A child psychiatrist (D.H.H.) interviewed all adolescents to confirm the diagnosis of comorbidities (Ha et al., 2006). All participants completed the Young Internet Addiction Scale (YIAS) (Young, 1996), a questionnaire used to assess the severity of their addiction to online gaming. The parents or main caretakers completed the Dupaul's ADHD scale-Korean version (K-ARS-P) to assess the patients’ ADHD symptoms (So et al., 2002). Tobacco and alcohol use were categorized as follows: no use, occasional use (less than 5 cigarettes a day or drinking less than 5 days per month on average), and or regular/heavy use (more than 5 cigarettes per day or binge drinking on 5 or more days per month) (Abuse, 2015, Kenford et al., 2005).
To classify a participant as having IGD, we used the criteria that were employed in our previous studies (Jeong et al., 2016, Han et al., 2011, Han et al., 2009, Kim et al., 2012). Patients who fulfilled the criteria of: (Karaca et al., 2017) who spend excessive time playing online games (more than 4 h per day/30 h per week as assessed by the parents or the main caretakers); (Chou et al., 2005) with YIAS scores >50; (Association, 2013) who exhibit irritable, anxious, and aggressive behavior when asked to stop playing online games; and (Carli et al., 2013) with impaired behaviors or distress, economic crisis, and maladaptive regular life patterns including disrupted diurnal rhythms, refusal to attend school, and unemployment were included in the study. Individuals (Karaca et al., 2017) with other axis I psychiatric diseases, (Chou et al., 2005) taking psychiatric medications for online addiction, (Association, 2013) full-scale intelligence quotient (IQ) <80, (Carli et al., 2013) substance abuse history except for alcohol or tobacco use, (Yen et al., 2017) with neurological or medical disorders, and (Kim et al., 2017) with claustrophobia were excluded. The Chung Ang University Hospital Institutional Review Board approved this study. A written informed consent was provided by each participant. In the case of participants aged below 18 years, a written informed consent was provided by the parents or the main caregivers and an assent was obtained from the adolescents.
Independent t-tests were used to compare the demographic and clinical variables, including age, IQ, intracranial volume (ICV), YIAS, and K-ARS-P scores among the two groups. Since game playing time, tobacco use, and alcohol consumption were recorded in categories, a chi-square test was conducted to compare the groups. Analyses of Pearson's correlations between K-ARS-P and YIAS scores were also conducted. All statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp, Armonk, NY, USA) with the significance level set at p < 0.05.
2.2. Magnetic Resonance Imaging Acquisition and Analysis
2.2.1. Data acquisition, Preprocessing, and Quality assurance
A sequence similar to that used previously was adopted in this study (Jeong et al., 2016). Multiple diffusion-weighted images (DWI) with 32 encoding directions and an additional image without diffusion weighting (i.e., b = 0 s/mm2) were acquired using a Philips Achieva 3.0 Tesla TX magnetic resonance imaging (MRI) scanner (Philips, Eindhoven, the Netherlands), with a standard single-shot, spin echo, echo planar acquisition sequence with eddy current balanced diffusion-weighted gradient pulses (b = 600 s/mm2, echo time (TE)/repetition time (TR) = 70 ms/9214 ms; matrix = 124 × 121 on 250 mm × 250 mm field of view; slices 2 mm without gap resulting in voxels of 0.97 × 0.97 × 2.0 mm). Volumetric T1-weighted anatomic reference images were acquired using a three-dimensional T1-weighted magnetization-prepared rapid gradient echo sequence (TE/TR = 3.8 ms/2 s; 256 × 256 matrix for 1.0 × 1.0 × 1.0 mm voxels; 180 slices). Preprocessing for DTI analysis, including eddy current correction and head motion, was performed using eddy_correct of FMRIB Software Library (FSL; Oxford, UK; http://www.fmrib.ox.ac.uk/fsl), by registering each diffusion-weighted image to the first b = 0 image with affine transformation. When correcting, the Euclidean distance for each patient head motion was determined to compare between groups (Tromp, 2016). There are several ways to preprocess the DWI images, and we chose to use a protocol similar to that in the previous articles reporting structural network alteration in ADHD (Hong et al., 2014, Beare et al., 2017, Cao et al., 2013). Each of the participants also passed both visual and automated quality-assessment protocols for DTI, temporal signal-to-noise ratio (tSNR) (Roalf et al., 2016), using a quality control toolbox (DTIprep (Oguz et al., 2014)). With regard to tSNR, which is a method used to quickly screen the overall data quality, the participants’ lowest value was 9.0272, which is above the suggested cutoff value (6.47) for poor data. DTIPrep quality control report for all participants have passed default thresholds, assuring that our data meets reasonable quality. In addition, participants had low in-scanner head motion (<1.2 mm mean Euclidean distance for each patient motion).
2.2.2. Whole Brain Tractography and Network Construction
DTI data were reconstructed in the DSI Studio (www.dsi-studio.labsolver.org). Whole-brain tractography was performed using the Fiber Assignment by Continuous Tracking algorithm (Yeh et al., 2013). The complete procedure is described in the Supplementary Material. Briefly, using the automated anatomical labeling (AAL) template (Tzourio-Mazoyer et al., 2002) and every participant's diffusion weighted image, a connectivity matrix populated with the number of streamlines from the tractography connecting each pair of 116 nodes of AAL atlas was mapped for each participant (node names are provided in Table S1 in the Supplementary Material). We also acquired a connectivity matrix filled with tract-averaged FA values, extracted from streamline bundles by averaging the FA values over all voxels intersected by at least one streamline. Since the gold standard for regional parcellation is currently not available, we also acquired a connectivity matrix using Destrieux atlas in FreeSurfer (Destrieux et al., 2010, Fischl et al., 2004).
2.2.3. Network-based statistic using streamline count connectivity matrix
Network-based statistic (NBS) is an approach used for identifying connected sub-networks showing significant differences between groups. Since NBS identifies a cluster of edges that are spatially interconnected and forms a graph component, it offers greater power to detect diffuse, but connected, differences (Zalesky et al., 2010). This NBS method has popularly been used to identify abnormal brain connectivity circuitry such as in ADHD and IGD (Hong et al., 2014, Beare et al., 2017, Wen and Hsieh, 2016, Cao et al., 2013). We used the automated anatomical labeling (AAL) and additional Destrieux atlas template as nodes for acquiring the connectivity matrix, which is in line with previous research on network studies in IGD and ADHD (Hong et al., 2014, Beare et al., 2017, Cao et al., 2013, Tzourio-Mazoyer et al., 2002, Hong et al., 2013). A more specific description of the NBS procedure is provided in the Supplementary Material.
The two alternative hypotheses (IGDADHD+ having stronger interconnected sub-network than IGDADHD- and vice versa) were evaluated independently. Analyses were also performed using addiction severity (YIAS score) as an additional covariate since it differed significantly between the groups. Since we wanted to isolate the network related to comorbid ADHD, we chose to compare the two IGD groups rather than compare IGDADHD+ with HC as we cannot discriminate the effect of IGD and that of ADHD. All these steps were performed using the NBS software package (http://www.nitrc.org/projects/nbs/) implemented on MATLAB 2017b.
In addition, we checked whether the edges detected at the lower threshold, P < 0.05, survived at a higher threshold level, P < 0.03 and P < 0.01; to prevent confusion, we used an uppercase, italicized letter P to indicate the threshold P values. If the edges persisted, the detected sub-network was considered to be a network having robustness (Beare et al., 2017). The BrainNet viewer (http://www.nitrc.org/projects/bnv/) was used to visualize the significant sub-networks and to make the figures (Xia et al., 2013).
2.2.4. Relationship between the FA values of the sub-network and symptom severity
After detection of the existence of sub-network using NBS, we investigated whether it could predict the patient's symptom scores. We used the mean edge FA values for further regression analysis. The FA values were used since broad areas of white matter FA alteration in ADHD and IGD have been repeatedly observed (Buchanan et al., 2014, Jones et al., 2013, van Ewijk et al., 2012). Additionally, the FA value is a normalized (between 0 and 1) and continuous measure (Abe et al., 2010), whereas the streamline counts are integer values, and FA leads were used to avoid potential binning artifacts associated with an integer scale (Hong et al., 2014, Hong et al., 2015). The complete sample and the within group (IGDADHD+ and IGDADHD−) relations were examined.
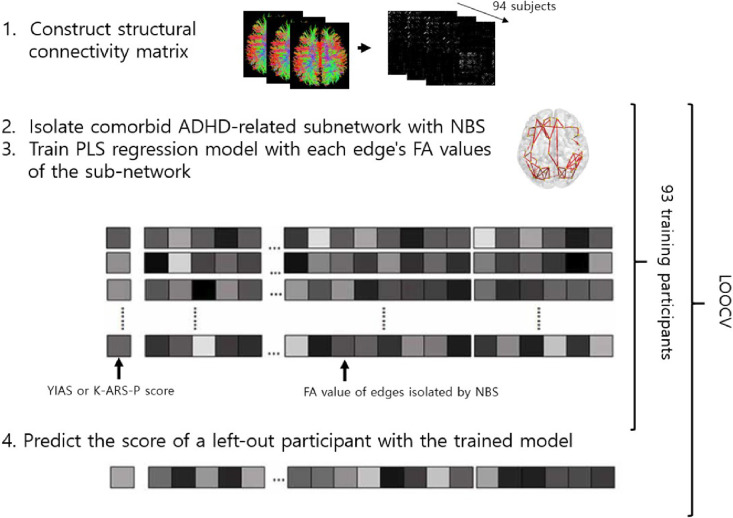
The partial least square (PLS) regression analysis, that linearly represents the independent variables with fewer components, was conducted to identify if the ADHD-related sub-networks can be used to predict and account for the severity of the symptom of the patients. We used the sub-networks identified at a threshold of P <0.05 as independent variables since they include all the edges identified at higher thresholds. We used the mean-centered K-ARS-P and YIAS scores as dependent variables and the tract-averaged FA values of every edge in the sub-networks as independent variables. To assure generalizability and avoid an over-fitted result of model performance, NBS and PLS regression models were constructed and tested using leave-one-out cross-validation (LOOCV) (Scheinost et al., 2019). A more detailed explanation of the PLS regression process is included in the Supplementary Material. The PLS regression analyses were also performed using the sub-network detected after adding addiction severity (YIAS score) as a covariate. We hypothesized that even though we controlled for the effect of YIAS in the streamline counts, the development status of the FA of the sub-network might still reflect the effect of addiction severity. The schematic summarization of the analysis process is shown in Fig. 1.
Fig. 1.
Schematic of the model performance validation procedure. During leave-one-out cross-validation (LOOCV), we first isolated the network of difference between IGDADHD+and IGDADHD−groups using NBS to elucidate a network related to comorbid ADHD. Next, FA values of each network edge were extracted and defined as predictors of the regression model. The PLS regression model was trained on data and tested with one left-out participant. The test was performed iteratively for all subjects (YIAS, Young Internet Addiction Scale; K-ARS-P, Dupaul's ADHD scale-Korean version).
3. Results
3.1. Demographics and clinical variables
Comparisons of the demographic variables are presented in Table 1. There was no significant difference in age, ICV, full-scale IQ, estimated motion, tobacco and alcohol usage, and game playing time reported by the participants. In accordance with a previous study (Yoo et al., 2004), the YIAS scores and K-ARS-P scores in the IGDADHD+ group were higher than those in the IGDADHD− group, and there was a significant correlation between the two scores in the complete sample and in the IGDADHD+ group (Pearson's r = 0.42, p <0.01 and Pearson's r = 0.33, p = 0.03, respectively, Figure S2 in Supplementary Material). Daily game playing time reported by the parents or the main caretakers was also higher in the IGDADHD+ group.
Table 1.
Demographic characteristics.
| IGDADHD+ (Tamm et al., 2012) | IGDADHD− (Li et al., 2010) | Healthy control (Yeh et al., 2013) | Statistics | Post hoc Tukey | |
|---|---|---|---|---|---|
| Age | 19.4 ± 3.9 | 21.1 ± 5.1 | 20.6 ± 4.1 | F = 1.832, p = 0.164 | |
| IQ | 105.8 ± 15.8 | 105.4 ± 13.7 | 105.8 ± 15.8 | F = 0.009, p = 0.991 | |
| ICV (mm3) | 1,385,850.9 ± 189,823.4 | 1,458,027.5 ± 139,577.3 | 1,394,009.0 ± 149,343.1 | F = 2.661, p = 0.074 | |
| YAIS | 68.3 ± 11.8 | 60.3 ± 6.6 | 26.6 ± 5.8 | F = 180.2, p < 0.001* | IGDADHD+> IGDADHD-> HC |
| K-ARS-P | 25.0 ± 9.9 | 11.4 ± 6.6 | 5.5 ± 5.4 | F = 69.7, p < 0.001* | IGDADHD+> IGDADHD-> HC |
| Euclidean distance | 0.376±0.22 | 0.314±0.14 | 0.376±0.22 | F = 1.879, p = 0.157 | |
| Game time/day† | χ2 = 6.22, p = 0.398 | ||||
| <2 h | 11 | 9 | 5 | ||
| 2–4 h | 16 | 22 | 17 | ||
| 4–8 h | 15 | 12 | 12 | ||
| >8 h | 4 | 5 | 0 | ||
| Game time/day ‡ | |||||
| 4–8 h | 12 | 24 | |||
| >8 h | 34 | 24 | |||
| Alcohol | χ2 = 1.83, p = 0.767 | ||||
| No use | 35 | 31 | 24 | ||
| Occasional use | 9 | 9 | 14 | ||
| Regular/heavy use | 2 | 3 | 1 | ||
| Tobacco | χ2 = 1.36, p = 0.851 | ||||
| No use | 31 | 34 | 26 | ||
| Occasional use | 11 | 11 | 7 | ||
| Regular/heavy use | 3 | 4 | 1 |
ICV: Intracranial volume; YIAS: Young Internet Addiction Scale; K-ARS-P: Korean Dupaul's ADHD scale, parents’ version, *next to the p-value indicates <0.05. †Game time reported by patients. ‡Game time reported by parents or main caretakers.
3.2. Sub-networks resulting from NBS
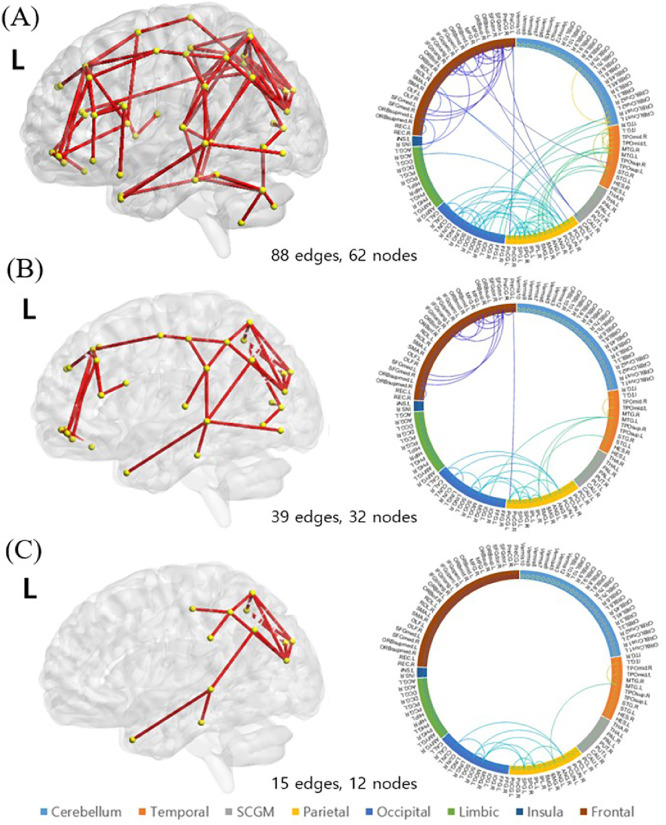
NBS yielded a sub-network showing significantly higher streamline counts in the IGDADHD+ than in the IGDADHD− group, namely, an ‘ADHD-related’ network. Summaries of the identified sub-networks with three levels of significance (FWE corrected (permutation) p = 0.001, 0.001, 0.002, respectively) are shown in Fig. 2. The sub-network at a threshold of P < 0.05 consisted of 88 edges, involving 62 different brain regions distributed in the frontal, parietal, and occipital areas (Fig. 2A). The opposite contrast was also tested, but no clusters reached a statistical significance after the FWE correction.
Fig. 2.
‘ADHD-related’ network. Affected structural connections in the IGDADHD+ group relative to the IGDADHD− participants under a series of probability thresholds ((A) at P < 0.05, (B) at P < 0.03, and (C) at P < 0.01). All clusters are a subset of the cluster identified at the previous threshold and can therefore be considered to be robust clusters. The corresponding circular plots of the network edges in (A) show that the edges are mainly connecting the frontal, parietal, and occipital regions. L indicates the left hemisphere.
In additional NBS analyses with the YIAS score as a covariate, we were still able to isolate a sub-network with a higher streamline count in the IGDADHD+ group with three levels of significance (FWE corrected (permutation) p = 0.006, 0.001, 0.018, respectively). In this case, we presume the ‘ADHD other than addiction’-related network (Figure S3 in the Supplementary Material). The remaining edges were distributed mostly in the parieto-occipital area, while most of the frontal and fronto-striatal connections regressed with addiction severity (Figure S3E and F).
For both the ‘ADHD-related’ network and the ‘ADHD other than addiction’-related network, the sub-networks at the higher thresholds were a subset of the edges detected at the lower P threshold; therefore, they can be called a “robust cluster.” The left occipital, temporal, and parietal connections were included in the relatively high threshold.
3.3. Relationship between the FA values of the sub-network and symptom severity
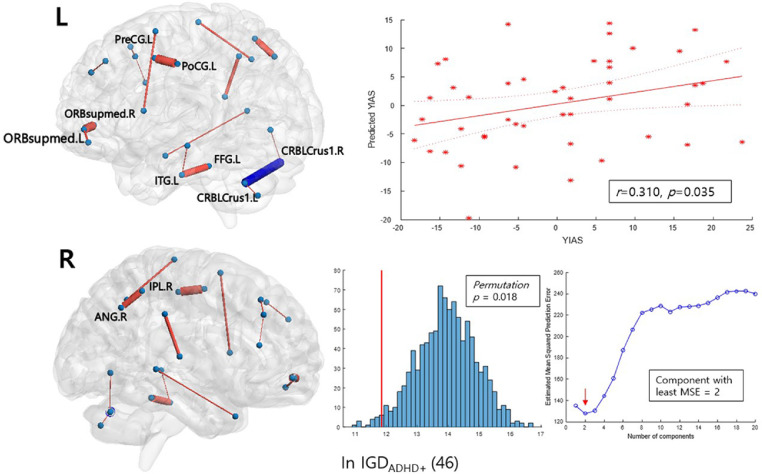
PLS regression revealed a significant linear relationship between the FA values of the edges in the sub-networks and the symptom scores. The results showed a significant prediction performance of the K-ARS-P score with the tract-averaged FA values of the ‘ADHD-related’ network in the entire sample of 94 patients but not within the IGDADHD+ or the IGDADHD−groups. With the FA values of the edges from NBS considered as independent variables, the regression analysis using 1 latent component explained 13.4% of the K-ARS-P score in a LOOCV (Table 2, first row and Figure S5A). When testing the PLS regression models explaining the YIAS scores, we did not found a positive association in the entire sample and within the IGDADHD− group. However, within the IGDADHD+ group, the ‘ADHD-related’ network was able to construct a model that significantly predicted the YIAS scores (Table 2, fifth row and Fig. 3). The regression model within the IGDADHD+ group with ‘ADHD-related’ network could explain around 1.9% of YIAS score variance in the IGDADHD+ group.
Table 2.
PLS regression modeling results with the K-ARS-P and YAIS scores as a function of the mean FA value of the ‘ADHD-related’ network edges.
| Pearson's Correlation | p-value | Predictive coefficient (q2) | permutation p | |
|---|---|---|---|---|
| K-ARS-P score | ||||
| Complete sample (94) | 0.368 | <0.001* | 0.134 | 0.001* |
| IGDADHD+ (Tamm et al., 2012) | −0.077 | 0.611 | −0.203 | 0.674 |
| IGDADHD- (Li et al., 2010) | −0.043 | 0.768 | −0.268 | 0.612 |
| YIAS score | ||||
| Complete sample (94) | 0.213 | 0.039* | −0.002 | 0.013* |
| IGDADHD+ (Tamm et al., 2012) | 0.310 | 0.035* | 0.019 | 0.018* |
| IGDADHD- (Li et al., 2010) | 0.207 | 0.156 | −0.224 | 0.077 |
p < 0.05.
Fig. 3.
Network edges predicting the YIAS score in the sub-network NBS analysis of the ‘ADHD-related’ network. The thickness of the edges corresponds to their mean coefficient in bootstrap resampling; the edges colored in red are those with a positive coefficient, while the blue edges have a negative coefficient. The mean and two standard errors for each edge are plotted in Figure S4C, in the Supplementary Material. L and R indicate the left and right brain sides, respectively. The scatter plots show a correlation between the mean-centered scores of real and the predicted scores from PLS regression and their fitted lines with 95% confidence intervals. The histograms show the performance of the PLSR model tested by comparing the models trained on the shuffled data with 1000 iterations. The red line represents the root mean squared error of prediction (RMSEP) of the original model. For each iteration, the RMSEP between the shuffled score and the predicted score trained on the shuffled data was calculated. The median RMSEP plot obtained from bootstrap LOOCV process denotes the number of the component we chose for the construction of the PLS model. PLS regression predicting the K-ARS-P score within the IGDADHD+ group did not yield a significant model.
Significantly contributing edges were detected using bootstrap resampling to estimate the coefficient p-values. When predicting YIAS scores in the IGDADHD+ group with the ‘ADHD-related’ network, 15 edges distributed in both cerebral hemispheres were found to have positive coefficients. The significant edges included the orbitofrontal, temporal, parietal, and occipital nodes. One edge connecting the bilateral cerebellar crus 1 was found to have a negative coefficient (Fig. 3).
We tested the same PLS regression analysis using the ‘ADHD other than addiction’-related network as a predictive variable and obtained similar results. Detailed results are shown in the Supplementary Material result section (Table S2 and Figure S5).
4. Discussion
4.1. ADHD-related network present in the IGDADHD+ group
This study examined the structural brain network differences between the IGDADHD+ group and IGDADHD−group and its association to addiction severity. Following the previously reported method of NBS, we isolated a particular sub-network of higher strength in the IGDADHD+ group: an ‘ADHD-related’ network. The sub-network encompasses the frontal, parietal and occipital area (Fig. 2). The PLS regression model predicted the K-ARS-P score based on the sub-network (Table 2, first row and Figure S5A). However, unlike previous studies comparing ADHD patients with healthy controls (Hong et al., 2014, Beare et al., 2017, Cao et al., 2013), wherein addiction was not a concern, we discovered that the IGDADHD+ group showed a significantly higher YIAS score. This may imply that the ‘ADHD-related’ network results not only from the ADHD diagnosis per se, but also from the difference in the addiction symptoms. This led us to run additional NBS analyses with the YIAS scores added as a covariate. We were still able to isolate a sub-network of a higher streamline count in the IGDADHD+ group, namely, an ‘ADHD other than addiction’-related network (Figure S3). In this case, we detected a sub-network with a distribution connecting the parieto-occipital regions, while the fronto-striatal connections covaried with the YIAS score (Figure S3E and F). PLS regression used to predict the K-ARS-P score from the ‘ADHD other than addiction’-related network also yielded a significant PLS model (Table S2, first and second rows).
Two studies have used this NBS approach to examine the alteration in structural connectivity in male-only cohorts of ADHD patients against healthy controls (Beare et al., 2017, Cao et al., 2013). Using multiple tractography schemes, Beare et al. reported a constantly detected sub-network of stronger connectivity in ADHD encompassing the entire brain including the fronto-striatal connections as well as the left occipital, temporal, and parietal regions (Beare et al., 2017). In addition, Cao et al. reported an increase in a sub-network primarily involving the orbitofrontal-striatal circuitry and the posterior regions of the right hemisphere (Cao et al., 2013). Although there may be a subtle discordance between these studies since ADHD is a heterogeneous disorder with interpersonal differences, the distribution of the ‘ADHD-related’ network more closely resembles that of previously reported sub-networks than the ‘ADHD other than addiction’-related network. This implies that previously reported sub-networks contain addictive symptom domains of ADHD and that the fronto-striatal connections in the ‘ADHD-related’ network are also reflective of addiction severity. This is expected from our hypothesis discussed in the literature review that the overlapping areas of increased connection between ADHD and addiction include the fronto-striatal region, where both disorders overlap with regard to impaired reward processing (Frodl, 2010, Tamm et al., 2012).
4.2. ADHD-related networks predict addiction severity in the IGDADHD+group
Similar to the reports of a previous study (Yoo et al., 2004), significant positive correlations were observed between the K-ARS-P score and YIAS score of our study population. After performing a within-group analysis, the significant correlation was only observed between the two scores of the IGDADHD+ group (Figure S2). This may imply that there is a symptomatic association between IGD and ADHD and that the association is more distinct in people diagnosed with ADHD. In the next step, we identified that a patient's addiction severity can be explained by the ‘ADHD-related’ network's edge-wise FA values. This was conducted to determine whether and how FA alteration from ADHD contributes to addiction severity.
The PLS regression result partially supported this idea. When we used PLS regression to predict YIAS scores from the edge-wise FA value of the ‘ADHD-related’ network, the regression was able to predict scores only within the IGDADHD+group, implying an association between the microstructural properties of the sub-networks and addiction severity in the IGDADHD+group. Therefore, the FA value of the ‘ADHD-related’ network is useful only in predicting YIAS score within the IGDADHD+group. This result suggests that the well-known close relationship between ADHD and IGD is partly explained by the microstructural integrity of the ‘ADHD-related’ network. Further studies should investigate whether ADHD is related to other types of addiction, using neuroimaging studies.
4.3. Anatomical distribution of edges with a significant PLS regression coefficient
In the PLS regression analysis conducted to predict the YIAS score from the ‘ADHD-related’ network, 16 edges with a significant contribution were detected, that is, 15 with positive coefficient edges in both cerebral hemispheres and one edge in the cerebellum with a negative coefficient (Fig. 3). The edges with the highest coefficients connect the left precentral–postcentral gyri, left superior frontal gyrus, medial orbital–right left superior frontal gyrus, medial orbital, left fusiform gyrus–inferior temporal gyrus, right inferior parietal lobule–right angular gyrus, and right supramarginal gyrus–right superior temporal gyrus.
The edges connecting the bilateral frontal medial orbital nodes pass through the anterior frontal white matter. Several studies reported an increased white matter FA in these areas in ADHD patients compared with healthy controls (Tamm et al., 2012–Li et al., 2010). Functional MRI studies reported dysfunctional ventrolateral and medial prefrontal activation and functional connectivity from the inferior and superior frontal gyri, potentially related to a lack of inhibitory control and impulsiveness in ADHD (Sebastian et al., 2014, van Rooij et al., 2015). Deficits in inhibitory response control are also suggested to contribute to the development of uncontrolled Internet game usage (Dong and Potenza, 2014). As greater FA values observed in neurodevelopmental disorders may represent inappropriate connections, such as abnormal reduction in the degree of neuronal branching (Hoeft et al., 2007), although direct evidence is lacking in this study, we carefully suggest that the impaired appropriate networking status of the orbitofrontal cortices may cause aberrant inhibitory function and contribute to addiction severity. This should be further investigated in future studies using tasks and graph theoretical analysis.
Somewhat unexpectedly, many significantly contributing edges were in the bilateral temporal and parietal areas. The significant edges including the fusiform gyrus and inferior temporal gyrus play a role in visual information processing, and the angular gyrus is involved in sensory integration (Seghier, 2013, Weiner and Zilles, 2016, Miyashita, 1993). Alteration of the FA in these areas may be related to the aberrant processing of visual information in ADHD patients (Peterson et al., 2011). Abnormally enhanced connection of tracts linking visual, auditory, and working memory is also a repeatedly found phenotype in IGD patients, since internet video game playing requires an active working memory system, including visual and auditory attention (Dong et al., 2018, Jeong et al., 2016). Although we should be aware of reverse inference (Poldrack, 2006), our results suggest that the edges included in this PLS regression model, in this respect, associate ADHD with addiction severity by aberrant visual/spatial and motoric processing. One edge presented a negative coefficient, which connects the bilateral cerebellar crus 1. In a previous study comparing the functional connectivity between IGD group with ADHD and IGD group without ADHD, the comorbid group showed an expanded connectivity between the posterior cingulate and cerebellum (Carpenter et al., 2017). Repeated detection of the cerebellum in connectivity studies implies that we should pay attention to the role of the cerebellum in ADHD and addiction.
5. Study Limitations
This study has several limitations. First, the set of patients included in the present study was a single-center and male-only sample seeking treatment for IGD; although IGD diagnosis in this study is stricter, it was not solely based on the DSM-5 diagnostic criteria for IGD. This may result in a generalization problem. Second, the cross-sectional design of the study cannot fully differentiate the causes and effects of ADHD in IGD. Since we did not include a cohort with ADHD without IGD (ADHDIGD−), we cannot completely differentiate the connections in the ‘ADHD-related’ network that are fully caused by ADHD or by differences in addiction severity. Moreover, the IGDADHD+ and IGDADHD− groups differ in IGD severity; thus, the networks identified as differentiating groups maybe also differed due to the severity of IGD (Kriegeskorte et al., 2009). We included a series of analyses in the Supplementary Material to show that the ADHD-related network and IGD severity are not associated by chance or circularity; however, further studies comparing the IGDADHD+ group with the ADHDIGD− group is needed. Third, some patients were adolescents and therefore the prematurity of the brain may cause inappropriate registration, even though age and ICV were used as covariates. Fourth, since the K-ARS-P questionnaire in this study is not significantly explained within groups and had low effect size compared with the PLS regression analysis used in previous studies (Meskaldji et al., 2016), it may suggest that the ADHD symptoms observed by the patient's parents did not solely successfully reflect the severity of addiction. Fifth, there is a need to use another high-resolution atlas and other tracking methods such as probabilistic tractography, since the graphic metrics of whole-brain networks can be different with a spatial scale of nodes (Zalesky et al., 2010). Sixth, quantified smoking and drinking history was not available and could not be matched, which can affect tract FA values.
6. Conclusions
There are two main findings and a clinical implication of this study. First, we identified the existence of a sub-network related to ADHD in IGDADHD+ patients by comparing the IGDADHD+group with the IGDADHD−group. The discovered sub-network is in line with that reported in previous studies and thought to be a phenotype of comorbid ADHD. Second, using edge-wise FA values in the ‘ADHD-related’ network as predictor variables, we were able to partly explain the IGD addiction severity of each patient in the IGDADHD+ group. The involved edges connect brain areas whose FA values relate to the altered inhibitory function of ADHD, as well as the aberrant visual/spatial integration and motoric processing of IGD. The results give a neurobiological underpinning that structural network alteration in comorbid ADHD is associated with severity of behavioral addiction. This finding serves as a basis for clinicians to understand and explain the importance of comorbidity when treating or evaluating patients with internet gaming addiction or ADHD. Although the effect size was small, our result justifies that treating ADHD may simultaneously help improve addiction.
Authors Contribution
DHH and SB recruited and evaluated patients and obtained the diffusion image; MK and DK preprocessed the diffusion image; MK designed the research and performed the statistical analysis; MK, DK, and BJ wrote the manuscript.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest relevant to this article.
Acknowledgments and Disclosures
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT (NRF-2016M3C7A1914448 and NRF-2017M3C7A1031331). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102263.
Contributor Information
Doug Hyun Han, Email: hduk70@gmail.com.
Bumseok Jeong, Email: bs.jeong@kaist.ac.kr.
Appendix. Supplementary materials
References
- Abe O, Takao H, Gonoi W, Sasaki H, Murakami M, Kabasawa H. Voxel-based analysis of the diffusion tensor. Neuroradiology. 2010;52(8):699–710. doi: 10.1007/s00234-010-0716-3. [DOI] [PubMed] [Google Scholar]
- Abuse S. Mental Health Services Administration (SAMHSA).(2015). 2015 National Survey on Drug Use and Health (NSDUH). Table 2.46 B—Alcohol Use, Binge Alcohol Use, and Heavy Alcohol Use in Past Month among Persons Aged 12 or Older, by Demographic Characteristics: Percentages, 2014 and 2015.
- Aoki Y, Cortese S, Castellanos FX. Research Review: Diffusion tensor imaging studies of attention‐deficit/hyperactivity disorder: meta‐analyses and reflections on head motion. Journal of Child Psychology and Psychiatry. 2018;59(3):193–202. doi: 10.1111/jcpp.12778. [DOI] [PubMed] [Google Scholar]
- Association AP. American Psychiatric Pub; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [DOI] [PubMed] [Google Scholar]
- Beare R, Adamson C, Bellgrove MA, Vilgis V, Vance A, Seal ML. Altered structural connectivity in ADHD: a network based analysis. Brain imaging and behavior. 2017;11(3):846–858. doi: 10.1007/s11682-016-9559-9. [DOI] [PubMed] [Google Scholar]
- Buchanan CR, Pernet CR, Gorgolewski KJ, Storkey AJ, Bastin ME. Test–retest reliability of structural brain networks from diffusion MRI. Neuroimage. 2014;86:231–243. doi: 10.1016/j.neuroimage.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Cao Q, Shu N, An L, Wang P, Sun L, Xia M-R. Probabilistic diffusion tractography and graph theory analysis reveal abnormal white matter structural connectivity networks in drug-naive boys with attention deficit/hyperactivity disorder. Journal of Neuroscience. 2013;33(26):676–687. doi: 10.1523/JNEUROSCI.4793-12.2013. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli V, Durkee T, Wasserman D, Hadlaczky G, Despalins R, Kramarz E. The association between pathological internet use and comorbid psychopathology: a systematic review. Psychopathology. 2013;46(1):1–13. doi: 10.1159/000337971. [DOI] [PubMed] [Google Scholar]
- Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M. Stan: A probabilistic programming language. Journal of statistical software. 2017;76(1) doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, Condron L, Belland JC. A review of the research on Internet addiction. Educational Psychology Review. 2005;17(4):363–388. [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, DeVito E, Huang J, Du X. Diffusion tensor imaging reveals thalamus and posterior cingulate cortex abnormalities in internet gaming addicts. Journal of psychiatric research. 2012;46(9):1212–1216. doi: 10.1016/j.jpsychires.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Potenza MN. A cognitive-behavioral model of Internet gaming disorder: theoretical underpinnings and clinical implications. Journal of psychiatric research. 2014;58:7–11. doi: 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Wu L, Wang Z, Wang Y, Du X, Potenza MN. Diffusion-weighted MRI measures suggest increased white-matter integrity in Internet gaming disorder: evidence from the comparison with recreational Internet game users. Addictive behaviors. 2018;81:32–38. doi: 10.1016/j.addbeh.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH. Automatically parcellating the human cerebral cortex. Cerebral cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frodl T. Comorbidity of ADHD and substance use disorder (SUD): a neuroimaging perspective. Journal of attention disorders. 2010;14(2):109–120. doi: 10.1177/1087054710365054. [DOI] [PubMed] [Google Scholar]
- Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for Internet addiction. The Journal of clinical psychiatry. 2006 doi: 10.4088/jcp.v67n0517. [DOI] [PubMed] [Google Scholar]
- Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. 2011. [DOI] [PubMed]
- Han DH, Lee YS, Na C, Ahn JY, Chung US, Daniels MA. The effect of methylphenidate on Internet video game play in children with attention-deficit/hyperactivity disorder. Comprehensive psychiatry. 2009;50(3):251–256. doi: 10.1016/j.comppsych.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience. 2007;27(44):960–965. doi: 10.1523/JNEUROSCI.3591-07.2007. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-B, Zalesky A, Cocchi L, Fornito A, Choi E-J, Kim H-H. Decreased functional brain connectivity in adolescents with internet addiction. PloS one. 2013;8(2):e57831. doi: 10.1371/journal.pone.0057831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-B, Zalesky A, Fornito A, Park S, Yang Y-H, Park M-H. Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biological psychiatry. 2014;76(8):656–663. doi: 10.1016/j.biopsych.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Park S, Yang YH, Park MH, Kim B. COMT genotype affects brain white matter pathways in attention‐deficit/hyperactivity disorder. Human brain mapping. 2015;36(1):367–377. doi: 10.1002/hbm.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong BS, Han DH, Kim SM, Lee SW, Renshaw PF. White matter connectivity and Internet gaming disorder. Addiction biology. 2016;21(3):732–742. doi: 10.1111/adb.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Karaca S, Saleh A, Canan F, Potenza MN. Comorbidity between behavioral addictions and attention deficit/hyperactivity disorder: a systematic review. International Journal of Mental Health and Addiction. 2017;15(3):701–724. [Google Scholar]
- Kenford SL, Wetter DW, Welsch SK, Smith SS, Fiore MC, Baker TB. Progression of college-age cigarette samplers: what influences outcome. Addictive behaviors. 2005;30(2):285–294. doi: 10.1016/j.addbeh.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee D, Lee J, Namkoong K, Jung Y-C. Association between childhood and adult attention deficit hyperactivity disorder symptoms in Korean young adults with Internet addiction. Journal of behavioral addictions. 2017;6(3):345–353. doi: 10.1556/2006.6.2017.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Han DH, Lee YS, Renshaw PF. Combined cognitive behavioral therapy and bupropion for the treatment of problematic on-line game play in adolescents with major depressive disorder. Computers in Human Behavior. 2012;28(5):1954–1959. [Google Scholar]
- Kim YS, Cheon KA, Kim BN, Chang SA, Yoo HJ, Kim JW. The reliability and validity of kiddie-schedule for affective disorders and schizophrenia-present and lifetime version-Korean version (K-SADS-PL-K) Yonsei Medical Journal. 2004;45(1):81–89. doi: 10.3349/ymj.2004.45.1.81. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Yen J-Y, Yen C-F, Chen C-S, Chen C-C. The association between Internet addiction and psychiatric disorder: a review of the literature. European Psychiatry. 2012;27(1):1–8. doi: 10.1016/j.eurpsy.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human brain mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature neuroscience. 2009;12(5):535. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X. Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Neuroendocrinology Letters. 2010;31(6):747. [PubMed] [Google Scholar]
- Meskaldji D-E, Preti MG, Bolton TA, Montandon M-L, Rodriguez C, Morgenthaler S. Prediction of long-term memory scores in MCI based on resting-state fMRI. NeuroImage: Clinical. 2016;12:785–795. doi: 10.1016/j.nicl.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annual review of neuroscience. 1993;16(1):245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G. DTIPrep: quality control of diffusion-weighted images. Frontiers in neuroinformatics. 2014;8:4. doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DJ, Ryan M, Rimrodt SL, Cutting LE, Denckla MB, Kaufmann WE. Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD) Journal of child neurology. 2011;26(10):1296–1302. doi: 10.1177/0883073811405662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in cognitive sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Roalf DR., Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Noble S, Horien C, Greene AS, Lake EM, Salehi M. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage. 2019 doi: 10.1016/j.neuroimage.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Krause-Utz A, Lieb K, Schmahl C, Tüscher O. Frontal dysfunctions of impulse control–a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Frontiers in human neuroscience. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean parent and teacher ADHD rating scale. Journal of Korean Neuropsychiatric Association. 2002;41(2):283–289. [Google Scholar]
- Tamm L, Barnea-Goraly N, Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. 2012;202(2):150–154. doi: 10.1016/j.pscychresns.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp D. A guide to quantifying head motion in DTI studies. The Winnower. 2016;6 88496. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2012;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- van Rooij D, Hartman CA, Mennes M, Oosterlaan J, Franke B, Rommelse N. Altered neural connectivity during response inhibition in adolescents with attention-deficit/hyperactivity disorder and their unaffected siblings. Neuroimage: clinical. 2015;7:325–335. doi: 10.1016/j.nicl.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62. doi: 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Hsieh S. Network-based analysis reveals functional connectivity related to internet addiction tendency. Frontiers in human neuroscience. 2016;10:6. doi: 10.3389/fnhum.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline J-B, Lourdusamy A, Banaschewski T, Barker GJ. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature neuroscience. 2012;15(6):920. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS one. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS one. 2013;8(11):e80713. doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J-Y, Liu T-L, Wang P-W, Chen C-S, Yen C-F, Ko C-H. Association between Internet gaming disorder and adult attention deficit and hyperactivity disorder and their correlates: Impulsivity and hostility. Addictive behaviors. 2017;64:308–313. doi: 10.1016/j.addbeh.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Cho SC, Ha J, Yune SK, Kim SJ, Hwang J. Attention deficit hyperactivity symptoms and internet addiction. Psychiatry and clinical neurosciences. 2004;58(5):487–494. doi: 10.1111/j.1440-1819.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- Young KS. Psychology of computer use: XL. Addictive use of the Internet: a case that breaks the stereotype. Psychological reports. 1996;79(3):899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yücel M, Pantelis C. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50(3):970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Zhai J, Luo L, Qiu L, Kang Y, Liu B, Yu D. The topological organization of white matter network in internet gaming disorder individuals. Brain imaging and behavior. 2017;11(6):1769–1778. doi: 10.1007/s11682-016-9652-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.